Summary
Introduction.
Cardiovascular Diseases (CD) have emerged as a leading cause of morbidity and mortality in HIV population. Some studies have reported higher carotid Intima Media Thickness (c-IMT), a measure of subclinical atherosclerosis (AT), in this cohort of patients.
Methods.
Here, we evaluate the role of Hepatic Steatosis (HS) as likely marker for AT in 128 HIV-infected patients without hepatitis C infection. c-IMT has been detected non-invasively by carotid ultrasonography to assess the progression of AT. HS has been evaluated using a process based on vibration-controlled transient elastography (Fibroscan) by a novel ultrasonic controlled attenuation parameter (CAP). The cut-off value for defining the presence of significant HS was CAP > 259 dBm-1.
Results.
AT has been detected in 26 patients (20.3%), whereas steatosis of grade 2 (S2) in 31 (24.2%). The variables statistically related to AT were age, obesity, diabetes, hypertension and S2. In the multivariate analysis, AT was only associated (p < 0.001) with age and S2. The optimal cut-off value indicated by ROC curve for predicting AT was CAP > 250 dB/m-1.
Discussion.
Our results highlight the presence of AT in HIVinfected persons and its association with fatty liver disease; therefore, HS assessment in HIV population results crucial to predict AT and CD.
Key words: Atherosclerosis, Hepatic steatosis, HIV, Cardiovascular diseases, Intima Media Thickness
Introduction
Since the advent of highly active anti-retroviral therapy (HAART), the prognosis of HIV infection has been dramatically altered, transforming it from an inexorably fatal disease into a manageable chronic condition [1, 2].
In this context, cardiovascular diseases (CD) result an important cause of morbidity and mortality [3, 4]. Atherosclerosis (AT), a complex, active and progressive disease with inflammation involved at every stage, is closely correlated to an increased risk of heart diseases [5].
Epidemiological data show a relationship between HIV and AT, in fact in the last few years AT prevalence has increasingly been found in this cohort of patients [6].
Several factors may contribute to its development among HIV-infected individuals. First and foremost the role of inflammation has been recognized as the key pathologic process [7, 8]. Furthermore, other determinants including dyslipidemia, lipodystrophy, insulin resistance, tobacco abuse, and metabolic and mitochondrial dysfunctions may be involved in AT disease [9-13]. Previous researches have demonstrated an association between various components of HAART and the development of AT, as well [14, 15]. Finally, HIV infection itself may lead AT via monocyte or T-cell activation and likewise concomitant infections in the HIV-infected subjects could also promote this process [16, 17].
Epidemiological studies have showed strong association between AT and hepatic steatosis (HS) [18, 19]. The latter is a clinical condition associated with inflammation and hepatocyte changes [20]. The different grade of steatosis (S) is defined as S0 = 0-10%; S1 = 11-33%; S2 = 34-66% and S3 = 67-100% of hepatocytes that have a fatty accumulation [21]; it can also be a co-factor in many chronic liver diseases that can lead to fibrosis, cirrhosis and hepatocellular carcinoma, as well as the possibility of developing metabolic alterations that can lead to AT [22, 23].
Mounting evidence suggests that HS is common among HIV-infected individuals with or without HCV co-infection [24, 25], though data on factors associated with steatosis in HIV-mono-infected patients are scarce [26].
To date, liver biopsy is considered the gold standard for the assessment of HS [27]. But recently, ultrasoundbased vibration-controlled transient elastography device has been developed to detect it [28] using controlled attenuation parameter (CAP). This tool is a non-invasive, quantitative, non-ionizing and inexpensive method that provides immediate results and it can be performed by an operator without specific radiological competence and individual interpretation. Furthermore, CAP is able to explore a liver volume ~ 100 times larger than liver biopsy [21].
Measurement of AT activity results fundamental for early detection of CD [29]. It can be detected non-invasively by carotid ultrasonography to assess carotid Intima Media Thickness (c-IMT) [30, 31]. IMT is a characteristic of arterial aging related to AT; the cellular and molecular alterations that underlie IMT are implicated in the development, progression or both of AT [32]. c-IMT is widely used as a validated marker for subclinical atherosclerosis in HIV-negative populations [33, 34]; moreover, its value greater than or equal to the 75th percentile for age, sex and ethnicity has been associated with an increased risk of CD [32]. For its low cost and the absence of exposure to radiation, this technology has also been used to identify the predictors of subclinical AT in HIV-infected individuals [10, 35, 36].
Few data are available on the relationship between AT and HS in HIV subjects and since fatty liver diseases are common among this population [37], determining the link with AT may be useful for predicting CD risk [38, 39].
Therefore, objective of our study is to evaluate this relationship in a cohort of HIV patients without concomitant hepatitis C virus (HCV) infection.
