Abstract
Objective:
Social relationships, such as committed partnerships, limit risky behaviors like heavy drinking, in part, because of increased social control. The current analyses examine whether involvement in committed relationships or social support extend beyond a main effect to limit genetic liability in heavy drinking (gene–environment interaction) during young adulthood.
Method:
Using data from the young adult wave of the Finnish Twin Study, FinnTwin12 (n = 3,269), we tested whether involvement in romantic partnerships or social support moderated genetic influences on heavy drinking using biometric twin modeling for gene–environment interaction.
Results:
Involvement in a romantic partnership was associated with a decline in genetic variance in both males and females, although the overall magnitude of genetic influence was greater in males. Sex differences emerged for social support: increased social support was associated with increased genetic influence for females and reduced genetic influence for males.
Conclusions:
These findings demonstrate that social relationships are important moderators of genetic influences on young adult alcohol use. Mechanisms of social control that are important in limiting genetic liability during adolescence extend into young adulthood. In addition, although some relationships limit genetic liability equally, others, such as extensive social networks, may operate differently across sex.
The causes of excessive alcohol use include genes, environment, and interactions between the two. Convergent findings from adolescent studies of gene–environment interaction (G × E) using twin samples support the social control/opportunity model of genetic risk for a range of substance use outcomes (Cooke et al., 2015; Dick et al., 2007b; Guo et al., 2009; Harden et al., 2008; Kendler et al., 2011; Miles et al., 2005). Under this model, genetic influences are greater when social control is reduced (e.g., low parental monitoring) or social opportunity is high (e.g., affiliations with deviant peers) because these environments provide individuals with more opportunity to express any genetic predisposition (Shanahan & Hofer, 2005).
Developmentally oriented twin studies have demonstrated that the relative importance of genetic influences on alcohol outcomes increases as individuals age, stabilizing in early adulthood (Bergen et al., 2007; Dick, 2011a). In addition, this period is characterized by significant variation in individuals’ environments (Rutter, 1996). The influence of certain social controls, both formal (e.g., legal prohibition) and informal (e.g., parental supervision), may be reduced or altogether removed. New social environments become important as individuals transition to adult roles (Elder et al., 2003; Rutter, 1996). The combination of increasing genetic influence, changes in social roles/expectations, and the development of future alcohol use patterns (Chen et al., 2011) makes young adulthood a critical period in understanding the contribution of genetic influences to alcohol use.
Whether the social control/opportunity model extends into young adulthood remains an open question. This is an important gap in the literature in view of the fact that genetic influences increase and new types of social environments become relevant for understanding substance use during this emerging/young adulthood period (Brown et al., 2009). Building off of the G × E research on adolescent alcohol use, we focus here on two important aspects of social relationships: romantic partnership and social support.
Social relationships are associated with a reduction in a variety of risky behaviors. Involvement in a romantic partnership is associated with reduced substance use (Duncan et al., 2006; Harris et al., 2010; Osler et al., 2008; Prescott & Kendler, 2001) and antisocial behaviors (Barnes & Beaver, 2012; Burt et al., 2010; Rönkä et al., 2002; Sampson & Laub, 2005). Social support is associated with lower levels of alcohol consumption (Hagihara et al., 2003; Peirce et al., 1996) and fewer alcohol-related problems (Jarnecke & South, 2014), and it can reduce the influence of stressful events on alcohol consumption (Steptoe et al., 1996).
Social relationships are thought to limit these behaviors, in part, through greater levels of social control. The expectations and obligations associated with many adult social relationships, such as romantic partnerships, are often at odds with patterns of excessive or illicit substance use (Umberson et al., 2010; Yamaguchi & Kandel, 1985). Thus, these contexts represent promising moderators of genetic predispositions toward alcohol use in a manner consistent with patterns in adolescence.
Romantic relationships become increasingly salient during young adulthood. Young adults who were cohabiting (Fleming et al., 2010) or in a relationship (Braithwaite et al., 2010; Whitton et al., 2013) consumed less alcohol than those who were single. Those in a relationship drank less as they progressed through college (Salvatore et al., 2014). Lower social support during the transition to young adulthood is also associated with a steeper decline in healthy behaviors, including avoidance of heavy episodic drinking (Frech, 2012). We hypothesize that social relationships may extend beyond main effects to moderate genetic influences on alcohol outcomes.
There is some evidence that these relationships alter genetic influences. Being in a marriage-like relationship attenuated genetic influences on alcohol consumption in adult women (Heath et al., 1989). Marriage also had a stronger protective effect against the development of alcohol use disorders in those who were at greatest genetic risk (Kendler et al., 2016). Perceived social support from romantic partners moderated genetic influences on alcohol problems so that under conditions of greater perceived support, genetic influences became more important (Jarnecke & South, 2014).
In addition, there is evidence of sex differences in the relationship between social support and genetic predisposition for substance use. Higher levels of reported “uplifts” from various support providers in one’s network moderated the genetic risk on alcohol dependence, such that the genetic effects became weaker as uplifts increased, but only among men (Perry et al., 2013). Greater social integration was associated with a lower probability of nicotine dependence among men with more high-risk genotypes (Perry, 2016). Men seem to benefit more in reduction of genetic risk from social support, similar to findings that men benefit more from main effects of social integration (Kawachi & Berkman, 2001).
We expanded on the adolescent alcohol G × E literature to examine romantic partnership and social support during young adulthood. We expected these relationships to limit genetic predisposition for heavy drinking because of the increased social control these relationships offer. In view of previous findings that romantic partnerships and social support both predict lower levels of alcohol misuse, we hypothesized that genetic influences on heavy drinking would be attenuated for those in a romantic relationship and those who experience higher levels of social support. Our expectations for social support differed from recent findings because we focused on the number of support providers—which reflects the size of an individual’s support network—rather than on perceived support examined previously (Jarnecke & South, 2014). Because these structural and functional characteristics of social support can have different influences (Cohen & Wills, 1985), we expected genetic variance to be attenuated at greater levels of social support owing to increased obligations associated with greater numbers of relationships. In addition, because previous research has demonstrated social relationships to be more important for men (Kawachi & Berkman, 2001), we expected any attenuation of genetic influences to be stronger among males (Salvatore et al., 2017).
Method
Sample
Data come from the youngest cohort of the Finnish Twin Cohort Study (FinnTwin12), which was established to examine genetic and environmental determinants of precursors to health-related behaviors, particularly the development of alcohol use disorder. Families were identified from Finland’s Population Registry, permitting comprehensive nationwide ascertainment of twins born from 1983 to 1987. Baseline collection occurred when twins were approximately 12 years old, with a sample of approximately 5,600 twins (87% participation) and their families (Kaprio, 2013). Follow-up surveys occurred at age 14, at age 17.5, and during young adulthood (age range: 20–26 years). Twin zygosity was determined using items developed for twin children (Goldsmith, 1991). Confirmation by multiple genetic markers revealed that 97% of same-sex pairs retained the original questionnaire-based zygosity classification (Knaapila et al., 2011). The Helsinki University Central Hospital District’s Ethical Committee and Indiana University’s Institutional Review Board approved the FinnTwin12 study.
