Abstract
Background
Many polyphenols have been proposed as broad-spectrum inhibitors of amyloid formation. To investigate structure–activity relationships relevant for the interaction of flavonoids with transthyretin (TTR), the protein associated with familial amyloid polyneuropathy (FAP), we compared the effects of major tea catechins and their larger polymers theaflavins, side-by-side, on TTR amyloid formation process.
Methods
Interaction of flavonoids with TTR and effect on TTR stability were assessed through binding assays and isoelectric focusing in polyacrylamide gel. TTR aggregation was studied, in vitro, by dynamic light scattering (DLS), transmission electron microscopy (TEM) and in cell culture, through cytotoxicity assays.
Results
Tested flavonoids bound to TTR and stabilized the TTR tetramer, with different potencies. The flavonoids also inhibited in vitro formation of TTR small oligomeric species and in cell culture inhibited pathways involving caspase-3 activation and ER stress that are induced by TTR oligomers. In all assays performed the galloyl esters presented higher potency to inhibit aggregation than the non-gallated flavonoids tested.
Conclusions
Our results highlight the presence of gallate ester moiety as key structural feature of flavonoids in chemical chaperoning of TTR aggregation. Upon binding to the native tetramer, gallated flavonoids redirect the TTR amyloidogenic pathway into unstructured nontoxic aggregation assemblies more efficiently than their non-gallated forms.
General significance
Our findings suggest that galloyl moieties greatly enhance flavonoid anti-amyloid chaperone activity and this should be taken into consideration in therapeutic candidate drug discovery.
Abbreviations: FAP, Familial Amyloidotic Polyneuropathy; TTR, transthyretin; EGCG, epigallocatechin gallate; DLS, dynamic light scattering; TEM, transmission electron microscopy
Keywords: Transthyretin, Amyloid, Neurodegenerative disease, Aggregation, Protein stability, Flavonoids
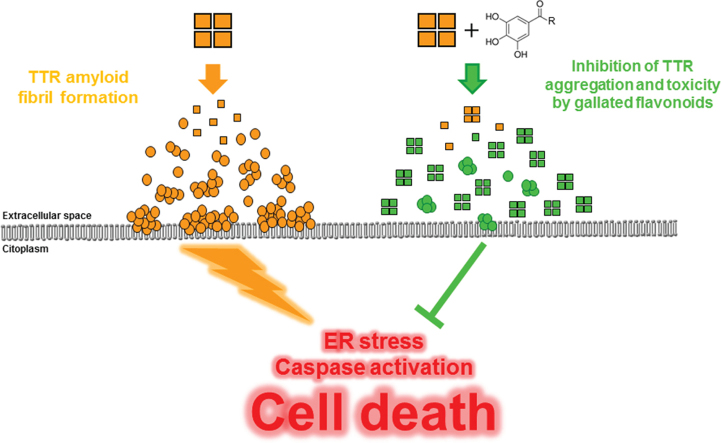
Graphical abstract
Highlights
-
•
Flavonoids are broad-spectrum inhibitors of TTR amyloid formation.
-
•
The galloyl moiety is essential for chemical chaperoning of TTR by flavonoids.
-
•
The galloyl moiety may be important for the design of new therapeutic agents for FAP.
1. Introduction
Transthyretin (TTR) is a tetramer composed by identical subunits of 127-amino acids and contains an extensive β-sheet structure [1]. TTR is predominantly synthesized in the liver and the choroid plexus of the brain. It circulates in plasma and cerebrospinal fluid where it transports thyroid hormones (namely, thyroxin-T4) and vitamin A (through a complex with retinol binding protein— RBP) [2]. More than one hundred TTR single point mutations have been reported (amyloidosismutations.com). Most TTR variants are pathogenic and associated with extracellular protein misfolding and aggregation that triggers inflammation, oxidative stress, matrix remodeling, unfolded-protein-response and endoplasmic reticulum (ER) pathways particularly along the gastrointestinal tract and peripheral nervous system [3], [4]. Among TTR point mutations promoting amyloidogenesis, the most common is TTR V30M which deposits diffusely in the peripheral nervous system, involving nerve trunks, plexuses, and sensory and autonomic ganglia leading to Familial Amyloidotic Polyneuropathy (FAP) [5], [6]. FAP has a wide geographic distribution with the largest foci in Portugal, Japan, and Sweden, and is estimated to affect ~5000–10,000 patients worldwide [7].
Since the TTR molecule has high degree of β-sheet structure, wild-type (WT) TTR protein is intrinsically prone to dissociate into non-native monomers that further aggregate into β-pleated sheet fibrils [1]. Deposition of TTR WT primarily in the heart and, occasionally, in carpal ligaments appears to cause senile systemic amyloidosis [SSA], nonhereditary, age-related form of TTR amyloidosis that affects the elderly [8]. As most circulating mutant TTR is synthesized in the liver, liver transplantation has been widely used to treat FAP since 1990s. Nevertheless, alternative and less invasive therapeutic approaches are mandatory.
Considering the original amyloid cascade hypothesis [9], initial efforts to develop more effective therapies for the treatment of amyloid-related diseases focused on inhibition and/or disruption of fibrils. With the recognition that intermediate oligomeric species, rather than mature fibrils, likely were the key culprits [10], the focus of research in the field shifted towards the development of small-molecule modulators that redirect the amyloidogenic cascade into unstructured “off-pathway” nontoxic intermediates [11].
Based on compound library screening or epidemiologic studies several closely related plant polyphenols, including catechins, theaflavins and tannins, have been proposed as broad inhibitors of protein aggregation and toxicity [11], [12], [13], [14]. The physicochemical features associated with the ability of flavonoids to inhibit amyloid fibril formation are most likely the presence of aromatic rings and the ability to form non-covalent interactions with different amino acids residues of the protein amyloidogenic core [15]. In fact, most flavonoids proved effective against amyloid beta (Aβ) abnormal misfolding have more than two aromatic rings essential for π–π stacking interactions with hydrophobic amino acid residues (Tyr, Phe) of Aβ and at least three hydroxyl groups that form hydrogen bonds with hydrophilic amino acid residues (His6, Ser8, Tyr10, His14, Lys16) of Aβ [15]. In addition, the planarity of the molecule is critical to increase surface contact with amyloid peptides [16], [17].
In the present study, we aimed to compare the effect of structurally related flavonoids, side-by-side, and advance our understanding on the mechanism of action of these phytochemicals on the TTR amyloid fibril formation process.
2. Materials and methods
2.1. Reagents
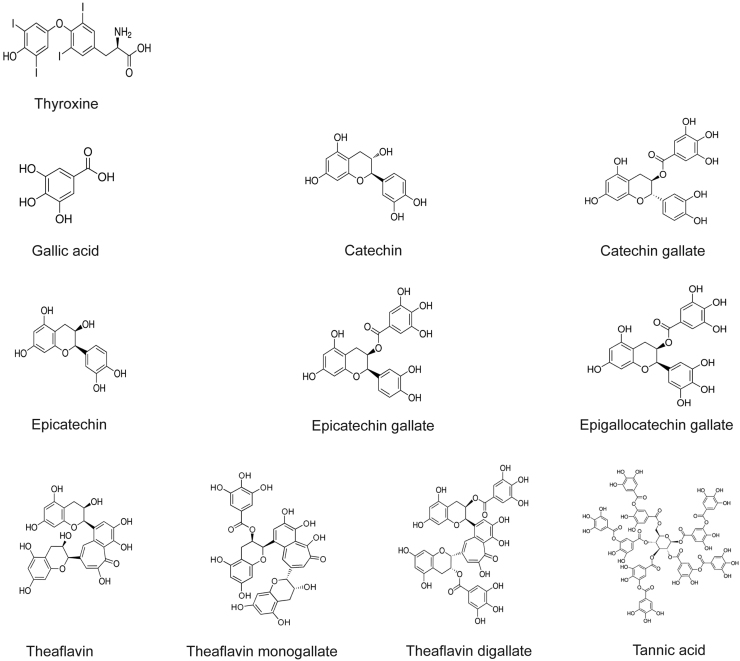
Gallic acid, catechin, catechin gallate, epicatechin, epicatechin gallate, epigallocatechin gallate (EGCG), theaflavin, theaflavin monogallate, theaflavin digallate and tannic acid were purchased from Sigma-Aldrich (St. Louis, MO, USA).
