Abstract
Intravenous (iv) infusion of high-dose ascorbic acid (AA) has been used as a treatment for cancer patients. The tumoricidal action of AA occurs due to its prooxidant effect. Erythorbic acid (EA), one of the AA epimers, has reduced vitamin C activity, while the antioxidant activity of EA is similar to that of AA. Currently, other physiological and pharmacological functions of EA are not well known. We examined the cytotoxicity of EA to murine colon carcinoma (colon-26) cells and the antitumor activity of EA in tumor-bearing mice. Cytotoxic activity of EA to colon-26 cells was evaluated by using the calcein-AM assay. EA showed the same cytotoxic activity to colon-26 cells as that of AA. The cytotoxicity of EA was shown to be caused by oxidative stress. Next, colon-26 tumor-bearing mice were iv administered EA and AA on alternate days for 4 times, and tumor growth rates were measured. Tumor growth was significantly inhibited by administration of high-dose EA in vivo as well as AA. Finally, the in vivo biodistribution and clearance of EA and AA were investigated in tumor-bearing mice. Endogenous AA in the tumor was consumed to resist oxidative stress caused by reactive oxygen species that was generated by administered EA. These results indicated that the oxidative stress-mediated antitumor activity is one of the pharmacological functions of high-dose iv EA.
Keywords: Cancer, Isoascorbic acid, Reactive oxygen species, Colon-26, Xenograft models
Highlights
-
•
High dose erythobic acid showed significant cytotoxicity to colon-26 cells.
-
•
Tumor growth was inhibited by administration of high-dose erythorbic acid in vivo.
-
•
High-dose iv erythorbic acid showed the oxidative stress-mediated antitumor activity.
-
•
The antitumor activities of erythorbic acid were the same as those of ascorbic acid.
-
•
Erythorbic acid can be used as an agent in infusion therapy for cancer.
1. Introduction
Ascorbic acid (AA, Fig. 1A) is a water-soluble compound known as vitamin C, and it is common knowledge that AA is an essential micronutrient for humans. AA shows various physiological and pharmacological activities, including collagen synthesis [1], drug metabolism [2], enhancing iron absorption [3] and antioxidant activity [4]. A number of recent publications report the prooxidant effects of antioxidants (e.g., resveratrol, quercetin, and curcumin) at high concentrations [5], [6], [7]. In the presence of transition metal ions such as iron and copper, high-concentration AA can exert prooxidant effects on a tumor, and it has been used by some practitioners as an intravenous (iv) infusion to treat cancer [8], [9]. Intravenous infusion of high-dose AA temporarily increases the plasma concentration of AA in patients to a level 100–1000-times than that of healthy levels (from 50–100 μM to 25–30 mM) [10], [11], while the upper limit value of oral administration was predicted to be approximately 220 μM [12]. Pharmacologic AA acts as a pro-oxidant agent. AA reduces ferric iron (Fe3+) to ferrous iron (Fe2+), and Fe2+ acts as an electron donor to O2, ultimately generating H2O2 in plasma and extracellular fluid. H2O2 then interacts with another transition metal to generate ROS, including the highly reactive hydroxyl radical [11], [13], [14], [15]. In normal cells that have antioxidant enzymes such as catalase and glutathione peroxidase, ROS are immediately detoxified [14]. On the other hand, many cancer cells have low levels of several antioxidant enzymes [16]. The mitochondria in many cancer cells may have increased sensitivity to ROS. Therefore, mitochondria of cancer cells that have low anti-oxidant capacity are damaged by ROS [14], resulting in cell death due to a decrease in ATP production [14], [17], [18]. High-dose iv AA therapy exhibits a cytotoxic activity selectively to cancer cells and has fewer adverse effects than other cancer treatments.
Fig. 1.
Structures and oxidative stress-induced cytotoxicity of AA and EA. (A) Structures of AA and EA. (B) In vitro cytotoxicity of EA and AA. Colon-26 cells were incubated in a medium containing EA or AA at indicated concentrations for 24 h. Vehicle-treated cells were arbitrarily set as 100% control viability. (C)–(E) Amount of ROS generated by EA or AA in colon-26 cells. They were then treated with EA or AA (2 mM) for indicated times (C, 15 min; D, 30 min; E, 60 min). Results are expressed as percentage (%) relative to each control value. All data represent means ± SD of three independent cultures (*P<0.05; **P<0.01, compared to control).
