Abstract
Objective:
Numerous studies have modeled the effects of stress in the laboratory, demonstrating that smokers who are exposed to experimental stressors exhibit significant increases in acute psychological distress. Whether these stress reactions are predictive of stress-induced smoking during an actual quit attempt, however, has not been examined. Furthermore, the possibility that such effects are particularly strong among smokers with higher ambient levels of distress has not been addressed.
Method:
Nicotine-dependent smokers (N = 60; 40 women, 20 men) completed the Brief Symptoms Index (BSI) and then participated in a laboratory stress task 1 week before a quit attempt. Acute psychological distress was measured immediately before and after exposure to stressful and neutral stimuli. After they quit, participants completed a smoking diary for 14 days in which they recorded the degree to which their smoking was precipitated by emotional stress.
Results:
Consistent with our hypotheses, BSI scores predicted both exaggerated laboratory stress responses (p < .005) and smoking that was attributable to stress during the 14-day postquit period (p < .01). Laboratory stress reactions were predictive of stress-induced smoking (p < .01), and acute psychological stress reactions mediated the effects of BSI on stress-induced smoking.
Conclusions:
Acute psychological stress reactivity is a potential mechanism underlying the effect of stress-induced smoking during a quit attempt.
Smoking is responsible for approximately 480,000 preventable deaths per year in the United States (U.S. Department of Health and Human Services, 2014). Although a majority of smokers express a desire to quit, smoking cessation remains elusive for most (U.S. Department of Health and Human Services, 2014). Even when using the most successful combinations of pharmacological and behavioral therapies, most individuals attempting to quit ultimately relapse (Hughes, 2003), suggesting an acute need to further explore mechanisms that might be amenable to intervention and support successful cessation.
Emotional stress, often characterized by symptoms of anxiety and depression, has long been recognized as a significant risk factor for relapse to smoking (Cohen & Lichtenstein, 1990). In animal models, nicotine can reduce stress behavior, whereas stress can cause a return to nicotine selfadministration (Anderson & Brunzell, 2012; Martin-Garcia et al., 2009). A variety of acute stressors—including the birth of a newborn (Notley et al., 2015; Wen et al., 2015), job loss (Falba et al., 2005), and military deployment (Boyko et al., 2015)—have been implicated in contributing to smoking relapse. In addition, there is a significant literature supporting a relationship between difficulty quitting smoking and symptoms of general psychological distress. For example, individuals with clinical depression or depressive symptoms are less likely to successfully maintain a quit attempt, particularly among women (Cooper et al., 2016; Linares Scott et al., 2009; Zvolensky et al., 2015), and even a decade-old history of major depression can halve a person’s odds of successfully maintaining long-term cessation (Zvolensky et al., 2015).
In addition to the effects of mood symptoms, smokers with a range of anxiety disorders have been shown to be at greater risk for lapse and relapse (Zvolensky et al., 2008), and anxiety symptoms have been correlated with a greater number of unsuccessful quit attempts (Zvolensky et al., 2009). Finally, numerous studies have shown that general feelings of psychological distress, even in the absence of a formal diagnosis of psychopathology, are predictive of poor smoking cessation outcomes (Leventhal & Zvolensky, 2015).
However, the mechanisms underlying the relationship between psychological distress and smoking during a cessation attempt remain understudied (Leventhal & Zvolensky, 2015). One possible mechanism may be individual variation in responses to acute stressors. Indeed, research has demonstrated that there is wide individual variation in reactivity to stress, recognizing that individuals exposed to similar stressors (e.g., caring for a newborn) may perceive the severity of the stressor differently (Wen et al., 2015). Similarly, Buchman et al. (2010) have shown that smoking characteristics affect variation in hypothalamic–pituitary axis (HPA) response to a social stressor.
People with elevated levels of general psychological distress may be particularly likely to appraise stressors as more severe, and there is some evidence that smokers with elevated levels of psychological distress report more severe withdrawal during a quit attempt (Breslau et al., 1992). Responses to acute stress are amenable to brief intervention (Davis et al., 2015; de Brouwer et al., 2011), making it particularly important to study how acute psychological distress may mediate the relationship between general psychological distress and smoking during a quit attempt.
A popular approach to studying stress reactivity is to model the effects of stress under laboratory conditions. Laboratory stress-induction paradigms reliably generate responses in (a) the sympathetic nervous system (Sinha, 2009), (b) the HPA axis (Sinha, 2009), and (c) self-report measures of acute psychological distress (Colamussi et al., 2007; Sinha, 2009). Laboratory stressors can similarly produce increases in cigarette cravings (Buchmann et al., 2010; Colamussi et al., 2007; Erblich & Michalowski, 2015) as well as cravings for other substances of abuse (Sinha, 2009).