2.2. Recombinant transthyretin
Recombinant wild-type TTR (TTR WT) and TTR variants, namely TTR V30M, TTR L55P and TTR Y78F, were produced in a bacterial expression system and purified as previously described [17].
2.3. Plasma samples
Whole blood from five heterozygote carriers of TTR V30M and from five control individuals was collected in the presence of EDTA and centrifuged for plasma separation. All donors gave informed consent.
2.4. Thyroxine competition assays
Displacement of T4 from TTR was assayed qualitatively by incubation of recombinant TTR WT (10 μg) or whole plasma (5 μL) with [125I]T4 [specific radioactivity 1250 /μg; Perkin-Elmer, MA, USA] in the presence of different flavonoids (10× molar excess relative to TTR tetramer) solubilized in DMSO. Subsequently, proteins were separated by PAGE [18], revealed by phosphor imaging and quantified using the ImageQuant program version 5.1.
Competition of flavonoids with T4 for the binding to TTR was quantitatively assayed by gel filtration as previously described [18]. Briefly, a solution of 30 nM TTR in 0.1 M Tris, 0.1 M NaCl and 0.001 M EDTA buffer, pH 8.0 was incubated with a trace amount of [125I]T4 (50,000 cpm) plus increasing concentrations of inhibitor (0–10 μM) overnight at 4 °C. [125I]T4 bound to TTR was separated from free T4 in a gel filtration column. All samples were run in duplicate.
2.5. Nitroblue tetrazolium [NBT] staining assay
The binding of different flavonoids to TTR was assayed by nitroblue tetrazolium (NBT) staining which detects quinone-modified proteins [19]. Recombinant TTR WT and TTR V30M (2 mg/mL) in PBS were incubated in the presence of 10× molar excess of each flavonoid for 2 h at 37 °C, or saline (as control). Then, samples were boiled with SDS loading buffer and proteins were separated by SDS-PAGE (15% polyacrylamide gel). The gels were electroblotted onto nitrocellulose membrane (GE Healthcare) and stained with Ponceau S [0.1% in 5% acetic acid] to confirm blotting. Subsequently the membranes were washed with water and stained with glycinate/NBT solution (0.24 mM NBT in 2 M potassium glycinate, pH 10) for 20 min.
2.6. Isoelectric focusing [IEF] in semi-dissociating conditions
Briefly, 30 μL of human plasma were incubated with 5 μL of 10 mM solution of each flavonoid for 1 h at 37 °C and subjected to native PAGE. The TTR gel band was excised and applied to a semi-dissociating (4 M urea) IEF gel containing 5% (v/v) ampholytes pH 4–6.5 (GE Healthcare) run at 1200 V for 6 h [20]. Proteins were stained with Coomassie Blue, the gels were scanned (GS-800 Calibrated Densitometer; Biorad) and subjected to densitometry using the Quantity One 1-D Analysis Software version 4.6.
2.7. Dynamic light scattering (DLS) and transmission electron microscopy (TEM)
A solution of TTR Y78F (2 mg/ml) in PBS with or without 10× molar excess of different flavonoids was filtered through 0.2 μm Anotop syringe filters (Whatman, England) and incubated at 37 °C for 4, 6 and 12 days. DLS measurements were performed at 25 °C in a Malvern Zetasizer Nano ZS (Malvern, Worcestershire, UK). Each sample was measured tree times; average distributions are presented. Aliquots from the samples studied by DLS were also analyzed by TEM as previously reported [21]. Sample aliquots (5 μL) were adsorbed to carbon-coated collodion film supported on 200-mesh copper grids. The grids were negatively stained with 1% uranyl acetate and visualized with a Zeiss microscope at 60 kV.
2.8. Cell culture and DoT-blot filter assay for aggregate detection
To evaluate the different flavonoids as TTR aggregation inhibitors we used a rat Schwannoma (RN22) (American Type Cell Collection) cell line stably transfected with TTR L55P cDNA [22]. Cells were grown till 80% cell confluence with the compounds at 1 μM concentration in cell medium (~5 days). Then, cells were incubated for further 24 h still in the presence of compounds but in serum free media. TTR in the medium was quantified by ELISA, and equal amounts of TTR (500 ng) were doted onto a 0.2 μm pore cellulose acetate membrane—dot-blot filter assay. TTR aggregates retained in the membrane were immunodetected using rabbit anti-human TTR (Dako, Glostrup Denmark) (1:500) followed by anti-rabbit HRP antibody (1:1500) and ECL® visualization (GE Healthcare, Buckinghamshire, UK). Quantification of dot-blots was performed with a Bio-Rad ChemiDoc XRS system using the IMAGELAB software. Experiments were repeated at least three times in triplicate.
2.9. Cell toxicity assays
Rat Schwannoma cells [RN22] were propagated and maintained as described previously [21]. Briefly, 80% confluent cells in Dulbecco's minimal essential medium with 1% fetal bovine serum were exposed for 16 h to 2 μM of TTR Y78F oligomers. These oligomers were obtained by incubation of soluble TTR Y78F either in the absence or presence of a 10x molar excess of different flavonoids at 37 °C, for 6 days. Then, cells were trypsinized and lysed using lysis buffer containing 5 mM EDTA, 2 mM EGTA, 20 mM MOPS, 1% Triton X-100, 1 mM PMSF and Protease Inhibitor Mix (GE Healthcare). After centrifugation (14,000 rpm for 20 min at 4 °C), the supernatant was collected and used for determination of caspase-3 activity in cell lysates and BiP detection by Western blot analysis.
Caspase-3 activation was determined using the CaspACE fluorimetric 96-well plate assay system (Sigma-Aldrich) according to manufacturer's instructions. Protein concentration in lysates was determined with the Bio-Rad protein assay reagent (Sigma-Aldrich).
Western blot was used to evaluate BiP protein levels in cell lysates. Fifty micrograms of total protein from each tissue were separated on 15% SDS-PAGE and transferred onto a nitrocellulose Hybond-C membrane using a Mini Trans-Blot Cell (Bio-Rad) system. The primary antibodies and the respective dilutions used were: rabbit polyclonal anti-BiP (1:1000) (Abcam, Cambridge, UK) and mouse polyclonal GAPDH (1:1000) (Santa Cruz Biotechnology, Santa Cruz, CA, USA). Detection was performed with ECL® (GE Healthcare, Buckinghamshire, UK). Quantification of blots was performed with a Bio-Rad ChemiDoc XRS system using the IMAGELAB software, and immunosignals were normalized with GAPDH expression.
2.10. Statistical analysis
Data are expressed as the mean±SEM. One-way ANOVA followed by Bonferroni's post-hoc comparisons tests were performed in all statistical analyzes here presented using GraphPad Prism 5.0. Statistics with a value of p<0.05 were considered significant.
