Key Points
Question
Does fat grafting improve patient-reported outcomes in women undergoing breast reconstruction?
Findings
In this cohort study that included 2048 patients, women who later underwent fat grafting reported significantly lower breast satisfaction, psychosocial well-being, and sexual well-being 1 year postoperatively, compared with those who did not receive subsequent fat grafting. However, following fat grafting, both cohorts had similar scores 2 years postoperatively.
Meaning
Women who needed fat grafting for contour irregularities or volume deficits and had initially lower scores later reported scores comparable to those of women who did not require fat grafting, once they had undergone fat transfer.
Abstract
Importance
Fat grafting has proven to be a useful adjunct to breast reconstruction for the treatment of contour irregularities and volume deficits, but the proposed US Food and Drug Administration regulations may severely limit the ability of plastic surgeons to continue its use in this clinical context.
Objective
To determine whether fat grafting is associated with patient-reported outcomes (PROs) in patients undergoing breast reconstruction.
Design, Setting, and Participants
A longitudinal, multicenter, prospective cohort study was conducted between February 1, 2012, and July 31, 2016, at the 11 sites associated with the Mastectomy Reconstruction Outcomes Consortium Study. Eligible patients included women 18 years or older presenting for breast reconstruction after mastectomy with 2 years or more of follow-up. All primary procedure types (implant based and flap based) were eligible. Patients were excluded if they had not completed breast mound reconstruction by 1 year after starting reconstruction.
Interventions
Fat grafting as an adjunct to breast mound reconstruction.
Main Outcomes and Measures
Primary end points were patient-reported outcome measures as assessed by the validated BREAST-Q survey, with higher scores on a 0- to 100-point scale indicating better health-related quality of life. Survey subscales included breast satisfaction, as well as psychosocial, physical, and sexual well-being. Patient-reported outcomes were compared between those who received and did not receive fat grafting.
Results
A total of 2048 women were included (mean [SD] age, 49.4 [10] years), with 165 (8.1%) undergoing fat grafting between years 1 and 2. One year postoperatively, patients who later underwent fat grafting reported significantly lower breast satisfaction (adjusted mean difference [AMD], −4.74; 95% CI, −8.21 to −1.28; P = .008), psychosocial well-being (AMD, −3.87; 95% CI, −7.33 to −0.40; P = .03), and sexual well-being (AMD, −5.59; 95% CI, −9.70 to −1.47; P = .008), compared with those who did not receive subsequent fat grafting. Following the procedure, the fat-grafted cohort reported similar breast satisfaction (AMD, −0.68; 95% CI, −4.42 to 3.06; P = .72), psychosocial well-being (AMD, −0.59; 95% CI, −3.92 to 2.74; P = .73), and sexual well-being (AMD, −2.94; 95% CI, −7.01 to 1.12; P = .15) 2 years postoperatively.
Conclusions and Relevance
Fat grafting may improve breast satisfaction, psychosocial well-being, and sexual well-being in patients undergoing breast reconstruction.
This cohort study examines satisfaction and quality-of life in women who undergo fat grafting for breast reconstruction after mastectomy.
Introduction
During the past 2 decades, fat grafting has revolutionized breast reconstruction, enabling plastic surgeons to significantly improve aesthetic outcomes. Contour irregularities and volume deficits in both autologous and implant-based reconstructions can both be addressed with autologous fat transfer. The concept was initially met with considerable skepticism, given concerns over differentiating between fat necrosis and cancer recurrence on imaging and possible stimulation of cancer development by transferred fat. However, subsequent studies have failed to validate either concern. The most recent guidelines on fat grafting released by the American Society of Plastic Surgeons conclude that “fat grafting does not increase the risk of breast cancer recurrence.”(p2)Furthermore, the society endorses fat grafting as enabling patients who undergo breast reconstruction to experience “moderate to significant aesthetic improvement,” noting that “patients are satisfied with the results.”(p1) Despite this endorsement, the US Food and Drug Administration (FDA) has proposed new guidelines for autologous fat grafting. The FDA recently noted that, since fat grafts do not mimic the “basic function” of native breast tissue, autologous fat may be regulated as a drug, device, and/or biological product under the Federal Food, Drug, and Cosmetic Act and/or Section 351 of the Public Health Service Act. Thus, well-designed research assessing the efficacy of fat grafting is essential not only for high-quality patient care, but also to meet growing regulatory concerns over these procedures.
Despite the widespread assumption that patients are pleased with the results of fat grafting, there have been few studies assessing the effects of these techniques on patient-reported outcomes (PROs). Previous reports have evaluated patient satisfaction but have not included other quality-of-life measures. Existing research has also been limited by lower-level study designs, often lacking control groups for comparison. Only 1 small, retrospective case series has evaluated both breast satisfaction and quality of life after fat grafting using the validated BREAST-Q survey. Given the limitations of the aforementioned studies, we sought to use a longitudinal, multicenter, prospective analysis to evaluate the association of autologous fat grafting with PROs in patients undergoing implant- or flap-based breast reconstruction.
