Abstract
With the rapid growth in fungal infections and drug-resistant fungal strains, antifungal vaccines have become an especially attractive strategy to tackle this important health problem. β-Glucans, a class of extracellular carbohydrate antigens abundantly and consistently expressed on fungal cell surfaces, are intriguing epitopes for antifungal vaccine development. β-Glucans have a conserved β-1,3-glucan backbone with sporadic β-1,3- or β-1,6-linked short glucans as branches at the 6-O-positions, and the branches may play a critical role in their immunologic functions. To study the immunologic properties of branched β-glucans and develop β-glucan-based antifungal vaccines, three branched β-glucan oligosaccharides with 6-O-linked β-1,6-tetraglucose, β-1,3-diglucose, and β-1,3-tetraglucose branches on a β-1,3-nonaglucan backbone, which mimic the structural epitopes of natural β-glucans, were synthesized and coupled with keyhole limpet hemocyanin (KLH) to form novel synthetic conjugate vaccines. These glycoconjugates were proved to elicit strong IgG antibody responses in mice. It was also discovered that the number, size, and structure of branches linked to the β-glucan backbone had a significant impact on the immunologic property. Moreover, antibodies induced by the synthetic oligosaccharide-KLH conjugates were able to recognize and bind to natural β-glucans and fungal cells. Most importantly, these conjugates elicited effective protection against systemic Candida albicans infection in mice. Thus, branched oligo-β-glucans were identified as functional epitopes for antifungal vaccine design and the corresponding protein conjugates as promising antifungal vaccine candidates.
Keywords: carbohydrate, β-glucan, glycoconjugate, vaccine, fungus
Graphical Abstract
1. Introduction
Fungal infections, mostly caused by Candida, have drastically increased in the past decades,1–3 which poses a serious health problem. On the other hand, the efficacies of antifungal drugs have been impeded by their severe side effects and the emergence of drug-resistant fungal strains. As a result, deep-seated fungal infections in nosocomial settings have high mortalities,2, 4 and alternative therapeutic strategies, such as antifungal vaccines, are desirable.5, 6
For antifungal vaccine development, polysaccharides,7 such as β-glucans,8, 9 in the cell wall glycocalyx of pathogenic fungi are attractive targets,10 as they are exposed on cells and can elicit strong immune response.8, 9 β-Glucans7, 11 are a class of polysaccharides composed of ca. 1,500 β-1,3-linked D-glucose units with 40–50 additional β-1,6- or β-1,3-linked glucans as branches at the main chain glucose 6-O-positions.12, 13 β-Glucans are highly conserved on all pathogenic fungal cells,11 because they play important functional roles, such as keeping the mechanical strength and integrity of fungal cells.14 Moreover, β-glucans are Dectin-1 ligands, which can help the uptake of their conjugates by dendritic cells, thereby enhancing their immunogenicity.15 Thus, β-glucans have incited great interest in antifungal vaccine development. For instance, the protein conjugate of a natural β-glucan was found to elicit effective protection against Candida albican and Aspergillus fumigatus infections in a mouse model.16
In the past two decades, antibacterial glycoconjugate vaccines have made great progresses,5–9 but there is still no clinically useful antifungal glycoconjugate vaccine. One of the reasons is that the correlations between the structures of fungal carbohydrate antigens, such as β-glucan, and their antigenicity are unclear. To decipher the structure-activity relationships of β-glucans for the design and optimization of antifungal vaccines, glycoconjugates composed of structurally defined and homogeneous synthetic oligosaccharides should be very useful. Such vaccines, compared to vaccines composed of natural polysaccharides and as demonstrated in antibacterial vaccines,17–22 also have other advantages, such as improved purity and reproducibility, easier quality control, and free of microbial contaminants.
Recently, several linear and a branched oligo-β-glucans were synthesized and studied. Their protein conjugates could elicit immune responses23–26 comparable to that elicited by the CRM197 conjugate of Laminarin (Lam), a natural β-glucan with sporadic 6-O-branches.16 However, it was found that only linear oligo-β-glucans,23, 25 not the branched one,23 elicited protective immunities against fungal infections in mouse. These results were in contrast to that of the Lam conjugate.16 Clearly, the immunologic role of β-glucan branches is undefined.
2. Results and Discussion
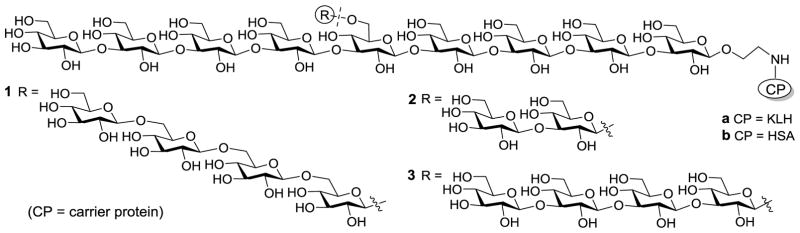
To better understand β-glucans and uncover the proper oligosaccharide epitopes for vaccine design, we prepared the keyhole limpet hemocyanin (KLH) conjugates 1a–3a (Figure 1) of three branched oligo-β-glucans with different β-1,6-tetraglucose (1a), β-1,3-diglucose (2a), and β-1,3-tetraglucose (3a) branches at the central sugar unit 6-O-position of the same β-1,3-nonaglucan backbone. They were designed to simulate and cover the immunologic determinant epitopes of natural β-glucans,19 such as that with short or long β-1,3-glucan and β-1,6-glucan branches. These glycoconjugates were tested in mice to study their immunologic properties and their capabilities to elicit protection against fungal infections. Herein, KLH was used as the carrier protein due to its easy accessibility and wide applications,27 although clinically it is not necessary the ideal carrier protein for antifungal vaccines. Nevertheless, it is suitable for immunologic and structure-immunogenicity relationship studies of the designed oligosaccharides and for revealing the proper antigens for vaccine development. They would also facilitate the comparison of 1a–3a with the reported KLH conjugates of linear oligo-β-glucans28 to gain more insights into structure-activity relationships of oligo-β-glucans. Moreover, the human serum albumin (HSA) conjugates 1b–3b (Figure 1) of the oligo-β-glucans were prepared, which were used as coating antigens for enzyme-linked immunosorbent assays (ELISA) of carbohydrate antigen-specific antibodies.
