Abstract
Introduction and Objective
Stone analysis should be performed in all first-time stone formers. The preferred analytical procedures are Fourier-transform infrared spectroscopy (FT-IR) or X-ray diffraction (XRD). However, due to limited resources, chemical analysis (CA) is still in use throughout the world. The aim of the study was to compare FT-IR and CA in well matched stone specimens and characterize the pros and cons of CA.
Methods
In a prospective bi-center study, urinary stones were retrieved from 60 consecutive endoscopic procedures. In order to assure that identical stone samples were sent for analyses, the samples were analyzed initially by micro-computed tomography to assess uniformity of each specimen before submitted for FTIR and CA.
Results
Overall, the results of CA did not match with the FTIR results in 56% of the cases. In 16% of the cases CA missed the major stone component and in 40% the minor stone component. 37 of the 60 specimens contained CaOx as major component by FTIR, and CA reported major CaOx in 47/60, resulting in high sensitivity, but very poor specificity. CA was relatively accurate for UA and cystine. CA missed struvite and calcium phosphate as a major component in all cases. In mixed stones the sensitivity of CA for the minor component was poor, generally less than 50%.
Conclusions
Urinary stone analysis using CA provides only limited data that should be interpreted carefully. Urinary stone analysis using CA is likely to result in clinically significant errors in its assessment of stone composition. Although the monetary costs of CA are relatively modest, this method does not provide the level of analytical specificity required for proper management of patients with metabolic stones.
Keywords: Nephrolithiasis, stone composition, chemical analysis, FT-IR
Introduction
The knowledge of stone composition may help direct preventive measures and therapeutic decisions in renal stone patients. A recommendation for stone analysis in any new stone former and some recurrent high-risk stone formers is now part of the EAU and the recently published AUA guidelines [1–2].
The common methods of stone analysis are polarization microscopy on grain preparations, chemical methods in the form of analysis kits (CA) and the more modern X-ray diffraction (XRD) and infrared spectroscopy- particularly the very quick FTIR technique [3]. While polarization microscopy can be very accurate it requires a high subjective experience and therefore CA gained popularity. Currently, the preferred analytical procedures are infrared spectroscopy or X-ray diffraction (XRD) [1–2]. However, while the use of CA for stone composition is declining it is still in use worldwide due to budget restraints as well as insufficient awareness of its limitations. For example, Hesse et al [4] reporting the results of quality control studies of urinary stone analysis in Germany showed that in 1980, 87% of the participating laboratories used CA. In 2001 a much smaller, but still a significant number (12.7%) of the laboratories were still using CA. In Israel, where the current study was performed, CA was the only available technique in the entire public health system until 2013 when the first FTIR system was installed in our center. Likewise, reports from other regions of the world show that CA is still utilized to assess urinary calculi composition [5–9].
For the purpose of CA, the stone substance is dissolved and individual ions are identified, from which the original substances may be deduced using specific calculation scales. The results of CA appear as individual ion percentages (i.e calcium, phosphate, etc.) as well as some deduced calculated mineral compositions. Due to the inherent difficulties of CA the referring physician has to interpret and integrate the different parameters in the CA report in order to predict what the actual stone composition was. The use of CA analysis is declining because analysis employing this method is very vulnerable to error [9–10] and yet it is important for those still using the technique to be fully aware of the limitations of CA.
The purpose of this study was to compare the results of CA and FTIR in order to characterize the specific errors that may be encountered using CA for stone composition. When comparing various stone composition analysis techniques significant discrepancies are found between the results [10–12]. Such discrepancies may be attributed to the different stone analysis techniques, but can also derive from stone samples which were not identical. Urinary stones are not homogeneous. Mixed stone composition is found in the majority of patients [13]. Therefore, an accurate comparison between two analytic techniques requires that the stone samples examined share the same composition [14].