Erythorbic acid (EA, Fig. 1A), a stereoisomer of AA, only differs from AA in the relative position of the hydrogen and hydroxyl groups on the fifth carbon atom. EA shows chemical properties similar to those of AA; however, the antiscorbutic activity of EA has been reported to be about one-twentieth of that of AA in vivo [19] and EA has only one-eighth of the activity of AA for stimulating collagen synthesis in vitro [20] because of the difference in quantity of intracellular uptake. It is believed that EA has a negligible vitamin C activity. On the other hand, EA has the same level of activity as that of AA for stimulating proline hydroxylation reaction [20]. Also, EA administration enhances iron absorption from Fe2+ sulfate more than does AA [21]. The antioxidant activity of EA is similar to that of AA, and EA has been widely used as an antioxidant in many processed foods [19], [21]. However, little is known about EA beyond these physiological functions.
Since EA has antioxidant activity similar to that of AA, it can be expected that high-dose iv EA will also act as a prooxidant like AA. However, there have been few studies in which the anticancer activity of high-dose iv EA was investigated. Administration of a relatively low dose of AA or EA intraperitoneally to a human mammary tumor xenograft mouse was reported not to inhibit tumor growth. However, when cupric sulfate was added to the injection fluid, tumor growth was depressed [22]. The aim of the present study was to find a new pharmacological function of EA. For this purpose, we assessed the antitumor activity of high-dose iv EA to murine colon carcinoma cells (colon-26) and cancer model mice. We also investigated the in vivo biodistribution and clearance of EA in tumor-bearing mice.
2. Materials and methods
2.1. Chemicals
Sodium erythorbate monohydrate (EA-Na·H2O) was obtained from Tokyo Chemical Industries (Tokyo, Japan). Sodium ascorbate (AA-Na), heparin, calcein-AM solution and 2′,7′-dichlorodihydrofluorescein diacetate (DCFH-DA) were purchased from Wako Pure Chemical Industries (Osaka, Japan). RPMI 1640 medium was purchased from Corning (NY, USA). Fetal bovine serum (FBS, heat-inactivated) was from HyClone (Logan, UT, USA). Triton X-100 was from Sigma (St. Louis, MO, USA). Dithiothreitol (DTT) was obtained from Nacalai tesque (Kyoto, Japan).
2.2. Cell lines
Murine colon carcinoma (colon-26) cells were purchased from RIKEN BRC CELL BANK (Tsukuba, Japan). Colon-26 cells were grown in RPMI 1640 with 10% FBS, 100 units/mL penicillin and 100 μg/mL streptomycin at 37 °C in 5% CO2. Experiments were performed when cell growth was approximately 80% confluent.
2.3. Evaluation of in vitro cytotoxicity of EA
Cytotoxicity of EA to colon-26 cells was assessed using calcein-AM. The cells were suspended in RPMI 1640 medium and seeded in a 96-well microplate at a density of 1.0×104 cells/100 μL/well and then incubated for 24 h at 37 °C in 5% CO2. After incubation, each medium was replaced with 90 μL of fresh medium and then 10 μL each of AA-Na and EA-Na were added and the cells incubated for 24 h. The cells were then washed with 100 μL of PBS(-) and added 100 μL of calcein-AM solution (5 μM). After 30 min of incubation, 20 μL of 0.6% Triton X-100 solution was added to each well. The fluorescence intensity (FI) of the cell lysate was recorded on a microplate reader (Varioskan Flash from Thermo Scientific, Ex. 485 nm, Em. 527 nm). Cell viability (%) was caluculated as (FI of treated-FI of blank )/(FI of control-FI of blank)×100. The difference in cell viability between the control and treatment was analyzed by Dunnett’s test (**P<0.01).