Figure 1.
The designed protein conjugates 1–3 of β-glucan oligosaccharides with β-1,6-tetraglucose (1), β-1,3-diglucose (2) and β-1,3-tetraglucose (3) branches at the 6-O-position of the central glucose unit of a nonasaccharide
Preparation of glycoconjugates
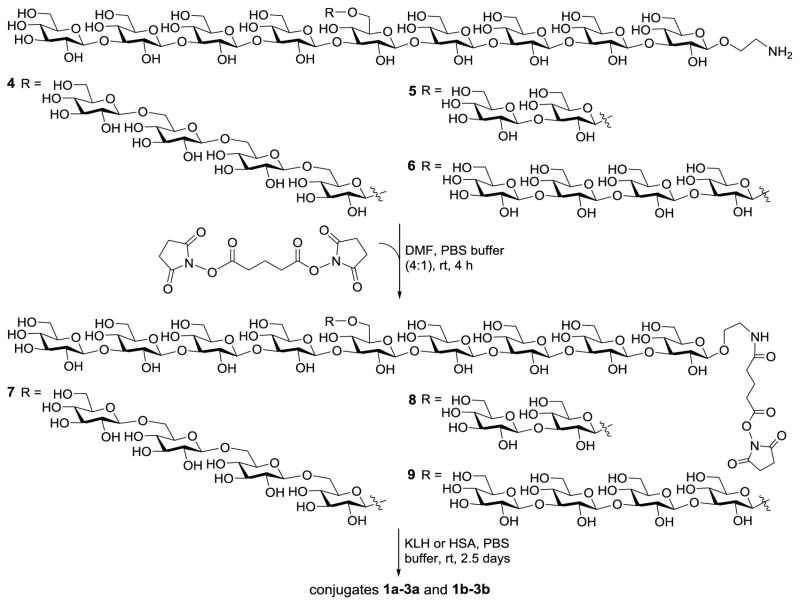
Oligosaccharides 4–6, which were prepared according to a reported procedure,29 were employed to couple with KLH and HSA through a bifunctional glutaryl linker (Scheme 1). This simple linker was selected since our previous studies proved that its conjugation reactions using activated glutaryl esters were easy and effective and it would not elicit antibodies against itself or affect the immunologic property of resultant glycoconjugates.30–32 Consequently, 4–6 were treated with excessive disuccinimidyl glutarate (DSG, 15 eq.) in DMF to form activated monoesters 7–9, which were readily purified via a precipitation process. Reaction of 7–9 with KLH and HSA in PBS afforded 1a,b–3a,b that were purified with a Biogel A0.5 column. We have demonstrated that active esters of N-hydroxysuccinimide were stable to PBS under natural conditions.32 The glucose contents of conjugates 1a,b–3a,b were assessed by the phenol-sulfuric acid method33 with KLH and HSA as blanks to exclude any potential interference caused by the glycans present in the proteins. The carbohydrate loadings of HSA conjugates 1b–3b were also assessed with MS (Supporting Information), based on the difference in molecular weights of a specific conjugate and its corresponding carrier protein. The two methods afforded similar results of carbohydrate loading for 1b, 2b and 3b, that is, 12.1%, 15.2%, and 15.5% with MS and 9.8%, 15.1%, and 13.7% with the phenol-sulfuric acid method, respectively. Again this work confirmed that the results of sugar analysis obtained with the phenol-sulfuric acid method were reliable32 and the method could be utilized to assess the carbohydrate loadings of the KLH conjugates 1a–3a, whose molecular weights were too big for MS analysis. The conjugation of oligo-β-glucans with KLH was further verified with SDS-PAGE, which showed the increase in molecular mass of 1a–3a as compared to KLH. The results have demonstrated that conjugation reactions between 7–9 and proteins was efficient and the antigen loading levels of 1a,b–3a,b were in the desirable range.34
Scheme 1.
Preparation of the β-glucan oligosaccharide–protein conjugates
Immunologic study of glycoconjugates
The immunologic properties of 1a–3a as vaccines were evaluated in female C57BL/6J mice. Each conjugate was mixed with the Freund’s complete adjuvant (CFA) to form an emulsion that was injected intramuscularly (i.m.) to each group of 5 mice. After the initial immunization, mice were boosted four times on days 14, 21, 28, and 38 via subcutaneous (s.c.) injection of the same emulsion. Blood samples were collected on day 0 before the initial immunization (blank control) and on days 21, 27, 38, and 48, respectively. Antisera were prepared from clotted blood samples according standard protocols and analyzed by ELISA with the corresponding HSA conjugates 1b–3b as capture reagents to detect the oligosaccharide-specific antibodies while avoiding the interference of anti-KLH antibodies.
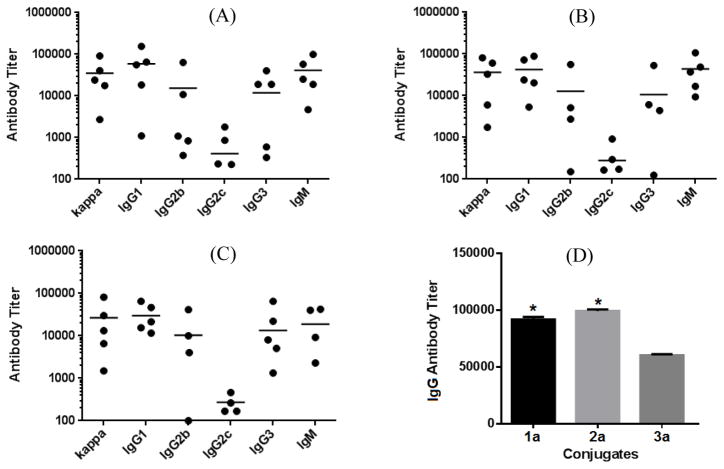
ELISA results (Figure 2) suggested that all three conjugates 1a–3a provoked high titers of antigen-specific total (anti-kappa) antibodies (Figure 2A–C). We further demonstrated by ELISA that the antisera obtained from mice immunized with KLH and CFA did not show any binding to capture reagents 1b–3b. Analysis of individual antibody isotypes revealed the production of high levels of IgM, IgG1, IgG2b, and IgG3 antibodies (Figure 2A–C), as well as a low level of IgG2c antibody. The induction of strong IgG, especially IgG1 and IgG2b, antibody responses indicated T cell-dependent immunity, because “to achieve class switch to IgG antibodies, the B cells need to interact with helper T cells.”18 Moreover, it was also reported that IgG1 and IgG2b antibodies had higher antigen binding affinity than other antibodies,35, 36 so these two isotypes of antibodies are considered protective37, 38 and should be functional at mediating the microorganism killing, although the mechanisms of action of anti-β-glucan antibodies are not completely understood.39 Nonetheless, the immunologic properties of 1a–3a as prophylactic vaccines are desirable.