Methods
The Mastectomy Reconstruction Outcomes Consortium (MROC) Study is a multicenter, prospective cohort study funded by the National Cancer Institute in 2011 to compare long-term outcomes among common techniques of breast reconstruction. Eligible patients included all women 18 years or older presenting for first-time breast reconstruction following mastectomy for cancer treatment or prophylaxis. For the present analysis, patients were recruited between February 1, 2012, and July 31, 2016. Fifty-one plastic surgeons (including E.G.W., B.J.M., and J.H.K.) practicing at 11 centers in Michigan; New York; Illinois; Ohio; Massachusetts; Washington, DC; Georgia; Texas; and Manitoba, Canada, contributed patients to this study. The study was approved by each site’s institutional review board or research ethics board: University of Michigan Health System, Ann Arbor; St Joseph Mercy Hospital, Ypsilanti, Michigan; Memorial Sloan-Kettering Cancer Center, New York, New York; Northwestern Memorial Hospital, Chicago, Illinois; Ohio State Medical Center, Columbus; Brigham and Women’s Hospital, Boston, Massachusetts; Georgetown University Medical Center, Washington, DC; Georgia Institute of Plastic Surgery, Savannah; MD Anderson Cancer Center, Houston, Texas; University of Manitoba, Winnipeg, Canada; and University of British Columbia, Vancouver, Canada. All patients provided written informed consent; there was no financial compensation.
For this analysis, we included patients with 2 years or more of follow-up after initiation of breast mound reconstruction, with all primary procedure types (implant based and flap based) being eligible. Women still awaiting expander-implant exchange at 1 year were excluded owing to the potential confounding effects of the exchange procedure. Implant procedures converted to flap reconstructions were also excluded. Finally, any patients with reconstructive failure (defined as removal of the reconstructive flap or implant without replacement) were not eligible for this analysis. To minimize the potential association of breast mound formation with patient-reported outcomes, we specifically designed our study to evaluate only patients who completed breast mound reconstruction by year 1 and then underwent fat grafting between years 1 and 2 to minimize confounding. Without making this distinction, we may have seen that the PROs of patients who underwent fat grafting improved, but we would not have been able to evaluate the association of fat grafting alone given the significant contribution that the formation of a breast mound has on PROs. Patients who did not complete the initial preoperative questionnaire were withdrawn from the study owing to their lack of baseline data.
Medical records for each patient were reviewed to obtain the demographic and clinical data used in our analysis. These reviews were conducted preoperatively and at 1 and 2 years postoperatively by each site’s project coordinator. Online survey panels were completed by participants preoperatively and at 1 week, 3 months, 1 year, and 2 years following the initial reconstructive procedure. For the purposes of the present analysis, we used the 1- and 2-year survey responses. All data were collected with Velos (Velos Inc), a web-based clinical trial management system.
The PROs were assessed using the previously validated BREAST-Q survey, with scores ranging from 0 to 100; higher scores indicate greater satisfaction or better health-related quality of life. Survey subscales analyzed included satisfaction with breast, as well as psychosocial, physical, and sexual well-being. The PROs were compared between 2 cohorts of patients: those who underwent fat grafting between years 1 and 2 and those who did not.
In addition to demographic and clinical variables, such as age, body mass index, and race, oncologic and reconstructive variables were also collected from the medical records. These variables included procedure type, indication for mastectomy, laterality, timing of reconstruction, radiotherapy, cancer recurrence, additional revision procedures between years 1 and 2, and complications. Procedure types were divided into 3 subgroups: (1) implant, (2) autologous, and (3) mixed. Mixed procedures referred to those in which patients received implant reconstruction on 1 side and autologous reconstruction on the other. Indications for mastectomy were categorized as either cancer treatment or prophylaxis. Timing of reconstruction was divided into 3 subgroups as well: (1) immediate, (2) delayed, and (3) mixed. Mixed reconstruction referred to patients who underwent bilateral mastectomies and received immediate reconstruction for 1 side and delayed reconstruction for the contralateral side. Likewise, radiotherapy was divided into 3 subgroups: (1) before reconstruction, (2) during or after reconstruction, and (3) none. Cancer recurrence was documented as either recurred or did not recur. Other revision procedures were documented for both cohorts as a binary (yes/no) variable. Finally, the occurrence of complications was recorded as a binary variable at years 1 and 2 for both the fat-grafted and non–fat-grafted cohorts. Complications included any adverse postoperative event requiring additional treatment.