3. Results
3.1. Binding of flavonoids
The structural similarities of polyphenols (Fig. 1) with thyroxine, the natural TTR ligand, lead us to investigate their ability to compete with T4 for the binding to TTR. The compounds were incubated with isolated TTR WT and with trace amounts of radiolabeled thyroxine ([125I]T4). After incubation, samples were analyzed by PAGE. Similar ex vivo assays were performed with TTR in whole plasma from control individuals and heterozygote carriers of TTR V30M. No significant alteration of the TTR band intensity was found when comparing the sample in the presence and absence of the different compounds, which indicates that none of the compounds compete with T4 for the binding to plasma TTR, neither isolated recombinant TTR nor TTR in whole plasma from control individuals (not shown). Competition of flavonoids with [125I]T4 for the binding to recombinant TTR WT was also tested by a quantitative assay [18]. The results obtained demonstrate that none of the tested compounds significantly competed with T4 for the binding to TTR indicating that they do not interfere with the transport of T4 by TTR (data not shown). These results were consistent with the ex vivo data from the PAGE binding assay.
Fig. 1.
Chemical structures of polyphenols.
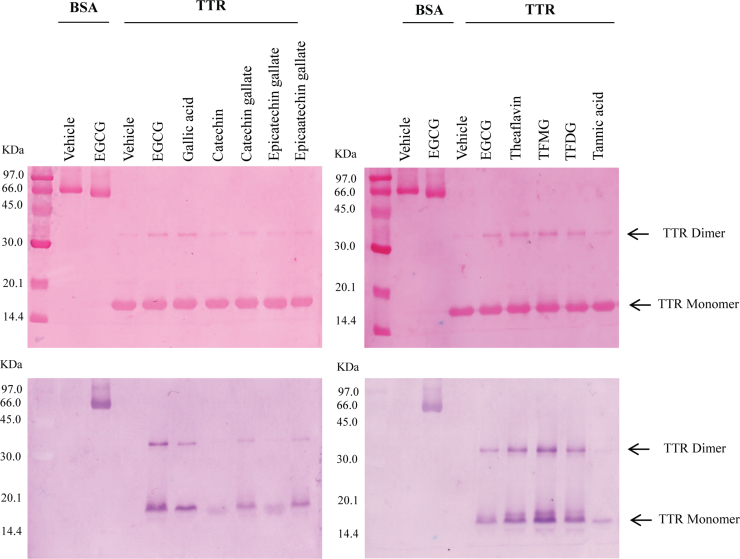
However, it has been reported that green tea polyphenols react with proteins forming complexes that are specifically detected with NBT/glycinate redox-cycling staining [23]. Therefore, to investigate flavonoids interaction with TTR WT and TTR V30M variant, each protein was incubated in the presence or absence (vehicle) of each polyphenol and subjected to SDS-PAGE followed by electroblotting. A bovine serum albumin (BSA) sample incubated with EGCG was included as positive control for protein–quinone complex formation. The membranes were stained with Ponceau S, to confirm blotting efficiency, followed by NBT/glycinate staining. Recombinant TTR WT (Fig. 2) incubated with different flavonoids showed purple protein bands of different intensity after NBT staining, while no color reaction occurred in vehicle treated samples. Similar results were obtained with TTR V30M (results not shown). Interestingly, TTR incubated with gallated flavonoids showed more intense protein bands as compared to samples treated with the non-gallated forms (ex. catechin gallate vs catechin, etc). Moreover, while samples treated with non-gallated flavonoids display a major purple protein band corresponding to the TTR monomer, in the presence of most gallated flavonoids an additional protein band consistent with the TTR dimer is observed. Therefore, NBT staining results indicate that the tested polyphenols bind to TTR and increase protein resistance to dissociation under the highly denaturing conditions of SDS-PAGE.
Fig. 2.
(A) Ponceau S staining of proteins treated or non-treated [vehicle] with polyphenols after electroblotting of SDS-PAGE gels onto nitrocellulose membranes. (B) NBT/glycinate staining of the membranes in (A).
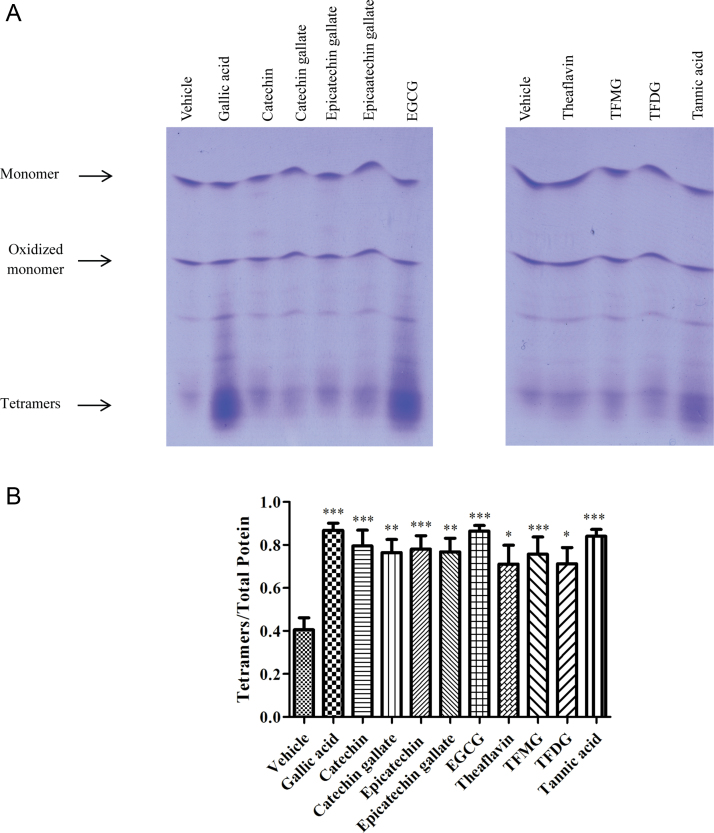
The effect of flavonoids on TTR tetramer stability was tested ex vivo by IEF under semi-dissociating conditions. EGCG, previously characterized as a TTR tetramer stabilizer, was used as reference in this assay [12], [21]. In the absence of flavonoids, plasma TTR presented a characteristic pattern of bands composed by monomer, oxidized monomer and several lower pI bands corresponding to different forms of tetramers (Fig. 3). The ratio tetramer/total TTR (tetramer+monomers) is typically higher (0.58±0.06) for plasma from normal individuals than for the heterozygote TTR V30M carriers plasma (0.40±0.06) (Fig. 3) [18].
Fig. 3.
(A) IEF analysis of plasma TTR stability after treatment with polyphenols. Plasmas from TTR V30M heterozygotic carriers were treated with polyphenols. Different molecular species visualized in the IEF gel after Coomassie Blue staining are indicated. These gels are representative of others run in parallel. (B) The histogram shows TTR tetramer/total TTR bands ratio obtained after densitometry of IEF gels corresponding to the analysis of 5 plasma samples from TTR V30M carriers]. *p<0.05; ** p<0.01; *** p<0.005.
The results showed that all tested flavonoids significantly stabilized TTR tetramers, both from WT and V30M variant, although with different stabilizing potencies. Overall, EGCG, gallic acid and tannic acid displayed the highest tetramers/total protein ratios, indicating that these compounds are the most potent TTR stabilizers under the tested conditions. Interestingly, all gallated flavonoids slightly altered the migration of TTR tetramers in the IEF gel.