Figure 2.
ELISA results of the day 48 antisera of individual mice inoculated with conjugates 1a (A), 2a (B), and 3a (C), with the antibody titer of each mouse shown as a dot and the group average as a black bar, as well as the total IgG antibody titers of the pooled day 48 antisera of each group of mice inoculated with 1a–3a (D). * Titer difference from that of 3a is statistically significant (P < 0.05).
It was also observed that 1a and 2a, which had antigens with a β-1,6-linked tetraglucose and β-1,3-linked diglucose branches, elicited similar titers of total IgG antibodies, 91,866 and 99,196 respectively, that were higher than the total IgG antibody titer of 3a (60,219) with a β-1,3-linked tetraglucose branch (Figure 3D). These results suggested that 1a and 2a were more immunogenic than 3a. Nonetheless, 3a induced robust and consistent immune responses in all tested mice.
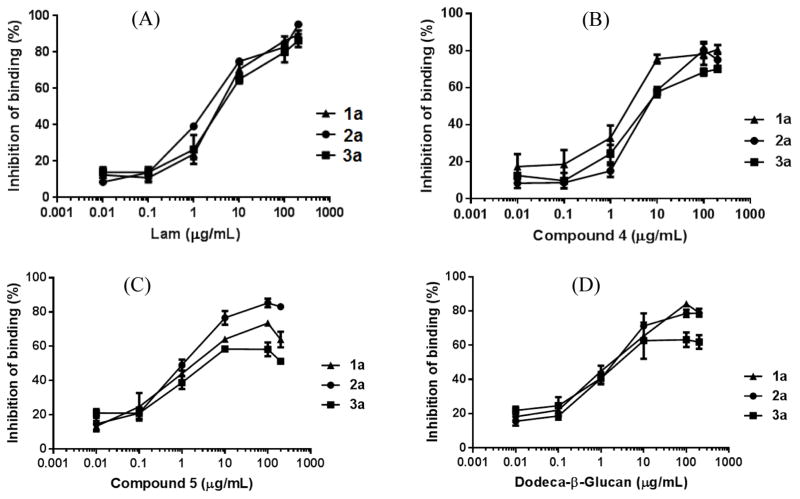
Figure 3.
Competitive inhibition of the binding between conjugates 1b–3b and the pooled antisera obtained with 1a–3a by Lam (A), 4 with a 1,6-linked branch (B), 5 with a 1,3-linked branch (C), and linear dodeca-β-glycan (D). The error bar shows the standard error of means of three parallel experiments.
Binding assays of the antisera to various β-glucans and fungal cells
To probe whether antibodies elicited by 1a–3a could recognize natural β-glucans, we analyzed the influence of Lam, a β-glucan carrying sporadic branches at the main chain 6-O-positions, on the binding between synthetic oligo-β-glucans and anti-1a-3a sera. Antisera (1:900 dilution) were mixed with various concentrations (0, 0.01, 0.1, 1, 10, 100, and 200 μg/mL) of Lam and then applied to ELISA with HSA conjugates 1b–3b as capture antigens. Antibody binding to Lam was shown by the decrease in the amount of antibodies bound to 1b–3b on the plates due to Lam-caused competitive binding inhibition, which was calculated according to the equation presented in the experimental section. Our results (Figure 3A) showed that Lam inhibited antibody binding to 1b–3b in a concentration-dependent manner with the 50% inhibition concentration (IC50) of 4.3, 2.2, and 7.8 μg/mL for 1a, 2a, and 3a, respectively, and at the 200 μg/mL concentration, the inhibition was >90% in all cases. Clearly, antibodies elicited by 1a–3a could recognize the structural epitopes of natural β-glucan, while Lam showed the strongest inhibition on the binding between the antiserum of 2a to the corresponding oligosaccharide in 2b.
We have also evaluated the influence of free oligosaccharides, including branched β-glucans 4 and 5 and linear dodeca-β-glucan, on the binding between antisera of 1a–3a and corresponding oligo-β-glucan antigens. It was shown that 4 had slightly stronger inhibition (IC50: 1.9 μg/mL) on the binding between 1b and antiserum 1a than on the binding between 2b and antiserum 2a (IC50: 8.0 μg/mL) or between 3b and antiserum 3a (IC50: 6.5 μg/mL) (Figure 3B). On the other hand, 5 had slightly stronger inhibition (IC50: 1.1 μg/mL) on the binding between 2b and antiserum 2a than on the binding between 1b and antiserum 1a (IC50: 2.0 μg/mL) or between 3b and antiserum 3a (IC50: 3.7 μg/mL) (Figure 3C). These results suggested that 1a–3a did elicit some antibodies specific to the unique structural motif of their oligosaccharide antigens, which are a β-1,6-linked glucan branch in 1a and a short disaccharide branch in 2a, but the majority of antibodies in the antisera were against a common motif. It was further disclosed that linear dodeca-β-glucan had similar and strong inhibition on the binding of all three antisera to respective antigens (IC50: 1.9, 1.8, and 2.4 μg/mL for 1a, 2a, and 3a, respectively, Figure 3D). The results of these preliminary studies led us to propose that the common structural motif recognized by the majority of antibodies elicited by 1a–3a was probably the main chain structure of β-glucans. This is a very interesting observation worth further detailed investigations.
