Abstract
A pirin-like protein from a marine denitrifying bacterium, Pseudomonas stutzeri Zobell has been heterologously expressed in E. coli and purified to homogeneity with metal-affinity and gel filtration chromatographies. The recombinant pirin-like protein has exhibited quercetinase activities upon the incorporation of a divalent metal ion, while its biological role remains unclear. In the case of Cu2+ the holo-protein demonstrated the highest activities and spectroscopic properties typical of type II Cu protein. A 3D-structual model constructed using the crystal structure of human pirin as temperate indicated that the metal biding site is constructed with 3His1Glu located in the consensus sequences in the N-terminal domain.
Abbreviations: 2,3QD, quercetin 2,3-dioxygenase; ABA, abscisic acid; IPTG, Isopropyl β-d-1-thiogalactopyranoside; CD, circular dichroism; EPR, electron paramagnetic resonance
Keywords: Pirin, Quercetinase, Flavonoid
Graphical abstract
Highlights
-
•
A pirin-like protein gene from Pseudomonas stutzeri has been expressed in E. coli.
-
•
The Cu2+-acted recombinant pirin-like protein exhibited quercetinase activities.
-
•
The Cu-pirin-like protein showed features typical of type II Cu protein.
1. Introduction
Flavonoids are one class of secondary metabolites of plants. Dietary plant flavonoids have been proposed to contribute to cancer prevention, neuro-protection, and cardiovascular health though their anti-oxidant, anti-inflammatory, pro-apoptotic, and anti-proliferative activities [1], [2]. Terrestrial plants secrete flavonoids such as flavone, flavonol, flavanone, flavanonol, flavan and isoflavon from their roots to send signals towards symbiotic bacteria or to avoid attacks from other bacteria [3], [4]. Recent studies have shown that marine plants also utilize flavonoids for protection [5], [6].
Quercetin (3,5,7,3′,4′-pentahydroxy-flavone) is produced in germinating seedlings by UV or blue light, and is widely contained in plants as glycosides, rutin and quercitrin [7], [8]. Quercetin is a polyphenolic compound together with anthocyanin and catechin etc. These polyphenolic compounds have received special attention for their anti-inflammatory actions arising from scavenging capacity of free radicals [9], [10].
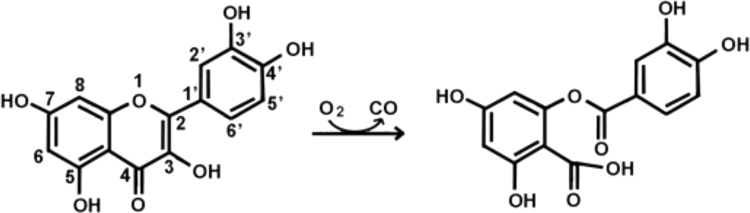
Quercetin is converted into the corresponding depside (phenolic ester 2-protocatechuoylphloroglucinol carboxylic acid) and carbon monoxide by quercetin 2,3-dioxygenase (2,3QD) or quercetinase (Scheme 1) [11]. Transformations of flavonols such as quercetin can be divided into three or four processes: microbial bioconversion, anaerobic or aerobic prokaryotic catabolism, and aerobic eukaryotic microbial catabolism [12]. Quercetinase has been found in various molds [13], [14], [15], [16], [17] and some bacteria such as Bacillus subtilis [18]. Beside catabolic roles ascribable to eukaryotic quercetinase, detoxification role of this enzyme against deleterious effects has also been postulated inside bacteria.
Scheme 1.
Oxygenolysis of quercetin catalyzed by quercetinase.
Amino acid sequence and higher order structure of quercetinases indicated that this enzyme has a strong resemblance with pirin [12], [19], which concerns in apoptosis and cellular stress in eukaryotic organisms and in seed germination and transcription of light- and ABA (abscisic acid)-regulated gene in plants. Both quercetinase and pirin belong to cupin superfamily which has one (monocupin) or two (bicupin) domains comprised of characteristic β-stranded motifs and intervening loops with the consensus sequences containing His and Glu residues [11]. It has been clarified that metal ions such as Cu2+, Fe2+, and Ni2+ are required to exert quercetinase activities, whilst it is not necessarily clear whether pirins play a role as sensor for metal ions [20]. While pirins and quercetinases have strong structural resemblances, their requirements for metal ions are different. This fact urged us to study quercetinase activity of pirin, although the bindings of divalent metal ions to pirins have already been reported [21].
