Abstract
Purpose
With multiple anti-vascular endothelial growth factor and steroid therapies available for diabetic macular edema (DME), there is a need for early determination of the best treatment for a particular patient to prevent irreversible vision loss from chronic DME. In this study, we classify patients as responders or non-responders to anti-vascular endothelial growth factor (VEGF) monotherapy in the treatment of DME after a single anti-VEGF injection.
Methods
The study was designed as a single center, retrospective, interventional case series. We included patients who received 3 consecutive monthly injections with the same anti-VEGF agent. We excluded patients who were treated for DME in the preceding 3 months with any form of anti-VEGF therapy. Visual acuity and central retinal thickness (CRT) data were followed for one year. Receiver operating characteristic (ROC) curve analysis was performed in order to identify cutoff values for identifying responders.
Results
107 eyes were reviewed, with 40 eyes of 34 patients meeting all inclusion criteria. Based on ROC curve analysis, a reduction in CRT by > 15% at 1-month, identified eyes that responded to treatment and had a >25% reduction in CRT at 3-months (sensitivity 0.75, specificity 0.92).
Conclusion
DME eyes that have early response to anti-VEGF treatment by reduction in CRT will have significant response to treatment by 3 months.
Keywords: Anti-VEGF, Diabetic Macular Edema, Diabetic Retinopathy, Receiver Operating Characteristics Curve, Vascular Endothelial Growth Factor
Introduction
Diabetic retinopathy is the leading cause of vision loss in the working-age population.1 Moderate visual loss in patients with diabetic retinopathy is most often caused by diabetic macular edema (DME).2 Macular edema treatment includes focal and grid laser photocoagulation3, 4 and pharmacologic therapy via intravitreal injections of anti-vascular endothelial growth factor (VEGF) agents5–9 and steroid medications.10, 11
Intravitreal anti-VEGF therapy is the most commonly used treatment approach in the management of DME because of its efficacy as validated by clinical trial data5, 9 and has become the first-line treatment for DME. It avoids the complications of cataract formation and increased intraocular pressure attributed to steroid treatments. Patients in the RISE and RIDE trials had, on average, an approximately 40% reduction in CRT on OCT within the first 3 months of initiating monthly treatment with ranibizumab (Lucentis®; Genentech, San Francisco, CA).5 While anti-VEGF therapy is often very effective, a subset of patients appear to be refractory and often require other forms of medical management, including alternative anti-VEGF therapies and/or steroids.12
Persistent DME (for more than 6 months) is often thought to induce permanent changes in vision that limit recovery even when the macular edema resolves. Patients in the RISE and RIDE trials who were initially treated with sham injections before crossing over to ranibizumab treatment did not experience similar visual acuity gains as those patients receiving ranibizumab immediately.13 This suggests that prompt and efficacious treatment of DME is important to maximize visual outcomes and that delay in effective treatment may adversely affect vision. Thus, there is a need to treat DME quickly and efficiently, and to determine optimal treatment paradigm for each patient early in the disease course. As most patients diagnosed with DME receive anti-VEGF therapy, early identification of anti-VEGF response status is important.
A method to identify responders to anti-VEGF therapy would aid physicians to tailor treatment to likely patient outcomes. Changes in CRT have been used to identify responders to anti-VEGF therapy for retinal vein occlusions.14 Specifically receiver operating characteristic (ROC) curves were used to identify the appropriate screening cutoff to identify at 1-month eyes that would be poor responders to anti-VEGF therapy even if treated with multiple intravitreal injections.14 We aim to develop a similar model to allow early identification of response to anti-VEGF treatment in eyes with diabetic macular edema.
Materials and Methods
The study was conducted with Institutional Review Board approval and adhered to the tenets of Declaration of Helsinki. The study was designed as a single center, retrospective, interventional case series. All research activities were performed at the Associated Retinal Consultants, P.C., Royal Oak, MI, USA.
