Abstract
The aim of the work was to evaluate whether or not there is glycolytic reprogramming in the neighboring cells of colorectal cancer (CRC). Using postoperative material we have compared the functional capacity of oxidative phosphorylation (OXPHOS) in CRC cells, their glycolytic activity and their inclination to aerobic glycolysis, with those of the surrounding and healthy colon tissue cells. Experiments showed that human CRC cannot be considered a hypoxic tumor, since the malignancy itself and cells surrounding it exhibited even higher rates of OXPHOS than healthy large intestine. The absence of acute hypoxia in colorectal carcinomas was also confirmed by their practically equal glucose-phosphorylating capacity as compared with surrounding non-tumorous tissue and by upregulation of VEGF family and their ligands. Studies indicated that human CRC cells in vivo exert a strong distant effect on the energy metabolism of neighboring cells, so that they acquire the bioenergetic parameters specific to the tumor itself. The growth of colorectal carcinomas was associated with potent downregulation of the creatine kinase system. As compared with healthy colon tissue, the tumor surrounding cells display upregulation of OXPHOS and have high values of basal and ADP activated respiration rates. Strong differences between the normal and CRC cells in the affinity of their mitochondria for ADP were revealed; the corresponding Km values were measured as 93.6±7.7 µM for CRC cells and 84.9±9.9 µM for nearby tissue; both these apparent Km (ADP) values were considerably (by almost 3 times) lower in comparison with healthy colon tissue cells (256±34 µM).
Abbreviations: AK, adenylate kinase; ANT, adenine nucleotide translocator; AP5A, diadenosine pentaphosphate; BSA, bovine serum albumin; BB-CK, – brain type creatine kinase; CAT, carboxyatractyloside; CIMP, CpG island methylator phenotype; COX, cytochrome c oxidase; CK, creatine kinase; CRC, colorectal cancer; ETC, electron transport chain; FDG, 18-fluorodeoxyglucose; HK, hexokinase; Km, Michaelis–Menten constant; uMtCK, ubiquitous mitochondrial creatine kinase; OXPHOS, oxidative phosphorylation; MI, Mitochondrial Interactosome; MOM, mitochondrial outer membrane; PCr, phosphocreatine; PET, positron emission tomography; PEP, phosphoenolpyruvate; PYK, pyruvate kinase; qPCR, real-time quantitative PCR; TMPD, N,N,N′,N′-tetramethyl-p-phenylenediamine; VEGF, vascular endothelial growth factor; VDAC, voltage dependent anion channel; V0, basal respiration level; Vm, maximal respiration rate
Keywords: Mitochondria, Respiration, ATP-synthasome, Human colorectal cancer, Energy metabolism, Glycolysis, OXPHOS
Highlights
-
•
Human colorectal cancer is not a pure hypoxic tumor of the Warburg phenotype.
-
•
The total hexokinase activity of CRC cells is close to that in nearby tissues.
-
•
In the tumor there is overexpression of VEGFs (A, B, and C) and their receptors.
-
•
CRC has higher rates of OXPHOS as compared with healthy tissue cells.
-
•
Tumor-surrounding cells cannot fuel via a lactate shunt the growth of CRC cells.
1. Introduction
Colorectal cancer (CRC) is a major cause of cancer death worldwide necessitating new strategies for treatment of this disease. Recent studies show that targeting cancer cell energy metabolism is possibly a new and very effective therapeutic approach for selective ablation of malignancies [39], [61], [70]. Intracellular ATP levels may be a key determinant of chemoresistance of human CRC cells [110]. There are some indications in literature that mitochondria (the main cell system for ATP generation) could play a supportive or possibly even a triggering role in metastasis of cancer cells [4].
The first half of the 20th century led to substantial breakthroughs in bioenergetics and mitochondrial research. During that time, Otto Heinrich Warburg observed abnormally high glycolysis and lactate production in cancer cells even in the presence of oxygen (later named as “aerobic glycolysis“), leading him to suggest that defects in mitochondrial functions are at the heart of malignant cell transformation [117].
The exact mechanisms mediating the strong tendency of some cancers to aerobic glycolysis remain still unclear. Several different hypothesis have been proposed to explain the causes of the Warburg effect, such as: 1) poor tumor vascularization leading to hypoxia-induced dysfunction of mitochondria [95] and stabilization of HIF-1α-a master of regulation of glycolytic fluxes [103], 2) post-translational modifications, 3) glutamine metabolism [26], [29]; 4) miRNA expression [42], 5) epigenetic changes [17], 6) nuclear and mitochondrial DNA mutations [10], [18] leading to mitochondrial dysfunction in cancer cells [77], and 7) oncogene activation and loss of tumor suppressor genes function [115].
A very attractive hypothesis for explanation of the Warburg phenomenon was proposed by Pedersen and colleagues [75]. They have suggested that in highly glycolytic malignant cells the overexpression of hexokinase-2 (HK-2) associated with its binding to voltage-dependent anion channel (VDAC, located on the outer mitochondrial membrane) plays a crucial role in mediating their high rate of aerobic glycolysis. In tumor cells, the interaction of HK-2 with VDAC induces very rapid phosphorylation of glucose through the use of mitochondrially-generated ATP. Also, the binding of HK-2 to mitochondria strongly (almost 5-fold) increases its affinity for ATP [12]. It is important to note that binding of HK-2 to VDAC maintains this channel in the open state [98] which further facilities the transport of adenine nucleotides across mitochondrial membranes in malignant cells. In cancer tissues, the high glycolytic activity requires an up-regulation of the key glycolytic enzymes including HK(s). Interestingly, the percentage of hexokinase binding to the mitochondria is also significantly increased in some cancer cells. For instance, in AS-20D liver tumor cells, the hexokinase protein level (mainly HK-2) was found to be more than 500 times higher than in normal liver cells, which mainly express HK-IV instead. Furthermore, around 80% of HK-2 is found to be associated with mitochondria [7]. Due to the frequent up-regulation of HK-2 in cancer cells and its important role in glycolytic pathway, this enzyme seems to be an attractive target for anticancer drug development. In line with this, Chen et al. [19] have shown treatment of cancer cells with 3-bromopyruvate (an inhibitor of glycolysis) caused a covalent modification of HK-2 protein that triggered its dissociation from mitochondria, leading to a specific release of apoptosis-inducing factor from the mitochondria to cytosol and cell death.
The regulation of mitochondrial function is a central issue in the bioenergetics of cancer cell. Studies performed during the past decade showed that interaction between cytoskeletal proteins and mitochondria is deeply involved in the regulation of mitochondrial function. A lot of experimental data demonstrate the importance of the structural factors in the intracellular arrangement of mitochondria and in the control of outer mitochondrial membrane permeability [104], [118], [40], [45], [47], [5], [6]. Potential candidates for the key roles in this regulation are the cytoskeletal proteins such as plectin and tubulin [118], [45], [47], [5], [6], [63]. It was hypothesized that in high-energy demand tissues there is a colocalization of β-tubulin isotype II with mitochondria (through VDAC) and it was suggested that it can be coupled with the adenine nucleotide translocase (ANT), mitochondrial creatine kinase (MtCK) and VDAC. This mitochondrial supercomplex (ANT-MtCK-VDAC) is responsible for the efficient intracellular energy transfer via the phosphocreatine (PCr) pathway. It is shown that the localization and function of β-tubulin isotypes varied in different muscle tissues and neoplastic cells [112], [118], [63], [78].
Recent investigations have clarified the benefits and selective advantages of aerobic glycolysis. Although glycolysis yields a lower amount of ATP compared to mitochondrial OXPHOS, several key benefits inherent in aerobic glycolysis drive cancer cells to favor glycolysis over mitochondrial oxidation [28]. Firstly, it was proposed [91] that the high rates, but low yields of ATP production through glycolysis, may give selective advantage under rivalry for shared energy sources. Moreover, the rate of ATP generation may be 100 times faster with glycolysis as compared with OXPHOS [36]. The low efficiency of ATP generation by glycolysis is nevertheless sufficient to meet intracellular demand. Secondly, besides ATP, cancer cells need additional metabolic intermediates and precursors that are decisive for the biosynthesis of macromolecules, the ultimate building blocks necessary to expand the tumor mass during its growth and proliferation [116]. Currently, human CRC is considered as a neoplasm of the Warburg phenotype with deregulated OXPHOS system. Positron emission tomography (PET) with 18-fluorodeoxyglucose (FDG) showed that the malignancy exhibits, as compared to surrounding normal intestine tissue, higher rates of glucose consumption [22] that in turn was associated with increased intratumoral levels of lactic acid [54], overexpression of GLUT-1 [48] and genes encoding glycolytic enzymes such as pyruvate kinase M2 (PKM2) [1], glyceraldehyde-3-phosphate dehydrogenase and enolase-1α [3], LDH5 [62], and HK-2 [52].
It is becoming evident that the upregulation of glycolysis exhibited by some cancer cells does not necessarily imply a strict anaerobic phenotype or a dysfunctional OXPHOS system. Rather, it is believed that the normal interplay between the glycolysis in the cytosol and OXPHOS in the mitochondria becomes disturbed or reprogrammed in tumor cells; the Crabtree effect was observed in cancer cells that exemplifies the intimate connection between glycolysis and the oxidative metabolism [90]. Moreover, recent studies have shown that not all tumor mitochondria display OXPHOS deficiency [111], [121], [30], [60], [95]. The OXPHOS system may be the principal ATP producer (>90%) for several malignant tumor cell types under normoxic conditions [111], [96], [97]. Therefore, drug therapy targeting OXPHOS has emerged as an important alternative for growth arrest of oxidative type tumors [39], [82], [96].
In our recent study, we clearly showed that CRC cannot be regarded as a tumor of purely Warburg phenotype and that in these cancer cells the OXPHOS system is the main energy source instead of aerobic glycolysis [58]. Although total glycolytic capacity of human CRC cells was found to be similar with normal cells, all their respiratory rates (both basal and ADP-activated) exceeded considerably those of healthy colon tissue samples. Furthermore, our studies indicated that the OXPHOS system may be even upregulated in CRC cells; the content of mitochondria in human CRC cells was found to be at least 2-times higher than that in healthy colon tissue cells [58].
Recently, a new framework of “Reprogramming the of Tumor Stroma metabolism” or “Reverse Warburg effect” was introduced in experimental oncology [108], [123], [68]. According to the paradigm, there is metabolic coupling between mitochondria in cancer cells and catabolism in stromal cells that promotes tumor growth and metastasis. In another words, cancer cells can induce the reprogramming of tumor microenvironment (fibroblasts, macrophages and other tumor-associated cells) towards the Warburg phenotype, so they donate the necessary fuels (l-lactate, ketone bodies, glutamine and others) to anabolic cancer cells, which metabolize these via the tricarboxylic acid cycle (TCA) and OXPHOS. Pioneering studies showed that such metabolic symbiosis may occur between breast cancer cells and the tumor stromal fibroblasts [107], [120], [73], and now this paradigm has extended to other malignancies like osteosarcoma, ovarian cancer, head and neck tumors, and cancer lymph node metastases [106], [25], [85]. However, in the case of human CRC the concept of “reverse Warburg” effect was not explored yet.
Though the above described form of “parasitic metabolism” in malignancies has only recently been proposed, transfer of energy precursors between cells to fuel growth is actually not a new discovery, but instead reflects the co-optation of normal physiological processes by tumor cells.
Taking into account the information presented above, the aim of the work was to estimate whether or not there is metabolic reprogramming in the human CRC surrounding cells. For this purpose, we used postoperative material, to estimate the glycolytic capacity of human CRC cells, their inclination towards aerobic glycolysis (coupling between HK processes and the OXPHOS system) and compared these parameters with those of healthy colon tissue and the tumor surrounding cells (nearby tissue). In addition, in situ experiments with the use of “permeabilized cell” techniques were carried out to compare the bioenergetic function of mitochondria in these tissues as well as the role of adenylate and creatine kinase systems in maintaining energy homeostasis.
2. Materials and methods
2.1. Reagents
Unless otherwise indicated, all chemicals were purchased from Sigma-Aldrich Chemical Com. (USA). Primary and secondary antibodies were obtained from Santa Cruz Biotechnology Inc. or Abcam PLC, rabbit polyclonal antibodies vs. VDAC were kindly donated by Dr. Catherine Brenner from Paris-Sud University, France.
2.2. Clinical materials and patients
CRC and normal tissue samples (0.1–0.5 g) were provided by the Oncology and Hematologic Clinic at the North Estonia Medical Centre, Tallinn. Pathology and histological reports were provided by the oncology clinic for each tissue sample. All patients examined (n=55, with ages ranging from 63 to 92 years) had local or locally advanced disease (T2-4, N0-1, M0-1). Only primary samples of adenocarcinoma were examined. The patients in the study had not received prior radiation or chemotherapy. All investigations were approved by the Medical Research Ethics Committee (National Institute for Health Development, Tallinn) in accordance with Helsinki Declaration and Convention of the Council of Europe on Human Rights and Biomedicine. Normal colon tissue samples were taken at sites distant from the tumor by 5 cm and they were controlled for presence of cancer cells. Nearby tissue is the junction area between cancer and normal mucosa. In addition, we performed molecular characterization of tissue samples from 35 patients (both tumor and normal) using microsatellite instability, CpG island methylator phenotype (CIMP) and 5-hydroxymethylation assay. CIMP is one of the mechanisms involved in colorectal carcinogenesis [38]. Our studies showed that both non-tumorous tissue samples (nearby and healthy) had a stable microsatellite profile and no CIMP phenotype. Also, the 5-hydroxymethylation expression was statistically significantly higher in both control and neighboring tissue samples as compared to tumor that was analyzed in 13 patients (Supplementary Fig. 1). 5-hydroxymethylation analysis was carried out according to manufacturer instructions provided with the MethylFlash Hydroxymethylated DNA Quantification Kit (Epigentek, USA).
2.3. Preparation of tumor fibers, permeabilization procedure, and assessment of mitochondrial respiration in situ
Numerous studies have demonstrated that isolated mitochondria behave differently from mitochondria in situ with respect to respiratory activity [101]. Therefore, we investigated mitochondrial respiratory activity of tumor and non-tumorous tissues in situ using the skinned fiber technique [101], [56], [58], [65], which allows analyzing the function of mitochondria in a cell in their natural surroundings. This technique leaves the links of these organelles with cytoskeletal structures intact. Immediately after surgery, the tissue samples treated as described previously [56] and the respiration of State 2 and ADP activated respiration was measured.
2.4. Mitochondrial respiration in saponin-permeabilized tissue samples
Rates of O2 consumption by skinned tissue fibers were assayed at 25 °C by an Oxygraph-2k high resolution respirometer (Oroboros Instruments, Innsbruck Austria) in pre-equilibrated respiration buffer (medium-B supplemented with 5 mM glutamate and 2 mM malate or 10 mM succinate as respiratory substrates) as described previously [65]. The solubility of oxygen at 25 °C was taken as 240 nmol/ml [37]. All respiration rates were normalized per mg dry weight of tissue.