Methods
PATIENTS
We performed a cross-sectional study by consecutively enrolling HIV-infected outpatients from January to June 2012. All patients approached for the study gave consent to participate.
Exclusion criteria were: age less than 18 years, HCV infection, liver cirrhosis, active psychiatric disorders, alcoholism and drug abuse.
At enrolment time the following demographic, clinical and laboratory variables were collected for each subject through a patient interview (using a predefined form) and a chart review: gender, age, ethnicity, duration of HIV infection, CD4 cell count, nadir CD4, HIV viral load, therapy, CD risk factors (current smoking habit; obesity, defined as a body mass index above or equal to 30 kg/m2; diabetes, defined as fasting plasma glucose (FPG) levels above 126 mg/dl; hypertension, defined as blood pressure (BP) above 140/90 mmHg and/or treatment with antihypertensive medications; dyslipidemia, characterized by increased plasma concentration of triglycerides, reduced high-density lipoprotein cholesterol and increased numbers of small, dense low-density lipoprotein particles).
ULTRASONOGRAPHY EXAMINATION
A Logiq 5 ultrasound scanner (General Electric Medical Systems, Wallingford, Connecticut, USA) was used to determine AT defined as c-IMT > 0.9 mm. The c-IMT was defined as the distance between media-adventitia and lumen-intima interfaces and it was measured at about 1 cm proximal to the bifurcation of the common carotid artery using a 7.5 MHz linear probe. The probe was placed so that the near and far walls were parallel to it and lumen diameter was maximized in the longitudinal plane. Mean common c-IMT was defined as mean IMT of the right and left common carotid arteries, calculated after 3 measurements on each side.
The patients were placed in the supine position with their head in the midline position, tilted slightly upwards and the heart in systole. Sonographic evaluations were performed by a single trained sonographer blinded to the patients' data [40].
Fibroscan examination
In all patients the liver steatosis was evaluated by CAP measuring ultrasonic attenuation in the liver at 3.5 MHz using signals acquired by the FibroScan M probe (Fibroscan 502, Echosens, Paris, France) based on vibration-controlled transient elastography. The principles have been described elsewhere [28]. An elastographic evaluation has been performed by a single trained sonographer blinded to patients' data. S2 was defined as CAP > 259 dBm-1 according to previously published data demonstrating that this cut-off was the most discriminating value [21].
STATISTICAL ANALYSES
The factors associated with AT were identified by logistic univariate regression analysis. The variables showing a p-value < 0.100 in the univariate analysis were evaluated in a multivariable analysis afterwards. The ROC (Receiver Operating Characteristic) curve and corresponding AUC (Area Under Curve) were calculated in order to evaluate the CAP cut-off value able to predict AT.
A two-tailed p-value < 0.05 was considered statistically significant. Statistical calculations were performed with MedCalc software, version 11.6.0.0.
Results
In total, 128 patients [71.9% males, median age 44 years (IQR 37-49), median CD4 543 cells/μl, (IQR 360-700), 83.5% with HIVRNA < 20 cp/ml] were enrolled.
At enrollment time, 93.7% of patients were receiving ART and were using it for an average of 8 years (IQR 4-13). Obesity was diagnosed in 8 patients (6.2%), diabetes mellitus in 7 (5.4%) and hypertension in 23 (17.9%). Instead, dyslipidemia was detected in a large proportion of patients (78.9%) (Tab. I).
Tab. I.
Baseline characteristics: Demographics, HIV factors, clinical characteristics of HIV patients (N = 128).
| Variable | n (%) | Range |
|---|---|---|
| Male sex | 92 (71.9) | |
| Age, years* | 44 | 37-49 |
| Ethnicity, Caucasian | 123 (96.0) | |
| Smoke use | 52 (40.6) | |
| Duration of HIV infection, years * | 11 | 5-16 |
| Current CD4, cells/mm3 * | 543 | 360-700 |
| Nadir CD4, cells/mm3 * | 233 | 112-313 |
| HIV RNA <20 cp/ml | 107 (83.5) | |
| Current cART | 128 (93.7) | |
| Duration of cART, years* | 8 | 4-13 |
| Obesity (BMI≥30 kg/m2) | 8 (6.2) | |
| Diabetes (FPG>126 mg/dl) | 7 (5.4) | |
| Hypertension (BP>140/90 mmHg) | 23 (17.9) | |
| Dyslipidemia | 101 (78.9) | |
| Atherosclerosis (IMT > 0.9 mm) | 26 (20.3) | |
| S2 | 31 (24.2) |
median (interquartile range); BMI, body mass index
26 (20.3%) patients had AT while 31 (24.2%) subjects had liver S2 (Table I). HS was observed in 12 patients (11.8%) considering the subjects (n = 102) without AT, whereas among those (n = 26) with AT, 19 patients had also S2 (73.1%). Subjects with AT showed a higher significant difference (p < 0.001) of S2 than those without AT.