3.2. Effect of polyphenols on TTR aggregation in vitro
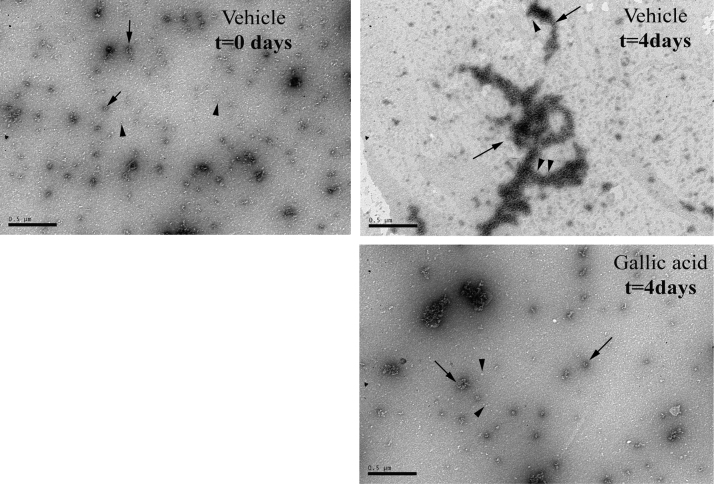
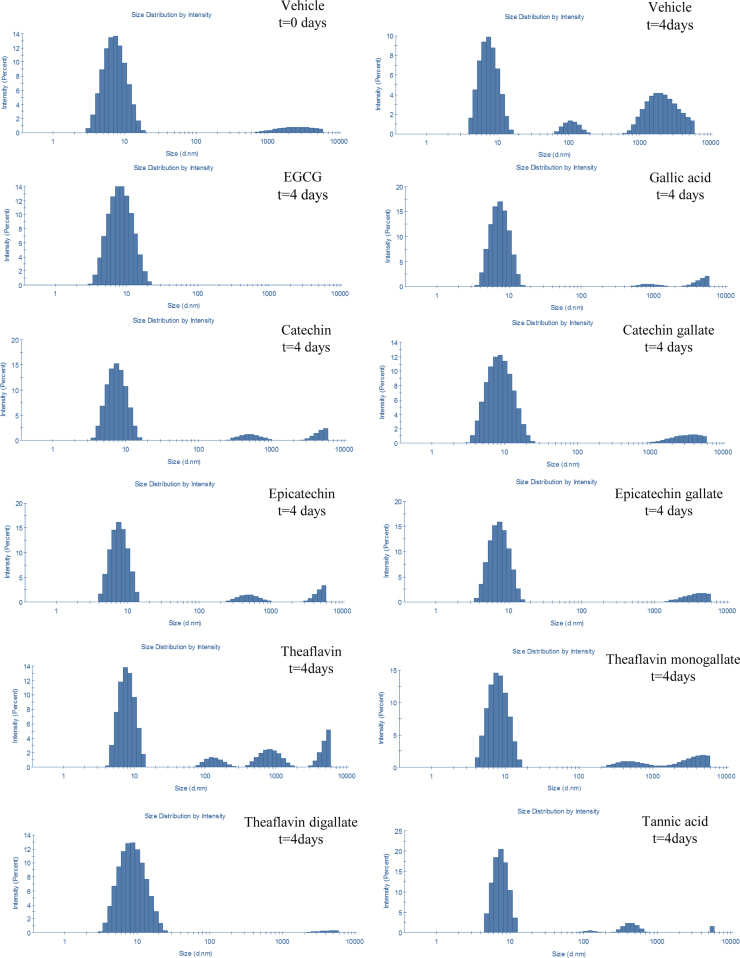
Next, flavonoids were tested for their effect on TTR aggregation using the TTR Y78F recombinant protein. This mutant aggregates under physiologic conditions, without agitation or acidification of the protein and its aggregation pathway has been characterized by TEM [12]. As shown in Fig. 4, after incubation for 4 days, TTR Y78F forms large protein aggregates and small fibrils. In this study, soluble TTR Y78F was incubated in the absence (vehicle) or presence of selected flavonoids and tested at different time points, up to 12 days. The samples were analyzed by DLS for measurement of the protein particles size distribution in solution. At the beginning of the experiment, the protein sample was composed mainly by particles of approximately 8 nm hydrodynamic diameter (dH) [Fig. 5], which is in accordance with previous reports for the TTR native tetrameric state [24], that evolved, after 4 days incubation, to small oligomeric species (105 nm dH) and later to large aggregates and fibrils (>1000 nm dH), as shown in Fig. 5. As expected from previous studies [21], EGCG incubated samples displayed a major particle population corresponding to native-state TTR indicating that EGCG stabilizes TTR tetramer, thus inhibiting protein aggregation. This inhibitory effect was observed during the entire incubation period. Although to different extent, all tested flavonoids inhibited the formation of small oligomeric species (50–200 nm dH) and mature fibrils (>1000 nm dH). Remarkably, EGCG (96.3±6.4%) and theaflavin digallate (98.3±0.4) sustained most TTR in its native conformation (~8 nm dH) during prolonged incubation (4 days) (Fig. 5; Table 1), supporting the notion that they inhibit the TTR amyloid cascade from the initial step in the pathway of self-assembly that is the irreversible dissociation of tetrameric TTR to unfolded monomers. In parallel, the same samples were analyzed by TEM for ultrastructure analysis of the protein species in solution. As expected from previous studies [12], TTR Y78F preparations treated with EGCG showed almost complete inhibition of TTR aggregation [data not shown]. Only soluble protein and small spherical aggregates, but no large oligomers or fibrils, could be observed for all time points analyzed [not shown]. A similar inhibitory effect was also observed in other flavonoid treated samples, particularly with samples treated with gallic acid or gallated forms of tea catechin and theaflavins (data not shown). These results were in accordance with those obtained from DLS.
Fig. 4.
TEM analysis of TTR aggregation. TTR Y78F was incubated at 37 °C under stagnant conditions for 4 days in the absence (vehicle) or presence of gallic acid. In panels referring to t=0 days for Vehicle and to t=4 days for gallic acid, arrowheads indicate soluble protein and arrows very small oligomers; in panel referring to t=4 days for vehicle, arrows indicate very large aggregates and arrowheads to small fibrils. Scale bar=500 nm.
Fig. 5.
DLS analysis of TTR aggregation. TTR Y78F was incubated at 37 °C under stagnant conditions for 4 days in the absence (vehicle) or presence of polyphenols. Representative graphs of each condition.
Table 1.
|
Particle intensity±SD (% ) |
|||
|---|---|---|---|
| Soluble TTR (~8 nm Dh) | TTR aggregates (>100 nm Dh) | ||
| t=0 days | |||
| Vehicle | 91.4±1.7 | 8.6±1.7 | |
| t=4 days | |||
| Vehicle | 54.9±4.8 | 38.6±3.0 | |
| Gallic acid | 84.2±9.4 | 15.8±9.4 | |
| Catechin | 84.9±4.8 | 15.1±4.8 | |
| Catechin gallate | 87.2±3.0 | 12.8±3.0 | |
| Epicatechin | 79.1±6.8 | 18.9±4.9 | |
| Epicatechin gallate | 89.2±0.5 | 10.8±0.5 | |
| EGCG | 96.3±6.4 | 3.7±6.4 | |
| Theaflavin | 67.3±4.0 | 26.3±3.3 | |
| Theaflavin monogallate | 78.9±5.9 | 19.1±4.9 | |
| Theaflavin digallate | 98.3±0.4 | 1.7±0.4 | |
| Tannic acid | 84.6±2.4 | 15.0±3.2 | |
3.3. Cell toxicity assays
Having characterized the inhibitory properties of the different flavonoids on TTR aggregation in vitro, the study continued with evaluation of the cytotoxicity of the TTR molecular species generated in the presence of phytochemicals. TTR Y78F was incubated in conditions to form oligomers in presence or absence of flavonoids. The resulting preparation was added to rat Schwannoma cells and incubated for 16 hours. Following, cells were lysed and Caspase-3 and BiP activation were evaluated. The results obtained are presented in Fig. 6. TTR oligomers added to cells considerably increased intracellular Caspase-3 activity as compared to addition of soluble TTR, as expected [25]. All tested flavonoids significantly impaired oligomer formation and thus toxicity of TTR Y78F preparation, although with different inhibitory efficiencies; gallic acid and galloyl esters from catechins or theaflavin presented stronger inhibitory effect on Caspase-3 activity as comparing to their ungallated forms. Under the tested conditions, EGCG was the most potent inhibitor of caspase-dependent pathways in TTR oligomers-induced apoptosis.