The degree to which these acute psychological distress reactions relate to actual smoking cessation outcomes, however, is not well established. Indeed, recent reviews have called the predictive validity of laboratory inductions into question (Perkins, 2009). A critical lacuna in this literature comes from a dearth of prospective studies linking laboratory-based findings to real-world findings. The body of research studying relationships between laboratory reactions to stress and the clinical course of substance abuse is still small, and there are particularly few studies addressing links between stress reactivity and real-time—or near-realtime—smoking episodes following a quit attempt.
In one retrospective study, Calhoun and colleagues (2007) exposed smokers with posttraumatic stress disorder to stressful scripts and found that increases in acute psychological distress were associated with shorter duration of previous quit attempts, whereas al’Absi and colleagues (2005) found that an attenuated adrenocortical response to a laboratory social stress task predicted shorter time to relapse among smokers trying to quit. However, considering the scope of research using laboratory stress inductions to study patterns of addiction, the number of studies investigating the predictive value of acute psychological distress reactions is still limited. There is particular need for further research measuring smoking behavior and its triggers as they happen during real-world cessation attempts to be able to analyze pathways by which stress reactivity in the laboratory might affect the cessation process.
The objective of the current study, therefore, was to examine the possibility that general psychological distress—as well as increased reactivity to acute stress—would predict stress-induced smoking following a quit attempt. In addition, we tested the possibility that acute psychological distress reactions would mediate the relationship between general psychological distress and stress-induced smoking. Last, we explored the possibility that general psychological distress, as well as increased acute psychological distress reactions, would predict the number of cigarettes smoked on each of the diary days.
Method
Participants
Healthy adult smokers (N = 60) interested in making a voluntary, unaided, “cold turkey” quit attempt were recruited from advertisements in and around a major urban medical center for a study aimed at better understanding the experience of smoking cessation. Participants were required to be at least 18 years old; be current smokers (breath carbon monoxide confirmed); meet diagnostic criteria for nicotine dependence according to the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (American Psychiatric Association, 1994); and smoke an average of 10 cigarettes per day for at least 5 years. In addition, all participants reported at least an 8 out of 10 on a single-item Contemplation Ladder (Biener & Abrams, 1991), suggesting high motivation to quit. Potential participants were excluded if they reported current treatment for nicotine dependence, current other substance abuse, a history of hospitalization for major mental illness, or current pregnancy. The research procedures were approved by the Institutional Review Board of the Icahn School of Medicine at Mount Sinai in New York City.
Procedure
Subjects first participated in a laboratory session scheduled approximately 1 week before a mutually agreed-upon target quit date. During this session, subjects provided informed consent, completed questionnaires (see below), and participated in a laboratory-based stress imagery task. In this task, participants underwent a guided-imagery stress induction that involved an experimenter reading a 60-second script describing the anticipation of painful dental work, followed by a 30-second silent period, during which time participants were instructed to continue to imagine the scene. Self-report measures of acute psychological distress (see below) were administered immediately before and after the induction. For comparison, participants also underwent a neutral imagery induction, which described changing a lightbulb. The stress and neutral imagery tasks were counterbalanced and separated by a 3-minute rest period, during which time participants viewed an aquatic video (Piferi et al., 2000). Similar methodology has successfully been used in previous studies and has reliably induced elevations in acute psychological distress (Colamussi et al., 2007; Saladin et al., 2015).
Approximately 1 week later, participants initiated an unaided, cold-turkey smoking cessation attempt. During the first 14 days of the attempt, participants were asked to complete a diary recording any smoking that may have occurred, as well as the triggers of those episodes (see Measures). Participants who did not successfully quit were offered referrals to smoking cessation resources available in the region. At the end of the study, participants returned to the laboratory to submit their diaries and were reimbursed $100 for their time.
Measures
Demographics.
Participants completed face-valid demographic and smoking history questionnaires. Demographic questions included age, gender, and ethnicity. Smoking history questions included age at initiation, typical number of cigarettes smoked per day, and number of years having smoked. These items were considered as potential covariates in the primary analyses described below.
Nicotine dependence.
To measure the intensity of participants’ addiction, they completed the Fagerström Test for Nicotine Dependence (FTND; Heatherton et al., 1991). This well-established six-item measure has good psychometric properties (Heatherton et al., 1991) and has been shown to predict a number of smoking cessation outcomes. The FTND was administered during the first study visit and was considered as a potential covariate in the primary analyses.
