Abstract
Paclitaxel (Px) is an effective chemotherapeutic agent for the treatment of various cancers. However, it is often associated with neurological side effects, including chemotherapy-associated cognitive impairment (CACI), such as “chemobrain”. Previously, we reported that endoplasmic reticulum (ER) stress is involved in Px-induced neurotoxicity, and immunoglobulin heavy chain binding protein (BiP) inducer X (BIX) alleviates Px-induced neurotoxicity. However, BIX has not been used in clinical practice yet. We recently reported that fluvoxamine (Flv) alleviates ER stress via induction of sigma-1 receptor (Sig-1R). The purpose of this study was to investigate whether Flv could alleviate Px-induced neurotoxicity in vitro. SK-N-SH cells were pre-treated for 12 h with or without 10 μg/ml Flv followed by treatment with 1 μM Px with or without co-existence of 10 μg/ml Flv for 24 h. To investigate the involvement of Sig-1R in alleviation effect on Px-induced neurotoxicity,1 μM NE100, an antagonist of Sig-1R, was added for 24 h. Neurotoxicity was assessed using the MTS viability assay and ER stress-mediated neurotoxicity was assessed by evaluating the expression of C/EBP homologous protein (CHOP), cleaved caspase 4, and cleaved caspase 3.
Pre-treatment with Flv significantly alleviated the induction of CHOP, cleaved caspase 4, and cleaved caspase 3 in SK-N-SH cells. At the same time, pre-treatment with Flv significantly induced Sig-1R in SK-N-SH cells. In addition, viability was significantly higher in Flv-treated cells than in untreated cells, which was reversed by treatment with NE100.
Our results suggest that Flv alleviates Px-induced neurotoxicity in part through the induction of Sig-1R. Our findings should contribute to one of the novel approaches for the alleviation of Px-induced neurotoxicity, including chemobrain.
Abbreviations: BiP, immunoglobulin heavy-chain binding protein; BIX, BiP inducer X; CACI, chemotherapy-associated cognitive impairments; CHOP, C/EBP homologous protein; CYP, cytochrome P450; ER, endoplasmic reticulum; Flv, fluvoxamine; JNK, c-Jun NH2-terminal kinase; Px, paclitaxel; QOL, quality of life; Sig-1R, sigma 1 receptor; SSRI, selective serotonin reuptake inhibitor; UPR, unfolded protein response
Keywords: Chemobrain, Paclitaxel, Endoplasmic reticulum stress, Fluvoxamine, Sigma 1 receptor, Selective serotonin reuptake inhibitor
Highlights
-
•
Paclitaxel (Px) induces neurotoxicity through endoplasmic reticulum (ER) stress.
-
•
Fluvoxamine (Flv) alleviates Px-induced ER-mediated neurotoxicity.
-
•
Sigma1 receptor is involved in alleviation of Px-induced neurotoxicity.
-
•
Flv could be a candidate drug for Px-induced neurological side effects.
1. Introduction
Cancer treatments, including chemotherapy, radiation therapy, and targeted biological therapies, have made great advances in the last century and have led to improved survival. However, their administration is often associated with several side effects and may reduce patient quality of life (QOL). Cognitive impairment is among the most frequently reported problems by patients during the treatment, especially in the context of chemotherapy. This cognitive impairment related to chemotherapy is known as “chemofog” or “chemobrain” and is well studied in breast cancer patients. Reports demonstrate that 15–75% of breast cancer survivors have cognitive impairment in the domain of memory, processing speed, attention, and executive function [1], [2], [3], [4]. Multiple hypotheses exist regarding chemobrain, including disruption of hippocampal cell proliferation and neurogenesis [5], chronic increases in inflammation [6], [7], increased oxidative stress [6], white matter disruption [8], [9], and long-term changes in cerebral blood flow and metabolism [10]. However, a detailed mechanism and intervention for CACI have not been established.
Paclitaxel (Px) is a taxane agent that binds microtubules, stabilizes microtubule dynamics, and arrests the cell at the mitotic phase [11]. However, Px often induces side effects such as arthralgia, myalgia, and ataxia. In addition, pronounced emotional distress, including depression, and reduced mental QOL through adjuvant treatment has recently been reported [12]. Though, it is believed that Px is prevented from penetrating into the brain, a positron emission tomography study demonstrated detectable levels of radiolabeled Px in the brain after intravenous administration [13], indicating that Px may directly influence the central nervous system.