Fig. 6.
Activation of caspase-3 in RN22 cells exposed to 2 μM TTR Y78F oligomers or TTR Y78F oligomers pretreated with. *** p<0.005.
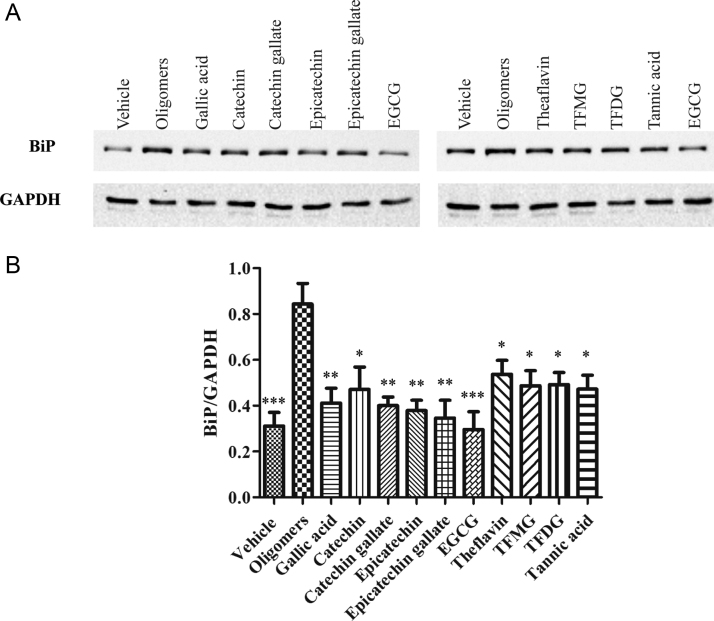
The same cell lysates were also tested for ER stress response triggered by TTR oligomers. As expected from previous studies [4], TTR oligomers induced an increase in the levels of ER-chaperone BiP comparing to soluble TTR, as depicted in Fig. 7. In contrast, pre-incubation of TTR with different flavonoids exerted a protective effect against ER-stress induction [Fig. 7]. These results are in accordance with those obtained for Caspase-3 determination.
Fig. 7.
(A) Anti-BiP Western blot of protein extracts from RN22 cells incubated with 2 μM TTR Y78F oligomers or TTR Y78F oligomers pretreated with polyphenols. (B) Histogram: normalized Bip/GAPDH density quantification. *p<0.05; **p<0.01; ***p<0.005.
3.4. Effect of flavonoids on TTR aggregation in cell culture
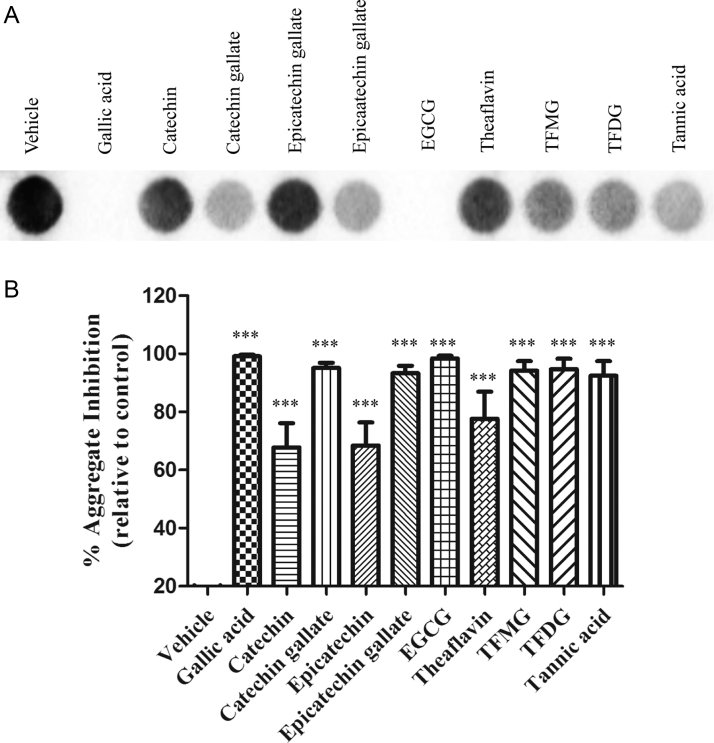
In view of the in vitro results described above we examined the impact of flavonoids on TTR abnormal misfolding and toxicity in a cell culture system. Thus, the effect of selected flavonoids on TTR aggregation was investigated in a rat Schwannoma cell line transfected with TTR L55P that secretes the TTR variant to the medium, where it forms small aggregates [22]. Conditioned medium was filtered through cellulose acetate membrane and the retained TTR aggregates were immunodetected with anti-TTR antibody. In this assay, we compared the conditioned media of cells incubated with flavonoids to the media of vehicle-treated cells. As expected, EGCG abolished TTR aggregation in conditioned medium (98.36%±0.90 aggregation inhibition), as evident in Fig. 8 by absence of signal in the corresponding dot-blot. This effect was surprisingly comparable to that obtained with gallic acid (99.10%±0.63). In addition, all the galloyl esters presented very similar and high levels of inhibition of aggregation as in the case of catechin gallate (95.13%±1.72), epicatechin gallate (93.27%±2.65), theaflavin monogallate (94.19%±3.28), theaflavin digallate [94.70%±3.60] and tannic acid (92.45%±4.98). Non-gallated flavonoids, including catechin, epicatechin and theaflavin also inhibited TTR aggregation although with lower inhibition potency (~70%).
Fig. 8.
(A) Immunodetection of TTR aggregates after dot-blot filter assay of the medium from TTR L55P transfected RN22 cells grown in the absence [vehicle] or in the presence of polyphenols. (B) Representation of the percentage of aggregation inhibition based on quantification of the dots obtained in (A).
4. Discussion
The current state of knowledge defines the amyloid cascade as a complex, multi-step process, where protein misfolding due to mutations, thermodynamics, aging or other stress factors result in abnormal protein self-assembly into higher-order structures. In TTR amyloidosis, deposition and accumulation of β-sheet-rich aggregated material in the extracellular compartment sets off a series of molecular events that result in cell death. Since TTR tetramer dissociation into partially unfolded monomers is the rate-limiting step in TTR amyloidogenic process, targeted therapies have mainly focused on small molecules that stabilize the tetramer, inhibiting TTR amyloid fibril formation. That is the case of ongoing therapeutic strategies for ameliorating TTR amyloidoses that include the use of small molecule TTR stabilizers such as tafamidis meglumine (Vyndaquel®) and diflunisal. In both cases Phase II/III clinical trials were recently completed for the treatment of FAP and demonstrated a slowing of disease progression in patients heterozygous for the V30M TTR mutation [26]. Recent studies have highlighted alternative approaches for intervention in amyloid formation and toxicity. In particular, plant-derived polyphenols, including catechins [11], [12], [14], theaflavins [13], tannins [27] and isoflavones [28] have been demonstrated to inhibit the formation of aggregate intermediates of different amyloid proteins in vitro and their associated cytotoxicity.