Micro CT is a research method that produces a three-dimensional image of the stone with microscopic resolution. Micro CT can reveal great detail in stone structure, and it is possible for mineral components to be identified by a combination of x-ray attenuation values and morphological appearance [14]. Because it is a non-destructive technique we have chosen to use it in the current study to verify the compositional similarity of the specimens sent out for FTIR and CA [15].
Materials and methods
After receiving institutional board approval we prospectively collected 60 urinary kidney stones from 60 consecutive patients that underwent endoscopic stone removal in our center. Not included in the study were patients in which the stone material available was judged to be too small to allow division into separate samples. Each stone sample was than washed and dried prior to further processing.
Micro CT analysis
All stone samples were initially evaluated by micro CT (Indiana University) to assess uniformity of each specimen. Each specimen was scanned using micro CT (Skyscan 1172 System, Bruker, Kontich, Belgium). Scanning parameters were 60 kV with rotation steps of 0.4°, and with source-to-camera distances to yield voxel sizes between 5 and 15 micrometers, depending on the specimen size (Figure 1). The resulting scans were studied for identification of mineral type(s) using x-ray attenuation values and mineral morphologies. Scrapings were also taken from selected regions of each specimen for verification of mineral type using Fourier-transform infrared spectroscopy (FT-IR; Bruker Alpha-T Spectrometer) using traditional KBr pellet methods. The true composition of each specimen was ascertained by micro CT and FT-IR results - a combination that is not available in any clinical laboratory.
Figure 1.
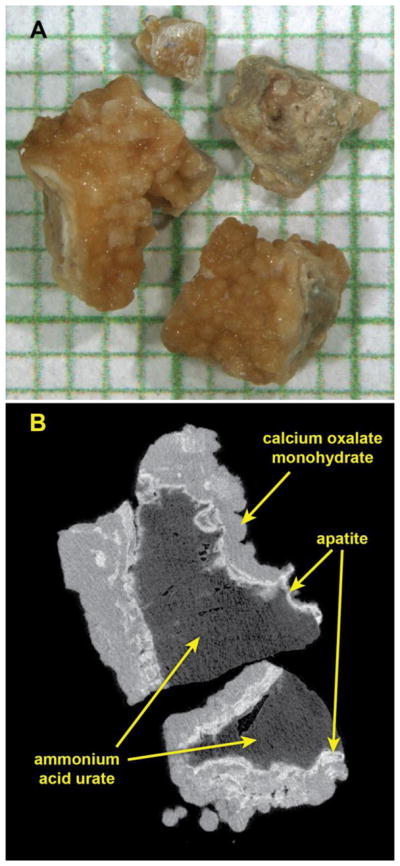
Micro CT characterization of a typical specimen of mixed mineral in this study. A: Photo of stone fragments on mm-grid paper. B: Micro CT image slice through two of the fragments. X-ray attenuation values indicated that these stone fragments contained a core of urate or uric acid (dark gray, determined by infrared spectroscopy to be urate), surrounded by a shell composed of calcium phosphate in the form of apatite (bright white regions) and calcium oxalate monohydrate (gray regions). This specimen was judged to be sufficiently uniform among fragments that it could be divided randomly and submitted for both clinical FT-IR and CA analysis.
Each specimen was divided in two, with one portion submitted for wet CA and the other for FT-IR, both carried out in the clinical laboratory (Rabin Medical Center). The hospital laboratory personnel were unaware of the micro CT and previous FT-IR results. For each stone the component with the highest percentage was reported as the major component and the next in line as the minor component. Stones composed of 90% or more of a single component were considered pure stones.
Chemical analysis
Semi-quantitative determination of several chemical components was performed with a commercial reagent kit (Urinary calculi analysis kit, Diasys, Diagnostic system GmBH, Holzheim, Germany). Weddelite and carbonate apatite are not analyzed in the kit, according to manufacturer’s information.
No differentiation is possible using the kit between Weddelite and Whewellite.
Briefly, the stone sample is crushed into powder. Several chemical reagents are added to the powder and titrimetric or colorimetric methods are used. For example, in the colorimetric method, the color complex formed by iron and sulfosalicylic acid is discharged by oxalate. The color of the solution is matched with a color scale to indicate the percentages of the different ion components.