2.4. DCFH-DA assay
The relative amount of reactive oxygen species (ROS) to the control value was assessed by using DCFH-DA. Colon-26 cells were suspended in RPMI 1640 medium and seeded in a 96-well microplate at a density of 1.0×104 cells/90 μL/well and then incubated for 24 h at 37 °C in 5% CO2. After incubation, 10 μL of DCFH-DA (5 μM) was added to each well and the cells were incubated for 20 min. The cells were then washed with 100 μL of fresh medium. The medium was replaced by 100 μL of AA-Na or EA-Na solution (2 mM) and the cells were incubated for the indicated periods. The cells were sonicated for several seconds with 100 μL of 0.1% Triton X-100. Fluorescence intensity of the cell lysate was measured by Varioskan Flash (Ex. 485 nm, Em. 527 nm). The difference in fluorescence intensity between the control and treatment was analyzed by Dunnett’s test (*P<0.05, **P<0.01).
2.5. Evaluation of in vivo antitumor activity of EA
The antiproliferative activity of EA was investigated in colon-26 xenograft mice. Colon-26 cells were implanted at a density of 1.0×106 cells in five-week-old female Balb/c mice (CLEA Japan, Tokyo, Japan) subcutaneously in the left and right dorsal areas. When transplanted tumor sizes were more than 10 mm in diameter, the tumors were excised and small pieces of the tumor (approximately 2-mm cubes) were engrafted subcutaneously into the left dorsal area of each five-week-old male CDF1 mice (Japan SLC, Shizuoka, Japan). When tumor sizes had reached 8–10 mm in diameter after implantation, the mice were used for studies of antitumor activity, biodistribution and clearance of EA-Na and AA-Na. EA and AA samples were dissolved in PBS (equimolecular amount of 300 mg/kg body weight of AA) was injected intravenously into colon-26 tumor-bearing mice on alternate days for 4 times. Tumor size was calculated from caliper measurements using volume=(length)×(width)2×0.5. The differences in tumor size between the treatment groups and control group were analyzed by Dunnett’s test (*P<0.05, **P<0.01). The experiments were approved by the Committee for Ethics in Animal Experiments of the Prefectural University of Hiroshima.
2.6. In vivo biodistribution and clearance of EA
EA and AA were injected intravenously into tumor-bearing CDF1 mice. The mice were sacrificed under anesthesia by isoflurane. The liver, kidney, tumor and blood were collected at 15, 30, 60, 180 and 360 min (n=4) after injection. A blood sample was collected by heart puncture into a heparin-coated syringe. Plasma was separated from whole blood by centrifugation at 10,000g for 10 min at 4 °C. The samples were stored at −80 °C until analysis. Samples were homogenized with 85% acetonitrile including 250 mg/L of DTT (100 mg wet tissue or 100 μL plasma/400 μL solution). Then all samples were centrifuged at 10,000g for 10 min at 4 °C and the supernatants were collected. The supernatants were analyzed by HPLC. Separation for EA and AA was achieved by isocratic elution of an HILIC column (ϕ4.6×250 mm, 5 μm, GL Sciences Inc., Tokyo) kept at 40 °C with 85% acetonitrile/66.7 mM ammonium acetate at a flow rate of 0.7 mL/min [23]. The absorbance at 260 nm was monitored. The mean concentration±standard error of the mean (SE) was calculated for each time point.
3. Results and discussion
3.1. In vitro cytotoxic activity of EA
The tumoricidal action of high-dose AA occurs due to generation of extracellular ROS such as H2O2 by reduction of catalytic metals [13]. Since the two isomers EA and AA are equally effective at reducing, it was expected that high-dose EA also exhibits cytotoxicity similar to that of AA. First, EA was tested for its effect on colon-26 cells using the calcein-AM assay (Fig. 1B). EA exhibited cytotoxic activity to colon-26 cells in a concentration-dependent manner, and there was a significant toxic effect at a concentration of more than 0.6 mM. The toxicity profiles of EA and AA were similar (EC50 of 0.67 and 0.75 mM, respectively). As expected, EA as well as AA exhibited cytotoxic activity to colon-26 cells at the high concentrations. It is thought that the cytotoxicity of EA is due to generation of ROS.