Statistical Analysis
Demographic and clinical variables were compared across the cohorts using a 2-tailed unpaired t test for continuous variables and the Pearson χ2 test for categorical variables. Patient-reported outcome measures at each time point were summarized as means (SDs) for each group. To compare differences in PROs between the 2 groups, mixed-effects regression models were used, with dependent variables being each PRO measure at postoperative years 1 and 2. Each model included an indicator for fat grafting between years 1 and 2 as the primary predictor and controlled for baseline PRO scores. Each model also adjusted for relevant clinical characteristics and included random intercepts for centers (hospitals) to account for between-center variability. Baseline and postoperative PRO measures were missing for some patients. To account for such missing data, multiple imputation with chained equations were used to create 10 complete imputed data sets, each of which was used to run the regression models specified above. The results were then combined, using Rubin’s rules. Adjusted means of PRO measures based on the model were then presented. All statistical analyses were performed with SAS, version 9.4 (SAS Institute), and statistical significance was set at P < .05.
Results
Demographic Data
Our total cohort included 2048 patients. Of these, 165 (8.1%) women underwent fat grafting between years 1 and 2, and 1883 did not (91.9%). More than half of the population received bilateral breast reconstruction (1159 [56.6%]), and most (1832 [89.5%]) underwent mastectomy for cancer treatment. With regard to procedure type, 1226 (59.9%) of patients received implant-based reconstruction, 786 (38.4%) received autologous procedures, and 36 (1.8%) underwent mixed procedures, while most reconstructions were immediate (1838 [89.7%]). The mean (SD) age of the cohort was 49.4 (10) years, and mean body mass index (calculated as weight in kilograms divided by height in meters squared) was 26.7. With regard to race and ethnicity, 1795 (87.6%) of the participants were white, 117 (5.7%) were African American, and 116 (5.7%) were Latino. Only 45 (2.2%) of the patients were current smokers.
Clinical and demographic data are summarized in Table 1. Patients who underwent fat grafting between years 1 and 2 were younger (47.5 [8.5] vs 49.5 [10.1] years; P = .01) and had a higher rate of complications (11 [6.7%] vs 48 [2.6%] years; P = .002) during that period than those who did not undergo fat grafting. Women undergoing fat grafting had a higher rate of concurrent revision procedures during the same period (123 [74.5%] vs 310 [16.5%]; P < .001), and patients who underwent fat grafting between years 1 and 2 were more likely to have undergone fat grafting before year 1 (50 [30.3%] vs 355 [18.9%]; P < .001). Patients who did not undergo fat grafting were more likely to have had implant-based reconstruction and less likely to have received radiotherapy, regardless of timing. Cancer recurrence during the study period did not differ significantly between the 2 groups—3 patients (1.8%) in the fat-grafted group and 38 patients (2.0%) in the non–fat-grafted group (P = .86).
Table 1. Clinical Characteristics of Patients by Fat-Grafting Status.
| Variable | Fat Graft Status Between Postoperative Years 1 and 2a | P Value | |
|---|---|---|---|
| Fat Grafted (n = 165) |
Non–Fat-Grafted (n = 1883) |
||
| Age, mean (SD), y | 47.5 (8.5) | 49.5 (10.1) | .01 |
| BMI, mean (SD) | 26.6 (5.1) | 26.7 (5.6) | .86 |
| Race, No. (%)a | |||
| White | 146 (89.0) | 1649 (88.6) | .91 |
| African American | 10 (6.1) | 107 (5.7) | |
| Other | 8 (4.9) | 105 (5.6) | |
| Ethnicity, No. (%)a | |||
| Hispanic/Latino | 6 (3.7) | 110 (6.0) | .24 |
| Non-Hispanic/Latino | 156 (96.3) | 1731 (94.0) | |
| Smoking history, No. (%)a | |||
| Nonsmoker | 107 (64.8) | 1211 (65.0) | .64 |
| Previous smoker | 56 (33.9) | 610 (32.7) | |
| Current smoker | 2 (1.2) | 43 (2.3) | |
| Procedure type, No. (%) | |||
| Implant | 87 (52.7) | 1139 (60.5) | .04 |
| Autologous | 72 (43.6) | 714 (37.9) | |
| Mixed | 6 (3.6) | 30 (1.6) | |
| Laterality, No. (%) | |||
| Unilateral | 65 (39.4) | 824 (43.8) | .28 |
| Bilateral | 100 (60.6) | 1059 (56.2) | |
| Indication for mastectomy, No. (%) | |||
| Therapeutic | 150 (90.9) | 1682 (89.3) | .53 |
| Prophylactic | 15 (9.1) | 201 (10.7) | |
| Timing of reconstruction, No. (%) | |||
| Immediate | 148 (89.7) | 1690 (89.8) | .98 |
| Delayed | 13 (7.9) | 143 (7.6) | |
| Mixed | 4 (2.4) | 50 (2.7) | |
| Radiotherapy, No. (%) | |||
| Before reconstruction | 22 (13.3) | 289 (15.3) | .006 |
| During/after reconstruction | 38 (23.0) | 262 (13.9) | |
| None | 105 (63.6) | 1332 (70.7) | |
| Fat grafting during postoperative year 1, No. (%) | |||
| Yes | 50 (30.3) | 355 (18.9) | <.001 |
| No | 115 (69.7) | 1528 (81.1) | |
| Other revision procedure done between postoperative years 1 and 2, No. (%) | |||
| Yes | 123 (74.5) | 310 (16.5) | <.001 |
| No | 42 (25.5) | 1573 (83.5) | |
| Cancer recurrence between postoperative years 1 and 2, No. (%) | |||
| Recurred | 3 (1.8) | 38 (2.0) | .86 |
| Did not recur | 162 (98.2) | 1845 (98.0) | |
| Complication during postoperative year 1, No. (%) | |||
| Yes | 54 (32.7) | 525 (27.9) | .19 |
| No | 111 (67.3) | 1358 (72.1) | |
| Complication between postoperative years 1 and 2, No. (%) | |||
| Yes | 11 (6.7) | 48 (2.5) | .002 |
| No | 154 (93.3) | 1835 (97.5) | |
Abbreviation: BMI, body mass index (calculated as weight in kilograms divided by height in meters squared).