After the results of ion components were obtained some mineral compositions were additionally deduced using dedicated calculation scales which are part of the analysis kit. The final CA report included percentages of the different ions and the results of the mineral calculation scales.
The results of the wet chemical analysis were compared to the results of FTIR and assessed in terms of sensitivity and specificity for major and minor minerals in each specimen.
FTIR analysis
Measurements were performed using a Bruker - ALPHA FTIR spectrometer (Karlsruhe, Germany), with resolution of 4cm-1 at a measuring range of 400 – 4000cm-1. The stone samples were ground to dust and placed for analysis. FTIR results were compared with the stone IR data spectra with Bruker’s BLG 1 & 2 spectral libraries.
Results
The stone composition distribution is presented in table 1. Calcium oxalate monohydrate and dihydrate were grouped together as the CA was not able to detect the difference. The predominant stone types were calcium and uric acid, comprising 65% and 20% of the stones, respectively.
Table 1.
Urinary stone composition as identified by FT-IR.
| Number of stones (%) | |
|---|---|
| CaOx | 37 (62) |
| CaP | 2 (3) |
| UA | 12 (20) |
| struvite | 5 (8) |
| cystine | 4 (7) |
Comparisons between the results of FTIR and CA for the major and minor stones components are presented in tables 2 and 3.
Table 2.
Comparison among major stone components as identified by FT-IR and wet chemical analysis (CA).
| FT-IR = n (%) | Pure = n (%) | CA = n (%) | Pure = n (%) | |
|---|---|---|---|---|
| CaOx | 37 (62) | 26 (70) | 47 (78) | 27 (57) |
| CaP | 2 (3) | 0 | 0 | - |
| UA | 12 (20) | 9 (75) | 9 (15) | 1 (11) |
| struvite | 5 (8) | 2 (40) | 0 | - |
| cystine | 4 (6) | 4 (100) | 4 (7) | 2 (50) |
Table 3.
Comparison among minor stone component as identified by FT-IR and CA.
| FT-IR = n (%) | CA = n (%) | |
|---|---|---|
| CaOx | 8 (13) | 10 (17) |
| CaP | 8 (13) | 9 (15) |
| UA | 3 (5) | 6 (10) |
| struvite | 0 | 5 (8) |
| cystine | 0 | 0 |
| none | 41 (68) | 30 (50) |
Overall, the results of CA did not match the FTIR results in 56% of the cases. In 16% of the cases CA missed the major stone component and in 40% the minor stone component. CA over-diagnosed calcium oxalate stones resulting in a high sensitivity rate (98%) but a very low specificity (25%). CA overestimated the number of mixed stones resulting in 10/60 stones (16%) erroneously identified as non-pure stones, mostly uric acid stones. The positive predictive value of CA for pure uric acid stones was only 10%. CA failed to identify struvite as the major stone component in all the 5 struvite stones. In these stones calcium oxalate was erroneously identified as the major stone component while struvite was found as the minor component. Likewise, CA failed to identify calcium phosphate as a major stone component and could not differentiate between the mineral phases of calcium oxalate and calcium phosphate stones. CA correctly diagnosed all cystine stones but reported false minor components in half of the cases.
The overall sensitivity rate of CA for the minor stone component was approximately 40% while the specificity was 89%.
Discussion
CA preceded the more modern physical techniques of FTIR and XRD. Quality assessment studies performed in the early ‘90’s suggested that the use of CA should be discontinued because of its unacceptable quality in approximately 40% of laboratories [16]. Likewise, Hesse et al [4], reporting the results of 44 quality control trials (1980–2001) found that chemical methods produced a very high proportion of errors (6.5–94%) with both pure substances and binary mixtures submitted for analysis. In the last two decades the use of CA is declining. However in a recent review of stone analysis techniques, CA is still considered one of the most widely used approaches for stone analysis [17].