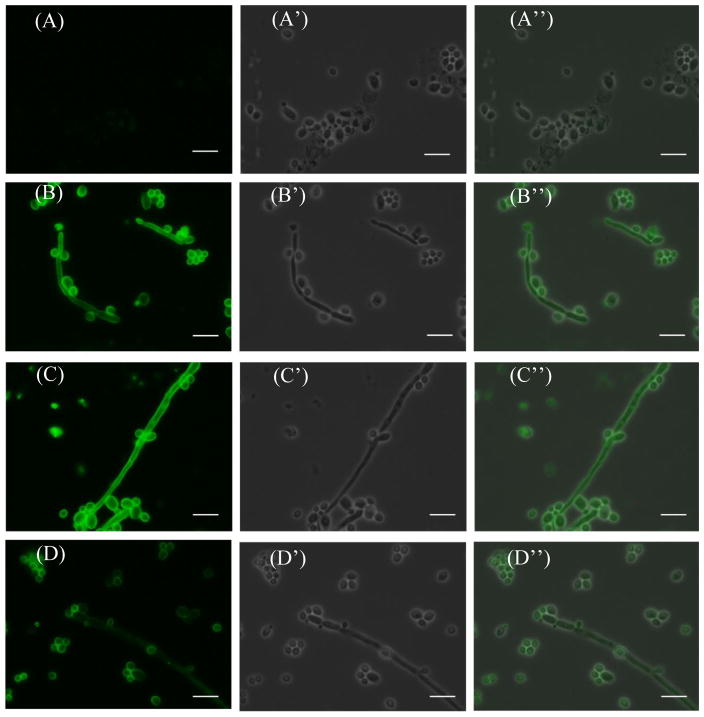
The binding of antisera with C. albicans (HKCA) cell was studied by immunofluorescence (IF) assay. Heat-killed HKCA cell was treated first with BSA blocking buffer to mask potentially nonspecific protein binding sites on the cell surface and then incubated with pooled antisera obtained with 1a–3a. Finally, the cell was stained with fluorescein isothiocyanate (FITC)-labeled goat anti-mouse kappa antibody and examined with a microscope. The results (depicted in Figure 4) showed that compared to the negative control (panel A), both the fungal particles and hyphal cells were uniformly IF-stained, indicating the strong binding of antisera to HKCA cell.
Figure 4.
IF staining results of the heat-killed C. albicans cells using sera obtained from mice (A) without vaccine treatment (negative control) or immunized with glycoconjugates (B) 1a, (C) 2a, and (D) 3a. Sub-panels A′ through D′ show the corresponding bright field images, and sub-panels APrime; through DPrime; show the merged images. The length of the white bar is 10 μm.
Protection against fungal infection
To validate the new conjugates as antifungal vaccines, 1a and 3a, whose carbohydrate antigens had the same length of side chains but different glycosyl linkages, were evaluated for their abilities to protect animals from fungal infection using a mouse model.16,23 The fungal cell used was Candida albicans (strain SC5314), one of the most common pathogenic fungi in clinic.40 Each group of 11 mice were immunized 4 times with 1a, 3a, or PBS buffer (negative control). Meanwhile, literature studies revealed that, similar to PBS, CFA alone did not elicit protection against C. albicans,16,23 and as mentioned above, KLH did not induce specific antibodies against C. albicans, which could also be considered as the negative controls for this study. Here, only 1a and 3a were selected for in vivo studies because their carbohydrate chains had the same length but different glycosidic linkages. After positive immune responses to 1a and 3a were confirmed, a pre-determined lethal dose of C. albicans cells (7.5×105 cells/mouse in 200 μL PBS) was i.v. injected into each mouse. The mice were monitored, and their survival time and rate are shown in Figure 5.
Figure 5.
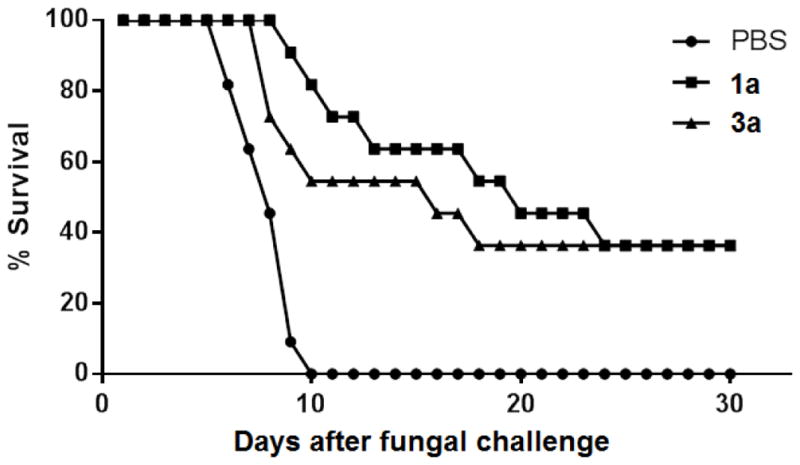
Survival rates of mice immunized with 1a and 1c or with PBS (the negative control) after i.v. injection of a lethal dose of C. albicans (7.5 ×105 cells per mouse and 11 mice per group).
As depicted in Figure 5, mice in the control group started to die on day 5 after C. albicans challenge, and all died of fungal infection in 10 days. No death occurred to the mice immunized with 1a and 3a until days 8 and 7, respectively, and the animal survival rate was about 82% for 1a and 55% for 3a on day 10. At the end of the experiment (30 days after fungal challenge), the survival rate for mice immunized with 1a and 3a was 37%, suggesting affective protection of the mice from C. albicans challenge. The results unambiguously confirmed that conjugates 1a and 3a elicited functional immunity that could effectively protect mice from C. albicans-caused infection. Moreover, 1a provided better protection against C. albicans than 3a at the beginning of infection, which was consistent with the discovery that 1a elicited higher titers of antibodies than 3a, but these two vaccines had similar long-term protection against C. albicans infection.