Data Acquisition
We performed a retrospective chart review of all patients seen between June 2011 and June 2014 with DME and undergoing at least 3 treatments of anti-VEGF therapy. Of the patients identified by billing codes, further inclusion criteria were applied. Inclusion criteria were: (1) 18 years of age or older, (2) either treatment-naïve DME or no treatment with any therapy (laser, steroid, or anti-VEGF) for the prior 3 months, and (3) underwent three consecutive monthly treatments with the same anti-VEGF agent. Patients that (1) changed anti-VEGF medication during the initial 3-month period or (2) had follow up periods less than 6 weeks after the initial 3 months were excluded. Patients’ best corrected visual acuity (BCVA) and CRT was recorded at baseline, 1-month, 2-months, 3-months and extended out to 6-months and 12-months. After 3-months of anti-VEGF monotherapy, patients were kept in the study even if the treating physician changed the type of anti-VEGF therapy or treatment modality. We recorded demographic information, Snellen BCVA as measured by Topcon autorefractor, CRT by macular OCT measurements, lens status, prior treatment history (including anti-VEGF therapy, steroid, or focal laser), and history of vitrectomy. OCT measurements of the foveal thickness were manually performed using the caliper functions in spectral domain OCT (SD-OCT) (Cirrus® [Carl Zeiss Meditec, Dublin, CA] or Spectralis® [Heidelberg Engineering, Heidelberg, Germany]).
Based on RISE and RIDE clinical trial data a 40% reduction of CRT was seen at 3 months on average.5 Based on this we conservatively used a 25% reduction of CRT at 3 months as a method of identifying anti-VEGF responders, which correlates with 2 standard deviations below the mean CRT reductions seen in these trials.5 Using this as a criterion, “responders” to anti-VEGF were defined as those with > 25% decrease in CRT at 3 months. We used reduction in CRT after a single anti-VEGF injection to identify responders. A series of different cutoff values for the initial reduction in CRT after one anti-VEGF treatment were used and sensitivity and specificity values for each were determined. These values were plotted using receiver operating characteristic curve analysis to determine the optimal screening cutoff with the highest combined sensitivity and specificity.
Statistical Analysis
BCVA was converted into logarithm of the minimum angle of resolution (logMAR) visual acuities by standard methods.15 One patient had vision worse than 20/400, and an approximation of 20/2000 for count fingers vision at two feet was used as has been previously published.15 Two-tailed paired t-tests were used for statistical analysis of eyes pre and post intervention, while two-tailed student’s t-tests assuming unequal variance were used to compare difference in the responder and non-responder subgroups (Microsoft Excel 2011, Redmond, WA). Statistical significance required a p-value <0.05. Given that the CRT change at 1 month is a continuous, uniformly distributed variable, we used an empirical (non-parametric) Receiver Operating Characteristics (ROC) curve analysis plotting 1 minus specificity (i.e., false positives) against sensitivity to determine a cutoff to identify poor responders after a single intravitreal anti-VEGF injection (StataCorp. 2013. Stata Statistical Software: Release 13. College Station, TX: StataCorp LP).16, 17 An advantage of this method is that no structural assumptions are made about the form of the plot, and the underlying distributions of the outcomes for the two groups do not need to be specified.18 The area under the curve (AUC), which indicates “the probability that a classifier will rank a randomly chosen positive instance higher than a randomly chosen negative one,”19 was calculated and compared against a chance AUC (0.5).
Results
A total of 107 patients were identified with DME treated with repeat anti-VEGF treatment, of which 40 eyes of 34 patients met all study inclusion criteria. Table 1 shows demographic data of all patients at baseline. The mean VA was 0.60 ± 0.38 (logMAR, 20/80 Snellen equivalent), and mean CRT was 434.58 ± 176.94 µm at baseline. Treatments prior to inclusion in this study included anti-VEGF therapy for 14 eyes, intravitreal steroid for 6 eyes, focal laser for 18 eyes, and vitrectomy for 2 eyes. There were 23 eyes that were treatment naïve. During the review period more eyes were treated with ranibizumab (34) than either bevacizumab (5) or aflibercept (1).
Table 1.
Baseline Demographics, Visual Acuity, OCT Characteristics, and Prior Treatment History in the Study Patient Cohort.