We used data from the young adult follow-up (Mage = 24.33, SD = 1.64), in which 66% of the original sample participated, and included all individuals who had initiated alcohol use (n = 3,269). Compared with those in the age 12 assessment, those in the young adult follow-up were more likely to be female, monozygotic (MZ) twins, and from better educated families in which the father was not unemployed. Importantly, parental alcohol problems were not associated with participation in the young adulthood assessment. Questions for social support were not asked of a subset of intensively studied individuals (Kaprio, 2013). Accordingly, the analyses for social support used a subsample (n = 2,103). We had complete information on 1,230 twin pairs in the G × E models for romantic partnership and 582 twin pairs in the G × E models for social support. These samples were similar across sex (full = 56.7% female, subsample = 57.7% female, p > .05). However, the full sample was less likely to be in a romantic partnership (full = 63.5% in relationship, subsample = 67.6% in relationship, p < .05) and was slightly younger (full Mage = 24.4 years, subsample Mage = 25.4 years, p < .001).
Measures
We assessed heavy drinking with a single question, “How often do you use alcohol in such a way that you get really drunk?” Responses were 0 (never), 1 (once a year), 2 (2–4 times a year), 3 (every other month), 4 (once a month), 5 (more than once a month), 6 (once a week), 7 (more than once a week), and 8 (daily). We transformed this ordinal measure in to a pseudo-continuous measure of days intoxicated per month (30 days), as described previously (Barr et al., 2016; Cooke et al., 2015), with possible responses ranging from 0 to 30. Heavy drinking was then log-transformed (plus a constant of one).
Participants were asked, “How long (in years) have you been together with your present partner?” Respondents who indicated that they were not dating were coded as 0. Those who indicated they were in a romantic relationship for any length were coded as 1. More detailed measures of relationship characteristics (e.g., relationship quality, partner’s alcohol misuse, living situation) are not available in the FinnTwin12 data.
Social support was created from two items that asked, “Do you know any people from whom you can get social support?” and, “Do you have anyone with whom you can share your innermost feelings and to whom you can confide in?” These measures are similar to items from the Social Support Questionnaire that assess the perceived number of support providers (Sarason et al., 1983). Responses for each were 1 (no persons), 2 (1–2 people), 3 (3–5 people), and 4 (6 or more people). Scores were combined and averaged (α = .71). Because the frequency of contact with a co-twin can moderate genetic influences on alcohol use (Kaprio et al., 1987), we excluded 114 individuals who were still living with their co-twin.
Analytic plan
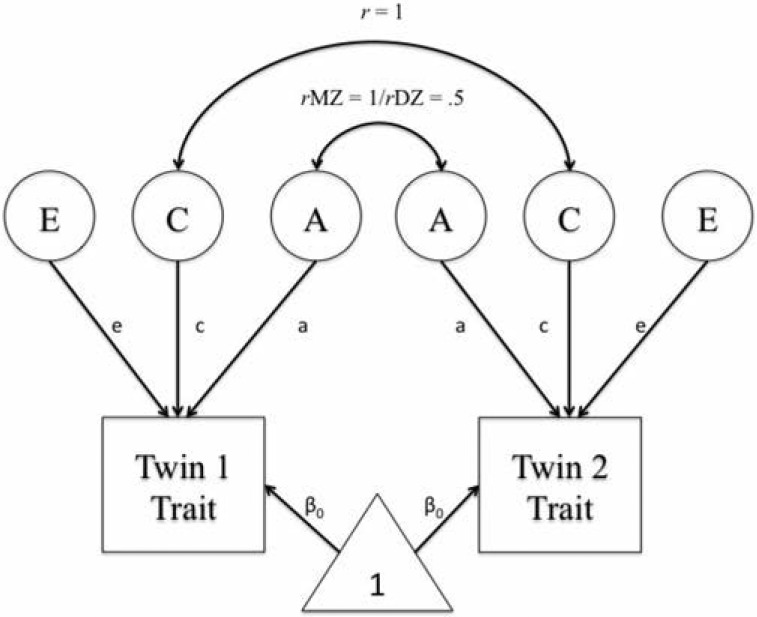
We used biometric twin modeling to partition the variance in heavy drinking into additive genetic factors (A), shared environmental influences between the twins (C), and environmental factors unique to each twin (E). A graphical example of the classic twin model is presented in Figure 1. Decomposition is possible because of assumptions regarding shared genetic and environmental contributions between MZ and dizygotic (DZ) twins. MZ twins share all of their genetic variation (r = 1), whereas DZ twins share on average half of their segregating genes (r = .5). The shared environmental component models the environmental influences that make siblings more similar to one another (r = 1). All twins were included in this analysis using full-information maximum likelihood (FIML).
Figure 1.
The classic univariate twin model depicting variance in traits partitioned into additive genetic (A), shared environmental (C), and unique environmental (E) components. Correlations between A fixed to 1 for monozygotic (MZ) twin pairs and .5 for dizygotic (DZ) twin pairs. Correlations between C fixed to 1 for both MZ and DZ twins.
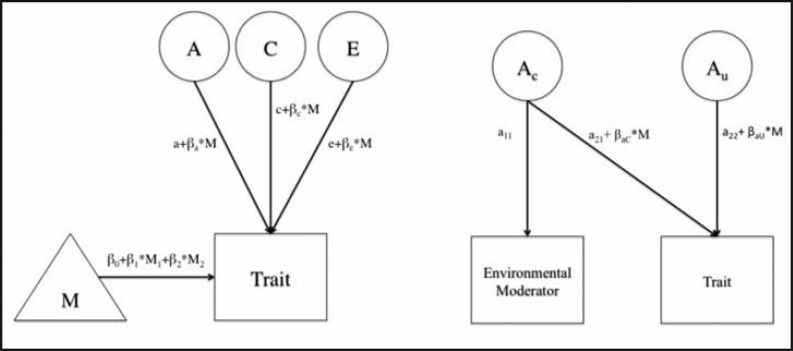
G × E can be detected by estimating how each variance component in a trait changes based on a measured environmental moderator (Figure 2), which is included on the path for each component (Purcell, 2002). G × E can also be detected using a more detailed bivariate model (abbreviated version with only A included for ease of display in Figure 2), which includes the unique (Au) and common (Ac) additive genetic components for the trait and the environmental moderator as well as the moderation (βaC and βaU) on path estimates. We first fit the G × E models in the bivariate format to determine if there was moderation on the shared variance between a trait and moderator (Purcell, 2002; van der Sluis et al., 2012). Because we found no evidence of moderation on the shared paths between heavy drinking and either moderator (results available on request), we focused exclusively on the results from the more powerful, extended univariate G × E models. The extended univariate model includes the moderator of each twin and their co-twin on the mean for heavy drinking to adjust for inflated false positives in moderation on A, which can arise when the moderator is correlated between twins (see van der Sluis et al., 2012, for a detailed review).