Some data missing.
Comparison of PRO Measures
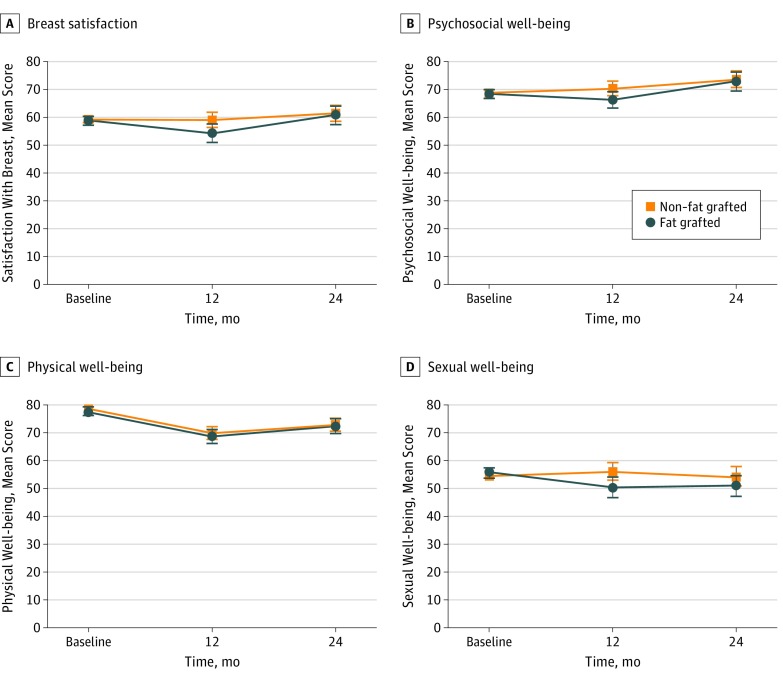
Table 2 summarizes unadjusted mean scores of the PRO measures for both groups at 3 separate time points: (1) before the operation (baseline), (2) 1 year after starting reconstruction (1 year postoperatively), and (3) 2 years after starting reconstruction (2 years postoperatively). Unadjusted means showed little to no group differences at baseline in breast satisfaction, psychosocial well-being, physical well-being, or sexual well-being. However, at 1 year postoperatively, unadjusted PRO scores tended to be higher for the non–fat-grafted group, compared with those who later underwent fat grafting between 1 and 2 years. Unadjusted means were comparable across the 2 cohorts at 2 years, at which point the fat-grafted group had completed fat transfer.
Table 2. Unadjusted and Adjusted Difference Scores of BREAST-Q PROs.