The endoplasmic reticulum (ER) stress response, also called the unfolded protein response (UPR), is a defense system that deals with the accumulation of unfolded proteins in the ER lumen. However, when ER stress is very severe, cells induce and/or activate C/EBP homologous protein (CHOP), the c-Jun NH2-terminal kinase (JNK) pathway, and caspase 4, which lead to apoptosis. Accumulating evidence demonstrates the importance of ER stress and of UPR in the pathophysiology of human neurological diseases, such as Parkinson's disease [14], [15], Alzheimer's disease [15], [16], [17], [18], and causes of cognitive dysfunction. Recently, we reported that ER stress is involved in Px-induced neurotoxicity [19]. In addition, we have also reported that immunoglobulin heavy-chain binding protein (BiP) inducer X (BIX) attenuates Px-induced neurotoxicity through alleviation of ER stress [19]. The effect of BIX in the alleviation of ER stress has been reported in many other situations [20], [21], [22], [23], [24], [25]. However, BIX has not been approved for clinical practice use yet and is only permitted for experimental use. Thus, the exploration of agents like BIX in drugs that have already been licensed for the clinical setting should be fast way to practical use.
Recently, we reported that fluvoxamine (Flv), a selective serotonin reuptake inhibitor (SSRI) that is widely used in clinical practice as an antidepressant, alleviates ER stress in vitro and in animal experiments [26].
In the present study, we investigated the effect of Flv on Px-induced neurotoxicity using SK-N-SH cells in vitro.
2. Materials and methods
2.1. Chemicals
Flv (Sigma-Aldrich, St. Louis, MO, USA) and NE100 (Santa Cruz Biotechnology, Dallas, Texas, USA) were dissolved in double-distilled water (DDW). Px (Sigma) was dissolved in dimethyl sulfoxide (DMSO).
2.2. Cell culture
SK-N-SH neuroblastoma cells were grown in Dulbecco's Modified Eagle's Medium (DMEM; GIBCO/Invitrogen Life Technologies, Paisley, UK) with 10% fetal bovine serum (FBS; JRH, Woodland, CA, USA). Cells were maintained at 37 °C in an incubator within an atmosphere of 5% carbon dioxide (CO2). Cells were routinely passaged using trypsin (0.25%)–EDTA (0.1%) solution in Hank's Balanced Salt Solution (HBSS; Himedia Laboratories Pvt. Ltd., Mumbai, India).
2.3. MTS cell viability assays
Cellular viability was assessed using CellTiter 96 Aqueous One Solution Cell Proliferation Assays (Promega, Madison, WI, USA). Briefly, SK-N-SH cells were seeded in 96-well plates. Cells were allowed to attach for 24 h. For evaluation of the toxicity of Flv on SK-N-SH cells, cells were treated with 10, 25, 50, 75, or 100 μg/ml Flv for 24 h at 37 °C. For evaluation of the alleviation effect of Flv on Px-induced neurotoxicity, SK-N-SH cells were pre-treated with or without 10 μg/ml Flv for 12 h followed by 1 μM Px treatment with or without 10 μg/ml Flv for 24 h. To confirm the involvement of Sig-1 R in alleviation effect on Px- induced neurotoxicity, SK-N-SH cells were incubated with 1 μM Px, 10 μg/ml Flv and 1 μM NE100 for 24 h. Next, 20 μl of MTS reagent was added to each well and cells were incubated for 2 h. Optical density was measured at 490 nm using a Micro Plate Reader (Bio-Rad, Hercules, CA, USA).
2.4. Western blots
SK-N-SH cells were pre-treated with or without 10 μg/ml Flv for 12 h followed by 1 μM Px treatment with or without 10 μg/ml Flv for 24 h at 37 °C. Cells were washed in Tris-buffered saline (TBS), harvested, and lysed in RIPA buffer (Thermo Fisher Scientific, Inc., Rockford, IL, USA) with a protease inhibitor cocktail (Roche, Mannheim, Germany), and a phosphatase inhibitor cocktail (Roche). Lysates were sonicated on ice three times for five seconds each, and then incubated for 15 min. After centrifugation for 20 min at 13,000 g, supernatants were retained and boiled in SDS sample buffer. Lysates (10 μg) were separated on SDS-polyacrylamide gels and transferred to polyvinylidene fluoride (PVDF) membranes (Bio-Rad, Hercules, CA, USA). Non-specific protein binding was blocked by incubating membranes for 1 h at room temperature in 5% w/v non-fat milk powder in TBS-T [50 mM Tris–HCl (pH 7.6), 150 mM NaCl, and 0.1% v/v Tween-20]. The membranes were incubated overnight at 4 °C with the following primary antibodies: anti-CHOP (1:1000; Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-caspase 4 (1:500; Medical and Biological laboratories, Nagoya, Japan), anti-caspase 3 (1:1000; Cell Signaling Technology, Boston, MA, USA), anti-sigma 1 receptor (Sig-1R) (1:250; Abcom, Cambridge, UK) and anti-GAPDH (1:1000; Thermo Fisher Scientific, Waltham, MA, USA). The membranes were then washed three times in TBS-T for 5 min. Finally, the membranes were incubated for 60 min at room temperature with HRP-conjugated anti-rabbit or anti-mouse antibodies (Promega, Madison, WI, USA). Protein bands were detected using the ECL Plus kit (GE Healthcare, Buckinghamshire, UK). The intensity of each band was quantified using NIH image J software.