| Baseline Demographics | |||
|---|---|---|---|
| Mean | StDev | Unit | |
| Age | 67.83 ± 9.78 | Years | |
| Visual Acuity | 0.60 ± 0.38 | LogMAR | |
| Central Retinal Thickness | 434.58 ± 176.94 | microns | |
| Category | Number | Percentage | |
| Gender | Male | 20 | 50.00% |
| Female | 20 | 50.00% | |
| Eye | Right | 27 | 67.50% |
| Left | 13 | 32.50% | |
| Treatment Naïve | Yes | 23 | 57.50% |
| No | 17 | 42.50% | |
| Anti-VEGF For First 3 Months of Study | Bevacizumab | 5 | 12.50% |
| Ranibizumab | 34 | 85.00% | |
| Aflibercept | 1 | 2.50% | |
| Anti-VEGF Prior to Study | Yes | 14 | 35.00% |
| No | 26 | 65.00% | |
| Intravitreal Steroid Prior to Study | Yes | 6 | 15.00% |
| No | 34 | 85.00% | |
| Focal Laser Prior to Study | Yes | 18 | 45.00% |
| No | 22 | 55.00% | |
| Vitrectomized Eyes | Yes | 2 | 5.00% |
| No | 38 | 95.00% | |
| Lens Status | Phakic | 13 | 32.50% |
| Pseudophakic | 27 | 67.50% | |
Within the first month, patients saw visual acuity improvement from 0.60 ± 0.37 (logMAR, 20/80 Snellen) to 0.53 ± 0.36 (logMAR, 20/68 Snellen; p = 0.030). The visual acuity continued to improve to 0.47 ± 0.42 (logMAR, 20/59 Snellen) and 0.40 ± 0.33 (logMAR, 20/50 Snellen) by 3-months (p = 0.010) and 12-months (p<0.001) respectively (Figure 1). This improvement in VA seemed to correlate with significant reduction in CRT that was noted at all time points measured (Figure 2). Notably CRT improved from 434.58 ± 176.94 µm at baseline to 389.00 ± 153.31 µm at 1-month (p = 0.012), 352.75 ± 152.36 µm at 3-months (p < 0.001), and 324.18 ± 136.53 µm at 12-months (p < 0.001).
Figure 1.
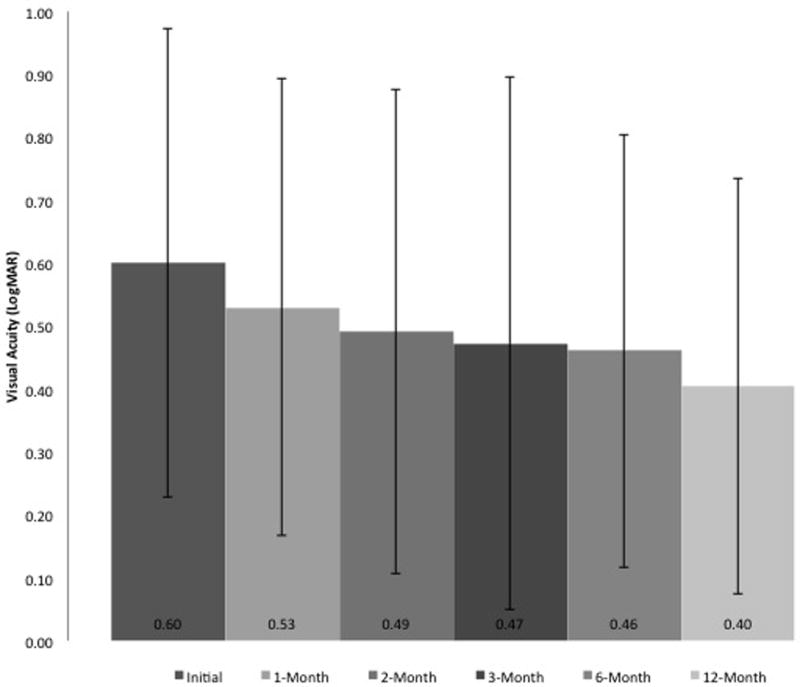
Visual acuity change in patients with diabetic macular edema on anti–vascular endothelial growth factor therapy during the first 12 months of treatment. LogMAR, logarithm of the minimum angle of resolution.
Figure 2.