We then evaluated the relative quantity of intracellular ROS to the control by using a fluorescent ROS-sensitive probe, DCFH-DA. Fig. 1C–E shows the change in intracellular ROS. At 15 min after exposure of cells to EA at the concentration of 2 mM, a slight increase in intracellular ROS was detected (Fig. 1C). At 30 min, the amount of ROS was significantly increased by EA treatment and reached approximately 3 times that of the control (Fig. 1D). There was little increase in ROS at 60 min, but the level was higher than that of the control (Fig. 1E). EA and AA generated similar levels of ROS at similar concentrations. The maximum generation of ROS was detected at 30 min. This result agrees with results reported by Verrax et al. showing that ROS was generated by exposure to a pharmacologic concentration of AA for 30 min [24]. These results indicate that EA has oxidative stress-mediated antitumor activity similar to that of AA in vitro.
3.2. In vivo antitumor activity of EA
To evaluate the inhibitory effects on tumor growth, we used a xenograft colon cancer model in CDF1 mice. The model mice were treated (iv injection) with a placebo (phosphate buffered saline: PBS), EA-Na or AA-Na (equimolecular amount of 300 mg/kg of AA) on alternate days for 4 times. As shown in Fig. 2, the difference between tumor growth in the placebo group and that in the EA-treated group was statistically significant at day 6. In the AA-treated group, the inhibition ratio of tumor growth was the same as that in the EA-treated group. A significant difference was observed in the tumor growth between the placebo group and the AA-treated group at day 5. It is expected that high-dose iv EA has the same antitumor activity as that of AA. The antitumor activity of AA only tended to decrease tumor growth but not completely inhibit, and this was always the case for AA as a single agent in some earlier works [11], [15]. In a clinical setting, depending on the condition of the patient, AA is administered at a dose of several dozen grams or more [25]. The plasma AA level must be raised up to 20 mM or more to obtain a sufficient therapeutic effect. Infusion of 0.9–1.5 g/kg AA resulted in elevation of AA level to 17–26 mM in plasma [10]. In the present study, the dosage was set low (300 mg/kg body weight) compared with that in clinical cases, and AA therefore showed weak activity [26]. Our results showed that tumor growth was significantly decreased by an intravenous shot of EA and AA, and stronger antitumor activity might have been shown if the mice had been given EA and AA by drip infusion.
Fig. 2.
Tumor growth in colon-26 tumor-bearing CDF1 mice treated with EA or AA. Tumor sizes were measured for 7 days during treatment. All data represent means±SE (n=6). (*P<0.05, **P<0.01, compared with the control).
3.3. In vivo biodistribution and clearance of EA
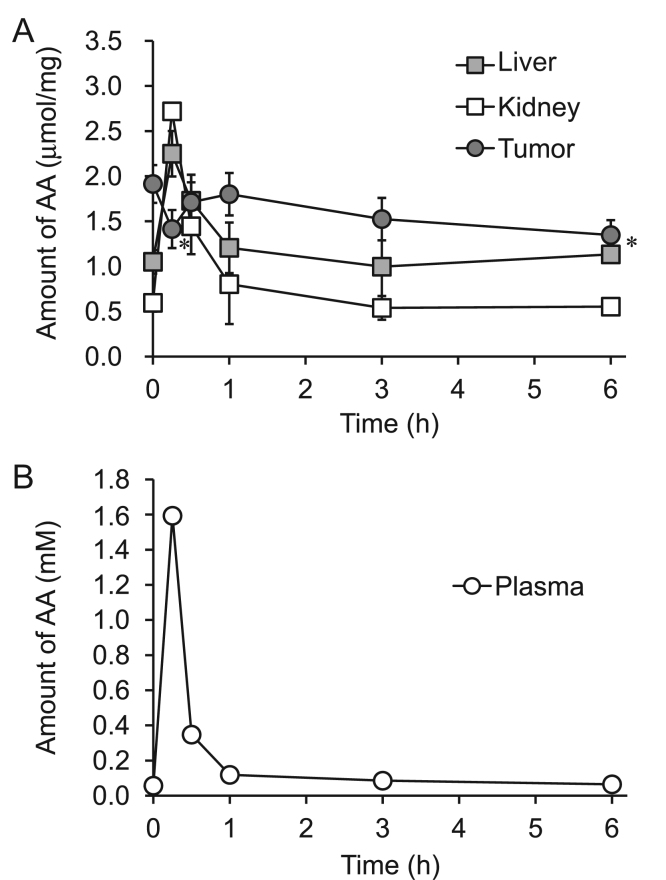