In univariate analysis, age (5.80, CI 1.8-19.5), obesity (15.0, CI 2.8-79.7), diabetes (6.0, CI 1.3-28.7), hypertension (3.90, CI 1.5-5.3), and liver S2 (20.3, CI 7.0-58.4) were significantly associated with subclinical AT (Tab. II).
Tab. II.
Factors associated with atherosclerosis.
| Variable | Univariate analysis OR (95% CI) | p | Multivariate analysis OR (95% CI) | p |
|---|---|---|---|---|
| Male sex | 1.78 (0.68-4.70) | 0.23 | ||
| Age | 5.80 (1.8-19.5) | 0.004 | 5.8 (2.2-24.5) | < 0.001 |
| CD4 at nadir (per 100 cells increase) | 1.08 (0.90-1.42) | 0.29 | ||
| CD4 cells count (per 100 cells increase) | 0.99 (0.78-1.13) | 0.66 | ||
| HIVRNA < 20 cp/ml | 0.44 (0.05-1.67) | 0.26 | ||
| Current smoking | 1.25 (0.5-13) | 0.62 | ||
| Obesity (BMI≥30 kg/m2) | 15.0 (2.8-79.7) | 0.005 | 5.82 (0.6-39.5) | 0.14 |
| Diabetes (FPG > 126 mg/dl) | 6.0 (1.3-28.7) | 0.02 | 2.5 (2.3-20.7) | 0.30 |
| Hypertension (BP > 140/90 mmHg) | 3.90 (1.5-5.3) | 0.005 | 2.14 (0.53-8.6) | 0.28 |
| Dyslipidemia | 3.80 (0.9-17.6) | 0.07 | 4.39 (0.45-42.7) | 0.20 |
| S2 | 20.3 (7.0-58.4) | <0.001 | 20.3 (4.5-60.1) | < 0.001 |
In the multivariate model, subclinical AT was associated with age [odds ratio (OR) 5.8; p < 0.001] and liver S2 (OR 20.3; p < 0.001). ROC curve indicated that the most discriminant CAP value for predicting AT was > 250 dB/m-1 (AUC = 0.92, sensitivity 92.31%, specificity 81.37%, p < 0.0001) (Fig. 1).
Fig. 1.
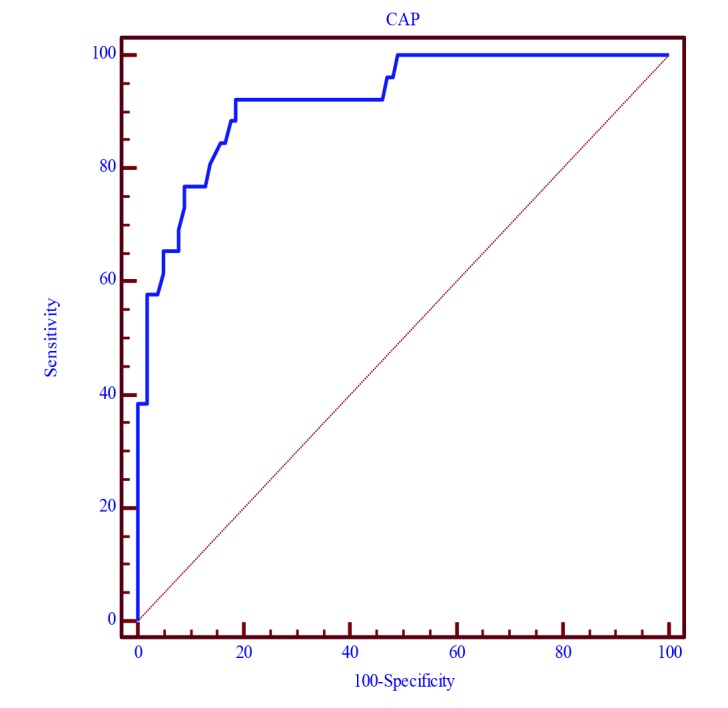
ROC curve.
Discussion
Several reports have shown that CD and in particular AT are common among HIV infected patients [29, 41, 42].
In this cross-sectional study, conducted in a cohort of HIV-infected subjects receiving care at the Infectious Disease Department in a tertiary hospital in southern Italy, 20.3% of the study population was affected by AT. These results, similar to those of other studies, underline the importance of CD in this group, also with regard to age; therefore, to address this issue is important for normalizing the life expectancy of HIV population [43, 44]. In our study, we have found that 24.2% of HIV-infected subjects had S2, 78.9% dyslipidemia and 17.9% were affected by hypertension. Our results regarding the univariate analysis have shown that age, diabetes, hypertension, obesity and S2 were associated with AT. This correlation did not remain significant in multivariate analysis unless age and S2.