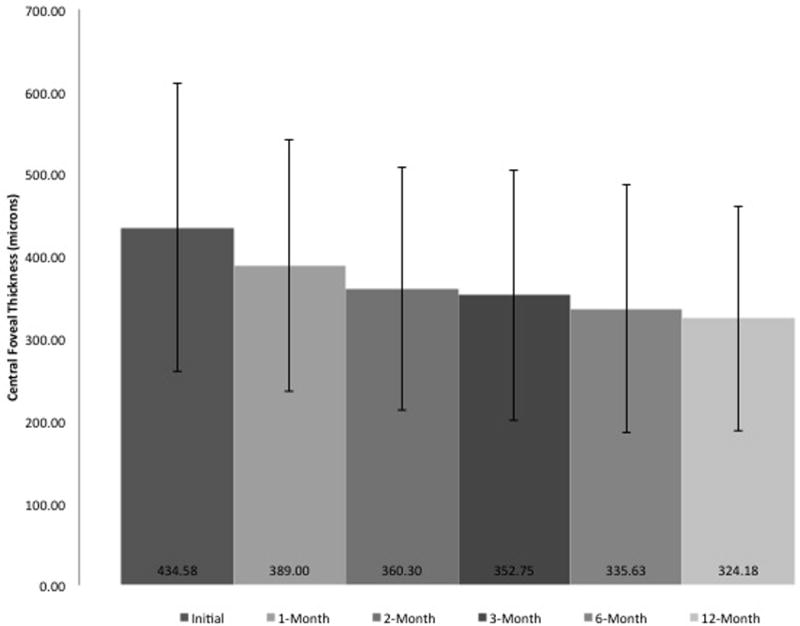
Change in central foveal thickness in patients with diabetic macular edema on anti–vascular endothelial growth factor therapy during the first 12 months of treatment.
We sought to identify responders with more than a 25% reduction in CRT at 3-months. To determine if responders could be separated from poor responders after a single anti-VEGF injection, sensitivity and specificity values were determined for all values of reduction in CRT as outlined in the methods above. This empirical ROC curve is illustrated in Figure 3.
Figure 3.
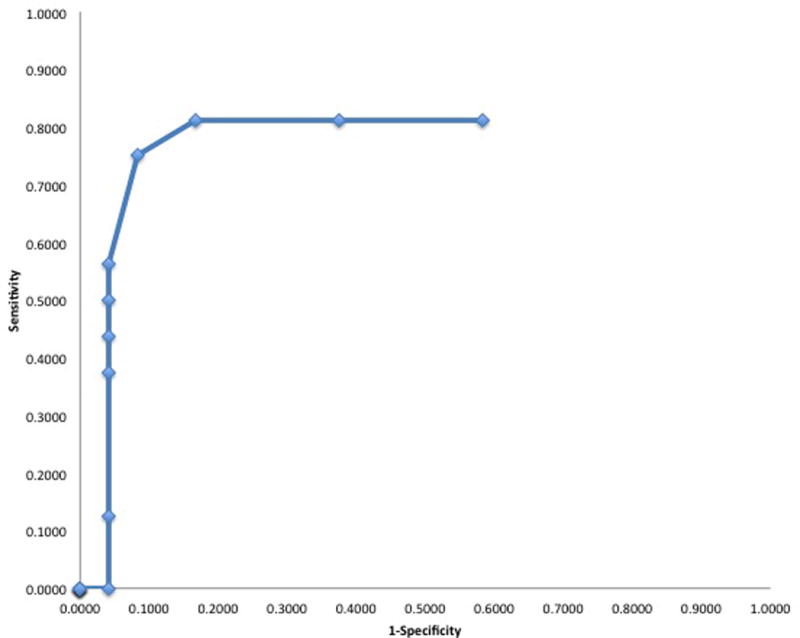
Empirical receiver operator curve for anatomic response after a single anti-VEGF injection in patients with diabetic macular edema on anti-VEGF therapy. VEGF, vascular endothelial growth factor.
Our analysis found if the treated patient had a >15% reduction in CRT at 1-month, they would often go on to have a 25% reduction at 3-months (sensitivity 0.75, specificity 0.92, positive predictive value 0.86, negative predictive value 0.85). Using this cutoff, we were able to correctly identify 16 out of 16 responders and 18 out of 24 poor responders to anti-VEGF therapy. Additionally, the area under curve (AUC) measure of 0.83±0.76 was significantly greater than chance (95% CI=0.68–0.97, p<0.001).
Discussion
The concept of responders to anti-VEGF therapy is not new to DME and has been examined recently due to the FDA approval of novel steroid implants.20 Multiple biochemical pathways that drive pathogenesis of diabetic macular edema help explain the presence of non-responders to anti-VEGF monotherapy. While DME is associated with increased anti-VEGF levels, multiple inflammatory pathways are now believed to be just as important in its pathophysiology.21 In other diseases, such as retinal vein occlusions that are also thought to have complex pathophysiology, visual outcomes were reduced in patients who had chronic persistent macular edema despite initiation of anti-VEGF therapy.22 Since chronic DME can lead to permanent reduction in visual acuity even after therapy is instituted,13 it highlights the need to start treatment early, and also identify responders and non-responders quickly.