Figure 2.
Figures show extended univariate model (left) and simplified bivariate model (right) with only the genetic components included for ease of display. Moderation is estimated through the β on each of the a, c, and e paths. In the bivariate case, the moderation can act on both the shared path between the moderator and trait (a21) and the path unique to the trait (a22). In the case when the extended univariate model is selected over the bivariate, all of the shared paths between trait and moderator (M) are collapsed into the means portion of the model.
After fitting the full univariate ACE moderation model, we tested simpler models without shared environmental influences (AE model) or without additive genetic influences (CE model). All models included scalar sex limitation so that parameters were estimated across sex. We tested for sex differences in moderation and variance components, constraining parameters to be equal across sex when possible. We then performed a global test of moderation by constraining the moderation path on each component to be zero. This provided an omnibus test before testing for moderation on individual paths. Age was included as a covariate on the mean. G × E models only included twin pairs with complete information, as FIML is not possible with missing observations on definition variables. All models were fit using the OpenMx package in R (Boker et al., 2011).
Results
Table 1 provides descriptive statistics, twin correlations, and bivariate correlations. Being in a relationship was negatively related to heavy drinking for both men (r = -.08, p < .01) and women (r = -.08, p < .001). The correlation between social support and heavy drinking was not significant for men (r = .06, p > .05) but was significant and positive for women (r = .12, p < .001). The twin correlations for heavy drinking in twin pairs suggested that heavy drinking was attributable to genetic and unique environmental influences.
Table 1.
Descriptive statistics and twin and bivariatea correlations
| Variable | MZF | DZF | MZM | DZM | DZO | |||
| Descriptive statistics | ||||||||
| Heavy drinking, M (SD) | 0.94 (1.30) | 1.01 (1.25) | 1.78 (1.99) | 1.86 (1.91) | 1.28 (1.58) | |||
| In romantic partnership, n (%) | 403 (65%) | 241 (54%) | 373 (70%) | 246 (57%) | 646 (66%) | |||
| Social support, M (SD) | 2.66 (0.62) | 2.48 (0.68) | 2.89 (0.62) | 2.61 (0.67) | 2.78 (0.66) | |||
| Correlations | MZF | DZF | MZM | DZM | DZO | 1. | 2. | 3. |
| 1. Heavy drinking | .47 | .24 | .58 | .22 | .20 | 1.00 | -.08** | .06 |
| n (twin pairs) | 334 | 292 | 257 | 266 | 605 | |||
| 2. Romantic partnership | .56 | .28 | .41 | .27 | -.04 | -.08*** | 1.00 | .08* |
| n (twin pairs) | 283 | 235 | 183 | 164 | 365 | |||
| 3. Social support | .43 | .28 | .49 | .08 | .23 | .12*** | .03 | 1.00 |
| n (twin pairs) | 120 | 118 | 68 | 75 | 201 |
Notes: MZF = monozygotic females; DZF = dizygotic females; MZM = monozygotic males; DZM = dizygotic males; DZO = dizygotic opposite sex.
Bivariate correlation matrix: males on the upper diagonal, females on the lower diagonal.
p < .05;
p < .01;
p < .001.
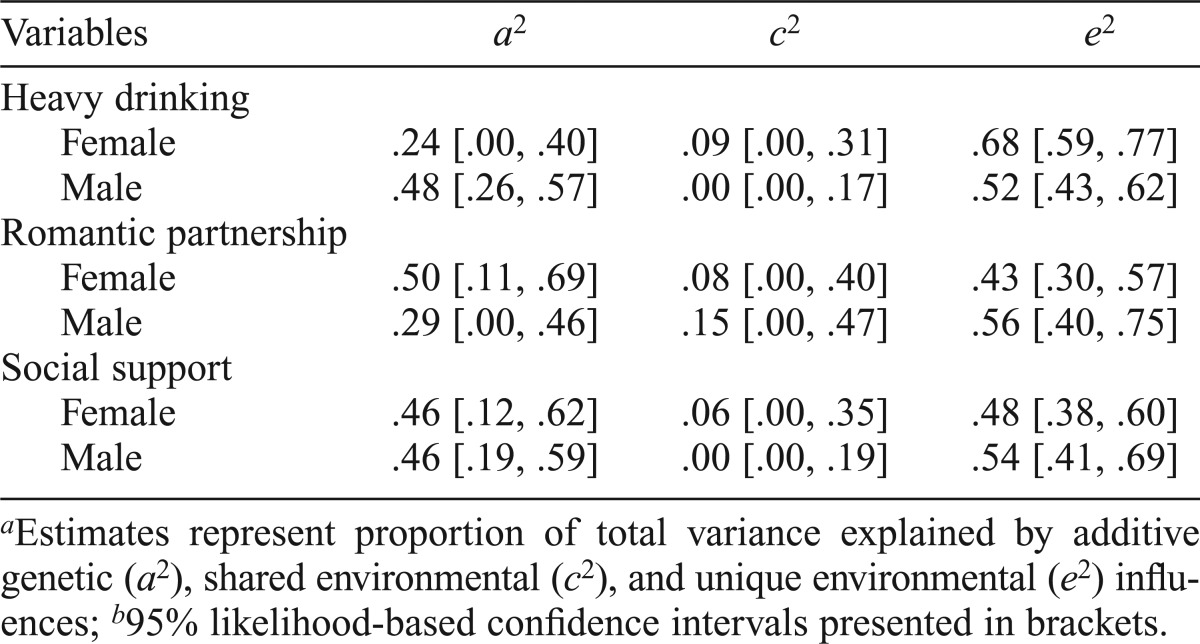
Table 2 presents the results from the sex-limited, univariate models for heavy drinking, relationship status, and social support. Heavy drinking had significantly higher heritability (the proportion of variance attributable to genetic influences) in men (a2 = .48) compared with women (a2 = .24), χ2(1) = 6.67, p < .01. Heritability for relationship status was higher in women (a2 = .50) compared with men (a2 = .29), but this difference was not significant, χ2(1) = 1.99, p = .158. The heritability estimates for social support were equivalent between men and women (a2 = .46), χ2(1) = 0.05, p = .814.
Table 2.
| Variables | a2 | c2 | e2 |
| Heavy drinking | |||
| Female | .24 [.00, .40] | .09 [.00, .31] | .68 [.59, .77] |
| Male | .48 [.26, .57] | .00 [.00, .17] | .52 [.43, .62] |
| Romantic partnership | |||
| Female | .50 [.11, .69] | .08 [.00, .40] | .43 [.30, .57] |
| Male | .29 [.00, .46] | .15 [.00, .47] | .56 [.40, .75] |
| Social support | |||
| Female | .46 [.12, .62] | .06 [.00, .35] | .48 [.38, .60] |
| Male | .46 [.19, .59] | .00 [.00, .19] | .54 [.41, .69] |
Estimates represent proportion of total variance explained by additive genetic (a2), shared environmental (c2), and unique environmental (e2) influences;
95% likelihood-based confidence intervals presented in brackets.