Among all polyphenols shown to interfere with amyloid protein misfolding and abnormal aggregation, tea flavonoid epigallocatechin gallate (EGCG) has received by far the most attention. EGCG inhibits aggregation and toxicity of various structurally and functionally distinct amyloid proteins, including α-synuclein [11], [14], Aβ [11], prion protein PrP [29] and TTR [12], [21]. While most currently available studies suggest that EGCG preferentially interacts with highly unstructured regions of intrinsically disordered molecules, work from our group has shown that EGCG can directly bind and stabilize TTR native tetrameric fold in vitro and in whole plasma without disturbing TTR physiological functions, in particular the transport of T4 [12], [21]. Although many efforts have been made to elucidate the molecular mechanism of natural occurring flavonoids against amyloidogenesis, the structure–activity relationship is still elusive and remains further explored.
Herein, we report the first biochemical study of major tea catechins and their larger polymers theaflavins to establish structure–activity relationships relevant for interaction with TTR and inhibition of TTR amyloidogenesis. We compared side-by-side the binding of different flavonoids to NBT/quinone-modified TTR and found that although they all form SDS-PAGE stable TTR-flavonoid complexes, a significant stronger signal was observed for samples incubated with catechins containing the galloyl moiety (gallic acid, catechin gallate, epicatechin gallate and EGCG) or theaflavins as compared to the ungallated catechins. In support of these observations, Ishii and colleagues [23] reported that EGCG presented higher affinity than the ungallated form (epigallocatechin) for the binding to human serum albumin, indicating that the galloyl moiety participated in the interaction of EGCG with albumin.
Previously, we have demonstrated that EGCG increases plasma TTR stability [12] and in this study we found that binding of EGCG structurally related flavonols to TTR could also impact on protein stability and that although all tested compounds significantly increased tetramer/total ratio, gallated monomeric (catechins) or polymeric (theaflavins) flavon-3-ols were the most potent tetramer stabilizers. Interestingly, gallated flavonols presented a significant alteration on the isoelectric focusing migration (isoelectric point) of TTR tetramers comparing to the ungallated forms. In addition, none of the tested flavonoids interferes with the transport of thyroxine by TTR. Thus, our data indicate that these flavonoids, particularly the gallated forms, strongly interact with TTR most likely at a region located at the surface of the molecule, probably at the interface of both TTR dimers, similarly to what has been described for EGCG [30].
In view of the in vitro results described above we examined the impact of flavonoids on TTR abnormal misfolding and toxicity in a cell culture system. Comparison of the effects of different flavonoids on inhibition of TTR aggregation and toxicity appears to correlate closely with i) the presence of gallate ester moiety in the catechin structure and ii) the number of hydroxyl groups in the B-ring catechin structure. Thus, the overall anti-amyloidogenic activity of flavonoids was: EGCG>gallic acid>catechin gallate=epicatechin gallate=theaflavin monogallate=theaflavin digallate=tannic acid>theaflavin=catechin=epicatechin.
Taken together, these results highlight the importance of the galloyl moiety on TTR anti-amyloidogenic activity associated with tea flavonoids. In support of this hypothesis, we observed a striking inhibition of TTR amyloidogenicity by gallic acid. This key finding is in agreement with previously reported data regarding the protective effects of flavonoid galloyl esters (i.e gallic acid, epicatechin gallate, EGCG) against β-amyloid induced toxicity using primary cultures of rat hippocampal cells as model [31]. Furthermore, the galloyl moiety seems to be required for major biological and pharmacological activities of tea flavonols, namely free radical-scavenging abilities [32] and antiproliferative activity of cancer cells [33], [34]. Stochastic conformational analysis in silico performed by Kuzuhara and colleagues revealed many conformations of EGCG and epicatechin gallate indicating that the mobility and flexibility of the galloyl moiety allow these compounds to take on multiple conformations that may be relevant for interaction with different molecular targets [35]. In addition, the presence of 3-trihydroxyl groups attached to the B-ring in EGCG enhances its anti-aggregation efficiency in comparison to those with dihydroxyl groups (catechin gallate and epicatechin gallate). Thus, the number of hydroxyl groups on the B-ring and D-Ring seems to impact on the anti-amyloidogenic potency of catechin gallate esters.
Although we present here the first direct evidence showing the structural-activity relationships of tea flavonoids on inhibition of TTR aggregation, it is most likely that multimodal activities of tea polyphenols, with emphasis on their neurorescue/neuroregenerative and mitochondrial stabilization actions, may potentiate their protective effects [36].
Pharmacokinetics and bioavailability of tea polyphenols in humans and rodents is poorly defined [37]. However it is known that gut absorption and metabolism of flavonoids varies depending on their chemical complexity. For instance, monomeric flavan-3-ols are principally absorbed in the small intestine while higher-molecular-weight polymers require prior metabolism into phenolic acids by the action of resident colonic microflora before absorption. Following absorption and passing through the circulatory system, metabolites are excreted in urine in amounts equivalent to about 40% of total flavonoid intake [38]. Taken this into account, different strategies aiming flavonoid bioavailability optimization have been proposed [39], including EGCG encapsulation in chitosan particles [40] or the design and semisynthesis O-acyl derivatives of EGCG [41] or co-treatment with piperine [42].
Neverthless, compelling evidence from epidemiologic observations and experimental studies in mouse models have indicated that green tea extracts (GTE) or EGCG consumption have beneficial effects in reducing the risk of neurodegeneration and dementia [43], [44], [45].
We have shown previously [46] that sub-chronic supplementation of FAP mice model with EGCG (100 mg/Kg/day) decreased TTR deposition along the gastrointestinal tract and peripheral nervous system (PNS). These results have recently been corroborated by an observational report on the effects of GTE consumption in patients with TTR cardiomyopathy showing an inhibitory effect of green tea and/or GTE on the progression of cardiac amyloidosis [47].
In conclusion, the current work provides strong support for the hypotheses that tea polyphenols, in particular galloyl esters, can act as chemical chaperones that inhibit or redirect otherwise aggregation-prone amyloidogenic intermediaries onto less hazardous species [21]. On basis of the structure-activity studies presented here, we identify the galloyl moiety as the key critical structure feature for TTR chaperoning by flavonoids. Our findings provide new evidence for comprehensive understanding of the mechanism of TTR toxicity inhibition by polyphenols and may open perspectives for the design and development of innovative disease-modifying drugs for the prevention and/or treatment of TTR-related amyloidosis.
Acknowledgments
This work was supported by FEDER funds through COMPETE and Fundação para a Ciência e Tecnologia [FCT] under the project FCOMP-01-0124-FEDER-021281 (PTDC/SAU-ORG/116645/2010), and through a Postdoctoral fellowship to Nelson Ferreira [SFRH/BPD/80356/2011] and a Researcher fellowship (Master) to Alda Pereira-Henriques (PTDC/SAU-ORG/116645/2010-PRO21901-BIM).