The measurements of cytokine levels in the aqueous and vitreous samples could be used to directly identify responders or non-responders to anti-VEGF therapy in DME, but no validated kit for this analysis is yet commercially available. Furthermore, these studies are expensive, time-consuming, require aqueous/vitreous tap that carries small but definitive risk of endophthalmitis and have results that are not immediately available to guide treatment decisions for the patients during their clinical visit. Therefore, we set out to determine if reduction in CRT on macular OCT after a single anti-VEGF injection could be used as a non-invasive, low-cost surrogate marker of treatment response to anti-VEGF monotherapy in DME.
Our ROC curve analysis determined optimal cutoff of a >15% reduction in CRT at one month as a way to identify responders to anti-VEGF therapy at 3-months with good sensitivity (0.75) and specificity (0.92). While an ideal test would have a sensitivity and specificity > 95%, rarely does a single medical test meet such a stringent standard. By comparison commonly accepted screening tests such as three repeated tests for fecal occult blood testing has a sensitivity of 0.69 and specificity of 0.73.23 Moreover, the area under curve (AUC) measure of 0.83±0.76 was significantly greater than chance (95% CI=0.68–0.97, p<0.001) and considered in the ‘excellent’ diagnostic range.19 So, while this may be an imperfect test to identify responders, it is immediately available and could be combined with other parameters, such as change in visual acuity or even cytokine analysis, in a larger sample. In addition, this technique of identifying responders has advantages. First, it would minimize the time before the clinician makes a decision to continue the same anti-VEGF treatment or switch to alternative therapies, such as a different anti-VEGF drug or steroid medications. Second, this method requires no additional intervention or imaging beyond current common practice patterns. Prospective data is needed to verify our results.
Visual acuity data is difficult to compare across trials, but CRT data is more readily comparable to RISE and RIDE data. Furthermore, there tends to be at best a modest correlation between central retinal thickness and visual acuity in patients with diabetes.25 Interestingly, we observed a near 25% reduction in CRT at 3 months, whereas a nearly 40% reduction in CRT was noted in the RISE and RIDE trials.13 We suspect this is largely due to selection bias in our study that focused on patients who had severe DME as we required a minimum of 3 injections to be included in this analysis. Patients with mild edema only requiring a single injection or infrequent injections were excluded. We believe our population was less uniform and not treatment-naïve compared to the population in RISE and RIDE studies. Similarly to RISE and RIDE, we noted a significant improvement in logMAR VA and CRT at 1-month (p=0.030 and p=0.012, respectively) which continued to improve further by 3-months (p=0.010 and p<0.001 respectively). After the initial 3-months or monthly anti-VEGF therapy, patients were followed for 12-months. All patients went on to receive additional anti-VEGF therapy. Twelve eyes went on to need some form of steroid therapy (intravitreal triamcinolone or intravitreal dexamethasone implant). The VA and CRT continued to improve during the 12-months of follow up (p<0.001 and p<0.001, respectively).
A recent post-hoc analysis of the Diabetic Retinopathy Clinical Research Network (DRCR.net) Protocol I data evaluated the predictability of early treatment response after ranibizumab injections.24 Unlike our current study that uses OCT data, the Protocol I analysis was based purely on visual acuity, and the predictability after 3 injections, rather than 1. The study showed that the letters gained after 3 injections correlated with letters gained at 1 and 3 years. This data supports and substantiates the findings of our study. Taken together, we show that OCT findings after 1 injection is predictive of OCT findings at 3 months, and Protocol I shows that the vision at that 3 month visit correlates with vision after 1- and 3-years. It is obviously not possible to conclude that anatomic outcomes after 1 injection is therefore predictive of vision 3-years later, but it would not be surprising. We believe that the treatment response as measured with anatomical data combined with visual acuity data may offer a more robust predictive framework for early identification of non-anti-VEGF responders.
The limitations of this study are inherent to its retrospective nature. Moderate sample size of 40 eyes limits the strength of the analysis. However, we took precautions by testing by parametric and non-parametric analysis when determining the AUC and found identical results. Additionally, as many definitions of treatment non-response exist in the literature, for the purposes of this study we chose to use a decrease in CRT at 3 months to define anti-VEGF response. As a result, the results of our study may be difficult to compare to others with different anatomic and visual criteria.