3. Conclusion
To assist rational design and optimization of β-glucan-based antifungal vaccine, it is crucial to have detailed information about the structure-antigenicity relationship of β-glucans. For this purpose, synthetic oligo-β-glucans and their conjugates can be especially useful, as they not only contain structurally homogeneous and defined antigens to facilitate structure-activity relationship study but also have other advantages as mentioned above, when compared to vaccines consisting of heterogeneous polysaccharides from natural sources. In this work, three oligo-β-glucans with different branches were coupled with KLH, and the resultant conjugates were evaluated in mice to gain insights into the impacts of side chains in β-glucans on their immunologic properties and to help identify the appropriate antigens for antifungal vaccine design.
The KLH conjugates of all three synthetic branched oligo-β-glucans elicited strong IgG antibody responses, indicating antibody class switch that is associated with long-lived antibody-mediated protection and T cell-dependent immunity,18 which is highly desirable for prophylactic vaccines. The results obtained here and in a previous study29 suggested that 1a–3a elicited similar pattern and strength of immune responses as the KLH conjugate of an optimized linear oligo-β-glucan, i.e., octa-β-glucan. In the previous paper, we only reported the results with Titermax Gold as the adjuvant, but we also evaluated the linear oligo-β-glucan-KLH conjugates in the presence of CFA, which gave very similar results. Thus, it was concluded that branched oligo-β-glucans should be at least as similarly promising antigens as, if not better than, linear oligo-β-glucans. It was also revealed that antibodies induced by 1a–3a could recognize and bind to natural β-glucans and fungal cells. Most importantly, 1a and 3a elicited protective immunities against systemically administered lethal C. albicans in mice. The immunologic results of 1a–3a were similar to that of Lam-CRM197 conjugate,16 even though a more immunogenic carrier protein CRM197 was used to construct this Lam conjugate. It is worth mentioning that there were differences in experimental details between our and the reported studies. Nonetheless, our results have proved that branched oligo-β-glucans can be useful for the design of functional antifungal vaccines.
Conjugates 1a and 2a provoked stronger IgG antibody responses than 3a, suggesting that both the structure and size of the side chains in branched oligo-β-glucans might have an impact on their immunogenicity. Nonetheless, both 1a and 3a were confirmed to elicit protection against C. albicans infection, and their long-term protection rates were similar. In contract, the CRM197 conjugate of an oligo-β-glucan with two β-linked glucose units as branches did not elicit obvious protection against fungal infection,23 even though it did provoke strong immune responses. These results indicated that the number and/or density of side chains in branched oligo-β-glucans might be also important for their immunologic properties. It seemed that to elicit protective immunity, branched oligo-β-glucans needed to carry fewer but longer than monosaccharide branches.
Although 1a provoked stronger immune responses than 3a, the two conjugates had similar long-term protection against C. albicans. Moreover, the long-term protection rate for 1a and 3a (both 37%) was only slightly higher than that (34%) of the KLH conjugate of linear β-octaglucan. The results suggested that so long as the conjugates provoked robust T cell-dependent immunity, they would be able to provide protection against C. albicans, even if they had different antibody titers. Based on current results, it is still early to conclude that, as antigens for antifungal vaccine development, branched oligo-β-glucans are significantly better than the optimized linear oligo-β-glucans or oligo-β-glucans with a β-1,6-linked branch are significantly better than those with a β-1,3-linked branch. Additionally, the readily accessible KLH was used to study the immunologic properties and structure-antigenicity relationships of the synthesized oligo-β-glucans to provide a proof of concept, but KLH is not the best carrier protein for antifungal vaccines. We expect that if more immunogenic carrier proteins, such as CRM197 or tetanus toxoid, are utilized to conjugate with the oligo-β-glucans, more potent vaccines and better protection results against fungi may be obtained to provide better understanding about oligo-β-glucans. Subsequently, we plan to couple the synthetic linear and branched oligo-β-glucans with tetanus toxoid or CRM197 and study them as vaccines, which will help gain more information about their structure-activity relationship and identify the best vaccines for clinical application.
In conclusion, three oligo-β-glucans with different branches were shown to elicit robust, β-glucan-specific, and protective immunities against C. albicans in mice. Branched oligo-β-glucans were identified as promising antigens for antifungal vaccine development. We have also revealed that the branches in β-glucans had an impact on its immunologic properties. Thus, it is rational to perform additional systematic studies by using redefined vaccines composed of the proper carrier protein and linear or branched oligo-β-glucans to gain more insights into their immunologic properties and functional mechanisms.
4. Methods
Preparation of HSA/KLH-oligosaccharide conjugates
Each synthetic oligosaccharide (5.0 mg) was dissolved in a mixture of DMF and 0.1M PBS (4:1, 0.5 mL), and to the solution was added DSG (15 eq). After the mixture was stirred at rt for 4 h, solvents were removed under vacuum. The resultant activated oligosaccharides were separated from excessive DSG through precipitation with EtOAc (4.5 mL) and washing with EtOAc 10 times. The products were mixed with HSA or KLH (in 30:1 molar ratio) in 0.1M PBS (0.35 mL) with stirring at rt for 3 days. The reaction mixtures were applied to a Biogel A0.5 column to remove excessive oligosaccharides with 0.1M PBS buffer (I 0.1, pH 7.8) as eluent. Fractions containing the glycoconjugates were combined and dialyzed against distilled water for 2 days. The solution was finally lyophilized to afford the glycoconjugates 1a,b–3a,b as white fluffy solids.