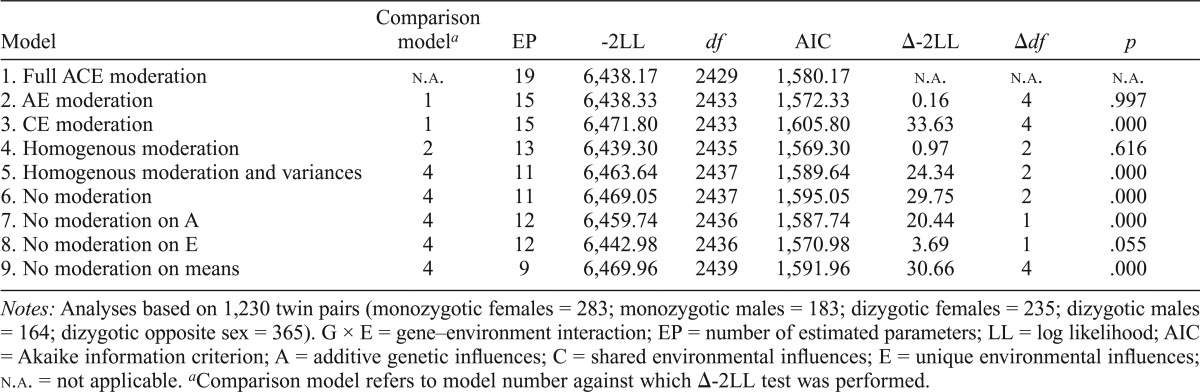
Table 3 presents the results for the G × E models for relationship status. Model 1 included all variance components, and parameters were freely estimated across sex. Dropping the shared environmental component (Model 2) did not alter model fit (Δ-2LL = 0.16, p = .997), although dropping the additive genetic component (Model 3) did (Δ-2LL = 33.63, p < .001), allowing us to use the simpler moderated AE model. Constraining the effect of relationship status to be equal across sexes did not result in poorer fit (Δ-2LL = 0.97, p = .616). However, we were unable to constrain the variance of the A and E components across sexes (Δ-2LL = 24.34, p < .001). Model 4 provided the comparison model for the tests of moderation.
Table 3.
Extended univariate G × E models for romantic partnership and heavy drinking
| Model | Comparison modela | EP | -2LL | df | AIC | Δ-2LL | Δdf | p |
| 1. Full ACE moderation | n.a. | 19 | 6,438.17 | 2429 | 1,580.17 | n.a. | n.a. | n.a. |
| 2. AE moderation | 1 | 15 | 6,438.33 | 2433 | 1,572.33 | 0.16 | 4 | .997 |
| 3. CE moderation | 1 | 15 | 6,471.80 | 2433 | 1,605.80 | 33.63 | 4 | .000 |
| 4. Homogenous moderation | 2 | 13 | 6,439.30 | 2435 | 1,569.30 | 0.97 | 2 | .616 |
| 5. Homogenous moderation and variances | 4 | 11 | 6,463.64 | 2437 | 1,589.64 | 24.34 | 2 | .000 |
| 6. No moderation | 4 | 11 | 6,469.05 | 2437 | 1,595.05 | 29.75 | 2 | .000 |
| 7. No moderation on A | 4 | 12 | 6,459.74 | 2436 | 1,587.74 | 20.44 | 1 | .000 |
| 8. No moderation on E | 4 | 12 | 6,442.98 | 2436 | 1,570.98 | 3.69 | 1 | .055 |
| 9. No moderation on means | 4 | 9 | 6,469.96 | 2439 | 1,591.96 | 30.66 | 4 | .000 |
Notes: Analyses based on 1,230 twin pairs (monozygotic females = 283; monozygotic males = 183; dizygotic females = 235; dizygotic males = 164; dizygotic opposite sex = 365). G × E = gene–environment interaction; EP = number of estimated parameters; LL = log likelihood; AIC = Akaike information criterion; A = additive genetic influences; C = shared environmental influences; E = unique environmental influences; n.a. = not applicable.
Comparison model refers to model number against which Δ-2LL test was performed.
Dropping the moderation effects on all of the paths resulted in a substantial decrease in model fit (Δ-2LL = 29.75, p < .001). Removing moderation on the A path (Δ-2LL = 20.44, p < .001) resulted in worse model fit. Dropping moderation on the E path, however, did not result in a significant decrease in fit (Δ-2LL = 3.69, p = .055). The final model dropped the effect of relationship status on the means (Model 9, Δ-2LL = 30.66, p < .001), demonstrating that being in a romantic partnership had a significant main effect on mean levels of heavy drinking for both women (βM = -.20) and men (βM = -.19).
Figure 3 provides a visual representation of the estimated change in variance components across relationship status from the full model (Model 4). Being involved in a relationship was associated with a reduction in the genetic influence on heavy drinking for both men and women. And although the pattern was similar across sexes, additive genetic influences on heavy drinking were stronger for men.
Figure 3.
Results from gene–environment interaction models for heavy drinking. Bars in first row represent changes in raw variance of components across relationship status. Shaded areas in second row represent change in proportion of total variance explained by each component. Moderation effects constrained to be equal across sex. Moderation on A is significant (p < .001). Moderation on E is not significant (p = .055).
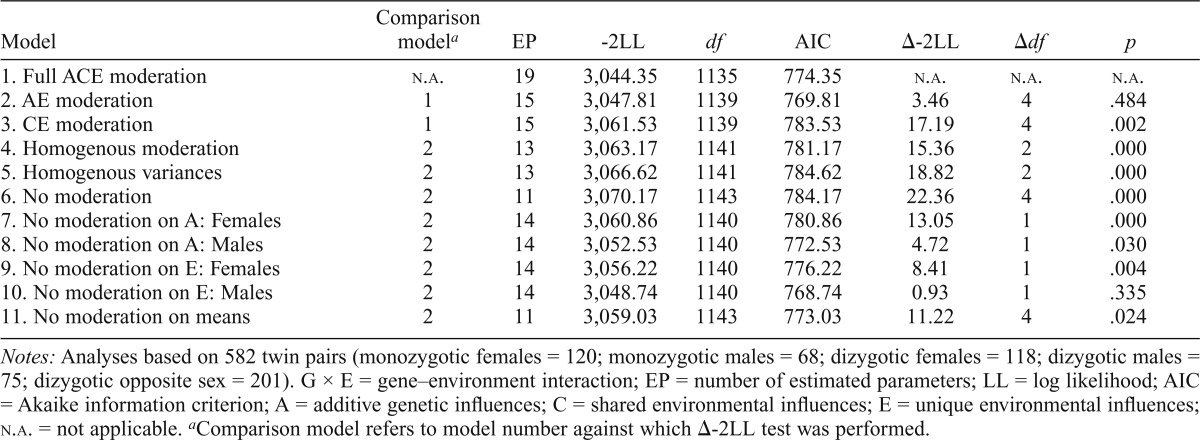
In the case of social support (Table 4), we could again limit the model to the moderated AE model, as the shared environment component did not contribute to model fit (Δ-2LL = 3.46, p = .484), whereas the additive genetic component did (Δ-2LL = 17.19, p = .002). However, neither the moderation effects (Δ-2LL = 15.36, p < .001) nor the variances (Δ-2LL = 18.82, p < .001) could be constrained across sexes, and the moderated AE model (Model 2) served as the reference for tests of moderation.