Metabolisms of flavonoids, especially of quercetin, by microorganisms in terrestrial plant rhizosphere, have been studied in some detail [12], [22]. However, few studies have been performed on defense of microorganisms from flavonoids secreted by aquatic plants. We have selected Pseudomonas stutzeri Zobell, formerly Pseudomonas perfectomarina (CCUG 16156=ATCC 14405) for the study on decomposition of flavonoids. P. stutzeri is a Gram-negative bacterium adapted for a variety of circumstances, and accordingly, very broad for its phenotypic and genotypic diversity. The Zobell strain of P. stutzeri is a marine isolate, and has been a model organism for denitrification because of its potential uses for agriculture, bioremediation and waste water treatment. However, quercetinase activities of this anaerobic denitrifier have never been studied yet in spite of detailed studies on the enzymes concerned in denitrification [23]. We planned to search and study a protein classified into pirin from P. stutzeri Zobell and its quercetinase activities.
2. Methods
2.1. Chemicals
Quercetin, fisetin, keamferol, and myricetin were purchased from Wako chemicals (Japan). Galangin and taxifolin were obtained from Sigma, morin from TCI (Japan), and luteolin from LKT laboratories (U.S.A.). Flavonoid stock solutions were prepared in dimethyl sulfoxide. All other chemicals were of analytical grade.
2.2. Construction of the expression system of the pirin-like protein in E. coli
The 5′-UTR region containing Shine-Dalgarno sequence and the open reading frame coding for the pirin-like protein with 6xHis-tag at C-terminus was amplified by PCR using two primers (P1: 5′-ggaattcggaggaagcgaagatggcccaacgggaaattc-3′, containing the EcoRI recognition site (underline), Shine-Dalgarno sequence (bold letters), and coding sequence for the N-terminal region of the pirin-like protein; P2: 5′-cgggatcctcaatgatgatgatgatgatgggccagtgtcccatcgcgaaa-3′, containing the BamHI site (underline) and the C-terminal region of the protein with 6xHis-tag (italic letters)) and a genomic DNA fragment of P. stutzeri Zobell as template.
The amplified gene fragment was digested with EcoRI and BamHI, and inserted into pUC18 vector to yield an expression plasmid pUC-2,3QD. To avoid co-expression of LacZ-fused pirin-like protein, a stop codon was introduced at an upstream of the SD sequence by PCR mutagenesis using two primers (5′-gaattcgtgaggaagcgaagatggcc-3′, 5′-cttcctcacgaattcgtaatcatggt-3′). The resultant plasmid was designated as pUC-2,3QD*.
2.3. Purification of the recombinant pirin-like protein
E. coli BL21 cells transformed with pUC-2,3QD* were cultured in LB medium supplemented with 50 μg/ml ampicillin at 37°C for overnight with shaking (150 rpm). The recombinant protein was induced with 0.2 mM IPTG. The cells were collected by centrifugation and disrupted by sonication in 20 mM Tris-H2SO4, pH 8.0. After centrifugation, the supernatant of the crude extract was applied onto a His-Accept resin column (Nacalai tesque, Japan) for Ni-affinity chromatography. After the column was washed with the same buffer containing 300 mM NaCl and 20 mM imidazole, proteins were eluted with the buffer containing 300 mM NaCl and 300 mM imidazole. The gel filtration using Superdex 200 (GE healthcare, U.S.A.) has been performed to obtain pure protein.
2.4. Determinations of protein concentration and amino acid sequence
Protein concentration was determined with the BCA protein assay kit (Pierce, U.S.A.) and bovine serum albumin as a standard protein. The N-terminal amino acid sequence of the recombinant protein was analyzed by a Shimadzu PPSQ-33A protein sequencer.
2.5. Construction of the 3D structure model
The 3D model structure for the P. stutzeri pirin-like protein was generated by SWISS-MODEL (http://swissmodel.expasy.org/) using human pirin (PDB ID: 4HLT) as a template.
2.6. Enzyme assays
Quercetinase activity of the pirin-like protein has been determined from the absorption change at 380nm (ε= 18,700 M−1 cm−1) for the reaction mixture containing 50 μM quercetin, 100 mM NaCl and 5%(V/V) DMSO in 50 mM Tris–HCl buffer at 25°C. One unit is defined as the amount of the enzyme catalyzing the decomposition of 1 μmol of quercetin (flavonol) per min. Production of carbon monoxide as co-product has been ascertained from the reaction with PdCl2 soaked on filter paper to give black solid precipitates of elemental palladium [17]. Kinetic parameters, Km and Vmax values were determined based on triplicate data by using Igor Pro ver. 5.03. The pH dependence study of quercetinase activity has been performed using 50 mM Britton–Robinson buffer. Thermal stability of the holo-protein has been studied by incubations at ambient temperatures for 30 min. In addition to quercetin, enzymatic activities for fisetin, galangin, kaemferol, morin, myricetin, taxifolin, and luteolin are also studied.