2.4.1. Analysis of OXPHOS coupling with hexokinase, adenylate and creatine kinase mediated processes
The coupling of mitochondrially-bound hexokinases with the OXPHOS system in permeabilized tumor and non-tumorous tissues was assayed by oxygraphy through stimulation of mitochondrial respiration by locally-generated ADP [58]. The glucose effect on mitochondrial respiration was expressed by glucose index (IGLU) that was calculated according to the equation: IGLU (%)=[(VGLU−VATP)/(VADP−VATP)]*100, where VADP is the rate of O2 consumption in the presence of 2 mM, VGLU-respiration rates with 10 mM glucose, and VATP is respiration rate with 0.1 mM ATP; i.e. this index reflects the degree glucose-mediated stimulation of mitochondrial respiration as compared to maximal ADP-activated rates of O2 consumption.
The adenylate kinase (AK) coupling with OXPHOS was estimated by respirometry using modified protocols of [43]. Then AK index (IAK) was calculated according to the equation: IAK=(VAMP−VAP5A)/VAP5A, where VAMP and VAP5A are mitochondrial respiration rates in the presence of 2 mM AMP and 0.2 mM diadenosine pentaphosphate (AP5A, an inhibitor of AK), respectively. This index shows the efficiency of the functional coupling between AK and mitochondrial OXPHOS.
The functional coupling between mitochondrial creatine kinase (CK) and OXPHOS system was estimated by respirometry essentially as described previously [114], [56].
2.5. Determination of enzymatic activities
HK activity was measured as the total glucose phosphorylating capacity of whole tissue extracts, using a standard glucose-6-phosphate dehydrogenase coupled spectrophotometric assay [94].
The CK activity was assessed spectrophotometrically at 25 °C in the direction of ATP formation [78]. One mU of CK activity represents the formation of 1 nmole of ATP per minute at 25 °C.
AK activity of whole-tissue extracts was measured at 25 °C by a coupled enzyme assay [33]. All enzymatic activities were normalized per mg of tissue protein. The protein content of tissue extracts was determined by a Pierce BCA Protein Assay Kit according to the manufacturer recommendations using BSA as a standard.
2.6. Western blot analysis of the levels of beta-tubulin isotypes and VDAC expression in postoperative tissue samples
Postoperative tissues were frozen in liquid N2 and crushed to a powder. The powder was then suspended in 20 volumes of microtubule lysing buffer containing 100 mM PIPES, 5 mM MgCl2, 1 mM EGTA, 30% glycerol, 0.1% IGEPAL, 0.1% Tween-20, 0.1% Triton X-100, 0.1% beta-mercaptoethanol, 1 mM ATP, 0.1 mM GTP and complete protease inhibitor cocktail (Roche); the recipe is according to Cytoskeleton Inc. The lysate was homogenized by Retsch Mixer Mill at 25 Hz for 2 min, and incubated for 30 min at 35 °C. The obtained tissue lysates were then clarified by centrifugation at 21,000 g for 40 min at 35 °C. The protein concentration in lysates was determined using the Pierce BCA Protein Kit. Proteins were separated by 12% SDS-PAGE and transferred onto PVDF membrane by Trans-Blot Semi-Dry Transfer system (Bio-Rad).
For determinations of the presence of beta-tubulin isotypes Abcam mono- and polyclonal antibodies (Anti beta I Tub (ab11312), Anti Tubb2A (ab170931), Anti beta III Tub (ab52901), Anti beta IV (ab11315)) were used. For VDAC detection, rabbit polyclonal antibody, kindly provided by Dr Catherine Brenner (Paris-Sud University, France) was used. Secondary antibodies were accordingly: anti-mouse (ab97046) and anti-rabbit (ab6721) HRP conjugates. After chemoluminescence reaction, the PVDF membranes were stained with Coomassie brilliant blue R250 to measure the total protein amount. The beta tubulin isoforms and VDAC signal intensities were calculated by ImageJ software and normalized to total protein intensities; staining with Coomassie is routinely used as loading control in Western blot analysis. Besides, after enzymatic chemiluminescence reaction and imaging, the PVDF membrane was washed once with Tris-buffered saline, re-colored with Coomassie brilliant blue for 5 min, distained and dried completely, and then was imaged again [119].
2.7. RNA isolation and real-time quantitative RT-PCR (qRT-PCR)
The technique of qRT-PCR was applied to estimate the expression of genes encoding main members of the vascular endothelial growth factor family (VEGFs) and their receptors (such as VEGF-A, -B, -C, FLT-1, FLT-4 KDR, NRP-1 and NRP-2) in human CRC, neighboring non-tumorous and healthy colon tissues. Total RNA from frozen postoperative tissue samples (n=47) was isolated by means of Trizol (Life Technologies) solution, followed by purification using the RNeasy Mini Kit (QIAGEN Sciences) with DNase treatment. Extracted RNA was dissolved in RNase-free water, quality and concentration were measured using Nanodrop and RNA was stored at −80 °C until cDNA synthesis. For cDNA synthesis 2 µg of total RNA was used. cDNA was synthesized using High Capacity cDNA Reverse Transcription Kit with RNase Inhibitor (Applied Biosystems). cDNA was used as a template for TaqMan® quantitative RT-PCR (qRT-PCR) analysis in the Roche LightCycler 480 system (Roche). TaqMan® Gene Expression Master Mix and FAM labeled TaqMan® (Applied Biosystems) gene assays were used to detect the mRNA expression level of the gene of interest and of actin as a reference gene. The following primers were used to determine the relative gene expression using qPCR: vegf-a (Hs00900055_m1), vegf-b(Hs00173634_m1),vegf-c (Hs00153458_m1), flt-1(Hs01052961_m1), flt-4 (Hs01047677_m1), kdr (Hs00911700_m1), nrp-1(Hs00826128_m1), nrp-2 (Hs00187290_m1). Reactions were carried out in four replicates. Data were analyzed by the 2(−Delta Delta C(T)) method [71], where the gene expression levels were normalized to the level of actin beta housekeeping gene. The data of studied genes following normal distribution were parametrically tested by unpaired Student t-test.
qRT-PCR analysis of the level of mRNA expression for CK(s), HK-1 and HK-2 was carried out as described in our previous work [58].
2.8. Statistical analysis
All results are presented as a mean value±standard error (SEM). They were analyzed by Student's t-test, and p-values<0.05 were considered statistically significant. Apparent Km values for ADP were measured by fitting experimental data to a non-linear regression or double reciprocal Lineweaver–Burk plot according to a Michaelis–Menten equation. In our studies, cutoff values were calculated by using of available SigmaPlot 11 software, Systat Software, Inc.
3. Results
3.1. Evaluation of the function of OXPHOS system in human CRC and surrounding non-tumorous tissues
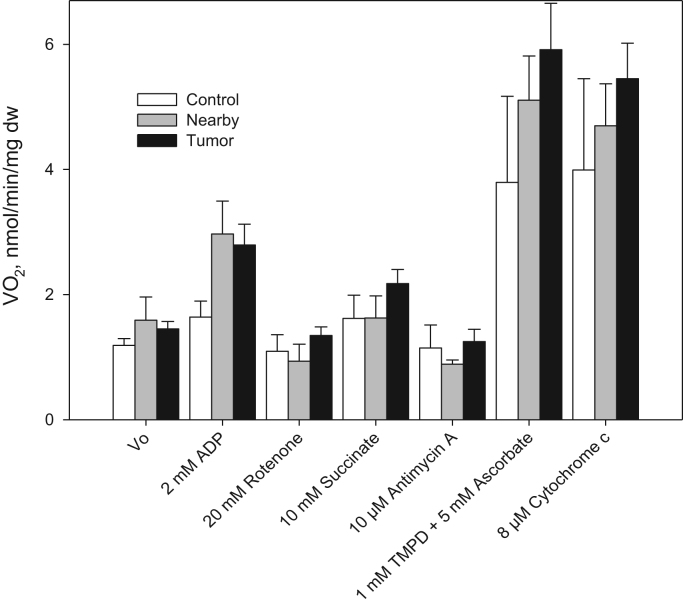
Our study was based on the hypothesis that epithelial CRC cells induce the Warburg effect (aerobic glycolysis) in neighboring tissue cells. In this situation, suppression of the mitochondrial function in association with the upregulation of glucose consumption could occur in nearby tissue cells. Perhaps, the increased glucose uptake registered by PET-FDG in CRC patients [22] is largely mediated by the higher glycolytic capacity of the tumor stroma as compared to its parenchymal cells. In addition, our prior studies on the function of OXPHOS system in CRC and healthy colon tissue cells are in line with the concept of reverse Warburg effect. In the current study we found that human CRC cells have fully-active respiratory chain (Fig. 1). CRC cells display higher rates of State 2 and State 3 oxygen consumption as compared with healthy colon tissue [58]; the measured rates of maximal ADP stimulated respiration (Vm) for tumor and normal tissues correlated well with their mitochondrial content [58]. Besides, it was found that both CRC and healthy colon tissues exhibit practically equal total glucose-phosphorylating activity (Table 1) [58]. These findings led us to the conclusion that human CRC is not a glycolytic tumor and in these cancer cells the OXPHOS system may be the main source of ATP. Thus, there is a probability that glycolytic stromal cells support the CRC cells growth by fueling their OXPHOS. But, the functional capacity of mitochondria in neighboring, to the malignancy growth area tissue was remained to be explored. Hereby, we have performed the corresponding comparative studies, and this is very important especially in the light of the concept of “reverse Warburg” effect.
Fig. 1.
Analysis of the mitochondrial respiratory chain function in permeabilized human colorectal cancer, junction area between cancer and normal mucosa (nearby) and healthy tissue samples. These studies were carried out in medium-B with 5 mM glutamate and 2 mM malate as respiratory substrates. TMPD is N,N,N′,N′-tetramethyl-phenylenediamine, and asc – ascorbate (bars are SEM, n=7, p<0.05). All respiratory substrates and inhibitors were added sequentially as indicated in the X-axis.
Table 1.
Enzymatic activities in human colorectal cancer and surrounding non-tumorous tissue samples.
| Enzyme activities, mU(s) per mg protein |
Tissues, mean±SE |
||
|---|---|---|---|
| Healthy colon tissue | Nearby | Colorectal cancer | |
| Hexokinase | 244±50 | 172±30, p=0.12 | 215±40, p=0.33 |
| Creatine kinase | 497±142 | 202±52, p<0.05 | 204±84, p<0.05 |
| Adenylate kinase | 257±35 | 256±35, p=0.49 | 411±43, p<0.05 |
Notes:p-values were calculated by Student test vs. control non-tumorous tissue data; number of examined samples was 11 – 16.
For this purpose, we analyzed the function of the mitochondrial electron transport chain (ETC) components in non-neoplastic, CRC, and nearby tissues using saponin-skinned samples. It was found (Fig. 1) that nearby tissue has relatively high rates of State 2 and ADP-activated respiration; in the presence 2 mM malate and 5 mM glutamate as respiratory substrates, the mean value V0 (State 2) was assayed as 1.59±0.37 nmol O2/min/mg dry tissue weight and it was increased after addition of 2 mM ADP by almost 2 times (2.97±0.53 nmol O2/min/mg dry tissue weight, p<0.05), confirming the presence of functionally-active mitochondria. The values of V0 and the respiration rate in the presence of 2 mM ADP for neighboring tissue exceeded notably (by ~1.5 times) the values obtained for skinned healthy colon tissue fibers. The ADP-activated respiration was strongly inhibited in all tissue samples upon addition of rotenone; this is a characteristic feature of cells with active Complex-I (Fig. 1). The differences of respiration rates between these three types of tissues are not statistically significant and it can be concluded that the development of colorectal cancer was not accompanied with suppression of complex I-dependent respiration as described in gastric cancer [92]. The next step was to estimate the Complex-II dependent respiration that can be achieved by addition of its substrate – succinate. We found that the addition of succinate (10 mM) leads to increase in the rates of O2 consumption suggesting that Complex-II of the mitochondrial respiratory chain is functionally active in these tissues cells. The ADP stimulated respiration was suppressed by addition of 10 μM antimycin-A (inhibitor for Complex-III); this result shows that the mitochondrial respiratory chain Complex III is also functionally active not only in unaffected tissue, but also in CRC cells. To activate cytochrome-c oxidase (COX) 1 mM N,N,N′,N′-tetramethyl-p-phenylenediamine (TMPD) jointly with 5 mM ascorbate were added and this resulted in a very strong increase in the rate of O2 consumption by all examined tissue samples (Fig. 1). The cytochrome c test is used to investigate the quality of the outer mitochondrial membrane after treatment of the tissue. Permeabilization of fibers does not alter the permeability of the outer mitochondrial membrane since addition of exogenous cytochrome c has no effect on respiration of the fibers [101]. The intactness of inner mitochondrial membrane was controlled by carboxyatractyloside (CAT). Upon addition of the inhibitor, the respiration rate decreased back to the basal respiration level due to inactivation of ANT [114].
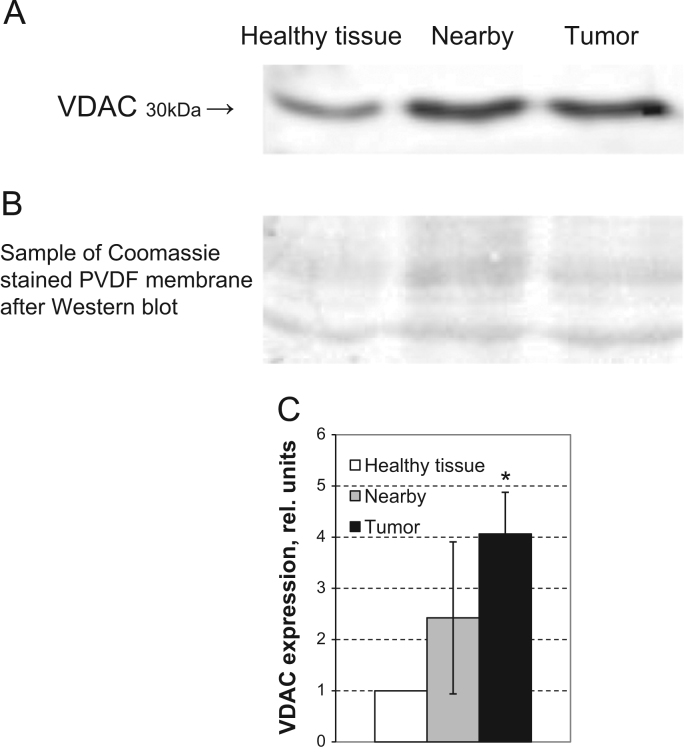
Respiratory control index (RCI) values for tumor and non-tumorous tissues were the following: ~2.0 for CRC,~1.8 for the neighboring tissue and~1.7 for unaffected colon tissue. The maximal rates of ADP-activated respiration (Vm) for cancerous and nearby tissue were found to exceed considerably those of healthy colon tissue (Table 2). This could be largely driven by the difference in the cellular content of mitochondria. In this connection, we estimated the content of mitochondria in tumorous and non-tumorous tissues through the analysis of VDAC protein expression. Western-blot analysis showed (Fig. 2) that CRC and neighboring tissue cells exhibited manifold higher levels of VDAC expression and, consequently, the number of mitochondria, as compared with healthy colon tissue, that in turn correlated directly with the measured Vm values (Table 2). In general, our estimations of the mitochondrial content in CRC and healthy intestinal tissues, as shown by increased VDAC expression in Western blot analysis, are similar with those obtained via immunocytochemical staining of mitochondrial outer membrane translocase (Tom20) protein [58]. In conclusion, our oxygraphic experiments clearly showed that the tissue neighboring to CRC contains a larger number of mitochondria, exhibits high rates of OXPHOS (close to carcinoma cells) and both these parameters exceed substantially the levels monitored for healthy colon tissue cells.