2.5. Statistical analyses
Data are presented as mean values±standard deviation (SD). Unpaired student's t-test or one-way analysis of variance (ANOVA) followed by Tukey–Kramer test were used to determine the levels of significance between sample means. All results are representative of at least three independent experiments.
3. Results
3.1. Flv toxicity on SK-N-SH cells
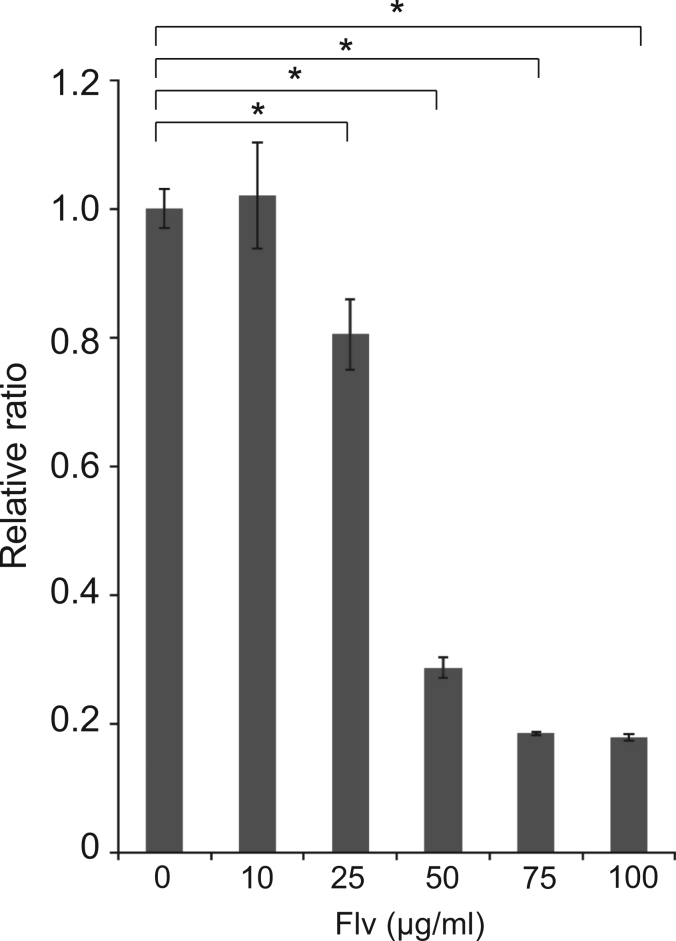
The toxicity of Flv on SK-N-SH cells was examined using an MTS assay. We used 10, 25, 50, 75, or 100 μg/ml Flv or a vehicle control to treat SK-N-SH cells. SK-N-SH cells treated with Flv showed 80% (25 μg/ml), 29% (50 μg/ml), 19% (75 μg/ml), and 18% (100 μg/ml) viability compared to the vehicle control cells ([p<0.001] at all doses) (Fig. 1). However, SK-N-SH cells treated with 10 μg/ml Flv did not show reduced viability (102%) compared to the vehicle control (Fig. 1). Based on these data, we used 10 μg/ml Flv in all subsequent experiments.
Fig. 1.
Flv toxicity in SK-N-SH cells. Flv was added to SK-N-SH cells at the indicated dose. Cell toxicity was assessed using an MTS assay 24 h later. DDW was applied as the vehicle control. The relative ratio compared with the absorbance of the vehicle control (0 μM) is indicated. Histograms shows the mean±SD from six independent experiments. ⁎p<0.001 (Student's t-test).