2.7. Spectroscopic characterizations of the CU-pirin-like protein
Absorption spectra have been measured on a Shimadzu UV-2600 spectrometer or on a JASCO V-560 spectrometer, to both of which a temperature controller has been attached, using quartz cells with 1 mm or 10 mm path-length. Circular dichroism (CD) spectra have been measured on a JASCO J-720 spectropolarimeter. Electron paramagnetic resonance (EPR) spectrum has been measured on a JEOL JES-RE1X X-band spectrometer at 77 K.
3. Results and discussion
3.1. Amino acid sequence and model structure of the pirin-like protein
We have cloned the gene coding for the pirin-like protein from the genome of P. stutzeri Zobell (ATCC 14405) by PCR based on the sequence obtained with genome walking prier to the construction of the expression system (data not shown). The sequence of cloned genome fragment is identical to the reported one (GenBank accession number of EHY79687.1).
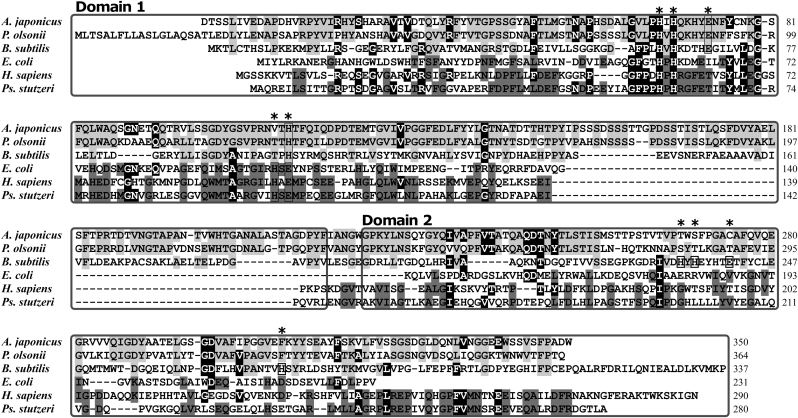
The amino acid sequence of the pirin-like protein from P. stutzeri Zobell (Fig. 1) shares 21–34% identities with the quercetinase sequences from P. olsonii, A. japonicus, A. flavus, A. niger, B. subtilis, and Streptomyces. Higher homologies in amino acid sequences are found for pirins, e.g. 37% and 39% for the E. coli and human proteins, respectively. Judging from the difference in sequence conservation patterns between quercetinase and pirin (Fig. 1), it is clear that the pirin-like protein from P. stutzeri belongs to the pirins. These sequence homologies indicated that the present P. stutzeri pirin-like protein is comprised of two domains, therefore belongs to bicupin. A modified characteristic consensus sequence for cupin superfamily, G(X)5HXH(X)3,4E(X)6G (motif 1, underlines are the ligands for metal ion) is present in the N-terminal domain (domain 1 in Fig. 1): GFPPHPHRGFETITYMLEG. On the other hand, another consensus sequence G(X)5PXG(X)2H(X)3N (motif 2), is also located in the N-terminal domain, although considerably modified for pirins: QWMTAARGVIHSEMP.
Fig. 1.
The amino acid sequence alignment of quercetinases and pirin-like proteins, quercetinases from A. japonicus (Acc: Q7SIC2), P. olsonii (Acc: ABV24349), B. subtilis (Acc: P42106), and pirins from E. coli (Acc: P46852), human (Homo sapiens, Acc: AAV38390), and P. stutzeri Zobell. The white letters on the black background are the common conserved residues of quercetinases and pirins. The conserved residues of quercetinases or pirins are shown by the light-gray and the dark-gray shadows, respectively. The coordination residues of metal ions are marked by asterisk and box.
The structural model constructed using the human pirin (PDB ID: 4HLT) as template (Supplemental Fig. 1) appears to be consisted of two–domains, each of which has the β-sandwich cupin fold as in the X-ray crystal structures of fungal (A. japonicas [24]) and bacterial (B. subtilis [25]) quercetinases or bacterial (E. coli [26]) and human [27] pirins. Supplemental Fig. 1 also indicates the presence of α helix in the C-terminal end. Analogous helix is also found in human pirin in harmony with a high homology in amino acid sequence (Fig. 1).