The elevated prevalence of CD and its correlation with age suggest that HIV patients may present accelerated vascular aging [44]. Nevertheless further studies are needed to clarify the mechanisms unrelated to natural aging process. Furthermore, the study has shown an association between carotid AT disease and S2, and the CAP value > 250 dB/m-1 discriminant in predicting AT has been detected. Thus, in patients with a value higher than the discriminant CAP value, an early evaluation of c-IMT may be strongly recommended.
Previous studies on the correlation between fatty liver disease and CD have displayed a prevalence of fatty liver of 37% and 13% in population with coronary artery disease [44, 45], versus 73.1% founded in our study.
The cause-effect relationship between these two factors still remains confusing, but the inflammatory state may be the common risk factor for AT [46].
The ultrasonographic system used to measure c-IMT seems a well-established method for assessing subclinical AT in the HIV-negative population and it is the only non-invasive imaging recommended by the American Heart Association for risk assessment for CD [47]. Moreover, c-IMT may be a useful methodology to evaluate the intima-media thickening implicated in the development and progression of atherosclerotic disease in HIV patients, as well [40, 48].
Our study have several strengths, in fact to the best of our knowledge, it is one of the few studies that examines the association between fatty liver disease and AT in HIV-infected persons.
Furthermore, the results of this paper may have important clinical implications. The elastography, used to detect HS, is a useful tool not dependent on operator skill and does not expose the patient to ionizing radiation. Other advantages of CAP include its simplicity, accurate quantification, inexpensive and sensitivity to lesser degrees of HS [49]. Therefore, its use in the normal clinical routine could be strongly recommended.
Weaknesses of the research include the cross-sectional design, therefore prospective studies might be appropriate to evaluate the progression of fatty liver disease in CD. Moreover, the sample size was limited to arrive at definite conclusions, thus the study needs to be confirmed in a larger population. The assessment of inflammatory markers to explain the association between HIV serostatus and c-IMT should be included in future studies also consequently to conflicting evidences founded [50].
In summary, HIV-infected populations have a high prevalence of subclinical AT. Moreover, AT is associated with fatty liver disease and HS assessment results crucial both in clinical practice for management of patients with chronic liver disease and for reducing risk factors, and in clinical research for epidemiological and therapeutic studies.
Early identification of CD risk in HIV patients could permit to modify lifestyle and to take therapeutic measures in order to prevent or delay the onset of chronic diseases difficult to manage.
ACKNOWLEDGMENTS
The authors declare that they have no conflict of interest.
References
- 1.Palella FJ, Jr, Delaney KM, Moorman AC, Loveless MO, Fuhrer J, Satten GA, Aschman DJ, Holmberg SD. Declining morbidity and mortality among patients with advanced human immunodeficiency virus infection. HIV Outpatient Study Investigators. N Engl J Med. 1998;338(13):853–860. doi: 10.1056/NEJM199803263381301. doi: 0.1056/ NEJM199803263381301. [DOI] [PubMed] [Google Scholar]
- 2.Weber R, Ruppik M, Rickenbach M, Spoerri A, Furrer H, Battegay M, Cavassini M, Calmy A, Bernasconi E, Schmid P, et al. Decreasing mortality and changing patterns of causes of death in the Swiss HIV Cohort Study. HIV Med. 2013;14(4):195–207. doi: 10.1111/j.1468-1293.2012.01051.x. doi: 10.1111/j.1468-1293.2012.01051.x. [DOI] [PubMed] [Google Scholar]
- 3.Lewden C, Chêne G, Morlat P, Raffi F, Dabis F, Leport C. Mortality rate of HIV-infected adults compared with the general population: long-term and CD4-lymphocyte-dependent results. Med Sci. 2008;24(10):804–806. doi: 10.1051/medsci/20082410804. doi: 10.1051/medsci/ 20082410804. [DOI] [PubMed] [Google Scholar]