| BREAST-Q Survey | Cohort | Unadjusted Scores, Mean (SD) | Adjusted Mean Difference (95% CI)a | |||||
|---|---|---|---|---|---|---|---|---|
| Baseline | 1 y Postoperative | 2 y Postoperative | 1 y Postoperative | P Value | 2 y Postoperative | P Value | ||
| Satisfaction with breast | Fat grafted | 58.7 (21.5) | 60.1 (16.7) | 65.6 (17.1) | −4.74 (−8.21 to −1.28) | .008 | −0.68 (−4.42 to 3.06) | .72 |
| No fat graft | 59.2 (22.5) | 66.1 (17.2) | 66.0 (18.3) | |||||
| Psychosocial well-being | Fat grafted | 68.4 (18.7) | 67.2 (19.3) | 73.2 (19.2) | −3.87 (−7.33 to −0.40) | .03 | −0.59 (−3.92 to 2.74) | .73 |
| No fat graft | 68.8 (18.5) | 73.5 (19.2) | 75.3 (19.1) | |||||
| Physical well-being | Fat grafted | 77.2 (16.0) | 72.5 (13.5) | 74.8 (15.2) | −1.23 (−3.71 to 1.25) | .33 | −0.50 (−3.36 to 2.36) | .73 |
| No fat graft | 78.4 (14.7) | 76.2 (14.9) | 76.8 (14.9) | |||||
| Sexual well-being | Fat grafted | 55.7 (20.3) | 48.0 (20.5) | 52.8 (20.9) | −5.59 (−9.70 to −1.47) | .008 | −2.94 (−7.01 to 1.12) | .15 |
| No fat graft | 54.4 (20.9) | 54.7 (21.0) | 55.4 (21.9) | |||||
Fat-grafted and non–fat-grafted differences based on mixed-effects regression models with each PRO measure at 1 or 2 years postoperatively as the dependent variable. Each model included an indicator for fat grafting between years 1 and 2 as the primary predictor, and included as covariates baseline PRO, age, body mass index, procedure type, laterality, indication for mastectomy, timing of reconstruction, radiotherapy, smoking history, race, ethnicity, fat grafting before year 1 PRO measures, concurrent revision procedure, cancer recurrence, and prior complication. Also included are random intercepts for study sites (hospitals) and an interaction variable between fat grafting and concurrent revision procedures. Analyses were performed and combined using 10 imputed data sets.
Adjusted mean differences (AMDs) of PROs between the 2 groups based on mixed-effects regression models are reported in Table 2. Controlling for covariates at 1 year postoperatively, patients who later underwent fat grafting reported significantly lower scores on satisfaction with breast (adjusted mean difference [AMD], −4.74; 95% CI, −8.21 to −1.28; P = .008), psychosocial well-being (AMD, −3.87; 95% CI, −7.33 to −0.40; P = .03), and sexual well-being (AMD, −5.59; 95% CI, −9.70 to −1.47; P = .008), compared with women who did not receive subsequent fat grafting. The difference in physical well-being 1 year postoperatively was not significant (AMD, −1.23; 95% CI, −3.71 to 1.25; P = .33). By contrast, there were no significant differences at 2 years between the fat-grafted and non–fat-grafted cohorts for any of the BREAST-Q subscales (Table 2, Figure). Patients who underwent fat grafting reported similar scores in satisfaction with breast (AMD, −0.68; 95% CI, −4.42 to 3.06; P = .72), psychosocial well-being (AMD, −0.59; 95% CI, −3.92 to 2.74; P = .73), physical well-being (AMD, −0.50; 95% CI, −3.36 to 2.36; P = .73), and sexual well-being (AMD, −2.94; 95% CI, −7.01 to 1.12; P = .15). In essence, the fat-grafted group had “caught up” with the non–fat-grafted group in their PRO scores by year 2.
Figure. Adjusted Difference Scores of BREAST-Q Patient-Reported Outcomes at Baseline, 1 Year, and 2 Years Postoperatively.
BREAST-Q subscales on breast satisfaction (A), psychosocial well-being (B), physical well-being (C), and sexual well-being (D) evaluated; higher scores indicate greater satisfaction or better health-related quality of life. Error bars indicate 95% CIs.
Discussion
Although fat grafting was originally described by Neuber in 1893, it has recently gained widespread acceptance for use in aesthetic and reconstructive breast surgery. As recently as the 1990s, issues with high resorption rates limited its use. Furthermore, fat necrosis, a common occurrence after fat injection, can be difficult to distinguish from cancer on mammography. As a result of these and other concerns, the American Society of Plastic Surgeons released a sobering position statement in 1987, concluding, “the committee is unanimous in deploring the use of autologous fat injection in breast augmentation, [as] much of the injected fat will not survive,” and predicted that “detection of early breast carcinoma through xerography and mammography will become difficult and the presence of disease may go undiscovered.”(p2) Use of fat grafting was further discouraged by evidence from both animal and human studies, suggesting an increased risk of breast cancer recurrence as a consequence of these procedures.
More recently, newer and higher-quality evidence has dispelled many of these traditional concerns regarding the safety of autologous fat grafting in breast reconstruction. Multiple studies have indicated that breast imaging and cancer screening can still be effectively managed following fat grafting. Although research on breast cancer risk and fat transfer is ongoing, recent reports have failed to demonstrate an association between breast cancer recurrence and fat grafting. Finally, the development of newer grafting techniques by Coleman has reduced the rates of reabsorption and fat necrosis. Despite this benefit, the FDA has released proposed guidelines that may significantly limit grafting in patients undergoing breast reconstruction.