Table 2.
The values of basal respiration rate (V0), maximal rate of respiration (Vm, was calculated from a titration curve after step-wise addition of ADP, up to 2 mM), apparent Km values for ADP for permeabilized human colorectal cancer and surrounding tissue samples as well as some healthy rat muscle tissues of different histological type.
| Tissue | V0 | KmappADP, µM | Vm | Source |
|---|---|---|---|---|
| Colorectal cancera | 1.99±0.26 | 93.6±7.7b | 3.82±0.32* | Our data |
| Nearby tissuec | 1.64±0.27 | 84.9±9.9b | 2.98±0.34* | Our data |
| Healthy colon tissuec | 1.13±0.12 | 256±34b | 1.92±0.14 | Our data |
| Rat heart fibers | 6.45±0.19 | 297±35 | 28.7±1.1 | [56], [64] |
| Rat soleus | 2.19±0.30 | 354±46 | 12.2±0.5 | [56], [64] |
| Rat gastrocnemius white | 1.23±0.13 | 14.4±2.6 | 7.0±0.5; 4.10±0.25 | [56], [64] |
significant difference vs. normal intestinal tissue, p<0.05.
All respiratory rates are given as nmol O2/min/mg dry weight of tissue.
These apparent Km values were determined by fitting experimental data to a non-linear regression equation (according to a Michaelis–Menten model).
Nearby and healthy intestinal tissue samples were taken at a site distant from the tumor locus by 2 and 5 cm, respectively.
Fig. 2.
(A) Western blot analysis of the level of voltage-dependent anion channel (VDAC) porin protein expression in human CRC, nearby and unaffected non-tumorous tissue. The VDAC bands in reference samples correspond to the molecular mass of 30 kDa. (B) Sample of Coomassie-stained PVDF membrane (cutoff between 35 and 63 kDa) after Western blot (loading control). (C) The level of VDAC expression in CRC and nearby tissues was normalized to that in unaffected (control) intestinal tissue. Mean values from 8 patients with clinically-diagnosed CRC; bars are SEM, * p<0.05.
3.2. Analysis of the coupling of hexokinase reactions with the OXPHOS system in CRC and surrounding tissues
Some tumor cells (e.g., breast cancer [44] along with HK-2 express type-1 hexokinase (HK-1) that like type-2 can bind to VDAC and could mediate their highly glycolytic type [87]. In our prior study, we have shown that human CRC cells are characterized by the presence of mitochondrially-bound HK-2 and that its interaction with VDAC may be responsible for increased rates of aerobic glycolysis in these cancer cells [58].
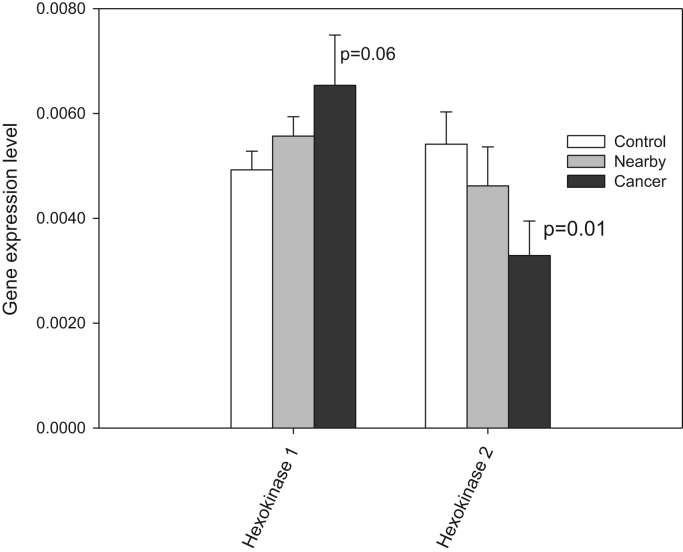
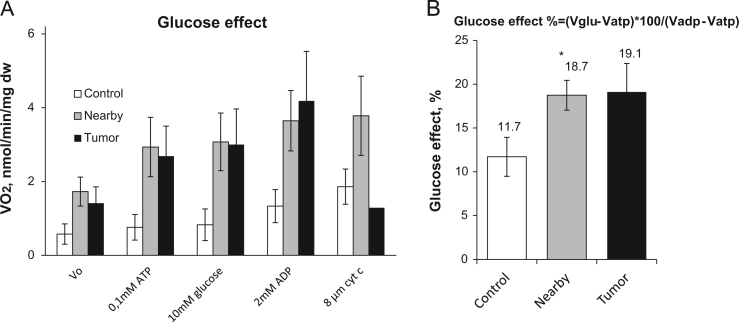
By using qRT-PCR, we estimated the levels of mRNA expression for HK-1 and -2 in CRC, adjacent and healthy colon tissue. It was found that HK-1 is expressed in each of the tissues examined in comparable levels (Fig. 3), only for HK 2 the statistical difference was significant for tumor and control tissue (p=0.01). Further, we performed oxygraphic analysis of the coupling of HK with the OXPHOS system in tumor, nearby and control tissues through the stimulation of OXPHOS by mitochondrially-bound HK(s) due to local generation of ADP. Experiments showed that the addition of 10 mM glucose to CRC and nearby tissue fibers in the presence of 0.1 mM Mg-ATP resulted in activation of mitochondrial respiration, but this HK coupling with OXPHOS was absent in healthy colon tissue (Fig. 4). In order to characterize the glucose effect on mitochondrial respiration, we used the glucose index (IGLU); it allows to compare the degree of stimulatory action of glucose (through ADP released in HK reactions) with maximal ADP (2 mM) activated respiration. As can be seen on Fig. 4B, the tissue neighboring to CRC has similar value of IGLU (18.7%), for the malignancy the index was measured as 19.1%, and healthy colon tissue had IGLU≈11.7%. HK activities are similar in CRC, nearby and healthy colon tissues (Table 2), it could be assumed that mitochondria of CRC and nearby tissue cells have a slightly higher capability for hexokinase binding in comparison with normal intestinal tissue cells and, as a consequence, slightly higher inclination to aerobic glycolysis. The revealed bioenergetic difference showing that this type of cancer cells could induce reprogramming of nearby tissue cells towards aerobic glycolysis.
Fig. 3.
Q-PCR analysis of the levels of mRNA expression for hexokinase 1 and 2 in human CRC and surroundings tissues; bars are SEM; p=0.06 for HK 1 and p=0.01 for HK 2. Average results for 29 CRC patients.
Fig. 4.
(A) Oxygraphic analysis of coupling of HK to OXPHOS in permeabilized CRC, nearby and control (healthy large intestine) tissue samples. The addition of 10 mM glucose to CRC fibers in the presence of 0.1 mM Mg-ATP caused a stimulatory effect on mitochondrial respiration in nearby and tumor tissue. (B) The comparison of glucose indexes of all permeabilized tissues samples; bars are SEM, n=5, * p<0.05.
3.3. Western blot analysis of the levels of beta-tubulin isotypes in healthy colon, CRC and the tumor surrounding tissue
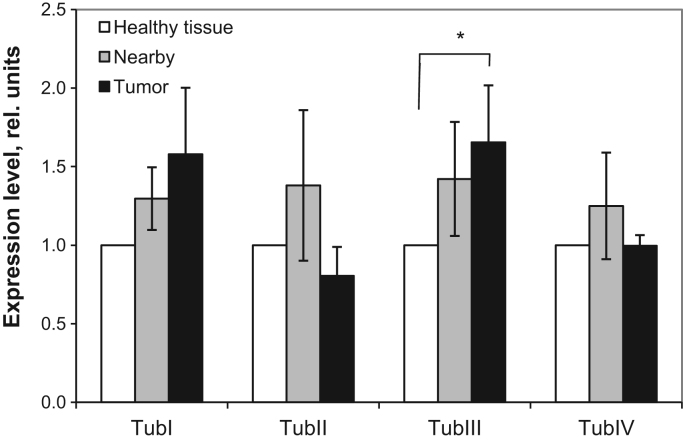
To understand possible reasons for the observed large difference between CRC and normal intestinal tissues in their respiratory parameters, we also examined the expression of main beta-tubulin isoforms in these tissues. Prior studies have shown that some β-tubulin isoforms may be involved in the regulation of OXPHOS and cellular cytoarchitecture in muscle cells of oxidative type. HK(s) can compete with tubulin for binding sites on the VDAC. It was already shown that in HL-1 tumor cells the downregulation of β2-tubulin expression can mediate their Warburg phenotype [45]. In this relation, we examined the spectrum of main beta-tubulin isoforms expression in CRC and surrounding non-tumorous tissues. Western blot analysis showed that there is not any statistically-significant difference in the levels of β-tubulin-I, II and IV expression between CRC, healthy colon and nearby tissues (Supplementary Fig 2). Only a slight (~ 1.5-fold) increase in the content of beta-III tubulin isotype was monitored in CRC in comparison with nearby and control tissues (Fig. 5)
Fig. 5.
Western blot analysis of the level of various β-tubulin isotypes: relative expression compared to control. Bars are SEM, n=7, ⁎p<0.05. Additional data on Supplementary Fig. 2.
3.4. Interrelationship between expression of vascular endothelial growth factors in CRC adjacent tissues and their respiratory activity
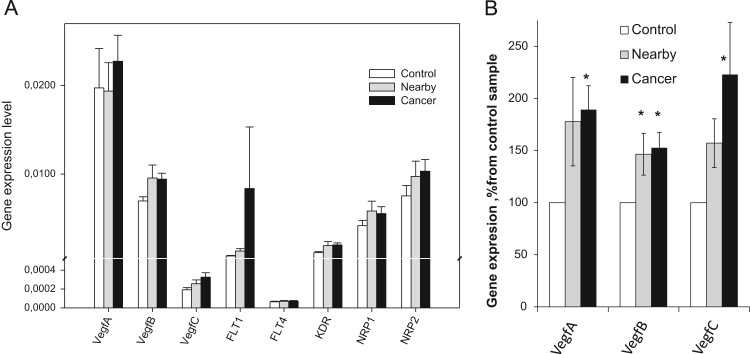
The ability of tumors to initiate the growth of new blood vessels is one of the key molecular events during carcinogenesis. It has been reported that the growth of solid tumors in vivo beyond 1–2 mm in diameter requires induction and maintenance of an angiogenetic response [93]. This abnormal vascularization of tumors may result in the development of microenvironments deprived of oxygen and nutrients [50]. As a consequence, hypoxic cancer cells use glycolysis, instead of OXPHOS, as a primary mechanism of ATP production. Moreover, severe prolonged hypoxia may affect OXPHOS or even cause irreversible damage of these organelles in cancer cells. But, our data suggest that human colorectal carcinomas are not hypoxic, since display high rates of OXPHOS (Fig. 1, and Table 2) due probably to extremely strong stimulation of angiogenesis. To check this assumption, we investigated the expression of genes encoding the VEGF family and its receptors (such as VEGF-A, -B, -C, FLT-1, FLT-4, KDR, NRP-1, and NRP-2) in human CRC, neighboring and healthy colon tissues. The role of VEGFs in promoting neovascularization and subsequent growth of tumors is well established [67]. Our experiments showed that the expression of gene encoding VEGF-A was predominant regardless of the examined tissue, since the levels of mRNA for VEGF-B and -C were found to be nearly 2-times lower (Fig. 6A). Among the VEGF receptors, the expression of FLT1 gene was the predominant in tumor, whereas the level of FLT4 is low in all tissue samples. Our studies suggest that a potent activation of angiogenesis, both in CRC and neighboring tissues can occur. The levels of VEGFs (A-C) expression in these tissues were found to be excessive, when the results standardized with respect to the control tissue for each patient separately (Fig. 6B). The ability of CRC cells to produce VEGFs, that will result in development of new blood and lymphatic vessels in the tumor growth area, is in good agreement with previous in vitro and in vivo studies performed in other laboratories [124], [15], [51], [8], [93]. In humans, angiogenesis is usually absent in most normal differentiated tissues and only very negligible levels of VEGFs can be observed. The relatively high levels of VEGFs expression in healthy intestinal tissue (Fig. 6) could be causally-linked with high regenerative activity of its epithelium.
Fig. 6.
(A) Q-PCR analysis of the mRNA expression for some vascular endothelial growth factors and their receptors in human CRC and surrounding tissue samples. (B) VEGF(A-C) gene expressions normalized to the unaffected tissue. Bars are SEM, n=29, * p<0.05.
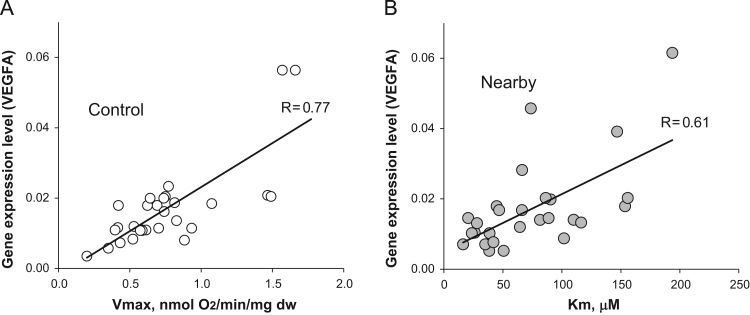
Besides, our data indicates that the process of neovascularization provides sufficient levels of nutrients and oxygen to support high rates of OXPHOS in CRC and its surrounding non-tumorous tissue cells (Table 2). So, among patients with CRC, a direct interrelationship between the level of VEGF-A expression and apparent Km (ADP) values was monitored for nearby tissue samples (Fig. 7B). A direct correlation between the level of VEGF-A expression and Vm values was found to occur in normal colon tissue samples (Fig. 7A). However, such correlation was not observed for CRC and nearby tissue samples. There was also no correlation between other vascular endothelial growth factors expression and these bioenergetics parameters.