- 4.Triant VA. HIV infection and coronary heart disease: an intersection of epidemics. J Infect Dis. 2012;205(3):S355–S361. doi: 10.1093/infdis/jis195. doi: 10.1093/infdis/jis195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burger-Kentischer A, Göbel H, Kleemann R, Zernecke A, Bucala R, Leng L, Finkelmeier D, Geiger G, Schaefer HE, Schober A, et al. Reduction of the aortic inflammatory response in spontaneous atherosclerosis by blockade of macrophage migration inhibitory factor (MIF) Atherosclerosis. 2006;184(1):28–38. doi: 10.1016/j.atherosclerosis.2005.03.028. doi: 10.1016/j.atherosclerosis.2005.03.028. [DOI] [PubMed] [Google Scholar]
- 6.Currier JS, Lundgren JD, Carr A, Klein D, Sabin CA, Sax PE, Schouten JT, Smieja M Working Group 2, author. Epidemiological evidence for cardiovascular disease in HIV-infected patients and relationship to highly active antiretroviral therapy. Circulation. 2008;118(2):e29–e35. doi: 10.1161/CIRCULATIONAHA.107.189624. doi: 10.1161/CIRCULATIONAHA. 107.189624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hansson GK. Inflammatory mechanisms in atherosclerosis. J Thromb Haemost. 2009;7(1):328–331. doi: 10.1111/j.1538-7836.2009.03416.x. doi: 10.1111/j.1538- 7836.2009.03416.x. [DOI] [PubMed] [Google Scholar]
- 8.Libby P, Ridker PM, Hansson GK. Inflammation in atherosclerosis. From pathophysiology to practice. J Am Coll Cardiol. 2009;54(23):2129–2138. doi: 10.1016/j.jacc.2009.09.009. for the Leducq Transatlantic Network on Atherothrombosis. doi: 10.1016/j.jacc.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Grinspoon SK, Grunfeld C, Kotler DP, Currier JS, Lundgren JD, Dubé MP, Lipshultz SE, Hsue PY, Squires K, Schambelan M, et al. State of the science conference: Initiative to decrease cardiovascular risk and increase quality of care for patients living with HIV/AIDS: executive summary. Circulation. 2008;118(2):198–210. doi: 10.1161/CIRCULATIONAHA.107.189622. doi: 10.1161/CIRCULATIONAHA. 107.189622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ho JE, Hsue PY. Cardiovascular manifestations of HIV infection. Heart. 2009;95(14):1193–1202. doi: 10.1136/hrt.2008.161463. doi: 10.1136/hrt.2008.161463. [DOI] [PubMed] [Google Scholar]
- 11.Stein JH, Tzou WS, DeCara JM, Hirsch AT, Mohler ER, 3rd, Ouyang P, Pearce GL, Davidson MH. Usefulness of increased skin cholesterol to identify individuals at increased cardiovascular risk (from the Predictor of Advanced Subclinical Atherosclerosis study) Am J Cardiol. 2008;101(7):986–991. doi: 10.1016/j.amjcard.2007.11.044. doi: 10.1016/j.amjcard.2007.11.044. [DOI] [PubMed] [Google Scholar]
- 12.Piconi S, Parisotto S, Rizzardini G, Passerini S, Meraviglia P, Schiavini M, Niero F, Biasin M, Bonfanti P, Ricci ED, et al. Atherosclerosis is associated with multiple pathogenic mechanisms in HIV-infected antiretroviral-naive or treated individuals. AIDS. 2013;27(3):381–389. doi: 10.1097/QAD.0b013e32835abcc9. doi: 10.1097/ QAD.0b013e32835abcc9. [DOI] [PubMed] [Google Scholar]
- 13.Albuquerque VM, Zírpoli JC, Barros Miranda-Filho D, Albuquerque Mde F, Montarroyos UR, Alencar Ximenes RA, Lacerda HR. Risk factors for subclinical atherosclerosis in HIVinfected patients under and over 40 years: a case-control study. BMC Infect Dis. 2013;13:274–274. doi: 10.1186/1471-2334-13-274. doi: 10.1186/1471-2334-13-274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Friis-Møller N, Worm SW. Can the risk of cardiovascular disease in HIV-infected patients be estimated from conventional risk prediction tools? Clin Infect Dis. 2007;45(8):1082–1084. doi: 10.1086/521936. doi: 10.1086/521936. [DOI] [PubMed] [Google Scholar]
- 15.Hakeem A, Bhatti S, Cilingiroglu M. The spectrum of atherosclerotic coronary artery disease in HIV patients. Curr Atheroscler Rep. 2010;12(2):119–124. doi: 10.1007/s11883-010-0089-4. doi: 10.1007/s11883-010-0089-4. [DOI] [PubMed] [Google Scholar]