3.2. Flv alleviates Px-induced ER stress mediated apoptosis
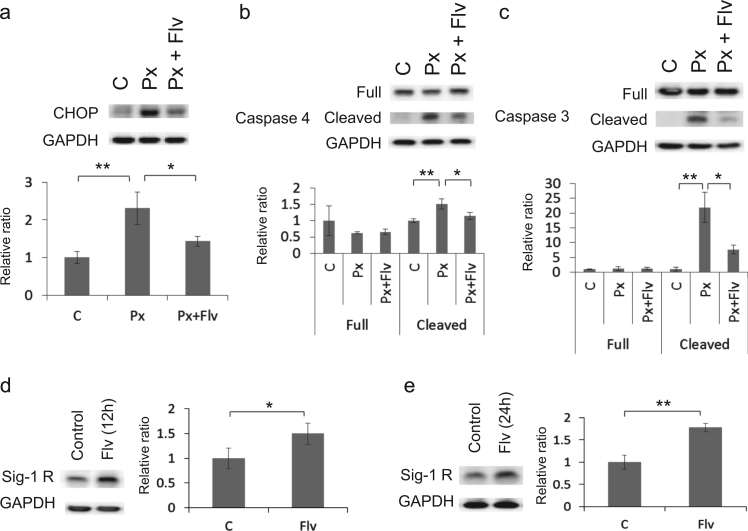
Next we investigated whether Flv could alleviate Px-induced ER stress-mediated apoptosis in SK-N-SH cells by monitoring CHOP, cleaved caspase 4, and cleaved caspase 3, an active form of each caspase. CHOP, cleaved caspase 4, and cleaved caspase 3 were induced in cells treated with Px compared to control cells (Fig. 2a–c, [p<0.01] at each comparison), which is consistent with our previous report [19], On the other hand, when cells were pre-treated with Flv followed by co-treatment with Px/Flv for 24 h, the induction of CHOP, cleaved caspase 4 and cleaved caspase 3 were alleviated compared to the Flv-untreated cells (Fig. 2a–c, p<0.05, p<0.05, and p<0.01, respectively). We next investigated the induction of Sig-1R by Flv in SK-N-SH cells. Flv has been reported not only as a potent Sig-1R agonist with stronger affinity than other SSRIs [27], but also as an inducer of Sig-1R [26]. Sig-1R was induced in cells treated with Flv for 12 h compared to untreated cells (Fig. 2d, p<0.05). This induction continued for at least 24 h (Fig. 2e, p<0.01).
Fig. 2.
Flv alleviates Px-induced ER stress-mediated apoptosis. (a–c) SK-N-SH cells were pre-treated with or without 10 μg/ml Flv for 12 h followed by a 1-μM Px treatment with or without 10 μg/ml Flv for 24 h. CHOP, caspase 4, and caspase 3 were detected by Western blotting. GAPDH was used as an internal control. ⁎p<0.05, ⁎⁎p<0.01 (one-way ANOVA followed by Tukey–Kramer test. (d and e) SK-N-SH cells were treated with 10 μg/ml Flv for 12 h and 24 h. Sig-1R was detected by Western blotting. GAPDH was used as an internal control. ⁎p<0.05, ⁎⁎p<0.01 (Student's t-test).
3.3. Flv alleviates Px-induced neurotoxicity through Sig-1R
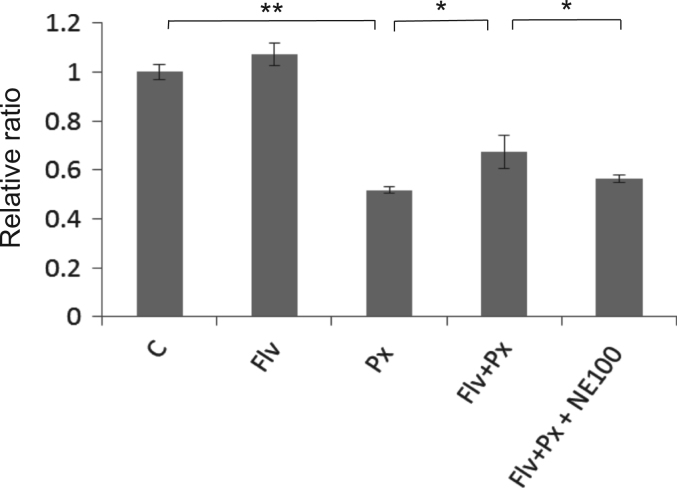
Finally, using a MTS assay, we quantitatively assessed whether Flv can alleviate Px-induced neurotoxicity. Similar to the results from Western blots, the viability that was decreased by Px treatment was recovered in Flv-pre-treated cells compared to Flv-untreated cells (Fig. 3, p<0.05). This recovery was reversed when cells were incubated with Px, Flv and NE100 (Fig. 3, p<0.05).
Fig. 3.
Flv alleviates Px-induced neurotoxicity through Sig-1R. SK-N-SH cells were pre-treated with or without 10 μg/ml Flv for 12 h followed by a 1 μM Px, 10 μg/ml Flv, and 1 μM Px/10 μg/ml Flv, with or without 1 μM NE100 treatment for 24 h. The viability was assessed using an MTS assay. The relative ratio of the absorbance compared with the vehicle control absorbance is indicated. Histograms show the mean±SD from six independent experiments. *p<0.05, **p<0.01 (one-way ANOVA followed by Tukey–Kramer test).