Fig. 7.
Interrelationships between the level of VEGF-A expression in tissue samples of CRC patients and their respiratory parameters; rates of maximal ADP-activated respiration (Vm) and Km values for ADP. (A) The relationship between the level of VEGF-A expression and Vm values for healthy (control) large intestine tissue samples. (B) Correlation between the level of VEGF-A and apparent Km (ADP) value for nearby non-tumorous tissue fibers. On these figures each data point corresponds to the individual patient. Note: such interrelationships were not registered for colorectal cancer samples.
3.5. Comparative analysis of regulation of mitochondrial respiration in CRC, nearby and healthy intestinal tissue cells
3.5.1. Coupling of OXPHOS with creatine kinase and adenylate kinase systems
Coupling of spatially separated intracellular ATP producing and ATP-consuming systems is fundamental for the bioenergetics of living organisms, ensuring a fail-safe operation of the energetic system over a broad range of cellular functional activities. The central cellular mechanism of functioning of these organized metabolic pathways is the functional coupling between the isoenzymes of the creatine kinase (CK) and/or adenylate (AK) kinase systems and mitochondrial adenine nucleotide translocase [100], [31], [32], [45], [46], [55]. Human colonocytes express all CK isoenzymes, can form PCr, and it was found that BB-CK which is characteristic for the brain, is also the predominant isoenzyme in normal colon tissue [55], [58], [59]. The revealed downregulation of uMtCK mRNA as well as oxygraphic analysis the coupling of MtCK with OXPHOS in CRC tissue let us to conclude that mitochondria of these carcinoma cells have a poor ability to synthesize PCr [58]. Our experiments showed that the nearby tissue is also characterized by downregulation of the CK system: a more than 2-fold fall in the total CK activity was monitored (Table 1). Our in situ experiments indicated that mitochondria in nearby tissue cells have a decreased ability for the production of PCr like it was observed for cancer tissue [58].
It is well-known that AK-catalyzed phosphotransfer plays one of the key roles in the maintenance of energy homeostasis in fully differentiated cells with a high-energy demand, such as neural, cardiac and some skeletal muscle cells [31]. To assess the role of AK in CRC cells, the presence of functional coupling between ANT and OXPHOS was investigated. Recently we revealed that in human CRC cells the AK system is up-regulated in comparison with healthy colon tissue, but the function of the system in nearby tissue cells remained to be explored [58].
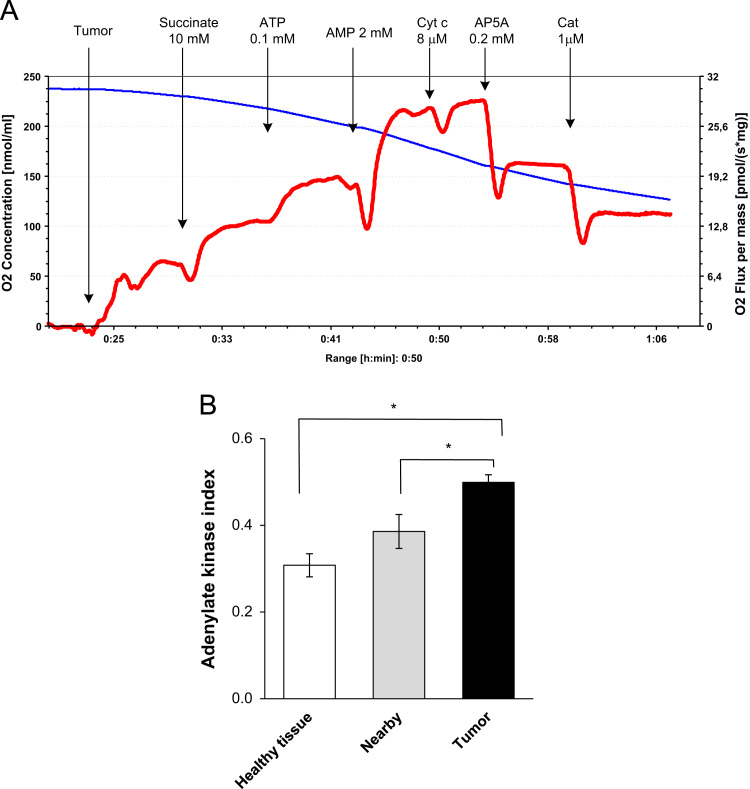
Experiments showed that the total AK activity of healthy intestine and nearby tissue extracts had similar values and they were ~40% lower as compared with CRC tissue (Table 1). These results indicate that the AK system, in contrast to CK system, is up-regulated in human colorectal carcinomas. Further, we compared the degree of functional coupling of AK-catalyzed processes with the OXPHOS system in CRC, nearby and non-tumor tissue samples. Fig. 8A depicts typical tracings of a change in the rates of O2 consumption, which were obtained during these studies. The addition of AMP (in the presence of 0.1 mM Mg-ATP) to permeabilized CRC fibers results in a remarkable (by ~40%) increase in the rate of O2 consumption by the samples (Fig. 8A). Addition of exogenous cytochrome c had no effect on ADP-dependent respiration indicting the intactness of MOM. Diadenosine pentaphosphate (AP5A, an inhibitor of AK) at a concentration of 0.2 mM suppressed the AMP-stimulated respiration up to initial level indicating thereby that it was largely mediated by ADP produced in AK reactions. Similar stimulatory effects of exogenously-added AMP on mitochondrial respiration were monitored for all non-tumorous tissues and they were notably (by about 1.5 times) lower as compared with CRC samples. For more precise estimating of the functional activity of AK processes and their coupling with the OXPHOS system both in cancerous and normal surrounding tissues, we applied the AK index (IAK) proposed by Gruno et al. [43]. The IAK measured for both non-neoplastic tissues (unaffected intestine: IAK=0.31±0.03; nearby tissue: IAK=0.39±0.04, n=10) were smaller as compared with CRC (IAK=0.50±0.02, n=12) (Fig. 8B). The reason of this difference may be the upregulation of AK system and a stronger interaction between the AK and OXPHOS system in comparison with nontumorous tissue. We assume that this could partly mediate by higher activity of AK2 in carcinoma cells in comparison with non-transformed cells. This will be checked in our further studies.
Fig. 8.
Oxygraphic analysis of the functional coupling between adenylate kinase (AK) catalyzed processes and OXPHOS in permeabilized CRC, nearby and adjacent healthy intestine tissue samples. (A) Representative tracing of rates of O2 consumption by CRC fibers; addition of 2 mM AMP in the presence of ATP led to activation of mitochondrial respiration due to formation of ADP in AK reactions. The involvement of AK(s) in stimulation of mitochondrial respiration was confirmed by subsequent addition of diadenosine pentaphosphate (AP5A)-an inhibitor of AK; as can be seen, the administration of AP5A resulted in a strong decrease in the rate of O2 consumption by CRC samples. Similar experiments were performed with healthy colon and nearby tissue samples. Outer mitochondrial intactness was controlled by effect of exogenously-added cytochrome c (Cyt); usually, the stimulating effect of Cyt on mitochondrial respiration was <10%. CAT is carboxyatractyloside – a selective inhibitor of adenine nucleotide translocator. (B) Efficiency of the coupling was estimated by means of AK index. Bars are SEM, n=10; * p<0.05.
3.5.2. Cutoff analysis of rates of maximal respiration, apparent Michaelis–Menten constant values for exogenously added ADP
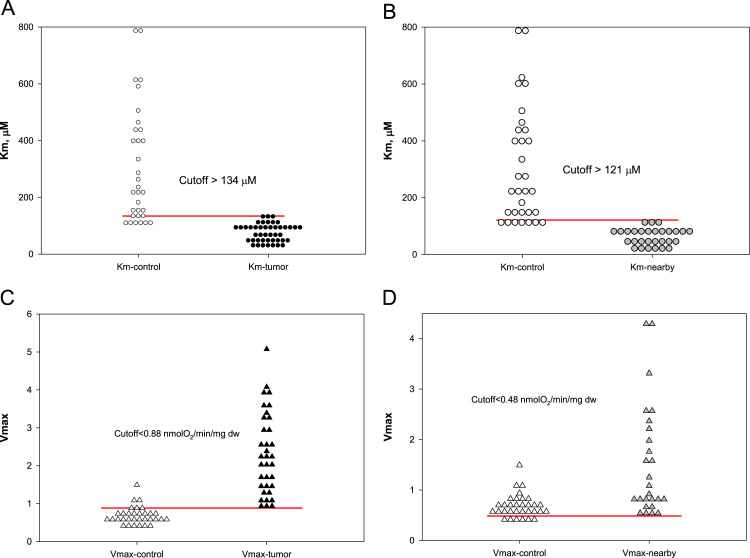
For a better understanding of the functioning and regulation of OXPHOS in human CRC cells and surrounding tissues, we measured their rates of maximal respiration as well as apparent Km values for exogenously-added ADP. The obtained results were also subjected to a cutoff analysis (based on random choice) for studies of distribution of these respiratory parameters in tumor, control and nearby tissues.
The analysis showed that there are strong differences in the regulation of mitochondrial respiration between CRC, nearby and unaffected tissue and it revealed the almost obvious signs the influence of tumor on mitochondrial function in neighboring tissue. Among the patients with CRC, the characteristic values of apparent Km for ADP for tumor tissue cells were found to be considerably smaller as compared with healthy large intestine (cutoff value>134 µM) (Fig. 9A). Similarly, decreased Km (ADP) values (very close to CRC cells) were also registered for nearby non-tumorous tissue cells; upon comparison of the data obtained for healthy and the tumor neighboring tissue the corresponding cutoff value was calculated as >121 µM (Fig. 9B). The analysis of this dataset led to the conclusion that Km normal values are above 120–130 µM, but pathological values will remain below this value. The maximal rates of ADP activated respiration Vm measured for permeabilized CRC and nearby tissue fibers were found to exceed significantly that for healthy intestinal cells (Table 2) due most probably to an increased amount of mitochondria in these tissue cells. Appropriate test cutoff values were calculated (Fig. 9C and D) and these could be used for diagnostics of CRC based on the measurements of corresponding respiratory rates in biopsy material. For control-tumor dataset the Vm cutoff value was calculated as 0.88 nmol O2/min per mg dry weight of tissue (Fig. 9C) This value is similar to the cutoff value found for the control-nearby dataset (0.48 nmol O2/min per mg dry weight of tissue) (Fig. 9D).
Fig. 9.
Cutoff analysis of apparent Km (ADP) for control-tumor (A), and control-nearby (B) permeabilized samples. Vm values cutoff analysis was also performed for these two datasets: (C) control-tumor and (D) control-nearby. For these calculations samples were taken from 46 patients.
Thus, the obtained data is showing that in situ the mitochondria of human CRC and neighboring normal tissue have increased affinity towards adenine nucleotides as compared with healthy colon tissue and these changes may appear in early stages of carcinogenesis. Besides, the results raise the question about the nature of tumor-associated factors which could influence the bioenergetic function of mitochondria in neighboring normal tissue. These factors are not related to hypoxia, since the Vm values characteristic for surrounding CRC tissue were even higher than those for healthy colon tissue (Fig. 9).
3.5.3. Assessment of the homogeneity of mitochondrial populations in CRC, nearby and control tissues
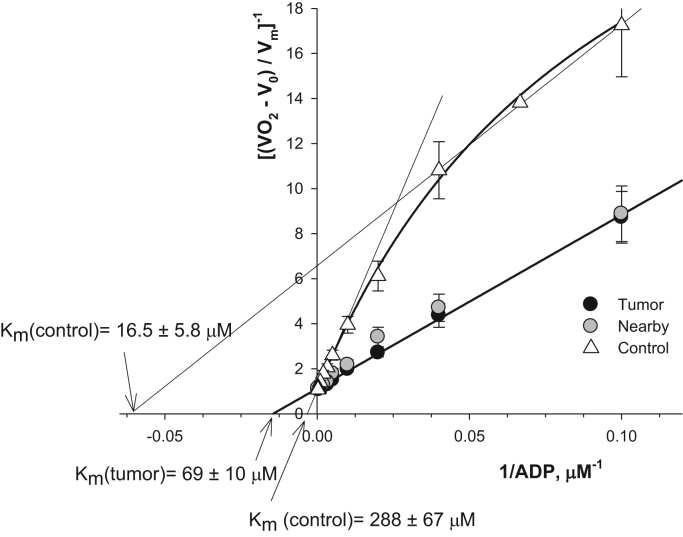
In addition, we evaluated heterogeneity of mitochondrial populations in CRC, nearby and normal tissue cells through analyzing the kinetics of respiration of skinned fibers with exogenously-added ADP as proposed by [101]. Distinct mitochondrial subpopulations could be present in these tissue cells and they could differ in their OXPHOS capacity. In our studies, permeabilized fibers were treated with increasing concentrations of ADP, and the measured rates of O2 consumption were plotted vs. ADP concentration in medium as double reciprocal Lineweaver–Burk plots, according to a Michaelis–Menten equation. A linearization approach permits us to calculate corresponding Vm and Km values. As has been shown by Saks and colleagues [101], the presence of two kinetic phases of respiration regulation on a graph curve is an indicator for the presence of two populations of mitochondria in a cell, which have different affinity for ADP and, correspondingly, various Km and Vm parameters. Our studies showed that there are not distinct mitochondrial subpopulations in CRC and neighboring tissue cells (Fig. 10). But, in healthy bowel, very clearly two populations of mitochondria with very different properties are revealed in double reciprocal plots. One population of mitochondria is characterized with a low Km value (16.5±5.8 µM) but Km for the second population is manifold higher (288±67 µM) (Fig. 10). The large difference in these apparent Km values for ADP (exceeding the factor of nearly 20 times) explains why in this case two populations of mitochondria are possible to see. These two populations of mitochondria, most probably, represent different types of fibers in this normal colon sample: mucosa and smooth muscle. Such a difference may be a reflection of the heterogeneity of healthy colon tissue cellular composition; high Km values could be attributed to mitochondria of the tissue cells with high OXPHOS rates. Similar values of Km for ADP were monitored by us for rat heart cardiomyocytes and soleus muscle cells. Low Km values for exogenously-added ADP (in the range of 20–40 µM) are usually characteristic for mitochondria of cells with high glycolytic rates, such as gastrocnemius white (see Table 2) or some poorly differentiated or tumor cells [47], [60]. In the nearby tissue two populations of mitochondria are absent and the apparent Km value for ADP is the same as for cancer tissue (Km=69±10 µM) (Fig. 10). Thus, we established that during colorectal carcinogenesis the amount of mitochondria rises together with profound changes in the regulation of the mitochondrial outer membrane permeability for ADP.