Table 4.
Extended univariate G × E models for social support and heavy drinking
| Model | Comparison modela | EP | -2LL | df | AIC | Δ-2LL | Δdf | p |
| 1. Full ACE moderation | n.a. | 19 | 3,044.35 | 1135 | 774.35 | n.a. | n.a. | n.a. |
| 2. AE moderation | 1 | 15 | 3,047.81 | 1139 | 769.81 | 3.46 | 4 | .484 |
| 3. CE moderation | 1 | 15 | 3,061.53 | 1139 | 783.53 | 17.19 | 4 | .002 |
| 4. Homogenous moderation | 2 | 13 | 3,063.17 | 1141 | 781.17 | 15.36 | 2 | .000 |
| 5. Homogenous variances | 2 | 13 | 3,066.62 | 1141 | 784.62 | 18.82 | 2 | .000 |
| 6. No moderation | 2 | 11 | 3,070.17 | 1143 | 784.17 | 22.36 | 4 | .000 |
| 7. No moderation on A: Females | 2 | 14 | 3,060.86 | 1140 | 780.86 | 13.05 | 1 | .000 |
| 8. No moderation on A: Males | 2 | 14 | 3,052.53 | 1140 | 772.53 | 4.72 | 1 | .030 |
| 9. No moderation on E: Females | 2 | 14 | 3,056.22 | 1140 | 776.22 | 8.41 | 1 | .004 |
| 10. No moderation on E: Males | 2 | 14 | 3,048.74 | 1140 | 768.74 | 0.93 | 1 | .335 |
| 11. No moderation on means | 2 | 11 | 3,059.03 | 1143 | 773.03 | 11.22 | 4 | .024 |
Notes: Analyses based on 582 twin pairs (monozygotic females =120; monozygotic males = 68; dizygotic females =118; dizygotic males = 75; dizygotic opposite sex = 201). G × E = gene–environment interaction; EP = number of estimated parameters; LL = log likelihood; AIC = Akaike information criterion; A = additive genetic influences; C = shared environmental influences; E = unique environmental influences; n.a. = not applicable.
Comparison model refers to model number against which Δ-2LL test was performed.
Dropping the moderation on all paths for both men and women was again highly significant (Δ-2LL = 22.36, p < .001), allowing us to test for moderation on the individual components. Dropping moderation on the A component for both women (Δ-2LL = 13.05, p < .001) and men (Δ-2LL = 4.72, p = .030) resulted in a significant decrease in model fit. Dropping the moderation effect on the E component led to a significant decrease in fit for women (Δ-2LL = 8.41, p = .004) but not for men. Model 11 dropped the main effect of social support on heavy drinking (Δ-2LL = 11.22, p = .024), revealing a weak, but significant, main effect for women (βM = .08) and men (βM = .09).
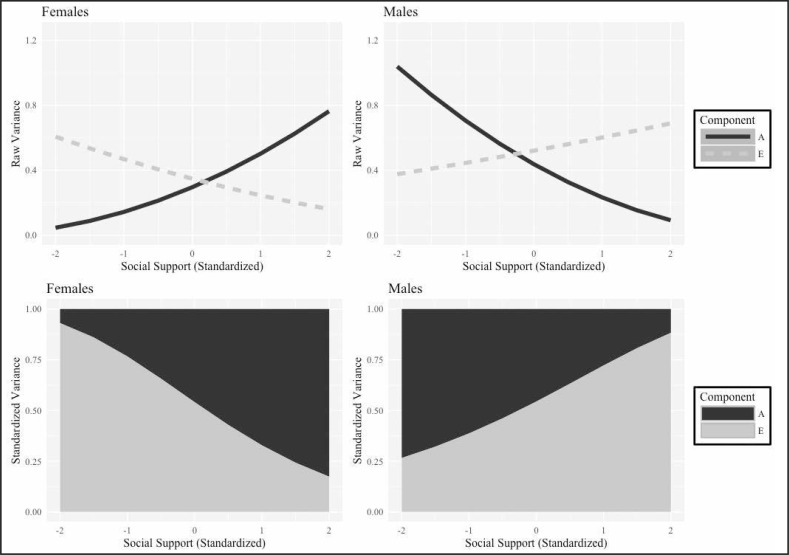
Figure 4 presents the change in variance components across social support from the moderated AE model (Model 2). Although the models for relationship status produced similar results across sex, the models for social support showed a different pattern of effects across sexes. For men, genetic variance declined as social support increased, although environmental variance did not change. For women, genetic variance increased as social support increased. Variance resulting from unique environmental factors worked in the opposite way, with environmental sources of variation decreasing as social support increased.
Figure 4.
Results from gene–environment interaction models for heavy drinking. Lines in first row represent changes in raw variance of components across social support (standardized). Shaded areas in second row represent change in proportion of total variance explained by each component. Moderation on A is significant for both women (p < .001) and men (p < .05). Moderation on E is only significant in women (p < .01).
Discussion
The goal of the current analyses was to examine whether social support and romantic partnering—important protective factors against alcohol misuse in research on social determinants of health (Duncan et al., 2006; Fleming et al., 2010; Frech, 2012; Hagihara et al., 2003)—altered the importance of genetic influences on heavy drinking. We expected that greater levels of social integration (i.e., being in a relationship or reporting greater levels of social support) would be associated with a decline in the genetic variance of heavy drinking. This expectation came from evidence that more socially integrated individuals experience greater levels of social control and monitoring than do those who are less integrated (Umberson et al., 2010). Because social control/opportunity has emerged as such a consistent mechanism limiting genetic predispositions in adolescence (Dick, 2011b), we expected this to extend into young adulthood.
We found that involvement in romantic partnerships constrained the genetic propensity for heavy drinking. For both men and women, being involved in a relationship was associated with reduced genetic influences compared with those who identified as not being in a relationship. Unique environmental influences on heavy drinking became more salient for those involved in a relationship compared with those who were not. Although these patterns were similar across sex, the overall magnitudes varied such that genetic influences on heavy drinking were stronger for men. These results are similar to previous G × E research focusing on marriage-like relationships and alcohol use (Heath et al. 1989) in adult female–female twin pairs. We add to previous findings by demonstrating these relationships are important for both men and women, even in young adulthood. It is important to note that the main effect of romantic partnership on heavy drinking was weak for both men and women (r = -.08). This could reflect the fact that data were collected during the highest developmental period for heavy drinking (Chen & Jacobson, 2012), where the protective effect of these relationships may be minimized. In addition, our measure of romantic partnership collapses different types of relationships. Because the effects of marriage and cohabitation are stronger than those of simply being in a relationship (Duncan et al., 2006), we are likely underestimating the influence of more serious relationships.