Little is known about the pharmacokinetics of AA after iv administration of AA under a cancer condition. Padayatty et al. examined AA pharmacokinetics only in blood of healthy people [12]. Kim et al. carried out an in vivo study of a radiotracer, 6-deoxy-6-iodo-l-ascorbic acid, in tumor-bearing mice, though they did not provide data for endogenous AA levels in the tumor [27]. Therefore, we studied the biodistribution and clearance of AA in tumor-bearing mice. Fig. 3 shows the distribution of AA in mouse liver, kidney, tumor (Fig. 3A) and plasma (Fig. 3B) for periods ranging from 15 to 360 min after iv injection of AA-Na (equimolecular of 300 mg/kg of AA). The AA levels in the liver (initial value: 1.1 μmol/mg), kidney (0.6 μmol/mg) and plasma (57.1 μM) substantially increased in the first 15 min. The maximum values of AA in the liver, kidney and plasma were 2.2, 2.7 μmol/mg and 1.6 mM, respectively. The increased levels in the first 15 min decreased to baseline levels after 1 h. In contrast, the AA levels in the tumor were decreased by administration of high-dose AA (Fig. 3A). Generally, a tumor takes up AA positively, and AA concentration in a tumor is maintained at a high level [28]. Therefore, it was expected that iv injection of high-dose AA would increase the level of AA in the tumor. Surprisingly, the amount of AA in the tumor was not increased but rather reduced at 15 min after administration of AA. Fifteen minutes after administration of 300 mg/kg iv AA, tumor levels had significantly decreased to 1.4 μmol/mg, while plasma levels were 1.6 mM, a concentration shown to be cytotoxic in vitro (Fig. 3B). Since exposure of colon-26 cells to AA increased intracellular ROS (Fig. 1C–E), it was thought that the cells were exposed to oxidative stress. Thus, at 15 min in the in vivo experiment, the tumor might have been exposed to oxidative stress (Fig. 3A). The results suggest that ROS were generated in the extracellular fluid by high dose iv AA. The resulting oxidative stress in the tumor cells was alleviated by oxidizing endogenous AA. After an initial recovery of endogenous AA levels, these decreased again (Fig. 3A), suggesting that ROS-induced damage either affected AA uptake from plasma or had resulted in a free radical cascade which continued to deplete endogenous AA levels. As shown in Fig. 1D, the greatest increase in intracellular ROS was detected at 30 min after exposure to AA in vitro. The time when the tumor received an oxdative stress in vivo was earlier than the time when the tumor cells showed the highest ROS concentration in vitro. It seems that ROS was generated in vivo more rapidly than in vitro because many transition metals exist in the body. These results suggest that the tumor may be injured by oxidative stress of ROS generated by AA.
Fig. 3.
In vivo biodistribution of AA in colon-26 tumor-bearing CDF1 mice. AA was injected intravenously into colon-26 tumor-bearing CDF1 mice. Liver, kidney, tumor (A) and plasma (B) samples from tumor-bearing mice were analyzed at the indicated times after injection. All data represent means±SE (n=4). *P<0.05, significant difference of tumor AA levels at each time point compared with at 0 min (Dunnett’s test).