Fig. 10.
The dependence of the normalized values of respiration rates of CRC, nearby and unaffected colon tissue permeabilized samples: double reciprocal Lineweaver–Burk plots (n=9).
4. Discussion
Previous studies have shown that during malignant transformation the emerging cancer cells acquire new behavioral features and profound changes in their energy metabolism, which in part resemble undifferentiated embryonic cells, such as: invasive and destructive growth, dissemination, higher glycolytic capacity even in the presence of O2 [49], [76], [83]. The arising shifts in bioenergetics of cancer cells provide them with high yields and rates of ATP production, which are sufficient to support high rates of biosynthetic processes and proliferation even in hypoxic environment [36]. Some signaling pathways underlying the Warburg phenotype of malignant tumors are now uncovered and some therapeutic strategies which target glycolysis in tumor cells have also been proposed [115], [74], [89]. Currently, however, little is known about the features of main energy producing systems and their functioning in human CRC cells, although a deeper understanding of the specificity of bioenergetic processes which occur in the malignant cells, is a prerequisite for the development of more effective treatment strategies. To date, most of the corresponding studies have been carried out on tumor cells grown in cultures, whose proliferative, metabolic and bioenergetic properties may differ cardinally from in vivo conditions. CRC is currently regarded as a tumor of the Warburg phenotype [22], [54], [62], but our recent studies clearly showed that in situ human CRC cells have the total glycolytic activity close to healthy tissue cells and that in these malignant cells there is up-regulation of OXPHOS associated with stimulation of mitochondrial biogenesis [58] (Table 1, Table 2). These data were in good agreement with the results of other researchers who showed that tumor cells are not intrinsically glycolytic and that not all tumors have mitochondria with OXPHOS deficiency. The OXPHOS system was reported to be the principal ATP producer (>90%) for several malignant tumor cell types under normoxic conditions [111], [81]. Therefore, drug therapy targeting OXPHOS has emerged as an important alternative for growth arrest of oxidative type tumors [81], [83], [96].
Recently, Witkiewicz and colleagues proposed a new conceptual model for metabolism of a cancer cell at the tissue level that sheds new light on the significance of aerobic glycolysis, linked with lactic acid production, in fueling tumor growth and metastasis, and acting as a paracrine onco-metabolite [123]. This phenomenon was called as the “reverse Warburg effect”, since aerobic glycolysis occurred in stromal fibroblasts, but not in epithelial cancer cells [123]. In our work, in situ experiments were carried out to reveal the presence of “parasitic” energy-transfer interactions between CRC and neighboring tissue cells. For this aim, we compared the glycolytic activity and the OXPHOS capacity of CRC tissue cells with those in neighboring and distant healthy intestinal tissue cells.
Taken together our in situ studies indicate that human CRC cells in vivo exert a strong effect on the energy metabolism of neighboring tissue cells, so that they acquire the bioenergetic parameters specific to the tumor itself. Nearby tissue samples are characterized with upregulated mitochondrial respiration: values of State 2 and State 3 respiration rates are close to the tumor cells (Table 2). It is also found that nearby tissue is not purely glycolytic (Table 1) and it contains active mitochondria with fully-functional respiratory chain complexes (Fig. 1). Further work is needed to clarify the nature of paracrine factors released by CRC cells, which could alter the energy metabolism in tumor neighboring tissue. These can be various transforming growth factors (TGFs), as a large proportion of colorectal cancers were found to be characterized by elevated TGF-β production [13]. The growth of colorectal carcinomas is also associated with downregulation of the CK system in nearby tissue cells (Table 2) as well as with profound alterations in function of their mitochondria. In nearby tissue the mitochondria exhibit increased affinity to exogenously-added ADP similarly to tumor mitochondria. The corresponding Km values were measured as 93.6±7.7 µM for CRC cells and 84.9±9.9 µM for nearby tissue; both these apparent Km (ADP) values were considerably (by almost 3 times) lower in comparison with distant healthy colon tissue cells (256±34 µM) (Table 1).
Our results indicate a very low probability that the nearby tissue cell populations’ fuel via a lactate shunt the OXPHOS system in CRC cells. We found that the postoperative samples derived from CRC and neighboring tissue displayed practically equal values of the glucose effect (Fig. 4), levels of HK-1 and HK-2 expression (Fig. 3), and total glucose-phosphorylating activity (Table 1).
Our studies demonstrate that human CRC cannot be considered as a pure hypoxic tumor, since the malignancy and its neighboring tissue exhibit high rates of OXPHOS (Table 2). The respiration rates for these tissues are even higher than in unaffected tissue. The possible absence of acute hypoxic nature in colorectal carcinomas is also confirmed by their practically equal glucose-phosphorylating capacity as compared with surrounding non-tumorous tissues (Table 1), although hypoxia is a well-known factor that strongly increases the dependence of cancer cells on glycolytic pathway for ATP generation. Pioneering studies by Folkman demonstrated the crucial role of angiogenesis in tumor growth [34]. In the presented work, we found that there is some upregulation of VEGFs (A, B, and C) and their receptors expression in CRC as well as in the tumor surrounding tissue (Fig. 6). These might support tumor growth by inducing neovascularization, providing thereby O2 and substrates for cancer cell proliferation. It was already proven that VEGF-B is abundantly expressed in tissues with highly-active energy metabolism, where it could support significant metabolic functions [11]. It has generally been assumed that in cancer cells, the upregulation of VEGFs is caused by a deficiency in oxygen through HIF-1α pathways [27]. Co-expression of HIF-1α with VEGFs has been already monitored in human CRC tissue, and to be associated with poor prognosis [14]. But our findings suggest that in human CRC cells HIF-1α is not the main factor in the induction of VEGFs formation. The opinion [109], [86] that HIF-1α negatively regulates mitochondrial biogenesis and O2 consumption in tumor cells is doubtful, as in our previous work we clearly showed (via TOM20 expression) that in human colorectal carcinomas mitochondrial biogenesis is upregulated [58]. Increased levels of VEGFs, as compared to healthy colon, were also registered by us in nearby tissue (Fig. 6B). Recent findings suggest that in some cases the tumor accessory cells, such as macrophages, may be the main source of VEGFs promoting thereby neovascularization and tumor proliferation [24]. In this relation, it was robustly demonstrated that some non-steroid anti-inflammatory drugs (e.g., aspirin) lower risk of colorectal cancer-mediated mortality [16]. Besides, it was reported [27] that cancer-associated fibroblasts can express increased levels of VEGFs, thereby contributing to angiogenesis and tumor progression. Multiple clinical trials using antiangiogenic agents blocking VEGFs have recently demonstrated high efficacy for patients with metastatic colorectal cancer [23]. Our results show a correlation between the level of VEGF-A expression and maximal mitochondrial respiration rate Vm occurring in healthy colon tissue (Fig. 7A), but in tumor nearby tissue this correlation is replaced by a relationship between VEGF-A expression and the mitochondrial outer membrane permeability for ADP (Fig. 7B). The reason for this is not clear yet.
Our study demonstrates that human CRC cells have higher rates of OXPHOS as compared to normal tissue cells (Table 2) [58]. This is surprising, since it has been reported [102], [122] that β-F1-ATPase catalytic subunit of the mitochondrial H+-ATP synthase is downregulated in human colorectal carcinomas, and this is associated with the upregulation of the ATPase inhibitory factor 1 expression [35]. These changes should be associated with severe suppression of OXPHOS capacity, but our results do not confirm this. Our results support the hypothesis proposed by Pedersen and coworkers [89] that in tumor cells the presence of mitochondrially-bound HK-2 can be responsible for their increased rates of aerobic glycolysis. We found that the addition of glucose to permeabilized fibers (in the presence of exogenous ATP) resulted in some stimulation of mitochondrial respiration, not only in CRC, but also in neighboring samples. This effect, on the contrary, was negligible for healthy colon tissue cells (Fig. 4). The minor effect of glucose on mitochondrial respiration of permeabilized fibers derived from unaffected tissue samples was initially confusing; all examined tissues are characterized by the presence of mitochondrially-bound HK-2 [58], but have different levels of HK-1 and HK-2 expression (Fig. 3). At the same time, it was reported that mitochondria of tumor cells have elevated binding capacity towards HK-1 and -2 as compared with normal cells due to an increased number of VDAC(s) per mitochondria and cholesterol content in the MOM [87]. Elevated levels of VDAC protein (a binding partner for HKs) were registered by us in human CRC tissue cells, as compared to healthy colon tissue (Fig. 2), and this may be a plausible explanation for the negligible stimulating effect of exogenously added glucose on mitochondrial respiration in the normal tissue cells (Fig. 4) in spite of similar with cancerous tissue the levels of HK 1 expression (Fig. 3). Although several isoforms of VDAC are known, they do have similar kinetic characteristics, which indicate that the contribution of VDAC to enhanced HK binding and glucose phosphorylation is due to quantitative differences in binding site availability [105]. The distribution and role of HK isoforms in CRC and surrounding tissues in situ need further studies.
CK plays a key role in the energy homeostasis of vertebrate cells. It has been shown by us and other researchers that during carcinogenesis there is a nearly 2 times decrease in the total CK activity (Table 1) associated with loss of BB-CK (predominant isoform of CK in normal intestinal tissue) MM-CK, sMtCK and uMtCK [55], [58]. Also, in our prior study [58] we showed that normal intestinal cells have elevated levels of uMtCK expression and these cells are characterized by, on contrary to CRC cells, tight coupling between the CK isoform and OXPHOS system like in cardiac and slow-twitch skeletal muscle cells [114], [47], [78]. Downregulation of the CK system (associated with loss of uMtCK) was registered by us and also others tumors with different histological type, such as human breast carcinoma [56], neuroblastoma [60] and sarcoma cells [88], [9]. Possible signaling pathways mediating the downregulation of CK system in HCC cells remain still unclear. It was proposed that the downregulation of BB-CK in human CRC cells may play an important role in the tumor progression [80]. In the presented work, we found that colorectal carcinomas exert a distant effect on neighboring cells resulting in downregulation of their CK system (Table 1). The revealed absence of stimulating effect of exogenous creatine on mitochondrial respiration in CRC and nearby samples, contrary to healthy intestinal tissue, shows that mitochondria in both these tissues lose their ability to produce PCr. Our findings, on the poor ability of human CRC cells mitochondria to produce the marked amount of PCr, are in good agreement with literature data [59]. Although during CRC progression the arising malignant cells were found to up-regulate their AK system (Table 1) and there is tight coupling of the system with OXPHOS (Fig. 8, perhaps partly via upregulation of mitochondrial AK2), the distant effect of the neoplasm on AK processes in surrounding tissue cells was found to be minor (Table 1 and Fig. 8). We assume that the revealed downregulation of CK system may be casually-linked with upregulation of the AK phosphotransfer system. Indeed, we found that in human CRC cells the total AK activity exceeded significantly from that of normal intestinal tissue cells (Table 1) and it has also been reported by Dzeja et al. [33] that suppression of CK-catalyzed phosphotransfer may result in increased phosphoryl transfer by AK in intact skeletal muscle. But in contrast to our findings concerning CRC, the total AK activity in human lung adenocarcinomas was found to be substantially less as compared with normal tissue [41].
In oxidative type tissues with high energy demand the OXPHOS system is organized into protein supercomplexes, called the Mitochondrial Interactosome (MI) [113], [45], [79], [99]. MI is a large trans-membrane complex consisting of ATP-Synthasome, MtCK, VDAC, and protein factors regulating the outer mitochondrial membrane. There also some indication that the mitochondrial supercomplex may include certain HK(s) [74] as well as AK2 and AK4 [69]. From our studies it becomes clear that during carcinogenesis the structure and function of MI in colonocytes undergoes severe and specific alterations. Moreover, our results strongly suggest that colorectal carcinomas exert, by a currently unknown way, a distant effect on the functioning of MI in neighboring tissue cells. These alterations provide the basis for successful proliferation and viability of malignant cells in the hostile environment, characterized by deficiency in oxygen and nutrients. It is important to emphasize that in tumor cells, in addition to the MI, substantial alterations were also found to occur in the organization of mitochondrial respiratory chain: some enzymes of the ETC can form large supercomplexes that in turn may promote the generation of ATP [2], [56], [57], [60]. Indeed, our studies showed that the MOM in CRC and nearby tissue cells has increased permeability towards adenine nucleotides and they display an increased affinity for exogenous ADP. Thus, the apparent Km values for exogenously-added ADP measured for CRC and the neighboring tissue cells were found to be considerably (by about 3 times) smaller in comparison with that for distant normal tissues samples (Table 2). Our results and an analysis of literature data allowed us to propose several possible explanations for this phenomenon. Firstly, our results indicate that CRC and neighboring tissue cells, as compared to control colon tissue, contain higher levels of VDAC (Fig. 2) and, consequently, they can bind much more HK-2 [87]. The presence of mitochondrially-bound HK-2 in CRC and control intestinal tissues was adjusted by our immunofluorescent studies [58]. This event can mediate lower Km for ADP, since the binding of HK to VDAC holds this channel in the opened state [98] or then some other unknown protein factors could be involved in this phenomenon. Secondly, the revealed downregulation of MtCK and the absence of creatine kinase energy transfer network in CRC and neighboring tissue cells could be also responsible for increased permeability of the MOM [114], [45]. Thirdly, it is known that some ß-tubulin isoforms can bind to the VDAC localized on MOM and suppress its permeability towards adenine nucleotides, mediating thereby higher Km values for ADP and high rates of PCr production [114], [47]. Western blot analysis showed similar levels of ß-tubulin isoforms (I, II, III and IV) expression in CRC, healthy colon and nearby tissue samples (Fig. 5) and no binding of ßII-tubulin to VDAC(s) was also registered in these tissues [58]. This result is not in accordance with the data obtained on pure oxidative cells (rat heart cardiomyocytes) [112], [46] and the question arises, what complex is interacting with VDAC and therefore possibly regulating its permeability. This question remains the key in understanding the regulation mechanism of ATP production in mitochondria, not only in cardiac and muscle cells, but also in cells of the CRC. Tubulin and its potential biding partner – VDAC can undergo a number of various posttranslational modifications [53], [87], which may deeply affect their interactions. Fourthly, humans encode three different VDACs; VDAC1, VDAC2, and VDAC3, which were found to display different binding capacity towards tubulin, and the VDAC3 permeability is not sensitive to tubulin regulation [72]. In this relation, we propose that in CRC and neighboring non-tumor tissue cells, having lowered apparent Km values for ADP (Table 2) the VDAC3 isoform may be predominant. In addition, remarkable differences in the profile of ANT isoforms expression between normal and cancerous tissue cells could be responsible for the increased permeability of MOM in tumor cells. Cancer cells express predominantly ANT2, which interacts with VDAC [20], [21], and this could result in a facilitated diffusion adenine nucleotides across the MOM.