General distress.
To measure general psychological distress, participants completed an abbreviated version of the classic Brief Symptoms Index (Derogatis, 2001) indexed to distress over the past month. This 18-item scale (BSI-18) has been used recently in a number of studies (e.g., Kowalkowski et al., 2014; Shuter et al., 2012) to reduce questionnaire burden and has been found to correlate strongly with the longer version (Andreu et al., 2008). Items on the BSI-18 (e.g., “Feeling Blue,” “Feeling Fearful,” “Feelings of worthlessness”) are summed to yield a “general distress” score. Each item is scored on a 0 (not at all) to 4 (extremely) scale. The instrument was administered during the first study visit.
Vividness of imagery.
After each of the two imaginal exposures (neutral, stress), participants completed a four-item, face-valid (e.g., “How vivid did your images seem?”, “How real did your images seem to you?”) 0–25 scale of imagery vividness. This instrument has been used in our previous work (Erblich & Bovbjerg, 2004; Erblich et al., 2005), and demonstrated strong internal consistency (Cronbach’s α ranging from .85 to .92 for the three administrations) in the current sample.
Acute psychological distress.
To assess acute psychological distress reactions to the stress imagery, we used a series of three visual analog scales (Bond & Lader, 1974) assessing (a) anxiety, (b) depression, and (c) general emotional upset. The scales consisted of a 100-mm line anchored on the left by “not at all” and on the right by “as much as can be.” Participants responded to the questions, “How are you feeling right now?” and “How did you feel during the scene?” (preand post-imagery, respectively) by striking a line across the continuum. Visual analog scale measures have been found to be a rapid, reliable, and valid way to assess transient changes in subjective feelings (Bond & Lader, 1974; Cella & Perry, 1986) and have been used extensively in other studies measuring changes in distress in response to experimental challenges (Colamussi et al., 2007; Krystal et al., 1993; Wright et al., 2006). The three items were averaged to form a composite three-item scale of acute distress. Internal consistency (Spearman-Brown) was excellent, ranging from .85 to .94 for the four administrations.
Stress-induced smoking.
During the first 14 days of the cessation attempt, participants completed a daily questionnaire assessing smoking behavior (number of cigarettes smoked) and the degree to which a variety of triggers gave rise to a given smoking episode. For each smoking episode, participants rated how much the episode was triggered by stress, boredom, or smoking cues. Participants were instructed to report on the first smoking episode of the day if they experienced more than one episode on a given day. Each trigger was rated on a face-valid scale from 0 (not at all) to 10 (extremely). Analogous triggers questionnaires have been successfully used in previous research (Ferguson & Shiftman, 2014; Shiftman et al., 2006).
Data analysis
To confirm the effects of the imagery task on acute psychological distress, we first conducted a repeated-measures analyses of variance (ANOVA) with Induction (stress, neutral) and Time (pre-stimulus, post-stimulus) as within-subjects factors, using SPSS, Version 23 (IBM Corp., Armonk, NY). Background variables, smoking characteristics, and imagery vividness were explored as potential covariates. Next, we conducted hierarchical linear modeling (HLM) analyses using WHLM software to evaluate the effects of general distress (BSI) and acute psychological distress reactions on the degree of stress-induced smoking across the 14-day diary interval.
For this analysis, the acute psychological distress reaction predictor was calculated as a pre-stimulus–adjusted change score (from pre-stressor to post-stressor), controlling for any change attributable to neutral imagery. HLM provided the benefit of avoiding listwise deletion of data from subjects (n = 16) who sporadically did not complete all 14 days of diary data.
Last, we tested the possibility that the relationship between BSI and stress-induced smoking was mediated by acute psychological distress reactions. To that end, we conducted bootstrap analyses of the indirect (mediated) effects of BSI on stress-induced smoking, using the PROCESS macro in SPSS (Hayes, 2013).
Results
Demographic and smoking variables
Sixty-seven percent of participants (n = 40) were women and 33% (n = 20) were men. The mean age of the sample was 41.6 years (SD = 6.4). Fifty-two percent of participants reported African American ethnicity, 18% reported White ethnicity, 15% reported Hispanic ethnicity, and the remaining 15% either reported other ethnic backgrounds or declined to report. Participants reported beginning to smoke at age 17.3 (SD = 5.8) and smoking an average of 17.9 (SD = 8.8) cigarettes per day for an average of 20.7 (SD = 9.2) years. Mean nicotine dependence score on the FTND was 6.0 (SD = 1.8), suggesting moderate levels of dependence. Preliminary analyses revealed that neither demographic nor smoking history characteristics were related to study variables (BSI-18, acute psychological distress, stress-induced smoking). Thus, they were not included as covariates in the analyses. We note, however, that results were comparable when these covariates were included.