Although there is a growing body of evidence confirming the safety of autologous fat grafting in breast reconstruction, fewer studies have addressed its efficacy. Many plastic surgeons report superior clinical outcomes with these techniques; however, there remain relatively few studies critically evaluating fat grafting outcomes using valid, reliable measures. In particular, there is a shortage of research assessing PROs, specifically patient satisfaction, body image, and health-related quality of life. A systematic review on fat grafting in oncoplastic breast reconstruction identified only 8 studies that assessed patient satisfaction (satisfied, neutral, or dissatisfied) after fat grafting as an adjunct for breast reconstruction. To our knowledge, the only study in the literature that examined PROs in addition to satisfaction in patients undergoing fat grafting was underpowered, with just 68 patients, and lacked a control group. Although surgeons can readily identify improvements in contour and volume deficits with fat transfer in breast reconstruction, PRO data are also needed to confirm the utility of these procedures. Patient-reported outcomes are now viewed by payers and policymakers (including the FDA) as key measures of the effectiveness and quality of care. These agencies recognize that postoperative outcomes, such as symptom severity, functional status, and even satisfaction with aesthetic appearance, can be reliably assessed by the patients.
In our analysis, autologous fat grafting was associated with improvements in all 4 BREAST-Q subscale scores between years 1 and 2 following the initial reconstructive procedures. While the fat-grafted cohort lagged significantly behind the non–fat-grafted control group in 3 of 4 PRO measures at year 1, these differences diminished to nonsignificant levels by year 2. Although these improvements might be attributable to factors other than fat grafting, our analyses controlled for a wide variety of potential confounders, including concurrent revision procedures, prior complications, and radiotherapy. In addition, baseline (before reconstruction) subscale scores were comparable for the fat-grafted and non–fat-grafted cohorts, suggesting that our results likely are not attributable to preexisting group differences. These findings constitute what we believe to be the first evidence from a large, multicenter, prospective outcome study demonstrating the effectiveness of autologous fat grafting for breast reconstruction. The study design was also strengthened by its reliance on a validated, condition-specific PRO instrument.
The use of the BREAST-Q survey, a condition-specific PRO instrument, was particularly important for this study. Introduced in 2011 after extensive field testing, the BREAST-Q was specifically designed and validated to evaluate PRO in breast surgery, with a distinct procedure module for breast reconstruction. Unlike more generic PRO instruments, the BREAST-Q assesses domains specific to patients who undergo breast reconstruction, including satisfaction, psychosocial functioning, and sexuality, since they relate to the reconstruction. As noted above, 4 BREAST-Q subscales were analyzed in this study: satisfaction with breasts, physical well-being, psychosocial well-being, and sexual well-being. For each subscale, scores are reported in a range of 0 to 100, with higher scores indicating better outcomes. The BREAST-Q has preoperative and postoperative versions, which are psychometrically linked to quantify change.
Limitations
The study had some inherent limitations. As with any nonrandomized study design, our findings may have been attributable to unknown confounders not controlled for in our analysis. Because health care practitioners and patients have strong preferences in surgical decision making, randomization in studies like ours is usually not feasible for practical and, perhaps, ethical reasons. The study was also limited by missing survey data at year 2, although the rate of missing data between the 2 groups was not significantly different At 2 years postoperatively, the fat-grafted group has 59 (35.8%) missing PRO data, and the non–fat-grafted group had 637 (33.8%) missing PRO data (P = .62). However, it is always possible that patients did not complete questionnaires because of dissatisfaction or other unknown effects. Next, despite the use of a multicenter study design, our findings may not be generalizable to all patients in all locations. For example, the 11 centers in MROC are primarily academic medical centers, except for 1 private practice. In addition, owing to the self-selected nature of centers for participation, we cannot make conclusions about potential geographic differences in our outcome variables. However, our model included random intercepts for centers (hospitals) to account for between-center variability, which helps to make our results more generalizable. Finally, we were unable to perform subgroup analysis between various cohorts owing to a loss of power when our sample population was divided into subgroups.
Conclusions
By providing multicenter, prospective data confirming the benefits of autologous fat grafting as a useful adjunct in breast reconstruction, we hope that this study will contribute to the ongoing discussion with payers and regulators over the safety and effectiveness of these procedures. Our findings should bolster the ongoing assertion that fat grafting is an important tool in breast reconstruction and that this option should remain available to reconstructive surgeons and to the patients they serve.