The increased level of β-III tubulin expression in colorectal carcinomas (Fig. 5) was also registered in other laboratories, and this may contribute to cancer cell invasion [66], [84].
5. Conclusion
This study revealed several important aspects in the bioenergetic metabolism of CRC and surrounding tissues cells. For a long time aerobic glycolysis has been considered the main energy source in CRC cells, but our results strongly suggest that CRC should not be considered a hypoxic tumor, since the malignancy exhibits high (even more than healthy colon tissue) rates of oxygen consumption, increased amount of mitochondria and practically equal glucose-phosphorylating capacity with the surrounding tissues. Obvious signs of stimulated neovascularization in CRC and nearby tissue are also evident. We observed strong differences in the function and regulation OXPHOS between CRC and normal intestinal tissue cells. During carcinogenesis, the amount of mitochondria is increasing in parallel to the change in the regulation of mitochondrial outer membrane permeability and phosphocreatine/creatine kinase shuttle that is completely replaced with AK mediated energy transport. The malignant cells are characterized by downregulation of CK, which is further associated with upregulation of the AK system and this could promote tumor growth and metastasis. The mitochondria of CRC cells lose the ability to produce PCr, reveal possibility of coupling of HK to OXPHOS and have increased affinity for ADP. In this aspect, more studies are required to determine the profile of AK, ANT and VDAC isoforms expression in human colorectal carcinomas. One of the most important findings from our studies is also that CRC cells exert a potent distant effect on the energy metabolism of nearby tissue cells. As compared with healthy colon tissue cells, nearby cells are also characterized by stimulated OXPHOS, downregulation of the CK system, which is associated with the loss of MtCK and increased permeability of the MOM against adenine nucleotides. Our data refused the initial hypothesis according to which the CRC surrounding cells could fuel via a lactate shunt the OXPHOS system in the malignancy cells.
Acknowledgments
This work was supported by institutional research funding IUT23-1 of the Estonian Ministry of Education and Research, the Estonian Science Foundation – Grant no. 8987, and by the project “Aid for research and development in healthcare technology” of Archimedes Foundation no. 3.2.1001.11-0027.
Footnotes
Supplementary data associated with this article can be found in the online version at doi:10.1016/j.bbrep.2015.08.020.
Appendix A. Supplementary material
Supplementary material Fig. 1. The 5-hydroxymethylation expression in human colorectal cancer and surrounding tissue samples (both nearby and healthy); bars are SEM, mean values from 13 patients. Fig. 2. Western blot analysis of the main β-tubulin isoforms expression in healthy colon, nearby and colorectal cancer tissues; here: TubI, TubII, TubIII and TubIV are βI-, βII-, βIII- and βIV-tubulin respectively. (PVDF membrane cutoff between 35 and 63 kDa).
Supplementary material
References
- 1.Abdullah M., Rani A.A., Simadibrata M., Fauzi A., Syam A.F. The value of fecal tumor M2 pyruvate kinase as a diagnostic tool for colorectal cancer screening. Acta Med Indones. 2012;44:94–99. [PubMed] [Google Scholar]
- 2.Acin-Perez R., Enriquez J.A. The function of the respiratory supercomplexes: The plasticity model. Biochim. Biophys. Acta (BBA) – Bioenerg. 2014;1837:444–450. doi: 10.1016/j.bbabio.2013.12.009. [DOI] [PubMed] [Google Scholar]
- 3.Altenberg B., Greulich K.O. Genes of glycolysis are ubiquitously overexpressed in 24 cancer classes. Genomics. 2004;84:1014–1020. doi: 10.1016/j.ygeno.2004.08.010. [DOI] [PubMed] [Google Scholar]
- 4.Amoedo N.D., Rodrigues M.F., Rumjanek F.D. Mitochondria: are mitochondria accessory to metastasis? Int. J. Biochem. Cell Biol. 2014;51:53–57. doi: 10.1016/j.biocel.2014.03.009. [DOI] [PubMed] [Google Scholar]
- 5.Anmann T., Guzun R., Beraud N., Pelloux S., Kuznetsov A.V., Kogerman L., Kaambre T., Sikk P., Paju K., Peet N. Different kinetics of the regulation of respiration in permeabilized cardiomyocytes and in HL-1 cardiac cells. Importance of cell structure/organization for respiration regulation. Biochim. Biophys. Acta. 2006;1757:1597–1606. doi: 10.1016/j.bbabio.2006.09.008. [DOI] [PubMed] [Google Scholar]
- 6.Appaix F., Kuznetsov A.V., Usson Y., Kay L., Andrienko T., Olivares J., Kaambre T., Sikk P., Margreiter R., Saks V. Possible role of cytoskeleton in intracellular arrangement and regulation of mitochondria. Exp. Physiol. 2003;88:175–190. doi: 10.1113/eph8802511. [DOI] [PubMed] [Google Scholar]
- 7.Arora K.K., Pedersen P.L. Functional significance of mitochondrial bound hexokinase in tumor cell metabolism. Evidence for preferential phosphorylation of glucose by intramitochondrially generated ATP. J Biol Chem. 1988;263:17422–17428. [PubMed] [Google Scholar]
- 8.Bagnasco L., Piras D., Parodi S., Bauer I., Zoppoli G., Patrone F., Ballestrero A. Role of angiogenesis inhibitors in colorectal cancer: sensitive and insensitive tumors. Curr. Cancer Drug Targets. 2012;12:303–315. doi: 10.2174/156800912800190929. [DOI] [PubMed] [Google Scholar]
- 9.Bera S., Wallimann T., Ray S., Ray M. Enzymes of creatine biosynthesis, arginine and methionine metabolism in normal and malignant cells. FEBS J. 2008;275:5899–5909. doi: 10.1111/j.1742-4658.2008.06718.x. [DOI] [PubMed] [Google Scholar]
- 10.Brandon M., Baldi P., Wallace D.C. Mitochondrial mutations in cancer. Oncogene. 2006;25:4647–4662. doi: 10.1038/sj.onc.1209607. [DOI] [PubMed] [Google Scholar]
- 11.Bry M., Kivela R., Leppanen V.M., Alitalo K. Vascular endothelial growth factor-B in physiology and disease. Physiol. Rev. 2014;94:779–794. doi: 10.1152/physrev.00028.2013. [DOI] [PubMed] [Google Scholar]
- 12.Bustamante E., Pedersen P.L. Mitochondrial hexokinase of rat hepatoma cells in culture: solubilization and kinetic properties. Biochemistry. 1980;19:4972–4977. doi: 10.1021/bi00563a006. [DOI] [PubMed] [Google Scholar]
- 13.Calon A., Espinet E., Palomo-Ponce S., Tauriello, Daniele V.F., Iglesias M., Céspedes, María V., Sevillano M., Nadal C., Jung P., Zhang, Xiang H.F. Dependency of Colorectal Cancer on a TGF-β-Driven Program in Stromal Cells for Metastasis Initiation. Cancer Cell. 2012;22:571–584. doi: 10.1016/j.ccr.2012.08.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cao D., Hou M., Guan Y.S., Jiang M., Yang Y., Gou H.F. Expression of HIF-1alpha and VEGF in colorectal cancer: association with clinical outcomes and prognostic implications. BMC Cancer. 2009;9:432. doi: 10.1186/1471-2407-9-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cecilia Subauste M., Kupriyanova T., Conn E., Ardi V., Quigley J., Deryugina E. Evaluation of metastatic and angiogenic potentials of human colon carcinoma cells in chick embryo model systems. Clin. Exp. Metastasis. 2009;26:1033–1047. doi: 10.1007/s10585-009-9293-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chan A.T., Ogino S., Fuchs C.S. Aspirin use and survival after diagnosis of colorectal cancer. JAMA. 2009;302:649–658. doi: 10.1001/jama.2009.1112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chandra D., Singh K.K. Genetic insights into OXPHOS defect and its role in cancer. Biochim. Biophys. Acta (BBA) – Bioenerg. 2011;1807:620–625. doi: 10.1016/j.bbabio.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chatterjee A., Mambo E., Sidransky D. Mitochondrial DNA mutations in human cancer. Oncogene. 2006;25:4663–4674. doi: 10.1038/sj.onc.1209604. [DOI] [PubMed] [Google Scholar]
- 19.Chen Z., Zhang H., Lu W., Huang P. Role of mitochondria-associated hexokinase II in cancer cell death induced by 3-bromopyruvate. Biochim. Biophys. Acta (BBA) – Bioenerg. 2009;1787:553–560. doi: 10.1016/j.bbabio.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chevrollier A., Loiseau D., Chabi B., Renier G., Douay O., Malthiery Y., Stepien G. ANT2 isoform required for cancer cell glycolysis. J. Bioenerg. Biomembr. 2005;37:307–316. doi: 10.1007/s10863-005-8642-5. [DOI] [PubMed] [Google Scholar]
- 21.Chevrollier A., Loiseau D., Reynier P., Stepien G. Adenine nucleotide translocase 2 is a key mitochondrial protein in cancer metabolism. Biochim. Biophys. Acta (BBA) – Bioenerg. 2011;1807:562–567. doi: 10.1016/j.bbabio.2010.10.008. [DOI] [PubMed] [Google Scholar]
- 22.Chung J.K., Lee Y.J., Kim C., Choi S.R., Kim M., Lee K., Jeong J.M., Lee D.S., Jang J.J., Lee M.C. Mechanisms related to [18F]fluorodeoxyglucose uptake of human colon cancers transplanted in nude mice. J. Nucl. Med. 1999;40:339–346. [PubMed] [Google Scholar]
- 23.Clarke J.M., Hurwitz H.I., Rangwala F. Understanding the mechanisms of action of antiangiogenic agents in metastatic colorectal cancer: a clinician's perspective. Cancer Treat. Rev. 2014;40:1065–1072. doi: 10.1016/j.ctrv.2014.07.001. [DOI] [PubMed] [Google Scholar]
- 24.Colegio O.R., Chu N.-Q., Szabo A.L., Chu T., Rhebergen A.M., Jairam V., Cyrus N., Brokowski C.E., Eisenbarth S.C., Phillips G.M. Functional polarization of tumour-associated macrophages by tumour-derived lactic acid. Nature. 2014;513:559–563. doi: 10.1038/nature13490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Curry J.M., Tuluc M., Whitaker-Menezes D., Ames J.A., Anantharaman A., Butera A., Leiby B., Cognetti D.M., Sotgia F., Lisanti M.P., Martinez-Outschoorn U.E. Cancer metabolism, stemness and tumor recurrence: MCT1 and MCT4 are functional biomarkers of metabolic symbiosis in head and neck cancer. Cell Cycle. 2013;12:1371–1384. doi: 10.4161/cc.24092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Daye D., Wellen K.E. Metabolic reprogramming in cancer: Unraveling the role of glutamine in tumorigenesis. Semin. Cell Dev. Biol. 2012;23:362–369. doi: 10.1016/j.semcdb.2012.02.002. [DOI] [PubMed] [Google Scholar]
- 27.De Francesco E.M., Lappano R., Santolla M.F., Marsico S., Caruso A., Maggiolini M. HIF-1alpha/GPER signaling mediates the expression of VEGF induced by hypoxia in breast cancer associated fibroblasts (CAFs) Breast Cancer Res. 2013;15:R64. doi: 10.1186/bcr3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.de Souza A.C., Justo G.Z., de Araujo D.R., Cavagis A.D. Defining the molecular basis of tumor metabolism: a continuing challenge since Warburg's discovery. Cell. Physiol. Biochem. 2011;28:771–792. doi: 10.1159/000335792. [DOI] [PubMed] [Google Scholar]
- 29.DeBerardinis R.J., Mancuso A., Daikhin E., Nissim I., Yudkoff M., Wehrli S., Thompson C.B. Beyond aerobic glycolysis: Transformed cells can engage in glutamine metabolism that exceeds the requirement for protein and nucleotide synthesis. Proc. Natl. Acad. Sci. 2007;104:19345–19350. doi: 10.1073/pnas.0709747104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Diers A.R., Broniowska K.A., Chang C.F., Hogg N. Pyruvate fuels mitochondrial respiration and proliferation of breast cancer cells: effect of monocarboxylate transporter inhibition. Biochem. J. 2012;444:561–571. doi: 10.1042/BJ20120294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Dzeja P., Terzic A. Adenylate kinase and AMP signaling networks: Metabolic monitoring, signal communication and body energy sensing. Int. J. Mol. Sci. 2009;10:1729–1772. doi: 10.3390/ijms10041729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dzeja P.P., Terzic A. Phosphotransfer networks and cellular energetics. J. Exp. Biol. 2003;206:2039–2047. doi: 10.1242/jeb.00426. [DOI] [PubMed] [Google Scholar]
- 33.Dzeja P.P., Vitkevicius K.T., Redfield M.M., Burnett J.C., Terzic A. Adenylate kinase-catalyzed phosphotransfer in the myocardium: increased contribution in heart failure. Circ. Res. 1999;84:1137–1143. doi: 10.1161/01.res.84.10.1137. [DOI] [PubMed] [Google Scholar]
- 34.Folkman J. Tumor angiogenesis: therapeutic implications. N. Engl. J. Med. 1971;285:1182–1186. doi: 10.1056/NEJM197111182852108. [DOI] [PubMed] [Google Scholar]
- 35.Formentini L., Sanchez-Arago M., Sanchez-Cenizo L., Cuezva J.M. The mitochondrial ATPase inhibitory factor 1 triggers a ROS-mediated retrograde prosurvival and proliferative response. Mol. Cell. 2012;45:731–742. doi: 10.1016/j.molcel.2012.01.008. [DOI] [PubMed] [Google Scholar]
- 36.Ganapathy-Kanniappan S., Geschwind J.F. Tumor glycolysis as a target for cancer therapy: progress and prospects. Mol. Cancer. 2013;12:152. doi: 10.1186/1476-4598-12-152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gnaiger E. Oxygen solubility in experimental media. OROBOROS Bioenerg. Newsl. 2001;6:1–6. [Google Scholar]