In summary, our study suggests that reductions in CRT can identify responders at one-month post initial anti-VEGF treatment for DME using a reduction of CRT by 15% as a cutoff value. This approach is readily available, cheap, non-invasive and can be combined with other measurable parameters to drive complex management of patients with DME.
Acknowledgments
MAW was supported by National Eye Institute, Bethesda, MD. K23 Mentored clinician scientist award K23EY023596-01
Footnotes
Disclosures: None of the authors report any disclosures or conflicts of interest.
References
- 1.Klein R, Klein BE, Moss SE, Cruickshanks KJ. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. XV. The long-term incidence of macular edema. Ophthalmology. 1995;102(1):7–16. doi: 10.1016/s0161-6420(95)31052-4. [DOI] [PubMed] [Google Scholar]
- 2.Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376(9735):124–36. doi: 10.1016/S0140-6736(09)62124-3. [DOI] [PubMed] [Google Scholar]
- 3.Diabetic Retinopathy Clinical Research N. Elman MJ, Aiello LP, et al. Randomized trial evaluating ranibizumab plus prompt or deferred laser or triamcinolone plus prompt laser for diabetic macular edema. Ophthalmology. 2010;117(6):1064–77. e35. doi: 10.1016/j.ophtha.2010.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Photocoagulation for diabetic macular edema. Early Treatment Diabetic Retinopathy Study report number 1. Early Treatment Diabetic Retinopathy Study research group. Arch Ophthalmol. 1985;103(12):1796–806. [PubMed] [Google Scholar]
- 5.Nguyen QD, Brown DM, Marcus DM, et al. Ranibizumab for diabetic macular edema: results from 2 phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119(4):789–801. doi: 10.1016/j.ophtha.2011.12.039. [DOI] [PubMed] [Google Scholar]
- 6.Mitchell P, Bandello F, Schmidt-Erfurth U, et al. The RESTORE study: ranibizumab monotherapy or combined with laser versus laser monotherapy for diabetic macular edema. Ophthalmology. 2011;118(4):615–25. doi: 10.1016/j.ophtha.2011.01.031. [DOI] [PubMed] [Google Scholar]
- 7.Do DV, Schmidt-Erfurth U, Gonzalez VH, et al. The DA VINCI Study: phase 2 primary results of VEGF Trap-Eye in patients with diabetic macular edema. Ophthalmology. 2011;118(9):1819–26. doi: 10.1016/j.ophtha.2011.02.018. [DOI] [PubMed] [Google Scholar]
- 8.Michaelides M, Kaines A, Hamilton RD, et al. A prospective randomized trial of intravitreal bevacizumab or laser therapy in the management of diabetic macular edema (BOLT study) 12-month data: report 2. Ophthalmology. 2010;117(6):1078–86. e2. doi: 10.1016/j.ophtha.2010.03.045. [DOI] [PubMed] [Google Scholar]
- 9.Brown DM, Schmidt-Erfurth U, Do DV, et al. Intravitreal Aflibercept for Diabetic Macular Edema: 100-Week Results From the VISTA and VIVID Studies. Ophthalmology. 2015;122(10):2044–52. doi: 10.1016/j.ophtha.2015.06.017. [DOI] [PubMed] [Google Scholar]
- 10.Boyer DS, Yoon YH, Belfort R, Jr, et al. Three-Year, Randomized, Sham-Controlled Trial of Dexamethasone Intravitreal Implant in Patients with Diabetic Macular Edema. Ophthalmology. 2014 doi: 10.1016/j.ophtha.2014.04.024. [DOI] [PubMed] [Google Scholar]
- 11.Gillies MC, Simpson JM, Gaston C, et al. Five-year results of a randomized trial with open-label extension of triamcinolone acetonide for refractory diabetic macular edema. Ophthalmology. 2009;116(11):2182–7. doi: 10.1016/j.ophtha.2009.04.049. [DOI] [PubMed] [Google Scholar]
- 12.Lazic R, Lukic M, Boras I, et al. Treatment of anti-vascular endothelial growth factor-resistant diabetic macular edema with dexamethasone intravitreal implant. Retina. 2014;34(4):719–24. doi: 10.1097/IAE.0b013e3182a48958. [DOI] [PubMed] [Google Scholar]