References
- 1.Nahabedian MY. Implant-based breast reconstruction: strategies to achieve optimal outcomes and minimize complications. J Surg Oncol. 2016;113(8):895-905. [DOI] [PubMed] [Google Scholar]
- 2.Harless C, Jacobson SR. Current strategies with 2-staged prosthetic breast reconstruction. Gland Surg. 2015;4(3):204-211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.de Blacam C, Momoh AO, Colakoglu S, Tobias AM, Lee BT. Evaluation of clinical outcomes and aesthetic results after autologous fat grafting for contour deformities of the reconstructed breast. Plast Reconstr Surg. 2011;128(5):411e-418e. [DOI] [PubMed] [Google Scholar]
- 4.Delay E, Guerid S. The role of fat grafting in breast reconstruction. Clin Plast Surg. 2015;42(3):315-323, vii. [DOI] [PubMed] [Google Scholar]
- 5.Chala LF, de Barros N, de Camargo Moraes P, et al. . Fat necrosis of the breast: mammographic, sonographic, computed tomography, and magnetic resonance imaging findings. Curr Probl Diagn Radiol. 2004;33(3):106-126. [DOI] [PubMed] [Google Scholar]
- 6.Petit JY, Lohsiriwat V, Clough KB, et al. . The oncologic outcome and immediate surgical complications of lipofilling in breast cancer patients: a multicenter study—Milan-Paris-Lyon experience of 646 lipofilling procedures. Plast Reconstr Surg. 2011;128(2):341-346. [DOI] [PubMed] [Google Scholar]
- 7.Petit JY, Rietjens M, Botteri E, et al. . Evaluation of fat grafting safety in patients with intraepithelial neoplasia: a matched-cohort study. Ann Oncol. 2013;24(6):1479-1484. [DOI] [PubMed] [Google Scholar]
- 8.Kneeshaw PJ, Lowry M, Manton D, Hubbard A, Drew PJ, Turnbull LW. Differentiation of benign from malignant breast disease associated with screening detected microcalcifications using dynamic contrast enhanced magnetic resonance imaging. Breast. 2006;15(1):29-38. [DOI] [PubMed] [Google Scholar]
- 9.Silva-Vergara C, Fontdevila J, Descarrega J, Burdio F, Yoon TS, Grande L. Oncological outcomes of lipofilling breast reconstruction: 195 consecutive cases and literature review. J Plast Reconstr Aesthet Surg. 2016;69(4):475-481. [DOI] [PubMed] [Google Scholar]
- 10.Kaoutzanis C, Xin M, Ballard TN, et al. . autologous fat grafting after breast reconstruction in postmastectomy patients: complications, biopsy rates, and locoregional cancer recurrence rates. Ann Plast Surg. 2016;76(3):270-275. [DOI] [PubMed] [Google Scholar]
- 11.Delay E, Garson S, Tousson G, Sinna R. Fat injection to the breast: technique, results, and indications based on 880 procedures over 10 years. Aesthet Surg J. 2009;29(5):360-376. [DOI] [PubMed] [Google Scholar]
- 12.Post-mastectomy Fat Graft/Fat Transfer ASPS Guiding Principles. https://www.plasticsurgery.org/Documents/Health-Policy/Principles/principle-2015-post-mastectomy-fat-grafting.pdf. Updated June 2015. Accessed October 21, 2016.
- 13.Human Cells, Tissues, and Cellular and Tissue-Based Products (HCT/Ps) from Adipose Tissue: Regulatory Considerations; Draft Guidance for Industry. http://www.fda.gov/downloads/biologicsbloodvaccines/guidancecomplianceregulatoryinformation/guidances/tissue/ucm427811.pdf. Updated December 2014. Accessed October 21, 2016.