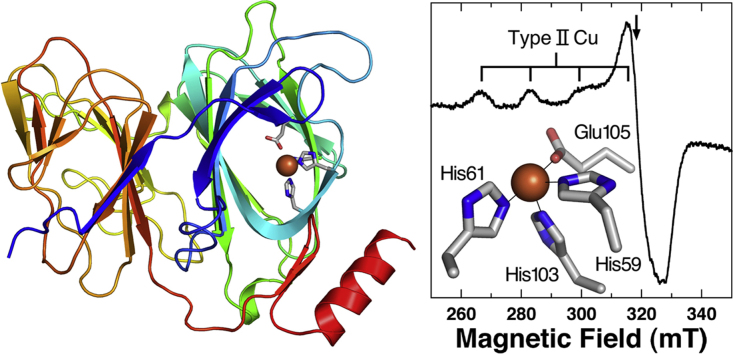
The CD spectrum in the UV region (Supplementary Fig. 2) indicated that the pirin-like protein is rich in β−structures and loops (estimated helix content is less than 20%, vide infra). The side chains of the His59, His61, His103 and Glu105 residues involved in the consensus sequence are closely located in the cavity formed in the N-terminal domain, which is smaller in size compared to those found in quercetinases. Thus, a potential binding site for divalent metal ions appears to be constructed with the conserved amino acid residues.
3.2. Construction of the expression system of the pirin-like protein and purification of the recombinant protein
To construct the expression system of the pirin-like protein, the open reading frame of the gene attached a 6X-His tag coding sequence at 3′-terminal end with 5′-UTR region containing SD-sequence was inserted into the cloning site of pUC18. However, the two protein molecules with analogous molecular masses (31 and 33 kDa) were expressed. Determination of the N-terminal amino acid sequence of 33 kDa protein indicated that the N-terminal 10 amino acids of LacZ protein encoded by the vector was fused to the 31 kDa protein. Both the LacZ-fused protein (33 kDa) and the target pirin-like protein (31 kDa) exhibit quercetinase activities upon the incorporation of divalent metal ions (not shown). However, it was impossible to separate them by chromatographies. Therefore, we inserted a stop codon just before the SD sequence by PCR mutagenesis, and succeeded in obtaining the recombinant protein to afford a single band on SDS-PAGE (Supplemental Fig. 3).
3.3. Requirement of metal ions for quercetinase activities
The pirin-like protein as isolated did not exhibit activities to quercetin and other flavonols, fisetin, galangin, kaempferol, morin, myricetin, taxifolin, and luteolin. Therefore, divalent metal ions were reacted with the apo-proteins. We could observe flavonol dioxygenase activities (quercetinase activities as a narrow sense) of the holo-proteins from the absorption changes for decompositions of substrates and also from the formation of CO by the Pd2+ reduction. Relative activities for every substrate were changed in the following order: Cu2+>Fe2+>Zn2+»Mn2+, Co2+, Ni2+~0. The specific activity of the Cu2+-acted pirin-like protein against quercetin was 1.2 U/mg. The fact that Zn2+ also exhibited oxygenase activities, although considerably low, indicates that the central metal ion does not perform a redox change but constructs the active center to activate flavonols and O2. According to the studies on quercetinase [11], [19], Cu2+ and Fe2+ are most frequently required divalent metal ions. Recently, other metal ions such as Ni2+, Co2+, and Mn2+-requiring quercetinases have also been reported [20]. Different activities shown by divalent metal ions would be due to differences in their Lewis acid character and steric requirement. Cu2+ ion favors the five- to six-coordinated structures with one or two elongated axial bonds according to small molecule studies. Otherwise, the tetrahedrally hindered four-coordinated structure is favored by Cu2+ ion [15], [24], [25], [26].
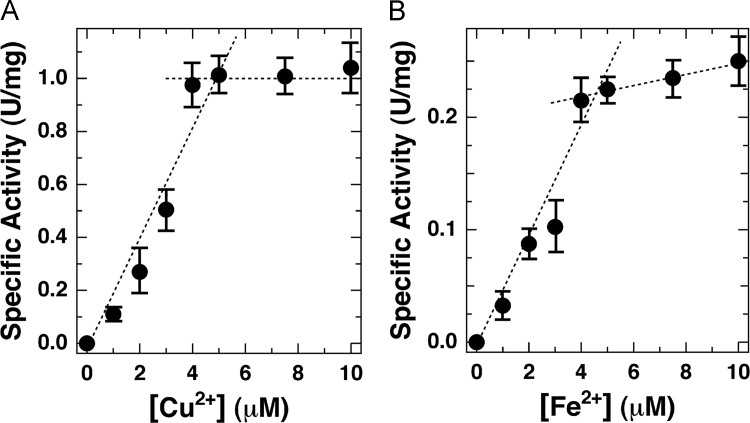
To explore how many metal ions are required for quercetinase activities, titrations for Cu2+ and Fe2+ have been performed. Figs. 2A and B unequivocally show that exactly one Cu2+ or Fe2+ ion is bound to a pirin-like protein. This experimental fact coincides with the prediction from the 3D-strcutrue model which shows a single potential metal binding site in the N-terminal domain of the pirin-like protein (Supplementary Fig. 1).
Fig. 2.