Subsequently, the pharmacokinetic of EA was investigated. The EA levels in tissues in the EA-treated group were measured (Fig. 4A and B). Amounts of EA in the liver, kidney and plasma had peaked at 15 min after administration of EA and then decreased. At 15 min, the uptake quantities of EA after injection of EA in the liver and plasma were 1.4 μmol/mg and 1.5 mM, respectively, and these amounts were the same as levels of AA of which were increased by AA administration. In contrast, the amount of EA in the kidney (4.4 μmol/mg, Fig. 4A) was larger than that of AA in AA-treated group (2.7 μmol/mg, Fig. 3A). This result suggests that EA is excreted faster than AA. Uptake of EA into the tumor was also observed. The amount of EA in the tumor was kept at a fixed value, though the amounts of EA in other tissues decreased with time. Interestingly, administration of EA affected the amounts of AA in the plasma and tumor (Fig. 4C and D). AA in the kidney and liver was not influenced (Fig. 4C), but plasma AA level was increased to 105.8 μM from the initial value of 56.0 μM at 15 min after EA injection (Fig. 4D). The increase level of AA in plasma had decreased to the initial level at 1 h. It cannot be assumed that the increment of AA by EA injection was due to efflux of AA from tissues by EA because AA levels in the liver and the kidney showed little change. The oxidized form of AA, dehydroascorbic acid (DHA), might be deoxidized to AA by reduction activity of EA. However, under phygiological conditions, since DHA concentrations in plasma remain extremely low [29], [30], it is difficult to think that the amount of AA was increased twice by reduction from DHA in plasma. It was reported that the AA level in plasma of cancer patients (29±27 μM) was reduced compared to that in a normal condition (50±29 μΜ) [31]. Thus, the DHA plasma level under a cancer condition might be higher than that under a normal condition. These results suggested that EA reduces DHA in plasma to AA. As shown in Fig. 4C, the AA level in the tumor decreased immediately after the injection of EA, and the level of AA had returned to the initial level at 60 min. After that, the content of AA in the tumor decreased slowly until 6 h, while the content of EA in the tumor increased slightly. The time-course profile of AA concentration in the tumor agrees well with that seen by AA injection (Fig. 3A: tumor). These results suggested that the decrease of AA in the tumor with EA injection was due to oxidative stress, not efflux of AA by competitive uptake of EA. On the other hand, the amounts of AA in the liver and kidney showed little change. Hence, it is thought that only the tumor was exposed to oxidative stress by injection of EA as well as AA. Therefore, it can be expected that EA shows cytotoxic activity selectively to tumor and has few adverse effects on normal tissues.
Fig. 4.
In vivo biodistribution of EA and influence of EA on quantity of AA in colon-26 tumor-bearing CDF1 mice. EA was injected intravenously into colon-26 tumor-bearing CDF1 mice. Amounts of EA (A) and (B) and AA (C) and (D) in the liver, kidney, tumor and plasma after administration of EA. All samples from tumor-bearing mice were analyzed at the indicated times after injection. All data represent means±SE (n=4).
4. Conclusions
The results of the present study showed that high-dose EA has antitumor activity in vitro and in vivo. Exposure of cells to EA increased intracellular ROS, and iv administration of EA to tumor-bearing mice temporarily decreased AA level in the tumor. These results indicate that the antitumor activity of EA was caused by damage to the tumor from ROS generated by its own oxidation. We propose oxidative stress-mediated antitumor activity as one of the unknown pharmacological functions of high-dose iv EA to colon-26 cells and cancer model mice.
Acknowledgment
The excellent technical assistance of Misses A. Iomori and K. Ikeda is gratefully acknowledged.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.bbrep.2015.07.018.
Appendix A. Supplementary material
Supplementary material