Results for social support provided partial support for our hypotheses. For men, the results supported our hypothesis and were in line with other recent findings using measured genotypes (Perry, 2016). A different pattern emerged for women in our study: higher levels of social support were associated with an increased genetic liability rather than limiting any underlying genetic predisposition for heavy drinking. We add to previous research in demonstrating that the influence of social support on genetic variance in alcohol misuse may differ across sex and dimensions of social support during young adulthood.
Why might greater levels of support be associated with increased genetic variance for women but not men? Social relationships can have a “dark side” (Umberson & Montez, 2010). Negative health outcomes, such as obesity (Christakis & Fowler, 2007) or alcohol consumption (Rosenquist et al., 2010), can spread across social networks, offering greater opportunity rather than greater control. In the current sample, women reported significantly more support providers (female mean = 2.81, male mean = 2.58, p < .001), increasing the likelihood of exposure to others who are drinking. Although peer effects tend to be stronger among men (Samek et al., 2015), previous analyses in FinnTwin12 have found peer effects to be stronger among women, especially those with opposite-sex friends (Dick et al., 2007a). Using data from a previous wave to test for possible sex differences in exposure to peer drinking, we found the correlation between age 17 peer alcohol use and social support at age 22 was stronger for females (r = .15) compared with males (r = .11), although this was not significant (p = .470).
Another possibility is that the questions for social support in FinnTwin12 are assessing emotional support. Because women generally provide more frequent support and maintain more intimate relationships than men (Kawachi & Berkman, 2001), greater levels of emotional support may also incur greater expectations and emotional labor, leading to greater responsibilities in maintaining social relationships. More support may create a burden for women that men do not experience. Therefore, men over benefit from involvement in support networks because of their lower reciprocal involvement in these relationships. This benefit may extend beyond main effects to altering genetic liability. Determining whether these sex differences are the result of greater exposure to peer influences or greater emotional burdens is an important step for future research.
In light of our results, we note several limitations. Although our results are robust to the possibility of gene–environment correlation (Purcell, 2002), other factors may explain selection into relationships or support networks. Using data from the age 17 survey, we ran a series of sensitivity analyses to determine predictors of relationship status or social support in young adulthood. Alcohol use, depressive symptoms, self-rated health, and self-esteem were not predictive of relationship status or social support. Those who indicated any drug use or spending more time with their co-twin had significantly lower odds of being in a relationship, and those with greater self-esteem reported significantly greater levels of social support in young adulthood. Although these results do not eliminate the possibility that other factors may account for selection into relationships, they do increase our confidence that the results in the G × E models are not explained by these potential confounds.
Second, the measure of relationship status does not tell us what aspects of being in a relationship matter when it comes to reducing the genetic risk for heavy drinking. Does relationship quality matter more than duration, or is it simply having a close confidant as some have suggested (Thoits, 1995)? Similarly, our measure of social support is made up of items that ask individuals about how many people they perceive as providing support.
Future work should explore the characteristics of these relationships and the behaviors of individuals with whom respondents have a relationship, as well as other environmental moderators such as employment, parenthood, and neighborhood characteristics. In addition, we should consider how these alter genetic influences concurrently and across time, as well as across different racial and ethnic groups. This will help us to better understand how socially patterned conditions can alter individual predispositions across the life course.
In summary, romantic partnership was associated with reduced heavy drinking and attenuated genetic influences for both men and women. Social support had no effect on mean levels of heavy drinking for men but was positively related to heavy drinking in women. For men, greater social support was associated with a reduced importance of genetic influences. For women, however, greater levels of support were associated with an elevated importance of genetic predispositions. These findings reiterate the need for understanding the developmentally situated environmental contexts that are important in relation to genetic predisposition if we hope to alleviate the harm caused by excessive alcohol use.
Footnotes
This study was supported by National Institute on Alcohol Abuse and Alcoholism Grants R01AA015416, K02AA018755, K01AA024152, and F32AA022269; Academy of Finland Grants 100499, 205585, 118555, 141054, 265240, 263278, and 264146; and Scientific and Technological Research Council of Turkey Grant 114C117. The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health, the Academy of Finland, or the Scientific and Technological Research Council of Turkey.
References
- Barnes J. C., Beaver K. M. Marriage and desistance from crime: A consideration of gene-environment correlation. Journal of Marriage and the Family. 2012;74:19–33. doi:10.1111/j.1741-3737.2011.00884.x. [Google Scholar]
- Barr P. B., Salvatore J. E., Maes H., Aliev F., Latvala A., Viken R., Dick D. M. Education and alcohol use: A study of gene–environment interaction in young adulthood. Social Science & Medicine. 2016;162:158–167. doi: 10.1016/j.socscimed.2016.06.031. doi:10.1016/j.socscimed.2016.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergen S. E., Gardner C. O., Kendler K. S. Age-related changes in heritability of behavioral phenotypes over adolescence and young adulthood: A meta-analysis. Twin Research and Human Genetics. 2007;10:423–433. doi: 10.1375/twin.10.3.423. doi:10.1375/twin.10.3.423. [DOI] [PubMed] [Google Scholar]
- Boker S., Neale M., Maes H., Wilde M., Spiegel M., Brick T., Fox J. OpenMx: An open source extended structural equation modeling framework. Psychometrika. 2011;76:306–317. doi: 10.1007/s11336-010-9200-6. doi:10.1007/s11336-010-9200-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braithwaite S. R., Delevi R., Fincham F. D. Romantic relationships and the physical and mental health of college students. Personal Relationships. 2010;17:1–12. doi:10.1111/j.1475-6811.2010.01248.x. [Google Scholar]