4. Discussion
In the present study, we investigated the neuroprotective effects of Flv on Px-induced neurotoxicity. We have clearly demonstrated that Flv alleviates the Px-induced ER stress-mediated apoptosis. We have also shown that the alleviation effect of Flv is due to the possible involvement of Sig-1R induction that is characteristic in Flv. These data suggest that Flv may be a candidate drug for the alleviation of Px-induced neurological side effects.
We have shown that a low dose of Flv (10 μg/ml) does not affect the viability of SK-N-SH cells. On the other hand, viability in both cells gradually decreases in a dose- dependent manner. Recently, the possible anti-cancer properties of antidepressants, including SSRIs, was reported, and increased interest within the scientific community [28], [29], [30]. Our results from experiments using a high dose of Flv (≧25 μg/ml) correspond with previous reports.
Px is a taxane agent and neurotoxicity is one of its most prominent side effects, leading to peripheral neuropathy in many patients. On the other hand, Px could also cause pronounced emotional distress, including depression [12], and/or cognitive dysfunction because detectable levels of radiolabeled Px was found in the brain by a positron emission tomography [13]. Several putative hypotheses regarding Px-induced neurotoxicity, such as abnormal aggregation of microtubules in neuronal cells and direct injury of neuronal cells through intrinsic toxicity are being considered. We have previously reported that Px-induced ER stress mediates neuronal apoptosis and BIX attenuates its neurotoxicity through alleviation of ER stress [19]. In the present study, we have demonstrated that pre-treatment with 10 μg/ml Flv, instead of BIX, significantly alleviates Px-induced CHOP, cleaved caspase 4, and cleaved caspase 3 (Fig. 2a–c). These results indicate that Flv alleviates Px-induced ER stress-mediated apoptosis. This tendency was also seen in the results of the MTS assay (Fig. 3). A similar result has been reported in human neuroblastoma SH-SY5Y cells treated with tunicamycin, an ER stress-inducing reagent [31]. However, the authors did not suggest how Flv alleviates ER stress. Recently, we have reported that Flv alleviates ER stress via induction of Sig1-R [26]. Sig-1R, which is expressed on ER membranes, has recently been discovered and shown to have neuro-protective activity as a molecular chaperone regulating protein folding and degradation at the ER [32], [33], [34], [35]. Furthermore, growing evidence suggests that Sig-1R plays an important role in neuronal plasticity, a process implicated in the pathophysiology of neuropsychiatric diseases, such as major depressive disorder and schizophrenia. Thus, Sig-1R receptor may be a novel therapeutic target for neuropsychiatric diseases and disorders [36], [37], [38], [39]. Similar to that of our previous report, the expression of Sig-1R was induced in SK-N-SH cells treated by 10 μg/ml Flv after 12 h (Fig. 2d) and the induction continued for at least 24 h (Fig. 2e). In addition, the alleviation effect of Flv on Px-induced neurotoxicity was reversed by NE100 (Fig. 3). Based on our previous and present results, Sig-1R is involved in the alleviation of Px-induced neurotoxicity.
Previous study demonstrated that Px-treated mice exhibit mechanical allodynia on days 3–15 of Px administration [40]. Interestingly, Flv administration for 5 days weakly but significantly attenuated Px-induced allodynia. Although, we only performed a cellular model and focused on the Px induced ER stress related neurotoxicity in the present study, our previous animal study has demonstrated that Flv treatment suppressed cerebral infarction size in mice after focal cerebral ischemia [26]. Because cerebral ischemia also induces ER stress [41], Flv could alleviate Px induced ER stress related neurotoxicity in the brain. Further in vivo studies, including cognitive behavioral evaluation in Px-injected mice, are needed to support our study.
In conclusion, we have shown that Flv alleviates Px-induced neurotoxicity in vitro and Sig-1R is involved in this alleviation mechanism. Flv has been commonly used in clinical practice, including in patients with breast cancer. Thus, a detailed evaluation of Flv effects (not only on depression but also other neurological symptoms, including cognitive function) will be required in clinical practice for the numerous applicable symptoms.
Acknowledgments
We thank Ms. Mieko Kitamura for technical assistance. This work was supported by a Grant-in-Aid for Scientific Research (C) (25461729) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan.
Footnotes
Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.bbrep.2015.09.014.