Titrations for quercetinase activities of the pirin-like protein from P. stutzeri Zobell as a function of increasing concentrations of added Cu2+ (Fig. 2A) and Fe2+ (Fig. 2B) ions. Conditions: protein concentration, 4 μM, in 10 mM potassium phosphate buffer (pH 6.0) supplemented with 0.1 M NaCl.
3.4. Enzymatic activities
The relative oxidation activities of the Cu-pirin-like protein against quercetin, myricetin, fisetin, kaempferol, galangin, morin, taxifolin, and luteolin are tabulated in Table 1 together with simultaneous qualitative analyzes of CO formation. The present Cu-pirin-like protein exhibited narrow substrate specificities, the high oxidation activities to quercetin and myricetin differing from other quercetinases. The kinetic parameters, Km and Vmax values for quercetin and myricetin are 13 μM and 1.2 U/mg, respectively, and 9.4 μM and 5.3 U/mg, respectively. The Km values are analogous to the values reported for quercetinases, while the Vmax values are considerably low (45–180 U/mg for fungal quercetinases) [12], presumably because quercetinase activity is not intrinsic to the present pirin-like protein. Expecting an increase in activity by enlarging the entrance of cavity to accommodate the bulky substrates, we performed a mutation at the non-coordinating Phe56 for Ala. However, enzymatic activities were significantly decreased presumably because the mutant molecule became unstable (data not shown).
Table 1.
Relative flavonol dioxygenase activities of the Cu-pirin-like protein and analyzes of CO formation.
| Substrates | Relative activity (%)a | CO formation | |
|---|---|---|---|
| Quercetin | 3,5,7,3′,4′-pentahydroxy-flavone | 100 | + |
| Myricetin | 3,5,7,3′,4′,5′-hexahydroxy-flavone | 460 | + |
| Fisetin | 3,7,3′,4′-tetrahydroxy-flavone | 28 | + |
| Kaempferol | 3,5,7,4′-tetrahydroxy-flavone | ~0 | − |
| Galangin | 3,5,7-trihydroxy-flavone | ~0 | − |
| Morin | 3,5,7,2′,4′-pentahydroxy-flavone | ~0 | − |
| Taxifolin | 3,5,7,3′,4′-pentahydroxy-2,3-dihydro-flavone | ~0 | − |
| Luteolin | 5,7,3′,4′-tetrahydroxy-flavone | ~0 | − |
Measurement conditions: protein concentration, 50 nM; 50 μM flavonol in 50 mM Tris–HCl buffer (pH 7.5) supplemented with 0.1 M NaCl and 5%(V/V) DMSO.
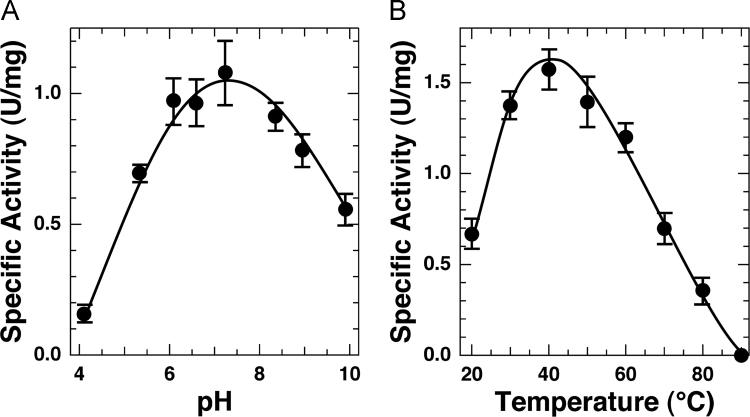
Considerably broad dependencies of activity in the pH range of 3.5–11.5, highest at pH ca. 7.0–7.5, are shown in Fig. 3A. The optimum pH value of the Cu-pirin-like protein is 1.0–1.5 pH units higher than those of the fungal quercetinases [12]. Fig. 3B indicates that the present Cu-pirin-like protein exhibits the highest activity at ca. 40°C but loses activity at 90°C.
Fig. 3.
(A) pH dependence and (B) optimum temperature of the quercetinase activity of the Cu-pirin-like protein from P. stutzeri Zobell. Quercetinase activity was determined at different pH with 50 mM Britton–Robinson buffer (Fig. 3A), and 50 mM Tris–HCl buffer, pH 7.5 (Fig. 3B).