- Brown S. A., McGue M., Maggs J., Schulenberg J., Hingson R., Swartzwelder S., Murphy S. Underage alcohol use: Summary of developmental processes and mechanisms: Ages 16–20. Alcohol Research & Health. 2009;32:41–52. [PMC free article] [PubMed] [Google Scholar]
- Burt S. A., Donnellan M. B., Humbad M. N., Hicks B. M., McGue M., Iacono W. G. Does marriage inhibit antisocial behavior? An examination of selection vs causation via a longitudinal twin design. Archives of General Psychiatry. 2010;67:1309–1315. doi: 10.1001/archgenpsychiatry.2010.159. doi:10.1001/archgenpsychiatry.2010.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P., Jacobson K. C. Developmental trajectories of substance use from early adolescence to young adulthood: Gender and racial/ethnic differences. Journal of Adolescent Health. 2012;50:154–163. doi: 10.1016/j.jadohealth.2011.05.013. doi:10.1016/j.jadohealth.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y. C., Prescott C. A., Walsh D., Patterson D. G., Riley B. P., Kendler K. S., Kuo P.-H. Different phenotypic and genotypic presentations in alcohol dependence: Age at onset matters. Journal of Studies on Alcohol and Drugs. 2011;72:752–762. doi: 10.15288/jsad.2011.72.752. doi:10.15288/jsad.2011.72.752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christakis N. A., Fowler J. H. The spread of obesity in a large social network over 32 years. The New England Journal of Medicine. 2007;357:370–379. doi: 10.1056/NEJMsa066082. doi:10.1056/NEJMsa066082. [DOI] [PubMed] [Google Scholar]
- Cohen S., Wills T. A. Stress, social support, and the buffering hypothesis. Psychological Bulletin. 1985;98:310–357. doi:10.1037/0033-2909.98.2.310. [PubMed] [Google Scholar]
- Cooke M. E., Meyers J. L., Latvala A., Korhonen T., Rose R. J., Kaprio J., Dick D. M. Gene-environment interaction effects of peer deviance, parental knowledge and stressful life events on adolescent alcohol use. Twin Research and Human Genetics. 2015;18:507–517. doi: 10.1017/thg.2015.56. doi:10.1017/thg.2015.56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick D. M. Developmental changes in genetic influences on alcohol use and dependence. Child Development Perspectives. 2011a;5:223–230. doi:10.1111/j.1750-8606.2011.00207.x. [Google Scholar]
- Dick D. M. Gene-environment interaction in psychological traits and disorders. Annual Review of Clinical Psychology. 2011b;7:383–409. doi: 10.1146/annurev-clinpsy-032210-104518. doi:10.1146/annurev-clinpsy-032210-104518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dick D. M., Pagan J. L., Holliday C., Viken R., Pulkkinen L., Kaprio J., Rose R. J. Gender differences in friends influences on adolescent drinking: A genetic epidemiological study. Alcoholism: Clinical and Experimental Research. 2007a;31:2012–2019. doi: 10.1111/j.1530-0277.2007.00523.x. doi:10.1111/j.1530-0277.2007.00523.x. [DOI] [PubMed] [Google Scholar]
- Dick D. M., Viken R., Purcell S., Kaprio J., Pulkkinen L., Rose R. J. Parental monitoring moderates the importance of genetic and environmental influences on adolescent smoking. Journal of Abnormal Psychology. 2007b;116:213–218. doi: 10.1037/0021-843X.116.1.213. doi:10.1037/0021-843X.116.1.213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan G. J., Wilkerson B., England P. Cleaning up their act: The effects of marriage and cohabitation on licit and illicit drug use. Demography. 2006;43:691–710. doi: 10.1353/dem.2006.0032. doi:10.1353/dem.2006.0032. [DOI] [PubMed] [Google Scholar]
- Elder G. H., Jr., Johnson M. K., Crosnoe R. The emergence and development of life course theory. In: Mortimer J. T., Shanahan M. J., editors. Handbook of the life course. New York, NY: Springer; 2003. pp. 3–19. [Google Scholar]
- Fleming C. B., White H. R., Catalano R. F. Romantic relationships and substance use in early adulthood: An examination of the influences of relationship type, partner substance use, and relationship quality. Journal of Health and Social Behavior. 2010;51:153–167. doi: 10.1177/0022146510368930. doi:10.1177/0022146510368930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frech A. Healthy behavior trajectories between adolescence and young adulthood. Advances in Life Course Research. 2012;17:59–68. doi: 10.1016/j.alcr.2012.01.003. doi:10.1016/j.alcr.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldsmith H. H. A zygosity questionnaire for young twins: A research note. Behavior Genetics. 1991;21:257–269. doi: 10.1007/BF01065819. doi:10.1007/BF01065819. [DOI] [PubMed] [Google Scholar]
- Guo G., Elder G. H., Cai T., Hamilton N. Gene-environment interactions: Peers’ alcohol use moderates genetic contribution to adolescent drinking behavior. Social Science Research. 2009;38:213–224. doi: 10.1016/j.ssresearch.2008.04.002. doi:10.1016/j.ssresearch.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagihara A., Miller A. S., Tarumi K., Nobutomo K. Social support has both positive and negative effects on the relationship of work stress and alcohol consumption. Stress & Health. 2003;19:205–215. doi: 10.15288/jsa.2003.64.874. doi:10.1002/smi.977. [DOI] [PubMed] [Google Scholar]
- Harden K. P., Hill J. E., Turkheimer E., Emery R. E. Gene-environment correlation and interaction in peer effects on adolescent alcohol and tobacco use. Behavior Genetics. 2008;38:339–347. doi: 10.1007/s10519-008-9202-7. doi:10.1007/s10519-008-9202-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris K. M., Lee H., DeLeone F. Y. Marriage and health in the transition to adulthood: Evidence for African Americans in the Add Health study. Journal of Family Issues. 2010;31:1106–1143. doi: 10.1177/0192513X10365823. doi:10.1177/0192513X10365823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heath A. C., Jardine R., Martin N. G. Interactive effects of genotype and social environment on alcohol consumption in female twins. Journal of Studies on Alcohol. 1989;50:38–48. doi: 10.15288/jsa.1989.50.38. doi:10.15288/jsa.1989.50.38. [DOI] [PubMed] [Google Scholar]
- Jarnecke A. M., South S. C. Genetic and environmental influences on alcohol use problems: Moderation by romantic partner support, but not family or friend support. Alcoholism: Clinical and Experimental Research. 2014;38:367–375. doi: 10.1111/acer.12263. doi:10.1111/acer.12263. [DOI] [PubMed] [Google Scholar]
- Kaprio J. The Finnish twin cohort study: An update. Twin Research and Human Genetics. 2013;16:157–162. doi: 10.1017/thg.2012.142. doi:10.1017/thg.2012.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaprio J., Koskenvuo M., Langinvainio H., Romanov K., Sarna S., Rose R. J. Genetic influences on use and abuse of alcohol: A study of 5638 adult Finnish twin brothers. Alcoholism: Clinical and Experimental Research. 1987;11:349–356. doi: 10.1111/j.1530-0277.1987.tb01324.x. doi:10.1111/j.1530-0277.1987.tb01324.x. [DOI] [PubMed] [Google Scholar]