- 13.Brown DM, Nguyen QD, Marcus DM, et al. Long-term outcomes of ranibizumab therapy for diabetic macular edema: the 36-month results from two phase III trials: RISE and RIDE. Ophthalmology. 2013;120(10):2013–22. doi: 10.1016/j.ophtha.2013.02.034. [DOI] [PubMed] [Google Scholar]
- 14.Wolfe JD, Shah AR, Yonekawa Y, et al. Receiver operating characteristic curve to predict anti-VEGF resistance in retinal vein occlusions and efficacy of Ozurdex. Eur J Ophthalmol. 2015:0. doi: 10.5301/ejo.5000686. [DOI] [PubMed] [Google Scholar]
- 15.Holladay JT. Proper method for calculating average visual acuity. J Refract Surg. 1997;13(4):388–91. doi: 10.3928/1081-597X-19970701-16. [DOI] [PubMed] [Google Scholar]
- 16.Linden A. Measuring diagnostic and predictive accuracy in disease management: an introduction to receiver operating characteristic (ROC) analysis. J Eval Clin Pract. 2006;12(2):132–9. doi: 10.1111/j.1365-2753.2005.00598.x. [DOI] [PubMed] [Google Scholar]
- 17.Obuchowski NA, Lieber ML, Wians FH., Jr ROC curves in clinical chemistry: uses, misuses, and possible solutions. Clin Chem. 2004;50(7):1118–25. doi: 10.1373/clinchem.2004.031823. [DOI] [PubMed] [Google Scholar]
- 18.Hsieh FT. Nonparametric and semiparametric estimation of the receiver operating characteristic curve. Annals of Statistics. 1996;24 [Google Scholar]
- 19.Fawcett T. An Introduction to ROC analysis. Pattern Recognition Letters. 2006;27 [Google Scholar]
- 20.Gutierrez-Benitez L, Millan E, Arias L, et al. Dexamethasone intravitreal implants for diabetic macular edema refractory to ranibizumab monotherapy or combination therapy. Arch Soc Esp Oftalmol. 2015;90(10):475–80. doi: 10.1016/j.oftal.2015.04.003. [DOI] [PubMed] [Google Scholar]
- 21.Das A, McGuire PG, Rangasamy S. Diabetic Macular Edema: Pathophysiology and Novel Therapeutic Targets. Ophthalmology. 2015;122(7):1375–94. doi: 10.1016/j.ophtha.2015.03.024. [DOI] [PubMed] [Google Scholar]
- 22.Bhisitkul RB, Campochiaro PA, Shapiro H, Rubio RG. Predictive value in retinal vein occlusions of early versus late or incomplete ranibizumab response defined by optical coherence tomography. Ophthalmology. 2013;120(5):1057–63. doi: 10.1016/j.ophtha.2012.11.011. [DOI] [PubMed] [Google Scholar]
- 23.Niv Y, Sperber AD. Sensitivity, specificity, and predictive value of fecal occult blood testing (Hemoccult II) for colorectal neoplasia in symptomatic patients: a prospective study with total colonoscopy. Am J Gastroenterol. 1995;90(11):1974–7. [PubMed] [Google Scholar]
- 24.Dugel P. Early anti-VEGF response and long-term efficacy (EARLY Analysis). American Academy of Ophthalmology Annual Meeting; Las Vegas NV. November 13, 2015. [Google Scholar]
- 25.Diabetic Retinopathy Clinical Research Network. Browning DJ, Glassman AR, Aiello LP, Beck RW, Brown DM, Fong DS, Bressler NM, Danis RP, Kinyoun JL, Nguyen QD, Bhavsar AR, Gottlieb J, Pieramici DJ, Rauser ME, Apte RS, Lim JI, Miskala PH. Relationship between optical coherence tomography-measured central retinal thickness and visual acuity in diabetic macular edema. Ophthalmology. 2007 Mar;114(3):525–36. doi: 10.1016/j.ophtha.2006.06.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wells J, Glassman A, Ayala A The Diabetic Retinopathy Clinical Research Network. Aflibercept, bevacizumab or ranibizumab for diabetic macular edema. N Engl J Med. 2015;26:1193–203. doi: 10.1056/NEJMoa1414264. 372:13. [DOI] [PMC free article] [PubMed] [Google Scholar]