Appendix A. Supplementary material
Supplementary material
Supplementary material
References
- 1.Ahles T.A., Saykin A.J., Furstenberg C.T., Cole B., Mott L.A., Skalla K., Whedon M.B., Bivens S., Mitchell T., Greenberg E.R., Silberfarb P.M. Neuropsychologic impact of standard-dose systemic chemotherapy in long-term survivors of breast cancer and lymphoma. J. Clin. Oncol. 2002;20:485–493. doi: 10.1200/JCO.2002.20.2.485. [DOI] [PubMed] [Google Scholar]
- 2.Brezden C.B., Phillips K.A., Abdolell M., Bunston T., Tannock I.F. Cognitive function in breast cancer patients receiving adjuvant chemotherapy. J. Clin. Oncol. 2000;18:2695–2701. doi: 10.1200/JCO.2000.18.14.2695. [DOI] [PubMed] [Google Scholar]
- 3.Joly F., Rigal O., Noal S., Giffard B. Cognitive dysfunction and cancer: which consequences in terms of disease management? Psychooncology. 2011;20:1251–1258. doi: 10.1002/pon.1903. [DOI] [PubMed] [Google Scholar]
- 4.Schagen S.B., van Dam F.S., Muller M.J., Boogerd W., Lindeboom J., Bruning P.F. Cognitive deficits after postoperative adjuvant chemotherapy for breast carcinoma. Cancer. 1999;85:640–650. doi: 10.1002/(sici)1097-0142(19990201)85:3<640::aid-cncr14>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 5.Monje M., Dietrich J. Cognitive side effects of cancer therapy demonstrate a functional role for adult neurogenesis. Behav. Brain Res. 2012;227:376–379. doi: 10.1016/j.bbr.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aluise C.D., Miriyala S., Noel T., Sultana R., Jungsuwadee P., Taylor T.J., Cai J., Pierce W.M., Vore M., Moscow J.A., St Clair D.K., Butterfield D.A. 2-Mercaptoethane sulfonate prevents doxorubicin-induced plasma protein oxidation and TNF-alpha release: implications for the reactive oxygen species-mediated mechanisms of chemobrain. Free Radic. Biol. Med. 2011;50:1630–1638. doi: 10.1016/j.freeradbiomed.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 7.Myers J.S. The possible role of cytokines in chemotherapy-induced cognitive deficits. Adv. Exp. Med. Biol. 2010;678:119–123. doi: 10.1007/978-1-4419-6306-2_15. [DOI] [PubMed] [Google Scholar]
- 8.Stemmer S.M., Stears J.C., Burton B.S., Jones R.B., Simon J.H. White matter changes in patients with breast cancer treated with high-dose chemotherapy and autologous bone marrow support. AJNR Am. J. Neuroradiol. 1994;15:1267–1273. [PMC free article] [PubMed] [Google Scholar]
- 9.Deprez S., Amant F., Smeets A., Peeters R., Leemans A., Van Hecke W., Verhoeven J.S., Christiaens M.R., Vandenberghe J., Vandenbulcke M., Sunaert S. Longitudinal assessment of chemotherapy-induced structural changes in cerebral white matter and its correlation with impaired cognitive functioning. J. Clin. Oncol. 2012;30:274–281. doi: 10.1200/JCO.2011.36.8571. [DOI] [PubMed] [Google Scholar]
- 10.Silverman D.H., Dy C.J., Castellon S.A., Lai J., Pio B.S., Abraham L., Waddell K., Petersen L., Phelps M.E., Ganz P.A. Altered frontocortical, cerebellar, and basal ganglia activity in adjuvant-treated breast cancer survivors 5–10 years after chemotherapy. Breast Cancer Res. Treat. 2007;103:303–311. doi: 10.1007/s10549-006-9380-z. [DOI] [PubMed] [Google Scholar]
- 11.Jordan M.A., Wilson L. Microtubules and actin filaments: dynamic targets for cancer chemotherapy. Curr. Opin. Cell Biol. 1998;10:123–130. doi: 10.1016/s0955-0674(98)80095-1. [DOI] [PubMed] [Google Scholar]
- 12.Thornton L.M., Carson W.E., 3rd, Shapiro C.L., Farrar W.B., Andersen B.L. Delayed emotional recovery after taxane-based chemotherapy. Cancer. 2008;113:638–647. doi: 10.1002/cncr.23589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gangloff A., Hsueh W.A., Kesner A.L., Kiesewetter D.O., Pio B.S., Pegram M.D., Beryt M., Townsend A., Czernin J., Phelps M.E., Silverman D.H. Estimation of paclitaxel biodistribution and uptake in human-derived xenografts in vivo with (18)F-fluoropaclitaxel. J. Nucl. Med. 2005;46:1866–1871. [PubMed] [Google Scholar]