References
- 1.Blake C.C.F., Geisow M.J., Oatley S.J. Structure of prealbumin: secondary, tertiary and quaternary interactions determined by Fourier refinement at 1.8 Å. J. Mol. Biol. 1978;121:339–356. doi: 10.1016/0022-2836(78)90368-6. [DOI] [PubMed] [Google Scholar]
- 2.Monaco H.L. Three-dimensional structure of the transthyretin-retinol-binding protein complex. Clin. Chem. Lab. Med. 2002;40:1229–1236. doi: 10.1515/CCLM.2002.213. [DOI] [PubMed] [Google Scholar]
- 3.Sousa M.M., Saraiva M.J. Neurodegeneration in familial amyloid polyneuropathy: from pathology to molecular signaling. Prog. Neurobiol. 2003;71:385–400. doi: 10.1016/j.pneurobio.2003.11.002. [DOI] [PubMed] [Google Scholar]
- 4.Teixeira P.F., Cerca F., Santos S.D., Saraiva M.J. Endoplasmic reticulum stress associated with extracellular aggregates. Evidence from transthyretin deposition in familial amyloid polyneuropathy. J. Biol. Chem. 2006;281:21998–22003. doi: 10.1074/jbc.M602302200. [DOI] [PubMed] [Google Scholar]
- 5.Saraiva M.J., Costa P.P., Goodman D.S. Studies on plasma transthyretin (prealbumin) in familial amyloidotic polyneuropathy, Portuguese type. J. Lab. Clin. Med. 1983;102:590–603. [PubMed] [Google Scholar]
- 6.Coimbra A., Andrade C. Familial amyloid polyneuropathy: an electron microscope study of the peripheral nerve in five cases. II. Nerve fibre changes. Brain. 1971;94:207–212. doi: 10.1093/brain/94.2.207. [DOI] [PubMed] [Google Scholar]
- 7.Said G., Grippon S., Kirkpatrick P. Tafamidis. Nat. Rev. Drug Discov. 2012;11:185–186. doi: 10.1038/nrd3675. [DOI] [PubMed] [Google Scholar]
- 8.Rapezzi C., Quarta C.C., Riva L., Longhi S., Gallelli I., Lorenzini M., Ciliberti P., Biagini E., Salvi F., Branzi A. Transthyretin-related amyloidoses and the heart: a clinical overview. Nat. Rev. Cardiol. 2010;7:398–408. doi: 10.1038/nrcardio.2010.67. [DOI] [PubMed] [Google Scholar]
- 9.Hardy J.A., Higgins G.A. Alzheimer’s disease: the amyloid cascade hypothesis. Science. 1992;256:184–185. doi: 10.1126/science.1566067. [DOI] [PubMed] [Google Scholar]
- 10.Hardy J., Selkoe D.J. The amyloid hypothesis of Alzheimer’s disease: progress and problems on the road to therapeutics. Science. 2002;297:353–356. doi: 10.1126/science.1072994. [DOI] [PubMed] [Google Scholar]
- 11.Ehrnhoefer D.E., Bieschke J., Boeddrich A., Herbst M., Masino L., Lurz R., Engemann S., Pastore A. Wanker EE EGCG redirects amyloidogenic polypeptides into unstructured, off-pathway oligomers. Nat. Struct. Mol. Biol. 2008;15:558–566. doi: 10.1038/nsmb.1437. [DOI] [PubMed] [Google Scholar]
- 12.Ferreira N., Cardoso I., Domingues M.R., Vitorino R., Bastos M., Bai G., Saraiva M.J., Almeida M.R. Binding of epigallocatechin-3-gallate to transthyretin modulates its amyloidogenicity. FEBS Lett. 2009;583:3569–3576. doi: 10.1016/j.febslet.2009.10.062. [DOI] [PubMed] [Google Scholar]
- 13.Grelle G., Otto A., Lorenz M., Frank R.F., Wanker E.E., Bieschke J. Black tea theaflavins inhibit formation of toxic amyloid-β and α-synuclein fibrils. Biochemistry. 2011;50:10624–10636. doi: 10.1021/bi2012383. [DOI] [PubMed] [Google Scholar]
- 14.Lorenzen N., Nielsen S.B., Yoshimura Y., Vad B.S., Andersen C.B., Betzer C., Kaspersen J.D., Christiansen G., Pedersen J.S., Jensen P.H., Mulder F.A., Otzen D.E. How epigallocatechin gallate can inhibit α-synuclein oligomer toxicity in vitro. J. Biol. Chem. 2014;289:21299–21310. doi: 10.1074/jbc.M114.554667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lakey-Beitia J., Berrocal R., Rao K.S., Durant A.A. Polyphenols as therapeutic molecules in Alzheimer’s disease through modulating amyloid pathways. Mol. Neurobiol. 2014:24826916. doi: 10.1007/s12035-014-8722-9. PubMed PMID. [DOI] [PubMed] [Google Scholar]
- 16.Porat Y., Abramowitz A., Gazit E. Inhibition of amyloid fibril formation by polyphenols: structural similarity and aromatic interactions as a common inhibition mechanism. Chem. Biol. Drug Des. 2006;67:27–37. doi: 10.1111/j.1747-0285.2005.00318.x. [DOI] [PubMed] [Google Scholar]
- 17.McCutchen S.L., Colon W., Kelly J.W. Transthyretin mutation Leu-55-Pro significantly alters tetramer stability and increases amyloidogenicity. Biochemistry. 1993;32:12119–12127. doi: 10.1021/bi00096a024. [DOI] [PubMed] [Google Scholar]
- 18.Almeida M.R., Macedo B., Cardoso I., Alves I., Valencia G., Arsequell G., Planas A., Saraiva M.J. Selective binding to transthyretin and tetramer stabilization in serum from patients with familial amyloidotic polyneuropathy by an iodinated diflunisal derivative. Biochem. J. 2004;381:351–356. doi: 10.1042/BJ20040011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Paz M.A., Flückiger R., Boak A., Kagan H.M., Gallop P.M. Specific detection of quinoproteins by redox-cycling staining. J. Biol. Chem. 1991;266:689–692. [PubMed] [Google Scholar]
- 20.Altland K., Winter P., Sauerborn M.K. Electrically neutral microheterogeneity of human plasma transthyretin (prealbumin) detected by isoelectric focusing in urea gradients. Electrophoresis. 1999;20:1349–1364. doi: 10.1002/(SICI)1522-2683(19990601)20:7<1349::AID-ELPS1349>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 21.Ferreira N., Saraiva M.J., Almeida M.R. Natural polyphenols inhibit different steps of the process of transthyretin (TTR) amyloid fibril formation. FEBS Lett. 2011;585:2424–2430. doi: 10.1016/j.febslet.2011.06.030. [DOI] [PubMed] [Google Scholar]
- 22.Cardoso I., Almeida M.R., Ferreira N., Arsequell G., Valencia G., Saraiva M.J. Comparative in vitro and ex vivo activities of selected inhibitors of transthyretin aggregation: relevance in drug design. Biochem. J. 2007;408:131–138. doi: 10.1042/BJ20070689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ishii T., Mori T., Tanaka T., Mizuno D., Yamaji R., Kumazawa S., Nakayama T., Akagawa M. Covalent modification of proteins by green tea polyphenol [-]-epigallocatechin-3-gallate through autoxidation. Free Radic. Biol. Med. 2008;45:1384–1394. doi: 10.1016/j.freeradbiomed.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 24.Hou X., Parkington H.C., Coleman H.A., Mechler A., Martin L.L., Aguilar M.I., Small D.H. Transthyretin oligomers induce calcium influx via voltage-gated calcium channels. J. Neurochem. 2007;100:446–457. doi: 10.1111/j.1471-4159.2006.04210.x. [DOI] [PubMed] [Google Scholar]