- Kawachi I., Berkman L. F. Social ties and mental health. Journal of Urban Health. 2001;78:458–467. doi: 10.1093/jurban/78.3.458. doi:10.1093/jurban/78.3.458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler K. S., Gardner C., Dick D. M. Predicting alcohol consumption in adolescence from alcohol-specific and general externalizing genetic risk factors, key environmental exposures and their interaction. Psychological Medicine. 2011;41:1507–1516. doi: 10.1017/S003329171000190X. doi:10.1017/S003329171000190X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kendler K. S., Lonn S. L., Salvatore J., Sundquist J., Sundquist K. Effect of marriage on risk for onset of alcohol use disorder: A longitudinal and co-relative analysis in a Swedish national sample. American Journal of Psychiatry. 2016;173:911–918. doi: 10.1176/appi.ajp.2016.15111373. doi:10.1176/appi.ajp.2016.15111373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knaapila A., Silventoinen K., Broms U., Rose R. J., Perola M., Kaprio J., Tuorila H. M. Food neophobia in young adults: Genetic architecture and relation to personality, pleasantness and use frequency of foods, and body mass index—A twin study. Behavior Genetics. 2011;41:512–521. doi: 10.1007/s10519-010-9403-8. [DOI] [PubMed] [Google Scholar]
- Miles D. R., Silberg J. L., Pickens R. W., Eaves L. J. Familial influences on alcohol use in adolescent female twins: Testing for genetic and environmental interactions. Journal of Studies on Alcohol. 2005;66:445–451. doi: 10.15288/jsa.2005.66.445. doi:10.15288/jsa.2005.66.445. [DOI] [PubMed] [Google Scholar]
- Osler M., McGue M., Lund R., Christensen K. Marital status and twins’ health and behavior: An analysis of middle-aged Danish twins. Psychosomatic Medicine. 2008;70:482–487. doi: 10.1097/PSY.0b013e31816f857b. doi:10.1097/PSY.0b013e31816f857b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peirce R. S., Frone M. R., Russell M., Cooper M. L. Financial stress, social support, and alcohol involvement: A longitudinal test of the buffering hypothesis in a general population survey. Health Psychology. 1996;15:38–47. doi: 10.1037//0278-6133.15.1.38. doi:10.1037/0278-6133.15.1.38. [DOI] [PubMed] [Google Scholar]
- Perry B. L. Gendering genetics: Biological contingencies in the protective effects of social integration for men and women. American Journal of Sociology. 2016;121:1655–1696. doi: 10.1086/685486. doi:10.1086/685486. [DOI] [PubMed] [Google Scholar]
- Perry B. L., Pescosolido B. A., Bucholz K., Edenberg H., Kramer J., Kuperman S., Nurnberger J. I., Jr Gender-specific gene-environment interaction in alcohol dependence: The impact of daily life events and GABRA2. Behavior Genetics. 2013;43:402–414. doi: 10.1007/s10519-013-9607-9. doi:10.1007/s10519-013-9607-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prescott C. A., Kendler K. S. Associations between marital status and alcohol consumption in a longitudinal study of female twins. Journal of Studies on Alcohol. 2001;62:589–604. doi: 10.15288/jsa.2001.62.589. doi:10.15288/jsa.2001.62.589. [DOI] [PubMed] [Google Scholar]
- Purcell S. Variance components models for gene-environment interaction in twin analysis. Twin Research and Human Genetics. 2002;5:554–571. doi: 10.1375/136905202762342026. doi:10.1375/136905202762342026. [DOI] [PubMed] [Google Scholar]
- Rönkä A., Oravala S., Pulkkinen L. “I met this wife of mine and things got onto a better track”: Turning points in risk development. Journal of Adolescence. 2002;25:47–63. doi: 10.1006/jado.2001.0448. doi:10.1006/jado.2001.0448. [DOI] [PubMed] [Google Scholar]
- Rosenquist J. N., Murabito J., Fowler J. H., Christakis N. A. The spread of alcohol consumption behavior in a large social network. Annals of Internal Medicine. 2010;152:426–433. doi: 10.1059/0003-4819-152-7-201004060-00007. doi:10.7326/0003-4819-152-7-201004060-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutter M. Transitions and turning points in developmental psychopathology: As applied to the age span between childhood and mid-adulthood. International Journal of Behavioral Development. 1996;19:603–626. doi:10.1177/016502549601900309. [Google Scholar]
- Salvatore J. E., Cho S. B., Dick D. M. Genes, environments, and sex differences in alcohol research. Journal of Studies on Alcohol and Drugs. 2017;78:494–501. doi: 10.15288/jsad.2017.78.494. doi:10.15288/jsad.2017.78.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvatore J. E., Kendler K. S., Dick D. M. Romantic relationship status and alcohol use and problems across the first year of college. Journal of Studies on Alcohol and Drugs. 2014;75:580–589. doi: 10.15288/jsad.2014.75.580. doi:10.15288/jsad.2014.75.580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samek D. R., Rueter M. A., Keyes M. A., McGue M., Iacono W. G. Parent involvement, sibling companionship, and adolescent substance use: A longitudinal, genetically informed design. Journal of Family Psychology. 2015;29:614–623. doi: 10.1037/fam0000097. doi:10.1037/fam0000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampson R. J., Laub J. H. A life-course view of the development of crime. Annals of the American Academy of Political and Social Science. 2005;602:12–45. doi:10.1177/0002716205280075. [Google Scholar]
- Sarason I. G., Levine H. M., Basham R. B., Sarason B. R. Assessing social support: The Social Support Questionnaire. Journal of Personality and Social Psychology. 1983;44:127–139. doi:10.1037/0022-3514.44.1.127. [Google Scholar]
- Shanahan M. J., Hofer S. M. Social context in gene-environment interactions: Retrospect and prospect. Journals of Gerontology. Series B, Psychological Sciences and Social Sciences, 60, Special Issue. 2005;1:65–76. doi: 10.1093/geronb/60.special_issue_1.65. doi:10.1093/geronb/60.Special_Issue_1.65. [DOI] [PubMed] [Google Scholar]
- Steptoe A., Wardle J., Pollard T. M., Canaan L., Davies G. J. Stress, social support and health-related behavior: A study of smoking, alcohol consumption and physical exercise. Journal of Psychosomatic Research. 1996;41:171–180. doi: 10.1016/0022-3999(96)00095-5. doi:10.1016/0022-3999(96)00095-5. [DOI] [PubMed] [Google Scholar]
- Thoits P. A. Stress, coping, and social support processes: Where are we? What next? Journal of Health and Social Behavior. 1995;35:53–79. doi:10.2307/2626957. [PubMed] [Google Scholar]
- Umberson D., Crosnoe R., Reczek C. Social relationships and health behavior across life course. Annual Review of Sociology. 2010;36:139–157. doi: 10.1146/annurev-soc-070308-120011. doi:10.1146/annurev-soc-070308-120011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umberson D., Montez J. K. Social relationships and health: A flashpoint for health policy. Journal of Health and Social Behavior, 51, Supplement. 2010:S54–S66. doi: 10.1177/0022146510383501. doi:10.1177/0022146510383501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van der Sluis S., Posthuma D., Dolan C. V. A note on false positives and power in G × E modelling of twin data. Behavior Genetics. 2012;42:170–186. doi: 10.1007/s10519-011-9480-3. doi:10.1007/s10519-011-9480-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitton S. W., Weitbrecht E. M., Kuryluk A. D., Bruner M. R. Committed dating relationships and mental health among college students. Journal of American College Health. 2013;61:176–183. doi: 10.1080/07448481.2013.773903. doi:10.1080/07448481.2013.773903. [DOI] [PubMed] [Google Scholar]
- Yamaguchi K., Kandel D. B. On the resolution of role incompatibility: A life event history analysis of family roles and marijuana use. American Journal of Sociology. 1985;90:1284–1325. doi:10.1086/228211. [Google Scholar]