Not surprisingly, as this was a cold-turkey, unaided cessation attempt, all of the participants reported smoking over the 14-day diary interval (mean cigarettes per day = 4.9, SD = 5.0). Of the 840 possible smoking events to be reported (14 days × 60 subjects), 720 events were recorded and included in the HLM model. Mean time to report the smoking event from its onset was 123.9 minutes (SD = 9.9). Mean stress-induced smoking score across all events was 3.7 (SD = 3.8), with a range of 0–10.
Effects of imaginal stress exposure
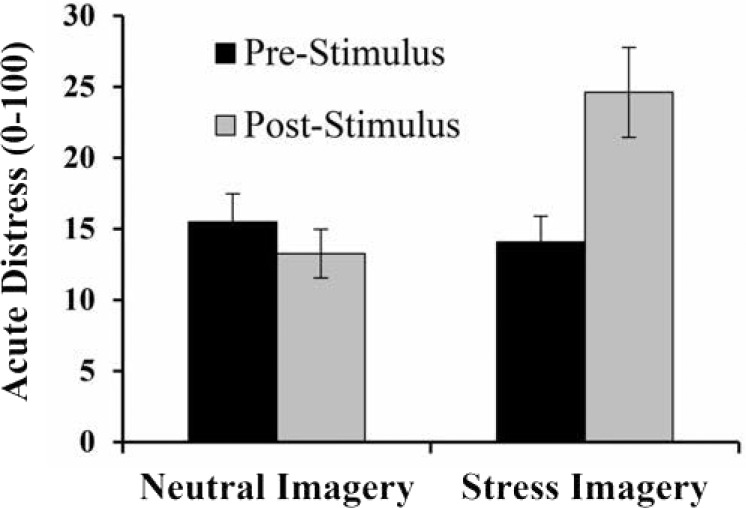
As indicated above, we first tested the hypothesis that the imaginal stress induction elicited increases in acute psychological distress. To that end, we performed a 2 (stress, neutral) × 2 (pre-stimulus, post-stimulus) repeated-measures ANOVA. Participants exhibited significant increases in acute psychological distress following the stress induction (pre-stimulus: Mdistress = 14.1, SD = 1.8; post-stimulus: Mdistress = 24.6, SD = 3.2) but not following the control induction (pre-stimulus: Mdistress = 15.4, SD = 2.0; post-stimulus: Mdistress = 13.3, SD = 1.7), F(1, 58) = 19.4, p < .0001, η2 = .25 (Figure 1).
Figure 1.
Mean (SE) distress scores before and after exposure to neutral and stress imagery. A 2 × 2 repeated-measures analysis of variance revealed a significant interaction, such that acute distress was higher following the stress imagery, but not following the neutral imagery (p < .0001).
Effects of general distress on laboratory reactivity
Next, we examined the possibility that participants with higher levels of general psychological distress exhibited elevations in acute psychological distress reactivity. To address this question, we conducted a repeated-measures ANOVA using pre-stimulus–adjusted change (from pre-stimulus to post-stimulus) scores for the neutral and stress inductions as a within-subjects factor of induction (neutral change, stress change—“acute stress reactivity”) and added BSI-18 score as a covariate. We decided to use change scores here, instead of including both pre-stimulus and post-stimulus raw scores in the analysis as another factor, so that we could directly examine and report the relationship between BSI-18 and reactivity (i.e., change) per se.
Consistent with the study hypothesis, there was a significant interaction between BSI-18 and induction, F(1, 55) = 9.6, p < .003, η2 = .15. As displayed in Figure 2, examination of parameter estimates revealed that higher BSI-18 scores were predictive of higher acute stress reactivity (b = 7.1, β = .37, p < .007) but not neutral reactivity (b = -1.4, β = -.13, p <.305).
Figure 2.
Regression of laboratory reactivity to stress and neutral exposures on general distress (Brief Symptoms Index, 18-item version [BSI-18]). BSI-18 significantly predicted increases reactivity to stress imagery (p < .007), but not to neutral imagery (p < .305).
Effects of acute stress reactivity and general distress on intensity of stress-induced smoking
Next, we tested the possibility that acute stress reactivity and BSI-18 predicted the degree to which smoking was induced by stress during the 14-day diary interval. To that end, we conducted HLM, with Day (1–14) as a Level 1 factor and BSI-18 and stress reactivity as Level 2 predictors. The degree to which smoking was induced by stress (0–10) served as the outcome.