Most of the studies comparing the different stone analysis techniques focused on quality control but did not address the clinical implications that may derive from an erroneous stone composition report. The aim of the current study was to help the urologists with limited access to more modern analytic techniques to better interpret the results of CA. Furthermore, even in places were FTIR and XRD are in use, a patient may present with historic results of CA stone composition.
Our results show, as expected, that the use of CA is associated with a high error rate in addition to the inherent limitation of the technique. This is probably due to the subjective nature of this method.
However, CA is fairly accurate in determining calcium oxalate as the major stone component. In 84% of the cases the results of CA matched with the FTIR results. Errors as to the major stone component occurred in the analysis of Struvite stones, Calcium phosphate stones and and uric acid stones.
CA always identified struvite as the minor component and usually calcium oxalate as the major component. Therefore, the clinician, taking the results as presented, may assume that the patient has a metabolic stone which was secondarily infected. According to our results it would be more practical to assume that any finding of struvite in the CA report should suggest the presence of an infection stone. Interestingly, a recent study examining the accuracy of stone analysis in modern commercial laboratories found a tremendous variability in the analysis of infection stones, with disagreement as to the presence of struvite in 25% of the cohort. The presence of a metabolic component mixed with struvite was also variably reported [15].
CA failed to recognize calcium phosphate as a major stone component. The percentage of calcium phosphate reported as a minor component was highly inaccurate. Likewise, in the study by Hesse et al. [4] CA performed poorly when calcium phosphate was identified in association with calcium oxalate. The proportion of calcium phosphate in a stone has significant clinical implication as it may suggest the presence of renal tubular acidosis [2]. Furthermore, CA does not differentiate between the mineral phases of calcium phosphate such as brushite which has a significant metabolic and clinical implication [18].
In 25% of the uric acid stones CA identified uric acid as the minor instead as the major component. Furthermore, while 9/12 were pure uric acid stones only 1 stone was categorized as pure by the CA. Of note, the highest percentage of uric acid in the color scale provided as part of the CA analysis kit is 80%! Therefore, if CA shows a high percentage of uric acid but suggests a mixed stone composition there is a high likelihood that the stone is actually a pure stone and the patient may respond well to urine alkalinization.
Our study shows that the results of CA for the minor stone component are not reliable with an error rate of 40%. Most pure uric acid stones and half of the cystine stones were reported as mixed stones. A significant over-diagnosis of calcium phosphate and calcium oxalate was noted. Therefore, proper interpretation of CA results should take into account the limitation of the technique and address minor stone components such as uric acid and struvite according to the principles outlined above. Our study has several limitations. The sample size was relatively small, perhaps explaining an unusually incidental high rate of cystine stones. As clinical data including metabolic studies were not part of our research we are not able to determine in which patients the errors of CA had a real clinical significance.
Conclusion
The use of CA to determine urinary calculi composition is associated with high error rate. CA report should not be interpreted by the clinician in the same way as the more modern techniques such as FTIR. CA is fairly accurate identifying calcium oxalate as the major stone component but is not reliable in determining minor components in mixed stones. However, any presence of struvite usually indicates an infection stone. Uric acid and cystine stones, usually pure stones often appear erroneously as mixed stones. Thus, careful integration of CA results and other clinical data is important before treatment decisions are made
Footnotes
Disclosures
On behalf of all authors, the corresponding author states that there is no conflict of interest. For this type of study, informed consent is not required. This article does not contain any studies with human participants performed by any of the authors.