3.5. Spectroscopic characterizations
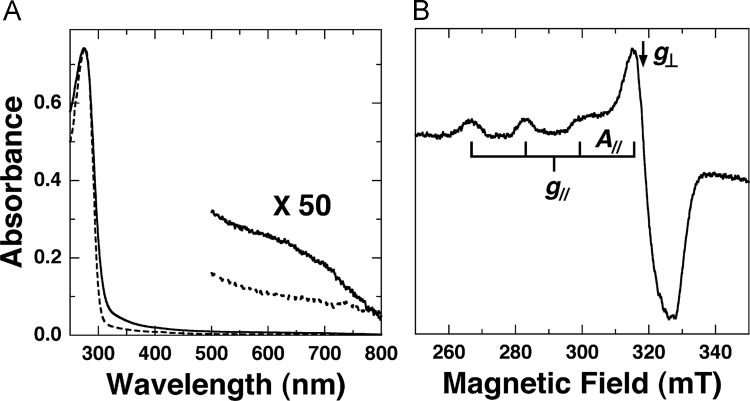
Absorption spectrum of the pirin-like protein as isolated is shown in Fig. 4A as broken line. Only one absorption band is observable at 280 nm (ε=35,000 M−1 cm−1), indicating the protein is in the apo-form (vide supra). With the action of excess Cu2+ ions on apo-protein, the absorption bands appeared at ca. 650 nm (not shown), the wavelength that Cu2+ ions in water never give the absorption band. The prolonged incubation of a stoichiometric amount of Cu2+ ion with the apo-protein molecule also gave the same spectrum (full line in Fig. 4A). The 650 nm band is assigned to the d–d band from the Cu2+ ion bound to the pirin-like protein. The intensity (ε=~100 M−1 cm−1) is in the range reported for Cu-proteins containing a type II Cu (ε<~500 M−1 cm−1) [28]. Absorption spectra of other Cu-quercetinases have not been published yet, and accordingly, comparison is not possible.
Fig. 4.
Absorption (A) and EPR (B) spectra of the Cu-pirin-like protein from P. stutzeri Zobell. Absorption spectrum of the apo protein is shown with broken line in Fig. 4A. Measurement conditions: protein concentration, 21 μM, in 50 mM Tris–HCl buffer (pH 7.5). The absorption spectrum was measured at room temperature using a 10 mm path length quartz cell and the EPR spectrum measured at 77 K with 9.2 GHz microwave.
The CD spectrum of the Cu-pirin-like protein is shown in Supplementary Fig. 2 as full solid line, indicating that protein conformation did not change practically after the incorporation of Cu2+ ion into protein molecule. The CD spectrum in the visible region afforded some CD bands at around 650 nm (shown elsewhere after ascertaining reproducibility).
Fig. 4B shows the EPR spectrum of the holo-protein. It appears that a single tetragonal Cu2+ species is present with the spin Hamiltonian parameters of g‖=2.26, g⊥=2.06, A‖=167 mT (17.6×10−3 cm−1) typical for type II Cu with the binding of 2N2O to 4 N atoms [29]. The presence or absence of water molecules or a hydroxide ion in an equatorial or the axial coordination position(s) is unclear from the EPR parameters. Nevertheless, the EPR parameters and the correlation of the g‖ vs. A‖ values are indicative for the binding of the conserved His59, His61, His103 and Glu105 to Cu2+ ion. Type II Cu EPR signals have been reported for the quercetinase from A. japonicus: g‖=2.33 and A‖=15 × 10−3 cm−1 in MES buffer, pH 6.0 (minor species: g‖=2.29 and A‖=13×10−3 cm−1) (Major and minor species have been considered to have the six-coordinated and distorted five-coordinated structures, respectively) [30]. In the present study, the Tris–HCl buffer, pH 8 was used to ensure the bindings of ligand groups to Cu2+. Therefore, the Cu2+-EPR signal with a highly planar character might have been obtained due to the binding of a hydroxide ion in the place of a water molecule, and minor species is practically negligible.
4. Conclusions
We constructed an expression system of a pirin-like protein from P. stutzeri Zobell in E. coli. The recombinant protein was expressed as the mixture of the target pirin-like protein and LacZ fusion at its N-terminus, but the pure recombinant protein molecules could be obtained by introducing the stop codon at the upstream region of the SD sequence. One divalent metal ion was incorporated into an apo-protein molecule and exhibited quercetinase activities, highest for Cu2+ ion. Furthermore, we performed detailed kinetic analysis for the pirin derived from a microbe for the first time and revealed that the present pirin-like protein has unique specificities considerably different from quercetinases reported hitherto. Absorption, CD, and EPR spectra of the Cu protein indicated that the coordination of all or some of His59, His61, His103 and Glu105 located in the consensus sequences for cupin superfamily. The biological role of the present pirin-like protein still remains unclear, but the fact that a divalent metal ion such as Cu2+ is required for quercetinase activities will be closely concerned with the involvement of this protein in a transcription process for regulation. In the next stage we will focus our study to explore this uninsured evolutional question, especially higher structural homology of the present pirin-like protein with the human pirin.