- 16.Friis-Møller N, Thiébaut R, Reiss P, Weber R, Monforte AD, Wit S, El-Sadr W, Fontas E, Worm S, Kirk O, et al. Predicting the risk of cardiovascular disease in HIV-infected patients: the data collection on adverse effects of anti-HIV drugs study. Eur J Cardiovasc Prev Rehabil. 2010;17(5):491–501. doi: 10.1097/HJR.0b013e328336a150. doi: 10.1097/ HJR.0b013e328336a150. [DOI] [PubMed] [Google Scholar]
- 17.Gillis J, Smieja M, Cescon A, Rourke SB, Burchell AN, Cooper C, Raboud JM OHTN Cohort Study Group, author. Risk of cardiovascular disease associated with HCV and HBV coinfection among antiretroviral-treated HIV-infected individuals. Antivir Ther. 2014;19(3):309–317. doi: 10.3851/IMP2724. doi: 10.3851/IMP2724. [DOI] [PubMed] [Google Scholar]
- 18.Polimeni L, Del Ben F, Baratta F, Perri L, Albanese F, Pastori D, Violi F, Angelico F. Oxidative stress: New insights on the association of non-alcoholic fatty liver disease and atherosclerosis. World J Hepatol. 2015;7(10):1325–1336. doi: 10.4254/wjh.v7.i10.1325. doi: 10.4254/wjh. v7.i10.1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cai J, Zhang S, Huang W. Association between nonalcoholic fatty liver disease and carotid atherosclerosis: a meta-analysis. Int J Clin Exp Med. 2015;8(5):7673–7678. [PMC free article] [PubMed] [Google Scholar]
- 20.Matteoni CA, Younossi ZM, Gramlich T, Boparai N, Liu YC, McCullough AJ. Nonalcoholic fatty liver disease: a spectrum of clinical and pathological severity. Gastroenterology. 1999;116(6):1413–1419. doi: 10.1016/s0016-5085(99)70506-8. doi: 10.1016/S0016-5085(99)70506-8. [DOI] [PubMed] [Google Scholar]
- 21.Sasso M, Tengher-Barna I, Ziol M, Miette V, Fournier C, Sandrin L, Poupon R, Cardoso AC, Marcellin P, Douvin C, et al. Novel controlled attenuation parameter for noninvasive assessment of steatosis using Fibroscan(®): validation in chronic hepatitis C. J Viral Hepat. 2012;19(4):244–253. doi: 10.1111/j.1365-2893.2011.01534.x. doi: 10.1111/j.1365-2893.2011.01534.x. [DOI] [PubMed] [Google Scholar]
- 22.Dam-Larsen S, Franzmann M, Andersen IB, Christoffersen P, Jensen LB, Sørensen TI, Becker U, Bendtsen F. Long term prognosis of fatty liver: risk of chronic liver disease and death. Gut. 2004;53(5):750–755. doi: 10.1136/gut.2003.019984. doi: 10.1136/gut.2003.019984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Targher G, Arcaro G. Non-alcoholic fatty liver disease and increased risk of cardiovascular disease. Atherosclerosis. 2007;191(2):235–240. doi: 10.1016/j.atherosclerosis.2006.08.021. doi: 10.1016/j.atherosclerosis. 2006.08.021. [DOI] [PubMed] [Google Scholar]
- 24.Li Vecchi V, Soresi M, Giannitrapani L, Di Carlo P, Mazzola G, Colletti P, Terranova A, Vizzini G, Montalto G. Prospective evaluation of hepatic steatosis in HIV-infected patients with or without hepatitis C virus co-infection. Int J Infect Dis. 2012;16(5):e397–e402. doi: 10.1016/j.ijid.2012.01.011. doi: 10.1016/j.ijid.2012.01.011. [DOI] [PubMed] [Google Scholar]
- 25.Macías J, González J, Tural C, Ortega-González E, Pulido F, Rubio R, Cifuentes C, Díaz-Menéndez M, Jou A, Rubio P, et al. Prevalence and factors associated with liver steatosis as measured by transient elastography with controlled attenuation parameter in HIV-infected patients. AIDS. 2014;28(9):1279–1287. doi: 10.1097/QAD.0000000000000248. doi: 10.1097/QAD.0000000000000248. [DOI] [PubMed] [Google Scholar]
- 26.Crum-Cianflone N, Dilay A, Collins G, Asher D, Campin R, Medina S, Goodman Z, Parker R, Lifson A, Capozza T, et al. Nonalcoholic fatty liver disease among HIV-infected persons. J Acquir Immune Defic Syndr. 2009;50(5):464–473. doi: 10.1097/QAI.0b013e318198a88a. doi: 10.1097/QAI.0b013e318198a88a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Manning DS, Afdhal NH. Diagnosis and quantitation of fibrosis. Gastroenterology. 2008;134(6):1670–1681. doi: 10.1053/j.gastro.2008.03.001. doi: 10.1053/j.gastro. 2008.03.001. [DOI] [PubMed] [Google Scholar]