- 38.Goel A., Nagasaka T., Arnold C.N., Inoue T., Hamilton C., Niedzwiecki D., Compton C., Mayer R.J., Goldberg R., Bertagnolli M.M., Boland C.R. The CpG island methylator phenotype and chromosomal instability are inversely correlated in sporadic colorectal cancer. Gastroenterology. 2007;132:127–138. doi: 10.1053/j.gastro.2006.09.018. [DOI] [PubMed] [Google Scholar]
- 39.Gogvadze V., Orrenius S., Zhivotovsky B. Mitochondria as targets for cancer chemotherapy. Semin. Cancer Biol. 2009;19:57–66. doi: 10.1016/j.semcancer.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 40.Gonzalez-Granillo M., Grichine A., Guzun R., Usson Y., Tepp K., Chekulayev V., Shevchuk I., Karu-Varikmaa M., Kuznetsov A.V., Grimm M. Studies of the role of tubulin beta II isotype in regulation of mitochondrial respiration in intracellular energetic units in cardiac cells. J. Mol. Cell. Cardiol. 2012;52:437–447. doi: 10.1016/j.yjmcc.2011.07.027. [DOI] [PubMed] [Google Scholar]
- 41.Greengard O., Head J.F., Goldberg S.L. Uridine kinase, adenylate kinase, and guanase in human lung tumors. Cancer Res. 1980;40:2295–2299. [PubMed] [Google Scholar]
- 42.Gregersen L., Jacobsen A., Frankel L., Wen J., Krogh A., Lund A. MicroRNA-143 down-regulates Hexokinase 2 in colon cancer cells. BMC Cancer. 2012;12:232. doi: 10.1186/1471-2407-12-232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gruno M., Peet N., Seppet E., Kadaja L., Paju K., Eimre M., Orlova E., Peetsalu M., Tein A., Soplepmann J. Oxidative phosphorylation and its coupling to mitochondrial creatine and adenylate kinases in human gastric mucosa. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2006;291:R936–R946. doi: 10.1152/ajpregu.00162.2006. [DOI] [PubMed] [Google Scholar]
- 44.Gudnason V., Ingvarsson S., Jonasdottir A., Andresdottir V., Egilsson V. Isoenzyme pattern and subcellular localization of hexokinases in human breast cancer and nonpathological breast tissue. Int. J. Cancer. 1984;34:63–66. doi: 10.1002/ijc.2910340111. [DOI] [PubMed] [Google Scholar]
- 45.Guzun R., Gonzalez-Granillo M., Karu-Varikmaa M., Grichine A., Usson Y., Kaambre T., Guerrero-Roesch K., Kuznetsov A., Schlattner U., Saks V. Regulation of respiration in muscle cells in vivo by VDAC through interaction with the cytoskeleton and MtCK within Mitochondrial Interactosome. Biochim. Biophys. Acta. 2012;1818:1545–1554. doi: 10.1016/j.bbamem.2011.12.034. [DOI] [PubMed] [Google Scholar]
- 46.Guzun R., Kaambre T., Bagur R., Grichine A., Usson Y., Varikmaa M., Anmann T., Tepp K., Timohhina N., Shevchuk I. Modular organization of cardiac energy metabolism: energy conversion, transfer and feedback regulation. Acta Physiol. 2015;213:84–106. doi: 10.1111/apha.12287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Guzun R., Karu-Varikmaa M., Gonzalez-Granillo M., Kuznetsov A.V., Michel L., Cottet-Rousselle C., Saaremae M., Kaambre T., Metsis M., Grimm M. Mitochondria-cytoskeleton interaction: distribution of beta-tubulins in cardiomyocytes and HL-1 cells. Biochim. Biophys. Acta. 2011;1807:458–469. doi: 10.1016/j.bbabio.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 48.Haber R.S., Rathan A., Weiser K.R., Pritsker A., Itzkowitz S.H., Bodian C., Slater G., Weiss A., Burstein D.E. GLUT1 glucose transporter expression in colorectal carcinoma: a marker for poor prognosis. Cancer. 1998;83:34–40. doi: 10.1002/(sici)1097-0142(19980701)83:1<34::aid-cncr5>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 49.Hanahan D., Weinberg R.A. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 50.Harris A.L. Hypoxia--a key regulatory factor in tumour growth. Nat. Rev. Cancer. 2002;2:38–47. doi: 10.1038/nrc704. [DOI] [PubMed] [Google Scholar]
- 51.Huang X., Withers B.R., Dickson R.C. Sphingolipids and lifespan regulation. Biochim. Biophys. Acta (BBA) – Mol. Cell Biol. Lipids. 2014;1841:657–664. doi: 10.1016/j.bbalip.2013.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Izuishi K., Yamamoto Y., Sano T., Takebayashi R., Nishiyama Y., Mori H., Masaki T., Morishita A., Suzuki Y. Molecular mechanism underlying the detection of colorectal cancer by 18F-2-fluoro-2-deoxy-d-glucose positron emission tomography. J. Gastrointest. Surg.: Off. J. Soc.r Surg. Aliment. Tract. 2012;16:394–400. doi: 10.1007/s11605-011-1727-z. [DOI] [PubMed] [Google Scholar]
- 53.Janke C., Bulinski J.C. Post-translational regulation of the microtubule cytoskeleton: mechanisms and functions. Nat. Rev. Mol. Cell. Biol. 2011;12:773–786. doi: 10.1038/nrm3227. [DOI] [PubMed] [Google Scholar]
- 54.Jimenez B., Mirnezami R., Kinross J., Cloarec O., Keun H.C., Holmes E., Goldin R.D., Ziprin P., Darzi A., Nicholson J.K. 1H HR-MAS NMR spectroscopy of tumor-induced local metabolic “field-effects” enables colorectal cancer staging and prognostication. J. Proteome Res. 2013;12:959–968. doi: 10.1021/pr3010106. [DOI] [PubMed] [Google Scholar]
- 55.Joseph J., Cardesa A., Carreras J. Creatine kinase activity and isoenzymes in lung, colon and liver carcinomas. Br. J. Cancer. 1997;76:600–605. doi: 10.1038/bjc.1997.432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Kaambre T., Chekulayev V., Shevchuk I., Karu-Varikmaa M., Timohhina N., Tepp K., Bogovskaja J., Kutner R., Valvere V., Saks V. Metabolic control analysis of cellular respiration in situ in intraoperational samples of human breast cancer. J. Bioenerg. Biomembr. 2012;44:539–558. doi: 10.1007/s10863-012-9457-9. [DOI] [PubMed] [Google Scholar]
- 57.Kaambre T., Chekulayev V., Shevchuk I., Tepp K., Timohhina N., Varikmaa M., Bagur R., Klepinin A., Anmann T., Koit A. Metabolic control analysis of respiration in human cancer tissue. Front. Physiol. 2013;4:1–6. doi: 10.3389/fphys.2013.00151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kaldma A., Klepinin A., Chekulayev V., Mado K., Shevchuk I., Timohhina N., Tepp K., Kandashvili M., Varikmaa M., Koit A. An in situ study of bioenergetic properties of human colorectal cancer: the regulation of mitochondrial respiration and distribution of flux control among the components of ATP synthasome. Int. J. Biochem. Cell Biol. 2014;55:171–186. doi: 10.1016/j.biocel.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 59.Kasimos J.N., Merchant T.E., Gierke L.W., Glonek T. 31P Magnetic Resonance Spectroscopy of Human Colon Cancer. Cancer Res. 1990;50:527–532. [PubMed] [Google Scholar]
- 60.Klepinin A., Chekulayev V., Timohhina N., Shevchuk I., Tepp K., Kaldma A., Koit A., Saks V., Kaambre T. Comparative analysis of some aspects of mitochondrial metabolism in differentiated and undifferentiated neuroblastoma cells. J. Bioenerg. Biomembr. 2014;46:17–31. doi: 10.1007/s10863-013-9529-5. [DOI] [PubMed] [Google Scholar]
- 61.Ko Y.H., Pedersen P.L., Geschwind J.F. Glucose catabolism in the rabbit VX2 tumor model for liver cancer: characterization and targeting hexokinase. Cancer Lett. 2001;173:83–91. doi: 10.1016/s0304-3835(01)00667-x. [DOI] [PubMed] [Google Scholar]
- 62.Koukourakis M.I., Giatromanolaki A., Simopoulos C., Polychronidis A., Sivridis E. Lactate dehydrogenase 5 (LDH5) relates to up-regulated hypoxia inducible factor pathway and metastasis in colorectal cancer. Clin. Exp. Metastasis. 2005;22:25–30. doi: 10.1007/s10585-005-2343-7. [DOI] [PubMed] [Google Scholar]
- 63.Kuznetsov A.V., Javadov S., Guzun R., Grimm M., Saks V. Cytoskeleton and regulation of mitochondrial function: the role of beta-tubulin II. Front. Physiol. 2013;4:1–7. doi: 10.3389/fphys.2013.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kuznetsov A.V., Tiivel T., Sikk P., Kaambre T., Kay L., Daneshrad Z., Rossi A., Kadaja L., Peet N., Seppet E., Saks V.A. Striking differences between the kinetics of regulation of respiration by ADP in slow-twitch and fast-twitch muscles in vivo. Eur. J. Biochem. 1996;241:909–915. doi: 10.1111/j.1432-1033.1996.00909.x. [DOI] [PubMed] [Google Scholar]
- 65.Kuznetsov A.V., Veksler V., Gellerich F.N., Saks V., Margreiter R., Kunz W.S. Analysis of mitochondrial function in situ in permeabilized muscle fibers, tissues and cells. Nat. Protoc. 2008;3:965–976. doi: 10.1038/nprot.2008.61. [DOI] [PubMed] [Google Scholar]
- 66.Leandro-García L.J., Leskelä S., Landa I., Montero-Conde C., López-Jiménez E., Letón R., Cascón A., Robledo M., Rodríguez-Antona C. Tumoral and tissue-specific expression of the major human β-tubulin isotypes. Cytoskeleton. 2010;67:214–223. doi: 10.1002/cm.20436. [DOI] [PubMed] [Google Scholar]
- 67.Lin E.Y., Li J.-F., Gnatovskiy L., Deng Y., Zhu L., Grzesik D.A., Qian H., Xue X.-n, Pollard J.W. Macrophages regulate the angiogenic switch in a mouse model of breast cancer. Cancer Res. 2006;66:11238–11246. doi: 10.1158/0008-5472.CAN-06-1278. [DOI] [PubMed] [Google Scholar]
- 68.Lisanti M.P., Martinez-Outschoorn U.E., Sotgia F. Oncogenes induce the cancer-associated fibroblast phenotype: Metabolic symbiosis and “fibroblast addiction” are new therapeutic targets for drug discovery. Cell Cycle. 2013;12:2740–2749. doi: 10.4161/cc.25695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Liu R., Ström A.-L., Zhai J., Gal J., Bao S., Gong W., Zhu H. Enzymatically inactive adenylate kinase 4 interacts with mitochondrial ADP/ATP translocase. Int. J. Biochem. Cell Biol. 2009;41:1371–1380. doi: 10.1016/j.biocel.2008.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Liu X.H., Zheng X.F., Wang Y.L. Inhibitive effect of 3-bromopyruvic acid on human breast cancer MCF-7 cells involves cell cycle arrest and apoptotic induction. Chin. Med. J. (Engl.) 2009;122:1681–1685. [PubMed] [Google Scholar]
- 71.Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT Method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 72.Maldonado E.N., Sheldon K.L., DeHart D.N., Patnaik J., Manevich Y., Townsend D.M., Bezrukov S.M., Rostovtseva T.K., Lemasters J.J. Voltage-dependent anion channels modulate mitochondrial metabolism in cancer cells: regulation by free tubulin and erastin. J. Biol. Chem. 2013;288:11920–11929. doi: 10.1074/jbc.M112.433847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Martinez-Outschoorn U.E., Lin Z., Trimmer C., Flomenberg N., Wang C., Pavlides S., Pestell R.G., Howell A., Sotgia F., Lisanti M.P. Cancer cells metabolically “fertilize” the tumor microenvironment with hydrogen peroxide, driving the Warburg effect: implications for PET imaging of human tumors. Cell Cycle. 2011;10:2504–2520. doi: 10.4161/cc.10.15.16585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Mathupala S.P., Ko Y.H., Pedersen P.L. Hexokinase II: cancer’s double-edged sword acting as both facilitator and gatekeeper of malignancy when bound to mitochondria. Oncogene. 2006;25:4777–4786. doi: 10.1038/sj.onc.1209603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Mathupala S.P., Ko Y.H., Pedersen P.L. Hexokinase-2 bound to mitochondria: cancer’s stygian link to the “Warburg Effect” and a pivotal target for effective therapy. Semin. Cancer Biol. 2009;19:17–24. doi: 10.1016/j.semcancer.2008.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mayevsky A. Mitochondrial function and energy metabolism in cancer cells: Past overview and future perspectives. Mitochondrion. 2009;9:165–179. doi: 10.1016/j.mito.2009.01.009. [DOI] [PubMed] [Google Scholar]
- 77.Miettinen M., Lasota J. Succinate dehydrogenase deficient gastrointestinal stromal tumors (GISTs) – a review. Int. J. Biochem. Cell Biol. 2014;53:514–519. doi: 10.1016/j.biocel.2014.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Monge C., Beraud N., Tepp K., Pelloux S., Chahboun S., Kaambre T., Kadaja L., Roosimaa M., Piirsoo A., Tourneur Y. Comparative analysis of the bioenergetics of adult cardiomyocytes and nonbeating HL-1 cells: respiratory chain activities, glycolytic enzyme profiles, and metabolic fluxes. Can. J. Physiol. Pharmacol. 2009;87:318–326. doi: 10.1139/Y09-018. [DOI] [PubMed] [Google Scholar]
- 79.Monge C., Guzun R., Tepp K., Timohhina N., Varikmaa M., Sikk P., Kaambre T., Saks V. Mitochondrial interactosome in health and disease: structural and functional aspects of molecular system bioenergetics of muscle and neuronal cells. In: Svensson O.L., editor. Mitochondria: Structure, Functions and Dysfunctions. Nova Science Pub Inc; New York: 2010. pp. 441–470. [Google Scholar]
- 80.Mooney S.M., Rajagopalan K., Williams B.H., Zeng Y., Christudass C.S., Li Y., Yin B., Kulkarni P., Getzenberg R.H. Creatine kinase brain overexpression protects colorectal cells from various metabolic and non-metabolic stresses. J. Cell. Biochem. 2011;112:1066–1075. doi: 10.1002/jcb.23020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Moreno-Sanchez R., Marin-Hernandez A., Saavedra E., Pardo J.P., Ralph S.J., Rodriguez-Enriquez S. Who controls the ATP supply in cancer cells? Biochemistry lessons to understand cancer energy metabolism. Int. J. Biochem. Cell Biol. 2014;50:10–23. doi: 10.1016/j.biocel.2014.01.025. [DOI] [PubMed] [Google Scholar]