Analysis of carbohydrate loadings of conjugates 1a,b–3a,b with the sulfuric acid-phenol method33
Aliquots of a standard D-glucose solution (1 mg/mL) in water were added in ten dry 10-mL test tubes in 5 μL increment to get standard samples containing 0 to 50 μg of glucose. In the meantime, an accurately weighed sample of a glycoconjugate 1a,b–3a,b (with the estimated glucose content in 0 to 50 μg range) and the corresponding protein were added in two other tubes. To the tubes were added 4% phenol (500 μL) and 96% sulfuric acid (2.5 mL). After stirring for 20 min, the solutions were transferred into cuvettes, and their absorptions at 490 nm wavelength (A490) were measured. A sugar calibration curve was created by plotting the A490 of standard samples against the glucose contents and was used to calculate glucose content of each conjugate based on its A490 after subtracting the A490 of corresponding protein sample (the blank): Carbohydrate loading (%) = sugar weight in a tested sample/total weight of the sample × 100%
Analysis of the carbohydrate loadings of conjugates 1a–3a with MS method
Conjugates 1a–3a, as well as the free carrier protein HSA, were applied to MALDI-TOF MS analysis to get their average molecular weights (MW). The carbohydrate loadings of conjugates were calculated according the following equation:
Immunization of mouse
Each glycoconjugate 1a–3a (2.07, 2.36 and 2.07 mg, respectively) was dissolved in 10×PBS (0.3 mL) and then diluted with water to form 2×PBS solution. It was mixed with CFA (1:1, v/v, 1.5 mL) according to the manufacturer’s protocol to form an emulsion. Each group of five female C57BL/6J mice (Jackson Laboratory) were initially immunized (day 1) via i.m. injection of an emulsion (0.1 mL) containing about 6 μg of the carbohydrate antigen. Thereafter, each mouse was boosted four times on days 14, 21, 28, and 38 by s.c. injection of the same emulsion. Mouse blood samples were collected via mouse leg veins on day 0 prior to initial immunization and on days 27, 38 and 48 after boost immunizations. Antisera were prepared from the clotted blood samples.
ELISA assay41
ELISA plates were treated with a solution (100 μl) of HSA conjugate 1a, 2a or 3a (2 μg/ml) dissolved in coating buffer (0.1M bicarbonate, pH 9.6) at 4 °C overnight. The plates were incubated at 37 °C for 1 h, washed three times with PBS containing 0.05% Tween-20 (PBST), and incubated with blocking buffer containing 1.0% bovine serum albumin (BSA) in PBS at rt for 1 h. After washing with PBST three times, to the plates was added three-fold diluted (from 1:300 to 1:656100) antiserum in PBS (100 μL/well), followed by incubation at 37 °C for 2 h. The plates were washed with PBST and incubated at rt for 1 h with 1:1000 diluted solutions of alkaline phosphatase-linked goat anti-mouse kappa, IgG1, IgG2b, IgG2c, IgG3 or IgM antibody (100 μL/well). The plates were developed with p-nitrophenylphosphate (PNPP) (1.67 mg/mL, 100 μL) for 30 min at rt and analyzed at 405 nm wavelength. The observed optical density (OD) was plotted against antiserum dilution values in logarithmic scale, and the best-fit line was used to calculate antibody titers that were defined as the dilution value at an OD value of 0.2.
Assay of Lam inhibition on antiserum binding to the synthetic oligosaccharides
ELISA plates were coated with HSA conjugates 1b–3b (2 μg/ml) dissolved in 0.1M coating buffer at 37 °C for 1 h. After being washed with PBST 3 times, the plates were incubated with BSA blocking buffer. The pooled antisera (1:900 dilution) were mixed with serially diluted PBS solutions of Lam (from 0.01 to 200 μg/ml), and the mixtures were added to the plates that were incubated at 37 °C for 2 h, washed, and incubated with 1:1000 diluted solution of AP-labeled goat anti-mouse kappa antibody (100 μL/well) at rt for 1 h. The plates were washed, developed with PNPP (1.67 mg/mL, 100 μL) at rt for 30 min, and analyzed at 405 nm wavelength.
where Aw/o is the absorbance without Lam and Aw is the absorbance in the presence of Lam.
Immunofluorescence assay
HKCA cells were smeared on IF microscope slides that were dried, washed with PBST, and treated with 3% BSA blocking buffer at 37 °C for 1 h. The slides were incubated with 1:3 diluted (in PBST) antiserum or normal serum at 37 °C for 2 h, followed by washing and incubation with FITC-labeled goat anti-mouse kappa at rt for 1 h. The slides were washed, mounted with the Fluoromount aqueous mounting medium, and studied with the Zeiss ApoTome Imaging System using 100x/1.30 Oil objective lens.
In vivo evaluation of 1a and 3a to protect mice against C. albicans infection
Each group of 11 female C57BL/6J mice were immunized with an emulsion of 1a or 3a (6 μg carbohydrate antigen per dose) or with PBS (control) on days 1, 14, 21, and 28. Thereafter, C. albicans (strain SC5314) cells (7.5 ×105/mouse), harvested from pre-cultured YEPD medium at 28 °C for 24 h, in 200 μL PBS were i.v. injected in the mice on day 38. The mice were monitored daily for 30 days after the systemic challenge with C. albican cell. Note: The animal protocols used for both immunologic and fungal challenge experiments were approved by the Institutional Animal Use and Care Committee of Second Military Medical University.
Supplementary Material
Acknowledgments
NIH/NCI (R01 CA95142) supported the synthesis and immunological studies, and the National Major Scientific and Technological Special Program for “New Drugs Development” (2012ZX09502001) and National High Technology Research and Development Program (2012AA021500) of China supported the animal studies of this work. We thank Dr. B. Ksebati for some 2D NMR measurements, and the 600 MHz NMR instrument was supported by an NSF grant (CHE-0840413). The authors declare no conflict of interest in this work.
Footnotes
Supporting Information: MS spectra of HSA conjugates 1–3b. This information is available free of charge from the Internet at http://www.acs.org/......