- 14.Hoozemans J.J., van Haastert E.S., Eikelenboom P., de Vos R.A., Rozemuller J.M., Scheper W. Activation of the unfolded protein response in Parkinson’s disease. Biochem. Biophys. Res. Commun. 2007;354:707–711. doi: 10.1016/j.bbrc.2007.01.043. [DOI] [PubMed] [Google Scholar]
- 15.Hoozemans J.J., van Haastert E.S., Nijholt D.A., Rozemuller A.J., Scheper W. Activation of the unfolded protein response is an early event in Alzheimer’s and Parkinson’s disease. Neurodegener. Dis. 2012;10:212–215. doi: 10.1159/000334536. [DOI] [PubMed] [Google Scholar]
- 16.Katayama T., Imaizumi K., Sato N., Miyoshi K., Kudo T., Hitomi J., Morihara T., Yoneda T., Gomi F., Mori Y., Nakano Y., Takeda J., Tsuda T., Itoyama Y., Murayama O., Takashima A., George-Hyslop P., St, Takeda M., Tohyama M. Presenilin-1 mutations downregulate the signalling pathway of the unfolded-protein response. Nat. Cell Biol. 1999;1:479–485. doi: 10.1038/70265. [DOI] [PubMed] [Google Scholar]
- 17.Kudo T., Okumura M., Imaizumi K., Araki W., Morihara T., Tanimukai H., Kamagata E., Tabuchi N., Kimura R., Kanayama D., Fukumori A., Tagami S., Okochi M., Kubo M., Tanii H., Tohyama M., Tabira T., Takeda M. Altered localization of amyloid precursor protein under endoplasmic reticulum stress. Biochem. Biophys. Res. Commun. 2006;344:525–530. doi: 10.1016/j.bbrc.2006.03.173. [DOI] [PubMed] [Google Scholar]
- 18.Sakagami Y., Kudo T., Tanimukai H., Kanayama D., Omi T., Horiguchi K., Okochi M., Imaizumi K., Takeda M. Involvement of endoplasmic reticulum stress in tauopathy. Biochem. Biophys. Res. Commun. 2013;430:500–504. doi: 10.1016/j.bbrc.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 19.Tanimukai H., Kanayama D., Omi T., Takeda M., Kudo T. Paclitaxel induces neurotoxicity through endoplasmic reticulum stress. Biochem. Biophys. Res. Commun. 2013;437:151–155. doi: 10.1016/j.bbrc.2013.06.057. [DOI] [PubMed] [Google Scholar]
- 20.Kudo T., Kanemoto S., Hara H., Morimoto N., Morihara T., Kimura R., Tabira T., Imaizumi K., Takeda M. A molecular chaperone inducer protects neurons from ER stress. Cell Death Differ. 2008;15:364–375. doi: 10.1038/sj.cdd.4402276. [DOI] [PubMed] [Google Scholar]
- 21.Oida Y., Izuta H., Oyagi A., Shimazawa M., Kudo T., Imaizumi K., Hara H. Induction of BiP, an ER-resident protein, prevents the neuronal death induced by transient forebrain ischemia in gerbil. Brain Res. 2008;1208:217–224. doi: 10.1016/j.brainres.2008.02.068. [DOI] [PubMed] [Google Scholar]
- 22.Inokuchi Y., Nakajima Y., Shimazawa M., Kurita T., Kubo M., Saito A., Sajiki H., Kudo T., Aihara M., Imaizumi K., Araie M., Hara H. Effect of an inducer of BiP, a molecular chaperone, on endoplasmic reticulum (ER) stress-induced retinal cell death. Investig. Ophthalmol. Vis. Sci. 2009;50:334–344. doi: 10.1167/iovs.08-2123. [DOI] [PubMed] [Google Scholar]
- 23.Hino S., Kondo S., Yoshinaga K., Saito A., Murakami T., Kanemoto S., Sekiya H., Chihara K., Aikawa Y., Hara H., Kudo T., Sekimoto T., Funamoto T., Chosa E., Imaizumi K. Regulation of ER molecular chaperone prevents bone loss in a murine model for osteoporosis. J. Bone Miner. Metab. 2010;28:131–138. doi: 10.1007/s00774-009-0117-z. [DOI] [PubMed] [Google Scholar]
- 24.Oida Y., Hamanaka J., Hyakkoku K., Shimazawa M., Kudo T., Imaizumi K., Yasuda T., Hara H. Post-treatment of a BiP inducer prevents cell death after middle cerebral artery occlusion in mice. Neurosci. Lett. 2010;484:43–46. doi: 10.1016/j.neulet.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 25.Nakanishi T., Shimazawa M., Sugitani S., Kudo T., Imai S., Inokuchi Y., Tsuruma K., Hara H. Role of endoplasmic reticulum stress in light-induced photoreceptor degeneration in mice. J. Neurochem. 2013;125:111–124. doi: 10.1111/jnc.12116. [DOI] [PubMed] [Google Scholar]