References
- 1.Skolarikos A, Straub M, Knoll T. Metabolic evaluation and recurrence Prevention for urinary stone patients: EAU guidelines. Eur Urol. 2015 Apr;67(4):750–763. doi: 10.1016/j.eururo.2014.10.029. [DOI] [PubMed] [Google Scholar]
- 2.Pearle MS, Goldfarb D, Assimos D, et al. Medical management of kidney stones: AUA guideline. 2014 doi: 10.1016/j.juro.2014.05.006. Available online: https://www.auanet.org/education/guidelines/management-kidney-stones.cfm. [DOI] [PubMed]
- 3.Schubert G. Stone analysis. Urol Res. 2006;34(2):146–150. doi: 10.1007/s00240-005-0028-y. [DOI] [PubMed] [Google Scholar]
- 4.Hesse A, Kruse R, Geilenkeuser W. Quality control in urinary stone analysis: results of 44 ring trials (1980–2001) Clin Chem Lab Med. 2005;43(3):298–303. doi: 10.1515/CCLM.2005.051. [DOI] [PubMed] [Google Scholar]
- 5.Young S, Jea WL, Joung SR, et al. Identification of uric acid stone with dual energy computed tomography in human. J Urol. 2011;185(supp):e895. [Google Scholar]
- 6.Davceva O, Nikolov G, Ivanovski O. Chemical composition of urinary tract stones in republic of Macedonia. European Urology Supplements. 2011;10(9):589–589. [Google Scholar]
- 7.Nikolon IG, Ivanovski O, Daudon M. Morphology and composition of kidney stones in patients with autosomal dominant polycystic kidney disease (ADPKD) European Urology Supplements. 2011;10(9):589–589. [Google Scholar]
- 8.Al-hunayan A, Abdul-halim H, Kehinde EO, et al. Mode of presentation and first line of management of non-recurrent urolithiasis in Kuwait. Int J Urol. 2004;11:963–968. doi: 10.1111/j.1442-2042.2004.00934.x. [DOI] [PubMed] [Google Scholar]
- 9.Hashim IA, Zawawi TH. Wet versus dry chemical analysis of renal stones. Irish Journal of Medical Science. 1999;168:114–118. doi: 10.1007/BF02946479. [DOI] [PubMed] [Google Scholar]
- 10.Gault MH, Ahmed M, Kalra J. Comparison of infrared and wet chemical analysis of urinary tract calculi. Clinica Chimica Acta. 1980;104:349–359. doi: 10.1016/0009-8981(80)90393-9. [DOI] [PubMed] [Google Scholar]
- 11.Primiano A, Persichilli S, Gambaro G, et al. FT-IR Analysis of Urinary Stones: A Helpful Tool for Clinician Comparison with the Chemical Spot Test. Disease Markers. 2014;2014 doi: 10.1155/2014/176165. Article ID 176165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Charafi S, Mohamed M, Costa-Bauza A. A comparative study of two renal stone analysis methods. Int J nephrol urol. 2010;(3):469–475. [Google Scholar]
- 13.Daudon M, Donsimoni R, Hennequin C, et al. Sex and age related composition of 10617 calculi analyzed by infrared-spectroscopy. Urol res. 1995;43:319. doi: 10.1007/BF00300021. [DOI] [PubMed] [Google Scholar]
- 14.Williams J, McAteer J, Evan A. Micro-computed tomography for analysis of urinary calculi. Urol Res. 2010;38:477–484. doi: 10.1007/s00240-010-0326-x. [DOI] [PubMed] [Google Scholar]
- 15.Krambeck A, Khan N, Jackson M. Inaccurate reporting of mineral composition by commercial stone analysis laboratories: implications for infection and metabolic stones. J Urol. 2010;184:1543–1549. doi: 10.1016/j.juro.2010.05.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rebentisch G, Muche J, Reinauer H. External quality assessment of analysis of urinary calculi--a new scheme based mainly on natural concrement materials. Scand J Clin Lab Invest Suppl. 1993;212:56–7. doi: 10.3109/00365519309085458. [DOI] [PubMed] [Google Scholar]
- 17.Basiri A, Taheri M, Taheri F. What is the state of the stone analysis techniques in urolithiasis? Urol J Spring. 2012;9(2):445–454. [PubMed] [Google Scholar]
- 18.Krambeck AE, Handa SE, Evan AP, Lingeman JE. Profile of the brushite stone former. J Urol. 2010;184(4):1367–1371. doi: 10.1016/j.juro.2010.05.094. [DOI] [PMC free article] [PubMed] [Google Scholar]