- 14.Groen JW, Negenborn VL, Twisk DJ, et al. . Autologous fat grafting in onco-plastic breast reconstruction: a systematic review on oncological and radiological safety, complications, volume retention and patient/surgeon satisfaction. J Plast Reconstr Aesthet Surg. 2016;69(6):742-764. [DOI] [PubMed] [Google Scholar]
- 15.Ho Quoc C, Piat JM, Carrabin N, Meruta A, Faure C, Delay E. Breast reconstruction with fat grafting and BRAVA pre-expansion: efficacy evaluation in 45 cases. Ann Chir Plast Esthet. 2016;61(3):183-189. [DOI] [PubMed] [Google Scholar]
- 16.Laporta R, Longo B, Sorotos M, Pagnoni M, Santanelli di Pompeo F. Breast reconstruction with delayed fat-graft-augmented DIEP flap in patients with insufficient donor-site volume. Aesthetic Plast Surg. 2015;39(3):339-349. [DOI] [PubMed] [Google Scholar]
- 17.Thekkinkattil DK, Salhab M, McManus PL. Feasibility of autologous fat transfer for replacement of implant volume in complicated implant-assisted latissimus dorsi flap breast reconstruction. Ann Plast Surg. 2015;74(4):397-402. [DOI] [PubMed] [Google Scholar]
- 18.Bonomi R, Betal D, Rapisarda IF, Kalra L, Sajid MS, Johri A. Role of lipomodelling in improving aesthetic outcomes in patients undergoing immediate and delayed reconstructive breast surgery. Eur J Surg Oncol. 2013;39(10):1039-1045. [DOI] [PubMed] [Google Scholar]
- 19.Cigna E, Ribuffo D, Sorvillo V, et al. . Secondary lipofilling after breast reconstruction with implants. Eur Rev Med Pharmacol Sci. 2012;16(12):1729-1734. [PubMed] [Google Scholar]
- 20.Serra-Renom JM, Muñoz-Olmo JL, Serra-Mestre JM. Fat grafting in postmastectomy breast reconstruction with expanders and prostheses in patients who have received radiotherapy: formation of new subcutaneous tissue. Plast Reconstr Surg. 2010;125(1):12-18. [DOI] [PubMed] [Google Scholar]
- 21.Bayti T, Panouilleres M, Tropet Y, Bonnetain F, Pauchot J. Fat grafting in breast reconstruction: retrospective study of satisfaction and quality of life about 68 patients. Ann Chir Plast Esthet. 2016;61(3):190-199. [DOI] [PubMed] [Google Scholar]
- 22.Pusic AL, Klassen AF, Scott AM, Klok JA, Cordeiro PG, Cano SJ. Development of a new patient-reported outcome measure for breast surgery: the BREAST-Q. Plast Reconstr Surg. 2009;124(2):345-353. [DOI] [PubMed] [Google Scholar]
- 23.Austin PC, Steyerberg EW. The number of subjects per variable required in linear regression analyses. J Clin Epidemiol. 2015;68(6):627-636. [DOI] [PubMed] [Google Scholar]
- 24.Rubin DB. Multiple Imputation for Nonresponse in Surveys. New York: John Wiley and Sons; 1987. [Google Scholar]
- 25.Ersek RA, Chang P, Salisbury MA. Lipo layering of autologous fat: an improved technique with promising results. Plast Reconstr Surg. 1998;101(3):820-826. [DOI] [PubMed] [Google Scholar]
- 26.Ersek RA. Transplantation of purified autologous fat: a 3-year follow-up is disappointing. Plast Reconstr Surg. 1991;87(2):219-227. [PubMed] [Google Scholar]
- 27.Carvajal J, Patiño JH. Mammographic findings after breast augmentation with autologous fat injection. Aesthet Surg J. 2008;28(2):153-162. [DOI] [PubMed] [Google Scholar]
- 28.Report on autologous fat transplantation. ASPRS Ad-Hoc Committee on New Procedures, September 30, 1987. Plast Surg Nurs. 1987;7(4):140-141. [PubMed] [Google Scholar]
- 29.Zhang Y, Daquinag A, Traktuev DO, et al. . White adipose tissue cells are recruited by experimental tumors and promote cancer progression in mouse models. Cancer Res. 2009;69(12):5259-5266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lindegren A, Chantereau MW, Bygdeson M, Azavedo E, Schultz I. Autologous fat transplantation to the reconstructed breast does not hinder assessment of mammography and ultrasound: a cohort study. World J Surg. 2016;40(5):1104-1111. [DOI] [PubMed] [Google Scholar]
- 31.Pinell-White XA, Etra J, Newell M, Tuscano D, Shin K, Losken A. Radiographic implications of fat grafting to the reconstructed breast. Breast J. 2015;21(5):520-525. [DOI] [PubMed] [Google Scholar]
- 32.Masia J, Bordoni D, Pons G, Liuzza C, Castagnetti F, Falco G. Oncological safety of breast cancer patients undergoing free-flap reconstruction and lipofilling. Eur J Surg Oncol. 2015;41(5):612-616. [DOI] [PubMed] [Google Scholar]
- 33.Kronowitz SJ, Mandujano CC, Liu J, et al. . Lipofilling of the breast does not increase the risk of recurrence of breast cancer: a matched controlled study. Plast Reconstr Surg. 2016;137(2):385-393. [DOI] [PubMed] [Google Scholar]
- 34.Coleman SR. Facial recontouring with lipostructure. Clin Plast Surg. 1997;24(2):347-367. [PubMed] [Google Scholar]
- 35.Pezold ML, Pusic AL, Cohen WA, et al. . Defining a research agenda for patient-reported outcomes in surgery: using a Delphi survey of stakeholders. JAMA Surg. 2016;151(10):930-936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Cano SJ, Klassen AF, Scott AM, Cordeiro PG, Pusic AL. The BREAST-Q: further validation in independent clinical samples. Plast Reconstr Surg. 2012;129(2):293-302. [DOI] [PubMed] [Google Scholar]
- 37.BREAST-Q Users’ Manual https://webcore.mskcc.org/breastq/qscore/qscore-manual.pdf. Updated July 2012. Accessed October 21, 2016.