- 26.Omi T., Tanimukai H., Kanayama D., Sakagami Y., Tagami S., Okochi M., Morihara T., Sato M., Yanagida K., Kitasyoji A., Hara H., Imaizumi K., Maurice T., Chevallier N., Marchal S., Takeda M., Kudo T. Fluvoxamine alleviates ER stress via induction of Sigma-1 receptor. Cell Death Dis. 2014;5:e1332. doi: 10.1038/cddis.2014.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Narita N., Hashimoto K., Tomitaka S., Minabe Y. Interactions of selective serotonin reuptake inhibitors with subtypes of sigma receptors in rat brain. Eur. J. Pharmacol. 1996;307:117–119. doi: 10.1016/0014-2999(96)00254-3. [DOI] [PubMed] [Google Scholar]
- 28.Lee C.S., Kim Y.J., Jang E.R., Kim W., Myung S.C. Fluoxetine induces apoptosis in ovarian carcinoma cell line OVCAR-3 through reactive oxygen species-dependent activation of nuclear factor-kappaB. Basic Clin. Pharmacol. Toxicol. 2010;106:446–453. doi: 10.1111/j.1742-7843.2009.00509.x. [DOI] [PubMed] [Google Scholar]
- 29.Levkovitz Y., Gil-Ad I., Zeldich E., Dayag M., Weizman A. Differential induction of apoptosis by antidepressants in glioma and neuroblastoma cell lines: evidence for p-c-Jun, cytochrome c, and caspase-3 involvement. J. Mol. Neurosci. 2005;27:29–42. doi: 10.1385/JMN:27:1:029. [DOI] [PubMed] [Google Scholar]
- 30.Kannen V., Hintzsche H., Zanette D.L., Silva W.A., Jr., Garcia S.B., Waaga-Gasser A.M., Stopper H. Antiproliferative effects of fluoxetine on colon cancer cells and in a colonic carcinogen mouse model. PLoS One. 2012;7:e50043. doi: 10.1371/journal.pone.0050043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hosoi T., Miyahara T., Kayano T., Yokoyama S., Ozawa K. Fluvoxamine attenuated endoplasmic reticulum stress-induced leptin resistance. Front. Endocrinol. 2012;3:12. doi: 10.3389/fendo.2012.00012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hayashi T., Maurice T., Su T.P. Ca(2+) signaling via sigma(1)-receptors: novel regulatory mechanism affecting intracellular Ca(2+) concentration. J. Pharmacol. Exp. Ther. 2000;293:788–798. [PubMed] [Google Scholar]
- 33.Hayashi T., Su T. The sigma receptor: evolution of the concept in neuropsychopharmacology. Curr. Neuropharmacol. 2005;3:267–280. doi: 10.2174/157015905774322516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hayashi T., Su T.P. Regulating ankyrin dynamics: Roles of sigma-1 receptors. Proc. Natl. Acad. Sci. USA. 2001;98:491–496. doi: 10.1073/pnas.98.2.491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hayashi T., Su T.P. Sigma-1 receptor chaperones at the ER-mitochondrion interface regulate Ca(2+) signaling and cell survival. Cell. 2007;131:596–610. doi: 10.1016/j.cell.2007.08.036. [DOI] [PubMed] [Google Scholar]
- 36.Hashimoto K., Ishiwata K. Sigma receptor ligands: possible application as therapeutic drugs and as radiopharmaceuticals. Curr. Pharm. Des. 2006;12:3857–3876. doi: 10.2174/138161206778559614. [DOI] [PubMed] [Google Scholar]
- 37.Hayashi T., Tsai S.Y., Mori T., Fujimoto M., Su T.P. Targeting ligand-operated chaperone sigma-1 receptors in the treatment of neuropsychiatric disorders. Expert Opin. Ther. Targets. 2011;15:557–577. doi: 10.1517/14728222.2011.560837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Niitsu T., Iyo M., Hashimoto K. Sigma-1 receptor agonists as therapeutic drugs for cognitive impairment in neuropsychiatric diseases. Curr. Pharm. Des. 2012;18:875–883. doi: 10.2174/138161212799436476. [DOI] [PubMed] [Google Scholar]
- 39.Stahl S.M. The sigma enigma: can sigma receptors provide a novel target for disorders of mood and cognition? J. Clin. Psychiatry. 2008;69:1673–1674. doi: 10.4088/jcp.v69n1101. [DOI] [PubMed] [Google Scholar]
- 40.Katsuyama S., Sato K., Yagi T., Kishikawa Y., Nakamura H. Effects of repeated milnacipran and fluvoxamine treatment on mechanical allodynia in a mouse paclitaxel-induced neuropathic pain model. Biomed. Res. 2013;34:105–111. doi: 10.2220/biomedres.34.105. [DOI] [PubMed] [Google Scholar]
- 41.DeGracia D.J., Montie H.L. Cerebral ischemia and the unfolded protein response. J. Neurochem. 2004;91:1–8. doi: 10.1111/j.1471-4159.2004.02703.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material
Supplementary material