References
- 1.Limper AH, Knox KS, Sarosi GA, Ampel NM, Bennett JE, Catanzaro A, Davies SF, Dismukes WE, Hage CA, Marr KA, Mody CH, Perfect JR, Stevens DA. An official American Thoracic Society statement: Treatment of fungal infections in adult pulmonary and critical care patients. Am J Respir Crit Care Med. 2011;183:96–128. doi: 10.1164/rccm.2008-740ST. [DOI] [PubMed] [Google Scholar]
- 2.Pfaller MA, Diekema DJ. Epidemiology of invasive candidiasis: a persistent public health problem. Clin Microbiol Rev. 2007;20:133–163. doi: 10.1128/CMR.00029-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lass-Florl C. The changing face of epidemiology of invasive fungal disease in Europe. Mycoses. 2009;52:197–205. doi: 10.1111/j.1439-0507.2009.01691.x. [DOI] [PubMed] [Google Scholar]
- 4.Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: Analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39:309–317. doi: 10.1086/421946. [DOI] [PubMed] [Google Scholar]
- 5.Cassone A, Casadevall A. Recent progress in vaccines against fungal diseases. Curr Opin Microbiol. 2012;15:427–433. doi: 10.1016/j.mib.2012.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edwards JE., Jr Fungal cell wall vaccines: an update. J Med Microbiol. 2012;61:895–903. doi: 10.1099/jmm.0.041665-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Masuoka J. Surface glycans of Candida albicans and other pathogenic fungi: physiological roles, clinical uses, and experimental challenges. Clin Microbiol Rev. 2004;17:281–310. doi: 10.1128/CMR.17.2.281-310.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cassone A. Development of vaccines for Candida albicans: fighting a skilled transformer. Nat Rev Microbiol. 2013;11:884–891. doi: 10.1038/nrmicro3156. [DOI] [PubMed] [Google Scholar]
- 9.Johnson MA, Bundle DR. Designing a new antifungal glycoconjugate vaccine. Chem Soc Rev. 2013;42:4327–4344. doi: 10.1039/c2cs35382b. [DOI] [PubMed] [Google Scholar]
- 10.Klis FM, de Koster CG, Brul S. A mass spectrometric view of the fungal wall proteome. Future Microbiol. 2011;6:941–951. doi: 10.2217/fmb.11.72. [DOI] [PubMed] [Google Scholar]
- 11.Bowman SM, Free SJ. The structure and synthesis of the fungal cell wall. BioEssays. 2006;28:799–808. doi: 10.1002/bies.20441. [DOI] [PubMed] [Google Scholar]
- 12.Fontaine T, Simenel C, Dubreucq G, Adam O, Delepierre M, Lemoine J, Vorgias CE, Diaquin M, Latge JP. Molecular organization of the alkali-insoluble fraction of Aspergillus fumigatus cell wall. J Biol Chem. 2000;275:27594–27607. doi: 10.1074/jbc.M909975199. [DOI] [PubMed] [Google Scholar]
- 13.Manners DJ, Masson AJ, Patterson JC. The structure of a β-(1→3)-d-glucan from yeast cell walls. Biochem J. 1973;135:19–30. doi: 10.1042/bj1350019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kollar R, Reinhold BB, Petrakova E, Yeh HJ, Ashwell G, Drgonova J, Kapteyn JC, Klis FM, Cabib E. Architecture of the yeast cell wall. Beta(1->6)-glucan interconnects mannoprotein, beta(1->)3-glucan, and chitin, J Biol Chem. 1997;272:17762–17775. doi: 10.1074/jbc.272.28.17762. [DOI] [PubMed] [Google Scholar]
- 15.Lipinski T, Fitieh A, StPierre J, Ostergaard HL, Bundle DR, Touret N. Enhanced immunogenicity of a tricomponent mannan tetanus toxoid conjugate vaccine targeted to DCs via Dectin-1 by incorporating β-glucan. J Immunol. 2013;190:4116–4128. doi: 10.4049/jimmunol.1202937. [DOI] [PubMed] [Google Scholar]
- 16.Torosantucci A, Bromuro C, Chiani P, De Bernardis F, Berti F, Galli C, Norelli F, Bellucci C, Polonelli L, Costantino P, Rappuoli R, Cassone A. A novel glyco-conjugate vaccine against fungal pathogens. J Exp Med. 2005;202:597–606. doi: 10.1084/jem.20050749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boltje TJ, Buskas T, Boons GJ. Opportunities and challenges in synthetic oligosaccharide and glycoconjugate research. Nat Chem. 2009;1:611–622. doi: 10.1038/nchem.399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peri F. Clustered carbohydrates in synthetic vaccines. Chem Soc Rev. 2013;42:4543–4556. doi: 10.1039/c2cs35422e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anish C, Schumann B, Pereira CL, Seeberger PH. Chemical biology approaches to designing defined carbohydrate vaccines. Chem Biol. 2014;21:38–50. doi: 10.1016/j.chembiol.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 20.Verez-Bencomo V, Fernandez-Santana V, Hardy E, Toledo ME, Rodriguez MC, Heynngnezz L, Rodriguez A, Baly A, Herrera L, Izquierdo M, Villar A, Valdes Y, Cosme K, Deler ML, Montane M, Garcia E, Ramos A, Aguilar A, Medina E, Torano G, Sosa I, Hernandez I, Martinez R, Muzachio A, Carmenates A, Costa L, Cardoso F, Campa C, Diaz M, Roy R. A synthetic conjugate polysaccharide vaccine against Haemophilus influenzae type b. Science. 2004;305:522–525. doi: 10.1126/science.1095209. [DOI] [PubMed] [Google Scholar]