- 28.Sasso M, Beaugrand M, Ledinghen V, Douvin C, Marcellin P, Poupon R, Sandrin L, Miette V. Controlled attenuation parameter (CAP): a novel VCTE™ guided ultrasonic attenuation measurement for the evaluation of hepatic steatosis: preliminary study and validation in a cohort of patients with chronic liver disease from various causes. Ultrasound Med Biol. 2010;36(11):1825–1835. doi: 10.1016/j.ultrasmedbio.2010.07.005. doi: 10.1016/j.ultrasmedbio. 2010.07.005. [DOI] [PubMed] [Google Scholar]
- 29.Mangili A, Gerrior J, Tang AM, O'Leary DH, Polak JK, Schaefer EJ, Gorbach SL, Wanke CA. Risk of cardiovascular disease in a cohort of HIV-infected adults: a study using carotid intima-media thickness and coronary artery calcium score. Clin Infect Dis. 2006;43(11):1482–1489. doi: 10.1086/509575. doi: 10.1086/509575. [DOI] [PubMed] [Google Scholar]
- 30.Currier JS, Kendall MA, Henry WK, Alston-Smith B, Torriani FJ, Tebas P, Li Y, Hodis HN. Progression of carotid artery intimamedia thickening in HIV-infected and uninfected adults. AIDS. 2007;21(9):1137–1145. doi: 10.1097/QAD.0b013e32811ebf79. doi: 10.1097/QAD.0b013e32811ebf79. [DOI] [PubMed] [Google Scholar]
- 31.Grunfeld C, Delaney JA, Wanke C, Currier JS, Scherzer R, Biggs ML, Tien PC, Shlipak MG, Sidney S, Polak JF, et al. Preclinical atherosclerosis due to HIV infection: carotid intima-medial thickness measurements from the FRAM study. AIDS. 2009;23(14):1841–1849. doi: 10.1097/QAD.0b013e32832d3b85. doi: 10.1097/ QAD.0b013e32832d3b85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Stein JH, Korcarz CE, Hurst RT, Lonn E, Kendall CB, Mohler ER, Najjar SS, Rembold CM, Post WS American Society of Echocardiography Carotid Intima-Media Thickness Task Force, author. Use of carotid ultrasound to identify subclinical vascular disease and evaluate cardiovascular disease risk: a consensus statement from the American Society of Echocardiography Carotid Intima-Media Thickness Task Force. Endorsed by the Society for Vascular Medicine. J Am Soc Echocardiogr. 2008;21(2):93–111. doi: 10.1016/j.echo.2007.11.011. doi: 10.1016/j. echo.2007.11.011. [DOI] [PubMed] [Google Scholar]
- 33.O'Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK., Jr Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med. 1999;340(1):14–22. doi: 10.1056/NEJM199901073400103. doi: 10.1056/NEJM199901073400103. [DOI] [PubMed] [Google Scholar]
- 34.Chambless LE, Heiss G, Folsom AR, Rosamond W, Szklo M, Sharrett AR, Clegg LX. Association of coronary heart disease incidence with carotid arterial wall thickness and major risk factors: the Atherosclerosis Risk in Communities (ARIC) Study, 1987-1993. Am J Epidemiol. 1999;146(6):483–494. doi: 10.1093/oxfordjournals.aje.a009302. doi: 10.1093/ oxfordjournals.aje.a009302. [DOI] [PubMed] [Google Scholar]
- 35.Longenecker CT, Hoit BD. Imaging atherosclerosis in HIV: carotid intima-media thickness and beyond. Transl Res. 2012;159(3):127–139. doi: 10.1016/j.trsl.2011.10.007. doi: 10.1016/j.trsl.2011.10.007. [DOI] [PubMed] [Google Scholar]
- 36.Hanna DB, Post WS, Deal JA, Hodis HN, Jacobson LP, Mack WJ, Anastos K, Gange SJ, Landay AL, Lazar JM, et al. HIV Infection Is Associated With Progression of Subclinical Carotid Atherosclerosis. Clin Infect Dis. 2015;64(4):640–650. doi: 10.1093/cid/civ325. doi: 10.1093/cid/civ325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Crum-Cianflone N, Collins G, Medina S, Asher D, Campin R, Bavaro M, Hale B, Hames C. Prevalence and factors associated with liver test abnormalities among human immunodeficiency virus-infected persons. Clin Gastroenterol Hepatol. 2010;8(2):183–191. doi: 10.1016/j.cgh.2009.09.025. doi: 10.1016/j.cgh.2009.09.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Guaraldi G, Squillace N, Stentarelli C, Orlando G, D'Amico R, Ligabue G, Fiocchi F, Zona S, Loria P, Esposito R, et al. Nonalcoholic fatty liver disease in HIV-infected patients referred to a metabolic clinic: prevalence, characteristics, and predictors. Clin Infect Dis. 2008;47(2):250–257. doi: 10.1086/589294. doi: 10.1086/589294. [DOI] [PubMed] [Google Scholar]