Supplementary material
Supplementary material
References
- 1.Tajima S., Pinnell S.R. Regulation of collagen synthesis by ascorbic acid. Ascorbic acid increases type I procollagen mRNA. Biochem. Biophys. Res. Commun. 1982;106:632–637. doi: 10.1016/0006-291x(82)91157-3. [DOI] [PubMed] [Google Scholar]
- 2.Sato P.H., Zannoni V.G. Ascorbic acid and hepatic drug metabolism. J. Pharmacol. Exp. Ther. 1976;198:295–307. [PubMed] [Google Scholar]
- 3.Hallberg L., Brune M., Rossander L. Effect of ascorbic acid on iron absorption from different types of meals. Studies with ascorbic-acid-rich foods and synthetic ascorbic acid given in different amounts with different meals. Hum. Nutr. Appl. Nutr. 1986;40:97–113. [PubMed] [Google Scholar]
- 4.Rose R.C., Bode A.M. Biology of free radical scavengers: an evaluation of ascorbate. FASEB J. 1993;7:1135–1142. [PubMed] [Google Scholar]
- 5.Azmi A.S., Bhat S.H., Hadi S.M. Resveratrol-Cu(II) induced DNA breakage in human peripheral lymphocytes: implications for anticancer properties. FEBS Lett. 2005;579:3131–3135. doi: 10.1016/j.febslet.2005.04.077. [DOI] [PubMed] [Google Scholar]
- 6.Simić A., Manojlović D., Segan D., Todorović M. Electrochemical behavior and antioxidant and prooxidant activity of natural phenolics. Molecules. 2007;12:2327–2340. doi: 10.3390/12102327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoshino M., Haneda M., Naruse M., Htay H.H., Tsubouchi R., Qiao S.L., Li W.H., Murakami K., Yokochi T. Prooxidant activity of curcumin: copper-dependent formation of 8-hydroxy-2′-deoxyguanosine in DNA and induction of apoptotic cell death. Toxicol. In Vitro. 2004;18:783–789. doi: 10.1016/j.tiv.2004.03.009. [DOI] [PubMed] [Google Scholar]
- 8.Du J., Cullen J.J., Buettner G.R. Ascorbic acid: chemistry, biology and the treatment of cancer. Biochim. Biophys. Acta. 1826;2012:443–457. doi: 10.1016/j.bbcan.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cameron E., Pauling L. Supplemental ascorbate in the supportive treatment of cancer: reevaluation of prolongation of survival times in terminal human cancer. Proc. Natl. Acad. Sci. USA. 1978;75:4538–4542. doi: 10.1073/pnas.75.9.4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoffer L.J., Levine M., Assouline S., Melnychuk D., Padayatty S.J., Rosadiuk K., Rousseau C., Robitaille L., Miller W.H., Jr Phase I clinical trial of i.v. ascorbic acid in advanced malignancy. Ann. Oncol. 2008;19:1969–1974. doi: 10.1093/annonc/mdn377. [DOI] [PubMed] [Google Scholar]
- 11.Parrow N.L., Leshin J.A., Levine M. Parenteral ascorbate as a cancer therapeutic: a reassessment based on pharmacokinetics. Antioxid. Redox Signal. 2013;19:2141–2156. doi: 10.1089/ars.2013.5372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Padayatty S.J., Sun H., Wang Y., Riordan H.D., Hewitt S.M., Katz A., Wesley R.A., Levine M. Vitamin C pharmacokinetics: implications for oral and intravenous use. Ann. Intern. Med. 2004;140:533–537. doi: 10.7326/0003-4819-140-7-200404060-00010. [DOI] [PubMed] [Google Scholar]
- 13.Chen Q., Espey M.G., Krishna M.C., Mitchell J.B., Corpe C.P., Buettner G.R., Shacter E., Levine M. Pharmacologic ascorbic acid concentrations selectively kill cancer cells: action as a pro-drug to deliver hydrogen peroxide to tissues. Proc. Natl. Acad. Sci. USA. 2005;102:13604–13609. doi: 10.1073/pnas.0506390102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chen Q., Espey M.G., Sun A.Y., Lee J.H., Krishna M.C., Shacter E., Choyke P.L., Pooput C., Kirk K.L., Buettner G.R., Levine M. Ascorbate in pharmacologic concentrations selectively generates ascorbate radical and hydrogen peroxide in extracellular fluid in vivo. Proc. Natl. Acad. Sci. USA. 2007;104:8749–8754. doi: 10.1073/pnas.0702854104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Q., Espey M.G., Sun A.Y., Pooput C., Kirk K.L., Krishna M.C., Khosh D.B., Drisko J., Levine M. Pharmacologic doses of ascorbate act as a prooxidant and decrease growth of aggressive tumor xenografts in mice. Proc. Natl. Acad. Sci. USA. 2008;105:11105–11109. doi: 10.1073/pnas.0804226105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ahmad I.M., Aykin-Burns N., Sim J.E., Walsh S.A., Higashikubo R., Buettner G.R., Venkataraman S., Mackey M.A., Flanagan S.W., Oberley L.W., Spitz D.R. Mitochondrial O2.- and H2O2 mediate glucose deprivation-induced stress in human cancer cells. J. Biol. Chem. 2005;280:4254–4263. doi: 10.1074/jbc.M411662200. [DOI] [PubMed] [Google Scholar]