Acknowledgments
T.W. has been supported from DGHE, the Ministry of National Education and Culture, Republic of Indonesia. The authors acknowledge Dr. H. Ashida at the Interdisciplinary Center for Science Research, Organization of Research, Shimane University, Japan for determinations of the N-terminal amino acid sequences of proteins. Prof. A. Odani of this university is also acknowledged for measurements of CD spectra. We thank Mr. D. Ushiyama for his technical assistance.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.bbrep.2015.08.001.
Appendix A. Supplementary material
Supplementary material
Supplementary material
References
- 1.Taerahara N. Flavonoids in foods; a review. Nat. Prod. Commun. 2015;10:521–528. [PubMed] [Google Scholar]
- 2.Ross J.A., Kasum C.M. Dietary flavonoids: bioavailability, metabolic effects, and safety. Annu. Rev. Nutr. 2002;22:19–34. doi: 10.1146/annurev.nutr.22.111401.144957. [DOI] [PubMed] [Google Scholar]
- 3.Okuunade A.L., Elvin-Lewis M.P., Lewis W.H. Natural antimycobacterial metabolites: current status. Phytochemistry. 2004;10:1017–1032. doi: 10.1016/j.phytochem.2004.02.013. [DOI] [PubMed] [Google Scholar]
- 4.Cesco S., Mimmo T., Tonon G. Plant-borne flavonoids released into the rhizosphere: impact on soil bio-activities related to plant nutrition. Biol. Fertil. Soils. 2012;48:123–149. [Google Scholar]
- 5.Sieg R.D., Kubanek J.J. Chemical ecology of marine angiosperms: opportunities at the interface of marine and terrestrial systems. Chem. Ecol. 2013;39:687–711. doi: 10.1007/s10886-013-0297-9. [DOI] [PubMed] [Google Scholar]
- 6.Engel S., Jensen P.R., Fenical W. Chemical ecology of marine microbial defense. J. Chem. Ecol. 2002;28:1971–1985. doi: 10.1023/a:1020793726898. [DOI] [PubMed] [Google Scholar]
- 7.Kubasek W.L., Shirley B.W., Mckillop A., Wang Z.H., Zhang Z., Kreft A.I. Regulation of flavonoid biosynthetic genes in germinating Arabidopsis seedlings. Plant Cell. 1992;4:1229–1236. doi: 10.1105/tpc.4.10.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fabjan N., Rode J., Kosir I.J., Wang Z.H., Zhang Z., Kreft A.I. Tartary buckwheat (Fagopyrum tataricum Gaertn.) as a source of dietary rutin and quercitrin. J. Agric. Food Chem. 2003;51:6452–6455. doi: 10.1021/jf034543e. [DOI] [PubMed] [Google Scholar]
- 9.Romano B., Pagano E., Montanaro V., Fortunato A.L., Milic N., Borrelli F. Novel insights into the pharmacology of flavonoids. Phytother. Res. 2013;27:1588–1596. doi: 10.1002/ptr.5023. [DOI] [PubMed] [Google Scholar]
- 10.Storniolo A., Raciti M., Cucuna A., Bizzarri M., Di Renzo L. Quercetin affects Hsp70/IRE1α mediated protection from death induced by endoplasmic reticulum stress. Oxid. Med. Cell. Longev. 2015:645157. doi: 10.1155/2015/645157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Solomon E.I., Heppner D.E., Johnston E.M., Ginsbach J.W., Cirera J., Qayyum M., Kieber-Emmons M.T., Kjaergaard C.H., Hadt R.G., Tian L. Copper active site in biology. Chem. Rev. 2014;114:3659–3853. doi: 10.1021/cr400327t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tranchimand S., Brouant P., Iacazio G. The rutin catabolic pathway with special emphasis on quercetinase. Biodegradation. 2010;21:833–859. doi: 10.1007/s10532-010-9359-7. [DOI] [PubMed] [Google Scholar]
- 13.Oka T., Simpson F.J., Krishnamurty H.G. Degradation of rutin by Aspergillus flavus. Studies on specificity, inhibition, and possible reaction mechanism of quercetinase. Can. J. Microbiol. 1972;18:493–508. doi: 10.1139/m72-076. [DOI] [PubMed] [Google Scholar]
- 14.Hund H.K., Breuer J., Lingens F., Hüttermann J., Kappl R., Fetzner S. Flavonol 2,4-dioxygenase from Aspergillus niger DSM 821, a type 2 CuII-containing glycoprotein. Eur. J. Biochem. 1999;263:871–878. doi: 10.1046/j.1432-1327.1999.00574.x. [DOI] [PubMed] [Google Scholar]