- 82.Moreno-Sanchez R., Rodriguez-Enriquez S., Marin-Hernandez A., Saavedra E. Energy metabolism in tumor cells. FEBS J. 2007;274:1393–1418. doi: 10.1111/j.1742-4658.2007.05686.x. [DOI] [PubMed] [Google Scholar]
- 83.Moreno-Sánchez R., Rodríguez-Enríquez S., Saavedra E., Marín-Hernández A., Gallardo-Pérez J.C. The bioenergetics of cancer: Is glycolysis the main ATP supplier in all tumor cells? BioFactors. 2009;35:209–225. doi: 10.1002/biof.31. [DOI] [PubMed] [Google Scholar]
- 84.Mozzetti S., Ferlini C., Concolino P., Filippetti F., Raspaglio G., Prislei S., Gallo D., Martinelli E., Ranelletti F.O., Ferrandina G., Scambia G. Class III beta-tubulin overexpression is a prominent mechanism of paclitaxel resistance in ovarian cancer patients. Clin. Cancer Res. 2005;11:298–305. [PubMed] [Google Scholar]
- 85.Nieman K.M., Kenny H.A., Penicka C.V., Ladanyi A., Buell-Gutbrod R., Zillhardt M.R., Romero I.L., Carey M.S., Mills G.B., Hotamisligil G.S. Adipocytes promote ovarian cancer metastasis and provide energy for rapid tumor growth. Nat. Med. 2011;17:1498–1503. doi: 10.1038/nm.2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Papandreou I., Cairns R.A., Fontana L., Lim A.L., Denko N.C. HIF-1 mediates adaptation to hypoxia by actively downregulating mitochondrial oxygen consumption. Cell Metab. 2006;3:187–197. doi: 10.1016/j.cmet.2006.01.012. [DOI] [PubMed] [Google Scholar]
- 87.Pastorino J., Hoek J. Regulation of hexokinase binding to VDAC. J. Bioenerg. Biomembr. 2008;40:171–182. doi: 10.1007/s10863-008-9148-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Patra S., Bera S., SinhaRoy S., Ghoshal S., Ray S., Basu A., Schlattner U., Wallimann T., Ray M. Progressive decrease of phosphocreatine, creatine and creatine kinase in skeletal muscle upon transformation to sarcoma. FEBS J. 2008;275:3236–3247. doi: 10.1111/j.1742-4658.2008.06475.x. [DOI] [PubMed] [Google Scholar]
- 89.Pedersen P.L. 3-bromopyruvate (3BP) a fast acting, promising, powerful, specific, and effective “small molecule” anti-cancer agent taken from labside to bedside: introduction to a special issue. J. Bioenerg. Biomembr. 2012;44:1–6. doi: 10.1007/s10863-012-9425-4. [DOI] [PubMed] [Google Scholar]
- 90.Pelicano H., Martin D.S., Xu R.H., Huang P. Glycolysis inhibition for anticancer treatment. Oncogene. 2006;25:4633–4646. doi: 10.1038/sj.onc.1209597. [DOI] [PubMed] [Google Scholar]
- 91.Pfeiffer T., Schuster S., Bonhoeffer S. Cooperation and competition in the evolution of ATP-producing pathways. Science. 2001;292:504–507. doi: 10.1126/science.1058079. [DOI] [PubMed] [Google Scholar]
- 92.Puurand M., Peet N., Piirsoo A., Peetsalu M., Soplepmann J., Sirotkina M., Peetsalu A., Hemminki A., Seppet E. Deficiency of the complex I of the mitochondrial respiratory chain but improved adenylate control over succinate-dependent respiration are human gastric cancer-specific phenomena. Mol. Cell. Biochem. 2012;370:69–78. doi: 10.1007/s11010-012-1399-3. [DOI] [PubMed] [Google Scholar]
- 93.Rak J., Mitsuhashi Y., Bayko L., Filmus J., Shirasawa S., Sasazuki T., Kerbel R.S. Mutant ras oncogenes upregulate VEGF/VPF expression: implications for induction and inhibition of tumor angiogenesis. Cancer Res. 1995;55:4575–4580. [PubMed] [Google Scholar]
- 94.Robey R.B., Raval B.J., Ma J., Santos A.V. Thrombin is a novel regulator of hexokinase activity in mesangial cells. Kidney Int. 2000;57:2308–2318. doi: 10.1046/j.1523-1755.2000.00091.x. [DOI] [PubMed] [Google Scholar]
- 95.Rodriguez-Enriquez S., Carreno-Fuentes L., Gallardo-Perez J.C., Saavedra E., Quezada H., Vega A., Marin-Hernandez A., Olin-Sandoval V., Torres-Marquez M.E., Moreno-Sanchez R. Oxidative phosphorylation is impaired by prolonged hypoxia in breast and possibly in cervix carcinoma. Int. J. Biochem. Cell Biol. 2010;42:1744–1751. doi: 10.1016/j.biocel.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 96.Rodriguez-Enriquez S., Gallardo-Perez J.C., Marin-Hernandez A., Aguilar-Ponce J.L., Mandujano-Tinoco E.A., Meneses A., Moreno-Sanchez R. Oxidative phosphorylation as a target to arrest malignant neoplasias. Curr. Med. Chem. 2011;18:3156–3167. doi: 10.2174/092986711796391561. [DOI] [PubMed] [Google Scholar]
- 97.Rodriguez-Enriquez S., Torres-Marquez M.E., Moreno-Sanchez R. Substrate oxidation and ATP supply in AS-30D hepatoma cells. Arch. Biochem. Biophys. 2000;375:21–30. doi: 10.1006/abbi.1999.1582. [DOI] [PubMed] [Google Scholar]
- 98.Rostovtseva T.K., Tan W., Colombini M. On the role of VDAC in apoptosis: fact and fiction. J. Bioenerg. Biomembr. 2005;37:129–142. doi: 10.1007/s10863-005-6566-8. [DOI] [PubMed] [Google Scholar]
- 99.Saks V., Guzun R., Timohhina N., Tepp K., Varikmaa M., Monge C., Beraud N., Kaambre T., Kuznetsov A., Kadaja L. Structure-function relationships in feedback regulation of energy fluxes in vivo in health and disease: mitochondrial interactosome. Biochim. Biophys. Acta. 2010;1797:678–697. doi: 10.1016/j.bbabio.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 100.Saks V., Kuznetsov A., Andrienko T., Usson Y., Appaix F., Guerrero K., Kaambre T., Sikk P., Lemba M., Vendelin M. Heterogeneity of ADP diffusion and regulation of respiration in cardiac cells. Biophys. J. 2003;84:3436–3456. doi: 10.1016/S0006-3495(03)70065-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Saks V.A., Veksler V.I., Kuznetsov A.V., Kay L., Sikk P., Tiivel T., Tranqui L., Olivares J., Winkler K., Wiedemann F., Kunz W.S. Permeabilized cell and skinned fiber techniques in studies of mitochondrial function in vivo. Mol. Cell. Biochem. 1998;184:81–100. [PubMed] [Google Scholar]
- 102.Sanchez-Cenizo L., Formentini L., Aldea M., Ortega A.D., Garcia-Huerta P., Sanchez-Arago M., Cuezva J.M. Up-regulation of the ATPase inhibitory factor 1 (IF1) of the mitochondrial H+-ATP synthase in human tumors mediates the metabolic shift of cancer cells to a Warburg phenotype. J. Biol. Chem. 2010;285:25308–25313. doi: 10.1074/jbc.M110.146480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Semenza G.L. Regulation of cancer cell metabolism by hypoxia-inducible factor 1. Semin. Cancer Biol. 2009;19:12–16. doi: 10.1016/j.semcancer.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 104.Seppet E.K., Eimre M., Anmann T., Seppet E., Peet N., Kaambre T., Paju K., Piirsoo A., Kuznetsov A.V., Vendelin M. Intracellular energetic units in healthy and diseased hearts. Exp. Clin. Cardiol. 2005;10:173–183. [PMC free article] [PubMed] [Google Scholar]
- 105.Shinohara Y., Ishida T., Hino M., Yamazaki N., Baba Y., Terada H. Characterization of porin isoforms expressed in tumor cells. Eur. J. Biochem. 2000;267:6067–6073. doi: 10.1046/j.1432-1327.2000.01687.x. [DOI] [PubMed] [Google Scholar]
- 106.Sotgia F., Martinez-Outschoorn U.E., Lisanti M.P. The reverse warburg effect in osteosarcoma. Oncotarget. 2014;5:7982–7983. doi: 10.18632/oncotarget.2352. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sotgia F., Martinez-Outschoorn U.E., Pavlides S., Howell A., Pestell R.G., Lisanti M.P. Understanding the Warburg effect and the prognostic value of stromal caveolin-1 as a marker of a lethal tumor microenvironment. Breast Cancer Res. 2011;13:213. doi: 10.1186/bcr2892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Sotgia F., Whitaker-Menezes D., Martinez-Outschoorn U.E., Flomenberg N., Birbe R., Witkiewicz A.K., Howell A., Philp N.J., Pestell R.G., Lisanti M.P. Mitochondrial metabolism in cancer metastasis: visualizing tumor cell mitochondria and the “reverse Warburg effect” in positive lymph node tissue. Cell Cycle. 2012;11:1445–1454. doi: 10.4161/cc.19841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Zhang H., Gao P., Fukuda R., Kumar G., Krishnamachary B., Zeller K.I., Dang C.V., Semenza G.L. HIF-1 inhibits mitochondrial biogenesis and cellular respiration in VHL-deficient renal cell carcinoma by repression of C-MYC activity. Cancer Cell. 2007;11:407–420. doi: 10.1016/j.ccr.2007.04.001. [DOI] [PubMed] [Google Scholar]
- 110.Zhou Y., Tozzi F., Chen J., Fan F., Xia L., Wang J., Gao G., Zhang A., Xia X., Brasher H. Intracellular ATP Levels Are a Pivotal Determinant of Chemoresistance in Colon Cancer Cells. Cancer Res. 2012;72:304–314. doi: 10.1158/0008-5472.CAN-11-1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Zu X.L., Guppy M. Cancer metabolism: facts, fantasy, and fiction. Biochem. Biophys. Res. Commun. 2004;313:459–465. doi: 10.1016/j.bbrc.2003.11.136. [DOI] [PubMed] [Google Scholar]
- 112.Tepp K., Mado K., Varikmaa M., Klepinin A., Timohhina N., Shevchuk I., Chekulayev V., Kuznetsov A.V., Guzun R., Kaambre T. The role of tubulin in the mitochondrial metabolism and arrangement in muscle cells. J. Bioenerg. Biomembr. 2014;46:421–434. doi: 10.1007/s10863-014-9579-3. [DOI] [PubMed] [Google Scholar]
- 113.Tepp K., Shevchuk I., Chekulayev V., Timohhina N., Kuznetsov A.V., Guzun R., Saks V., Kaambre T. High efficiency of energy flux controls within mitochondrial interactosome in cardiac intracellular energetic units. Biochim. Biophys. Acta. 2011;1807:1549–1561. doi: 10.1016/j.bbabio.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 114.Timohhina N., Guzun R., Tepp K., Monge C., Varikmaa M., Vija H., Sikk P., Kaambre T., Sackett D., Saks V. Direct measurement of energy fluxes from mitochondria into cytoplasm in permeabilized cardiac cells in situ: some evidence for mitochondrial interactosome. J. Bioenerg. Biomembr. 2009;41:259–275. doi: 10.1007/s10863-009-9224-8. [DOI] [PubMed] [Google Scholar]
- 115.Upadhyay M., Samal J., Kandpal M., Singh O.V., Vivekanandan P. The Warburg effect: insights from the past decade. Pharmacol. Ther. 2013;137:318–330. doi: 10.1016/j.pharmthera.2012.11.003. [DOI] [PubMed] [Google Scholar]
- 116.Vander Heiden M.G., Cantley L.C., Thompson C.B. Understanding the warburg effect: the metabolic requirements of cell proliferation. Science. 2009;324:1029–1033. doi: 10.1126/science.1160809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Warburg O., Wind F., Negelein E. The metabolism of tumors in the body. J. Gen. Physiol. 1927;8:519–530. doi: 10.1085/jgp.8.6.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Varikmaa M., Bagur R., Kaambre T., Grichine A., Timohhina N., Tepp K., Shevchuk I., Chekulayev V., Metsis M., Boucher F. Role of mitochondria-cytoskeleton interactions in respiration regulation and mitochondrial organization in striated muscles. Biochim. Biophys. Acta. 2014;1837:232–245. doi: 10.1016/j.bbabio.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 119.Welinder C., Ekblad L. Coomassie staining as loading control in western blot analysis. J. Proteome Res. 2011;10:1416–1419. doi: 10.1021/pr1011476. [DOI] [PubMed] [Google Scholar]
- 120.Whitaker-Menezes D., Martinez-Outschoorn U.E., Lin Z., Ertel A., Flomenberg N., Witkiewicz A.K., Birbe R.C., Howell A., Pavlides S., Gandara R. Evidence for a stromal-epithelial “lactate shuttle” in human tumors: MCT4 is a marker of oxidative stress in cancer-associated fibroblasts. Cell Cycle. 2011;10:1772–1783. doi: 10.4161/cc.10.11.15659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Viale A., Pettazzoni P., Lyssiotis C.A., Ying H., Sanchez N., Marchesini M., Carugo A., Green T., Seth S., Giuliani V. Oncogene ablation-resistant pancreatic cancer cells depend on mitochondrial function. Nature. 2014;514:628–632. doi: 10.1038/nature13611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Willers I.M., Isidoro A., Ortega A.D., Fernandez P.L., Cuezva J.M. Selective inhibition of beta-F1-ATPase mRNA translation in human tumours. Biochem. J. 2010;426:319–326. doi: 10.1042/BJ20091570. [DOI] [PubMed] [Google Scholar]
- 123.Witkiewicz A.K., Whitaker-Menezes D., Dasgupta A., Philp N.J., Lin Z., Gandara R., Sneddon S., Martinez-Outschoorn U.E., Sotgia F., Lisanti M.P. Using the “reverse Warburg effect” to identify high-risk breast cancer patients: Stromal MCT4 predicts poor clinical outcome in triple-negative breast cancers. Cell Cycle. 2012;11:1108–1117. doi: 10.4161/cc.11.6.19530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Xu J., Wang J., Xu B., Ge H., Zhou X., Fang J.-Y. Colorectal Cancer Cells Refractory to Anti-VEGF Treatment Are Vulnerable to Glycolytic Blockade due to Persistent Impairment of Mitochondria. Mol. Cancer Ther. 2013;12:717–724. doi: 10.1158/1535-7163.MCT-12-1016-T. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material Fig. 1. The 5-hydroxymethylation expression in human colorectal cancer and surrounding tissue samples (both nearby and healthy); bars are SEM, mean values from 13 patients. Fig. 2. Western blot analysis of the main β-tubulin isoforms expression in healthy colon, nearby and colorectal cancer tissues; here: TubI, TubII, TubIII and TubIV are βI-, βII-, βIII- and βIV-tubulin respectively. (PVDF membrane cutoff between 35 and 63 kDa).
Supplementary material