- 25.Sousa M.M., Cardoso I., Fernandes R., Guimarães A., Saraiva M.J. Deposition of transthyretin in early stages of familial amyloidotic polyneuropathy: evidence for toxicity of nonfibrillar aggregates. Am. J. Pathol. 2001;3:1993–2000. doi: 10.1016/s0002-9440(10)63050-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ueda M., Ando Y. Recent advances in transthyretin amyloidosis therapy. Transl. Neurodegener. 2014;3:19. doi: 10.1186/2047-9158-3-19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ono K., Hasegawa K., Naiki H., Yamada M. Anti-amyloidogenic activity of tannic acid and its activity to destabilize Alzheimer's beta-amyloid fibrils in vitro. Biochim. Biophys. Acta. 2004;1690:193–202. doi: 10.1016/j.bbadis.2004.06.008. [DOI] [PubMed] [Google Scholar]
- 28.Green N.S., Foss T.R., Kelly J.W. Genistein, a natural product from soy, is a potent inhibitor of transthyretin amyloidosis. Proc. Natl. Acad. Sci. USA. 2005;102:14545–14550. doi: 10.1073/pnas.0501609102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rambold A.S., Miesbauer M., Olschewski D., Seidel R., Riemer C., Smale L., Brumm L., Levy M., Gazit E., Oesterhelt D., Baier M., Becker C.F., Engelhard M., Winklhofer K.F., Tatzelt J. Green tea extracts interfere with the stress-protective activity of PrP and the formation of PrP. J. Neurochem. 2008;107:218–229. doi: 10.1111/j.1471-4159.2008.05611.x. [DOI] [PubMed] [Google Scholar]
- 30.Miyata M., Sato T., Kugimiya M., Sho M., Nakamura T., Ikemizu S., Chirifu M., Mizuguchi M., Nabeshima Y., Suwa Y., Morioka H., Arimori T., Suico M.A., Shuto T., Sako Y., Momohara M., Koga T., Morino-Koga S., Yamagata Y., Kai H. The crystal structure of the green tea polyphenol [-]-epigallocatechin gallate-transthyretin complex reveals a novel binding site distinct from the thyroxine binding site. Biochemistry. 2010;49:6104–6114. doi: 10.1021/bi1004409. [DOI] [PubMed] [Google Scholar]
- 31.Bastianetto S., Yao Z.X., Papadopoulos V., Quirion R. Neuroprotective effects of green and black teas and their catechin gallate esters against beta-amyloid-induced toxicity. Eur. J. Neurosci. 2006;23:55–64. doi: 10.1111/j.1460-9568.2005.04532.x. [DOI] [PubMed] [Google Scholar]
- 32.Nanjo F., Mori M., Goto K., Hara Y. Radical scavenging activity of tea catechins and their related compounds. Biosci. Biotechnol. Biochem. 1999;63:1621–1623. doi: 10.1271/bbb.63.1621. [DOI] [PubMed] [Google Scholar]
- 33.Salucci M., Stivala L.A., Maiani G., Bugianesi R., Vannini V. Flavonoids uptake and their effect on cell cycle of human colon adenocarcinoma cells (Caco2) Br. J. Cancer. 2002;86:1645–1651. doi: 10.1038/sj.bjc.6600295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Dodo K., Minato T., Hashimoto Y. Structure-activity relationship of bis-galloyl derivatives related to [-]-epigallocatechin gallate. Chem. Pharm. Bull. 2009;57:190–194. doi: 10.1248/cpb.57.190. [DOI] [PubMed] [Google Scholar]
- 35.Kuzuhara T., Kise D., Shirakawa Y., Sasada K., Suganuma M., Fujiki H. Generation of mouse monoclonal antibody against (-)-epigallocatechin gallate. Biol. Pharm. Bull. 2008;31:816–819. doi: 10.1248/bpb.31.816. [DOI] [PubMed] [Google Scholar]
- 36.Mandel S.A., Amit T., Weinreb O., Reznichenko L., Youdim M.B. Simultaneous manipulation of multiple brain targets by green tea catechins: a potential neuroprotective strategy for Alzheimer and Parkinson diseases. CNS Neurosci. Ther. 2008;14:352–365. doi: 10.1111/j.1755-5949.2008.00060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Manach C., Williamson G., Morand C., Scalbert A., Rémésy C. Bioavailability and bioefficacy of polyphenols in humans. I. Review of 97 bioavailability studies. Am. J. Clin. Nutr. 2005;81:230S–242S. doi: 10.1093/ajcn/81.1.230S. [DOI] [PubMed] [Google Scholar]
- 38.Roowi S., Stalmach A., Mullen W., Lean M.E., Edwards C.A., Crozier A. Green tea flavan-3-ols: colonic degradation and urinary excretion of catabolites by humans. J. Agric. Food Chem. 2010;58(2):1296–1304. doi: 10.1021/jf9032975. [DOI] [PubMed] [Google Scholar]
- 39.Mereles D., Hunstein W. Epigallocatechin-3-gallate (EGCG) for clinical trials: more pitfalls than promises? Int. J. Mol. Sci. 2011;12(9):5592–5603. doi: 10.3390/ijms12095592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dube A., Nicolazzo J.A., Larson I. Chitosan nanoparticles enhance the intestinal absorption of the green tea catechins (+)-catechin and (-)-epigallocatechin gallate. Eur. J. Pharm. Sci. 2010;41(2):219–225. doi: 10.1016/j.ejps.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 41.Vyas S., Sharma M., Sharma P.D., Singh T.V. Design, semisynthesis, and evaluation of O-acyl derivatives of (-)-epigallocatechin-3-gallate as antitumor agents. J. Agric. Food Chem. 2007;55(15):6319–6324. doi: 10.1021/jf070519f. [DOI] [PubMed] [Google Scholar]
- 42.Lambert J.D., Hong J., Kim D.H., Mishin V.M., Yang C.S. Piperine enhances thebioavailability of the tea polyphenol (-)-epigallocatechin-3-gallate in mice. J. Nutr. 2004;134(8):1948–1952. doi: 10.1093/jn/134.8.1948. [DOI] [PubMed] [Google Scholar]
- 43.Checkoway H., Powers K., Smith-Weller T., Franklin G.M., Longstreth W.T., Jr, Swanson P.D. Parkinson’s disease risks associated with cigarette smoking, alcohol consumption, and caffeine intake. Am. J. Epidemiol. 2002;155:732–738. doi: 10.1093/aje/155.8.732. [DOI] [PubMed] [Google Scholar]
- 44.Rezai-Zadeh K., Shytle D., Sun N., Mori T., Hou H., Jeanniton D., Ehrhart J., Townsend K., Zeng J., Morgan D., Hardy J., Town T., Tan J. Green tea epigallocatechin-3-gallate (EGCG) modulates amyloid precursor protein cleavage and reduces cerebral amyloidosis in Alzheimer transgenic mice. J. Neurosci. 2005;25:8807–8814. doi: 10.1523/JNEUROSCI.1521-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lee Y.J., Choi D.Y., Yun Y.P., Han S.B., Oh K.W., Hong J.T. Epigallocatechin-3-gallate prevents systemic inflammation-induced memory deficiency and amyloidogenesis via its anti-neuroinflammatory properties. J. Nutr. Biochem. 2013;24:298–310. doi: 10.1016/j.jnutbio.2012.06.011. [DOI] [PubMed] [Google Scholar]
- 46.Ferreira N., Saraiva M.J., Almeida M.R. Epigallocatechin-3-gallate as a potential therapeutic drug for TTR-related amyloidosis: “in vivo” evidence from FAP mice models. PLoS One. 2012;7:e29933. doi: 10.1371/journal.pone.0029933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kristen A.V., Lehrke S., Buss S., Mereles D., Steen H., Ehlermann P., Hardt S., Giannitsis E., Schreiner R., Haberkorn U., Schnabel P.A., Linke R.P., Röcken C., Wanker E.E., Dengler T.J., Altland K., Katus H.A. Green tea halts progression of cardiac transthyretin amyloidosis: an observational report. Clin. Res. Cardiol. 2012;101:805–813. doi: 10.1007/s00392-012-0463-z. [DOI] [PMC free article] [PubMed] [Google Scholar]