- 17.Hyslop P.A., Hinshaw D.B., Halsey Jr. W.A., Schraufstätter I.U., Sauerheber R.D., Spragg R.G., Jackson J.H., Cochrane C.G. Mechanisms of oxidant-mediated cell injury. The glycolytic and mitochondrial pathways of ADP phosphorylation are major intracellular targets inactivated by hydrogen peroxide. J. Biol. Chem. 1988;263:1665–1675. [PubMed] [Google Scholar]
- 18.Hampton M.B., Orrenius S. Dual regulation of caspase activity by hydrogen peroxide: implications for apoptosis. FEBS Lett. 1997;414:552–556. doi: 10.1016/s0014-5793(97)01068-5. [DOI] [PubMed] [Google Scholar]
- 19.Suzuki E., Kurata T., Tomita M., Arakawa N. Vitamin C activity of erythorbic acid in ascorbic acid-deficient guinea pigs. J. Nutr. Sci. Vitaminol. 1995;41:17–24. doi: 10.3177/jnsv.41.17. [DOI] [PubMed] [Google Scholar]
- 20.Kipp D.E., Schwarz R.I. Effectiveness of isoascorbate versus ascorbate as an inducer of collagen synthesis in primary avian tendon cells. J. Nutr. 1990;120:185–189. doi: 10.1093/jn/120.2.185. [DOI] [PubMed] [Google Scholar]
- 21.Fidler M.C., Davidsson L., Zeder C., Hurrell R.F. Erythorbic acid is a potent enhancer of nonheme-iron absorption. Am. J. Clin. Nutr. 2004;79:99–102. doi: 10.1093/ajcn/79.1.99. [DOI] [PubMed] [Google Scholar]
- 22.Tsao C.S. Inhibiting effect of ascorbic acid on the growth of human mammary tumor xenografts. Am. J. Clin. Nutr. 1991;54:1274–1280. doi: 10.1093/ajcn/54.6.1274s. [DOI] [PubMed] [Google Scholar]
- 23.Tai A., Gohda E. Determination of ascorbic acid and its related compounds in foods and beverages by hydrophilic interaction liquid chromatography. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2007;853:214–220. doi: 10.1016/j.jchromb.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 24.Verrax J., Calderon P.B. Pharmacologic concentrations of ascorbate are achieved by parenteral administration and exhibit antitumoral effects. Free Radic. Biol. Med. 2009;47:32–40. doi: 10.1016/j.freeradbiomed.2009.02.016. [DOI] [PubMed] [Google Scholar]
- 25.Padayatty S.J., Riordan H.D., Hewitt S.M., Katz A., Hoffer L.J., Levine M. Intravenously administered vitamin C as cancer therapy: three cases. Can. Med. Assoc. J. 2006;174:937–942. doi: 10.1503/cmaj.050346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Riordan H.D., Riordan N.H., Jackson J.A., Casciari J.J., Hunninghake R., González M.J., Mora E.M., Miranda-massari J.R., Rosario N., Rivera A. Intravenous vitamin C as a chemotherapy agent: a report on clinical cases. P. R. Health Sci.J. 2004;23:115–118. [PubMed] [Google Scholar]
- 27.Kim J., Yamamoto F., Gondo S., Yanase T., Mukai T., Maeda M. 6-Deoxy-6-[131I]iodo-l-ascorbic acid for the in vivo study of ascorbate: autoradiography, biodistribution in normal and hypolipidemic rats, and in tumor-bearing nude mice. Biol. Pharm. Bull. 2009;32:1906–1911. doi: 10.1248/bpb.32.1906. [DOI] [PubMed] [Google Scholar]
- 28.Agus D.B., Vera J.C., Golde D.W. Stromal cell oxidation: a mechanism by which tumors obtain vitamin C. Cancer Res. 1999;59:4555–4558. [PubMed] [Google Scholar]
- 29.VanderJagt D.J., Garry P.J., Bhagavan H.N. Ascorbic acid intake and plasma levels in healthy elderly people. Am. J. Clin. Nutr. 1987;46:290–294. doi: 10.1093/ajcn/46.2.290. [DOI] [PubMed] [Google Scholar]
- 30.Dhariwal K.R., Hartzell W.O., Levine M. Ascorbic acid and dehydroascorbic acid measurements in human plasma and serum. Am. J. Clin. Nutr. 1991;54:712–716. doi: 10.1093/ajcn/54.4.712. [DOI] [PubMed] [Google Scholar]
- 31.Gackowski D., Banaszkiewicz Z., Rozalski R., Jawien A., Olinski R. Persistent oxidative stress in colorectal carcinoma patients. Int. J. Cancer. 2002;101:395–397. doi: 10.1002/ijc.10610. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material
Supplementary material