- 21.Fernandez-Santana V, Cardoso F, Rodriguez A, Carmenate T, Pena L, Valdes Y, Hardy E, Mawas F, Heynngnezz L, Rodriguez MC, Figueroa I, Chang JN, Toledo ME, Musacchio A, Hernandez I, Izquierdo M, Cosme K, Roy R, Verez-Bencomo V. Antigenicity and immunogenicity of a synthetic oligosaccharide-protein conjugate vaccine against Haemophilus influenzae type b. Infec Immun. 2004;72:7115–7123. doi: 10.1128/IAI.72.12.7115-7123.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Astronomo RD, Burton DR. Carbohydrate vaccines: developing sweet solutions to sticky situations? Nat Rev Drug Discov. 2010;9:308–324. doi: 10.1038/nrd3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bromuro C, Romano M, Chiani P, Berti F, Tontini M, Proietti D, Mori E, Torosantucci A, Costantino P, Rappuoli R, Cassone A. Beta-glucan-CRM197 conjugates as candidates antifungal vaccines. Vaccine. 2010;28:2615–2623. doi: 10.1016/j.vaccine.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 24.Adamoa R, Tontinia M, Brogionia G, Romanoa MR, Costantinia G, Danielia E, Proiettia D, Bertia F, Costantinoa P. Synthesis of Laminarin Fragments and Evaluation of a β-(1,3) Glucan Hexasaccaride-CRM197 Conjugate as Vaccine Candidate against Candida albicans. J Carbohydr Chem. 2011;30:249–280. [Google Scholar]
- 25.Adamo R, Hu QY, Torosantucci A, Crotti S, Brogioni G, Allan M, Chiani P, Bromuro C, Quinn D, Tontini M, Berti F. Deciphering the structure–immunogenicity relationship of anti-Candida glycoconjugate vaccines. Chem Sci. 2014;5:4302–4311. [Google Scholar]
- 26.Hu QY, Allan M, Adamo R, Quinn D, Zhai H, Wu G, Clark K, Zhou J, Ortiz S, Wang B, Danieli E, Crotti S, Tontini M, Brogioni G, Berti F. Synthesis of a well-defined glycoconjugate vaccine by a tyrosine-selective conjugation strategy. Chem Sci. 2013;4:3827–3832. [Google Scholar]
- 27.Yin Z, Huang X. Recent Development in Carbohydrate Based Anti-cancer Vaccines. J Carbohydr Chem. 2012;31:143–186. doi: 10.1080/07328303.2012.659364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Liao G, Zhou Z, Burgular S, Liao J, Yuan C, Wu Q, Guo Z. Synthesis and immunological studies of linear oligosaccharides of β-glucan as antigens for antifungal vaccine development. Bioconjug Chem. 2015;26:466–476. doi: 10.1021/bc500575a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liao G, Burgula S, Zhou Z, Guo Z. A convergent synthesis of 6-O-branched β-glucan oligosaccharides. Eur J Org Chem. 2015:2942–2951. doi: 10.1002/ejoc.201500229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang Q, Ekanayaka SA, Wu J, Zhang J, Guo Z. Synthetic and immunological studies of 5′-N-phenylacetyl sTn to develop carbohydrate-based cancer vaccines and to explore the impacts of linkage between carbohydrate antigens and carrier proteins. Bioconjugate Chem. 2008;19:2060–2068. doi: 10.1021/bc800243f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Buskas T, Li Y, Boons GJ. The immunogenicity of the tumor-associated antigen Lewis (y) may be suppressed by a bi-functional cross-linker required for coupling to a carrier protein. Chem Eur J. 2004;10:3517–3524. doi: 10.1002/chem.200400074. [DOI] [PubMed] [Google Scholar]
- 32.Liao G, Zhou Z, Guo Z. Synthesis and immunological study of α-2,9-oligosialic acid conjugates as anti-group C meningitis vaccines. Chem Commun. 2015;51:9647–9650. doi: 10.1039/c5cc01794g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Wrolstad RE, Acree TE, Decker EA. Colorimetric Quantification of Carbohydrates. In: Wrolstad RE, Acree TE, Decker EA, Penner MH, Reid DS, Schwartz SJ, Shoemaker CF, Smith DM, Sporns P, editors. Current Protocols in Food Analytical Chemistry. John Wiley & Sons, Inc; 2001. pp. E1.1.1–E1.1.8. [Google Scholar]
- 34.Jennings HJ, Sood RK. Synthetic glycoconjugates as human vaccines. In: Lee YC, Lee RT, editors. Neoglycoconjugates: Preparation and Applications. Academic Press; San Diego: 1994. pp. 325–371. [Google Scholar]
- 35.Fossati-Jimack L, Ioan-Facsinay A, Reininger L, Chicheportiche Y, Watanabe N, Saito T, Hofhuis FM, Gessner JE, Schiller C, Schmidt RE, Honjo T, Verbeek JS, Izui S. Markedly different pathogenicity of four immunoglobulin G isotype-switch variants of an antierythrocyte autoantibody is based on their capacity to interact in vivo with the low-affinity Fcgamma receptor III. J Exp Med. 2000;191:1293–1302. doi: 10.1084/jem.191.8.1293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mond JJ, Lees A, Snapper CM. T cell-independent antigens type 2. Annu Rev Immunol. 1995;13:655–692. doi: 10.1146/annurev.iy.13.040195.003255. [DOI] [PubMed] [Google Scholar]
- 37.Yuan RR, Spira G, Oh J, Paizi M, Casadevall A, Scharff MD. Isotype switching increases efficacy of antibody protection against Cryptococcus neoformans infection in mice. Infec Immun. 1998;66:1057–1062. doi: 10.1128/iai.66.3.1057-1062.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Varshney AK, Wang X, Aguilar JL, Scharff MD, Fries BC. Isotype switching increases efficacy of antibody protection against staphylococcal enterotoxin B-induced lethal shock and Staphylococcus aureus sepsis in mice. MBio. 2014;5:e01007–01014. doi: 10.1128/mBio.01007-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Magliani W, Conti S, Giovati L, Maffei DL, Polonelli L. Anti-beta-glucan-like immunoprotective candidacidal antiidiotypic antibodies. Front Biosci. 2008;13:6920–6937. doi: 10.2741/3199. [DOI] [PubMed] [Google Scholar]
- 40.Kim J, Sudbery P. Candida albicans, a major human fungal pathogen. J Microbiol. 2011;49:171–177. doi: 10.1007/s12275-011-1064-7. [DOI] [PubMed] [Google Scholar]
- 41.Zhou ZF, Mondal M, Liao GC, Guo ZW. Synthesis and evaluation of monophosphoryl lipid A derivatives as fully synthetic self-adjuvanting glycoconjugate cancer vaccine carriers. Org Biomol Chem. 2014;12:3238–3245. doi: 10.1039/c4ob00390j. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.