- 15.Steiner R.A., Kalk K.H., Dijkstra B.W. Anaerobic enzyme-substrate structures provide insight into the reaction mechanism of the copper dependent quercetin 2,3-dioxygenase. Proc. Natl. Acad. Sci. USA. 2002;99:16625–16630. doi: 10.1073/pnas.262506299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tranchimand S., Ertel G., Gaydou V., Gaudin C., Tron T., Iacazio G. Biochemical and molecular characterization of a quercetinase from Penicillium olsonii. Biochimie. 2008;90:781–789. doi: 10.1016/j.biochi.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 17.Merkens H., Sielker S., Rose K., Fetzner S. A new monocupin quercetinase of Streptomyces sp. FLA: Identification and heterologous expression of the queD Gene and activity of the recombinant enzyme towards different flavonols. Arch. Microbiol. 2007;187:475–487. doi: 10.1007/s00203-007-0215-z. [DOI] [PubMed] [Google Scholar]
- 18.Hirooka K., Fujita Y. Excess production of Bacillus subtilis quercetin 2,3-dioxygenase affects cell viability in the presence of quercetin. Biosci. Biotechnol. Biochem. 2010;74:1030–1038. doi: 10.1271/bbb.90928. [DOI] [PubMed] [Google Scholar]
- 19.Fetzner S. Ring-opening dioxygenases with a cupin fold. Appl. Environ. Microbiol. 2012;78:2505–2514. doi: 10.1128/AEM.07651-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nianios D., Thierbach S., Ateimer L., Lulchev P., Klostermeier D., Fetzner S. Nickel quercetinase, a “promiscuous” metalloenzyme: metal incorporation and metal ligand substitution studies. BMC Biochemistry. 2015;16 doi: 10.1186/s12858-015-0039-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Orozco-Nunnelly D.A., Muhammad D., Mezzich R., Lee B.-S., Jayathilaka L., Kaufman L.S., Warpeha K.M. Pirin1 (PRN1) is a multifunctional protein that regulates quercetin, and impacts specific light and UV responses in the seed-to-seedling transition of Arabidopsis thaliana. PLOS One. 2014;9:e93371–e93372. doi: 10.1371/journal.pone.0093371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cao H., Chen X., Jassbi A.R., Xiao J. Microbial biotransformation of bioactive flavonoids. Biotechnol Adv. 2015;33:214–223. doi: 10.1016/j.biotechadv.2014.10.012. [DOI] [PubMed] [Google Scholar]
- 23.Zumft W.G. Cell biology and molecular basis of denitrification. Microbiol. Mol. Biol. Rev. 1997;61:533–616. doi: 10.1128/mmbr.61.4.533-616.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Steiner R.A., Kooter I.M., Dijkstra B. Functional analysis of the copper-dependent quercetin 2,3-dioxygenase. 1. Ligand-induced coordination changes probed by X-ray crystallography: inhibition, ordering effect, and mechanistic insights. Biochemistry. 2002;41:7955–7962. doi: 10.1021/bi0159736. [DOI] [PubMed] [Google Scholar]
- 25.Gopal B., Madan L.L., Betz S.F., Kossoakoff A.A. The crystal structure of a quercetin 2,3-dioxygenenase from Bacilus subtilis suggests modification of enzyme activity by a change in the metal ion at the active site(s) Biochemistry. 2005;44:193–201. doi: 10.1021/bi0484421. [DOI] [PubMed] [Google Scholar]
- 26.Adams M., Jia Z. Structural and biochemical analysis reveal pirins to possess quercetinase activity. J. Biol. Chem. 2005;280:28675–28682. doi: 10.1074/jbc.M501034200. [DOI] [PubMed] [Google Scholar]
- 27.Miyazaki I., Simizu S., Okumura H., Takagi S., Osada H. A small-molecule inhibitor shows that pirin regulates migration of melanoma cells. Nat. Chem. Biol. 2010;6:667–673. doi: 10.1038/nchembio.423. [DOI] [PubMed] [Google Scholar]
- 28.Halcrow M.A., Knowles P.F., Philips S.E.V. Copper Proteins in the Transport and Activation of Dioxygen, and the Reduction of Inorganic Molecules. In: Bertini I., Sigel A., Sigel H., editors. Basel; 2001. pp. 709–762. [Google Scholar]
- 29.Vänngård T. Biological applications of electron spin resonance. In: Swartz H.M., Bolton J.R., Bord D.C., editors. Biological Applications of Electron Spin Resonance. John Wiley & Sons; 1972. pp. 411–447. [Google Scholar]
- 30.Kooter I.M., Steiner R.A., Dijstra B.W., van Noort P.I., Egmond M.R., Huber M. EPR characterization of the mononuclear Cu-containing Aspergillus japonicus quercetin 2,3-dioxygenase reveals dramatic changes upon anaerobic binding of substrates. Eur. J. Biochem. 2002;269:1971–2979. doi: 10.1046/j.1432-1033.2002.02973.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material