- 39.Mangili A, Jacobson DL, Gerrior J, Polak JF, Gorbach SL, Wanke CA. Metabolic syndrome and subclinical atherosclerosis in patients infected with HIV. Clin Infect Dis. 2007;44(10):1368–1374. doi: 10.1086/516616. doi: 10.1086/516616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Grima P, Guido M, Chiavaroli R, Donno A, Tana M, Zizza A. Comparison of intima-media thickness and ophthalmic artery resistance index for assessing subclinical atherosclerosis in HIV-1-infected patients. Cardiovasc Ultrasound. 2011;9:9–9. doi: 10.1186/1476-7120-9-9. doi: 10.1186/1476-7120-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Saint Martin L, Vandhuick O, Guillo P, Bellein V, Bressollette L, Roudaut N, Amaral A, Pasquier E. Premature atherosclerosis in HIV positive patients and cumulated time of exposure to antiretroviral therapy (SHIVA study) Atherosclerosis. 2006;185:361–367. doi: 10.1016/j.atherosclerosis.2005.06.049. doi: 10.1016/j.atherosclerosis.2005.06.049. [DOI] [PubMed] [Google Scholar]
- 42.Mercié P, Thiébaut R, Aurillac-Lavignolle V, Pellegrin JL, Yvorra-Vives MC, Cipriano C, Neau D, Morlat P, Ragnaud JM, Dupon M, et al. Carotid intima-media thickness is slightly increased over time in HIV-1-infected patients. HIV Med. 2005;6(6):380–387. doi: 10.1111/j.1468-1293.2005.00324.x. doi: 10.1111/j.1468-1293.2005.00324.x. [DOI] [PubMed] [Google Scholar]
- 43.Kingsley LA, Cuervo-Rojas J, Muñoz A, Palella FJ, Post W, Witt MD, Budoff M, Kuller L. Subclinical coronary atherosclerosis, HIV infection and antiretroviral therapy: Multicenter AIDS Cohort Study. AIDS. 2008;22(13):1589–1599. doi: 10.1097/QAD.0b013e328306a6c5. doi: 10.1097/ QAD.0b013e328306a6c5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Crum-Cianflone N, Krause D, Wessman D, Medina S, Stepenosky J, Brandt C, Boswell G. Fatty liver disease is associated with underlying cardiovascular disease in HIV-infected persons(*) HIV Med. 2011;12(8):463–471. doi: 10.1111/j.1468-1293.2010.00904.x. doi: 10.1111/j.1468- 1293.2010.00904.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Guaraldi G, Zona S, Alexopoulos N, Orlando G, Carli F, Ligabue G, Fiocchi F, Lattanzi A, Rossi R, Modena MG, et al. Coronary aging in HIV-infected patients. Clin Infect Dis. 2009;49(11):1756–1762. doi: 10.1086/648080. doi: 10.1086/648080. [DOI] [PubMed] [Google Scholar]
- 46.McKimmie RL, Daniel KR, Carr JJ, Bowden DW, Freedman BI, Register TC, Hsu FC, Lohman KK, Weinberg RB, Wagenknecht LE. Hepatic steatosis and subclinical cardiovascular disease in a cohort enriched for type 2 diabetes: the Diabetes Heart Study. Am J Gastroenterol. 2008;103(12):3029–3035. doi: 10.1111/j.1572-0241.2008.02188.x. doi: 10.1111/j.1572-0241.2008.02188.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Smith SC, Jr, Greenland P, Grundy SM. AHA Conference Proceedings. Prevention conference V: Beyond secondary prevention: Identifying the high-risk patient for primary prevention: executive summary. American Heart Association. Circulation. 2000;101(1):111–116. doi: 10.1161/01.cir.101.1.111. doi: 10.1161/01.CIR.101.1.111. [DOI] [PubMed] [Google Scholar]
- 48.Miller LH, Coppola JT. Non invasive assessment of HIV-related coronary artery disease. Curr HIV/AIDS Rep. 2011;8(2):114–121. doi: 10.1007/s11904-011-0074-8. doi: 10.1007/s11904-011-0074-8. [DOI] [PubMed] [Google Scholar]
- 49.Lédinghen V, Vergniol J, Capdepont M, Chermak F, Hiriart JB, Cassinotto C, Merrouche W, Foucher J, Brigitte Le B. Controlled attenuation parameter (CAP) for the diagnosis of steatosis: a prospective study of 5323 examinations. J Hepatol. 2014;60(5):1026–1031. doi: 10.1016/j.jhep.2013.12.018. doi: 10.1016/j.jhep.2013.12.018. [DOI] [PubMed] [Google Scholar]
- 50.Hanna DB, Guo M, Bůžková P, Miller TL, Post WS, Stein JH, Currier JS, Kronmal RA, Freiberg MS, Bennett SN, et al. HIV infection and carotid artery intima-media thickness: Pooled analyses across five cohorts of the NHLBI HIV-CVD Collaborative. Clin Infect Dis. 2016;63(2):249–256. doi: 10.1093/cid/ciw261. doi: 10.1093/ cid/ciw261. [DOI] [PMC free article] [PubMed] [Google Scholar]