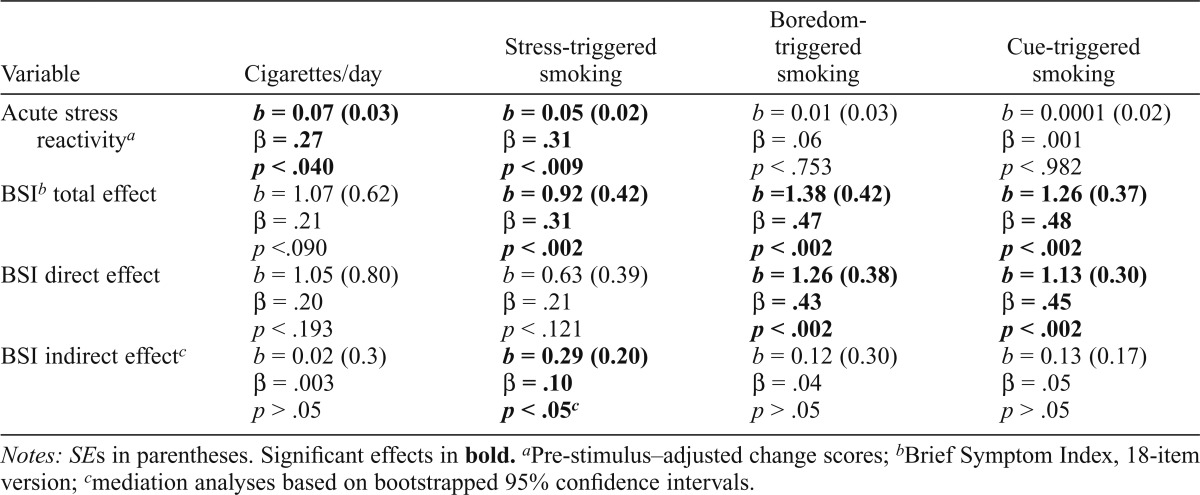
As indicated in Table 1, findings revealed that BSI-18 predicted significant elevations in stress-induced smoking (b = 0.92, β = .31, p < .002). Similarly, acute stress reactivity predicted significant elevations in stress-induced smoking (b = 0.05, β = .31, p < .009). Reactivity to the neutral induction, however, did not predict stress-induced smoking (p < .757).
Table 1.
Mediational models of Brief Symptom Index (BSI), acute stress reactivity, and smoking outcomes
| Variable | Cigarettes/day | Stress-triggered smoking | Boredom-triggered smoking | Cue-triggered smoking |
| Acute stress reactivitya | b = 0.07 (0.03) | b = 0.05 (0.02) | b = 0.01 (0.03) | b = 0.0001 (0.02) |
| β = .27 | β = .31 | β = .06 | β = .001 | |
| p < .040 | p < .009 | P < .753 | p < .982 | |
| BSIb total effect | b = 1.07 (0.62) | b = 0.92 (0.42) | b =1.38 (0.42) | b = 1.26 (0.37) |
| β = .21 | β = .31 | β = .47 | β = .48 | |
| p <.090 | p < .002 | p < .002 | p < .002 | |
| BSI direct effect | b = 1.05 (0.80) | b = 0.63 (0.39) | b = 1.26 (0.38) | b = 1.13 (0.30) |
| β = .20 | β = .21 | β = .43 | β = .45 | |
| p < .193 | p < .121 | p < .002 | p < .002 | |
| BSI indirect effectc | b = 0.02 (0.3) | b = 0.29 (0.20) | b = 0.12(0.30) | b = 0.13 (0.17) |
| β = .003 | β = .10 | β = .04 | β = .05 | |
| p > .05 | p < .05c | p > .05 | p > .05 |
Notes: SEs in parentheses. Significant effects in bold.
Pre-stimulus–adjusted change scores;
Brief Symptom Index, 18-item version;
mediation analyses based on bootstrapped 95% confidence intervals.
Laboratory stress reactivity was not predictive of the degree to which smoking was triggered by smoking cues (p < .982) or boredom (p < .753), demonstrating that the stress reactivity effects were specific to stress-induced smoking. Interestingly, however, BSI-18 was related to cue-induced smoking (b = 1.26, β = .48, p < .002) and boredom-induced smoking (b = 1.38, β = .47, p < .002), suggesting a broader influence of general distress.
Last, we explored the possibility that general psychological distress, as well as increased acute stress reactivity, would predict the number of cigarettes smoked each day. Findings revealed that laboratory stress reactivity significantly predicted the number of cigarettes smoked per day (b = 0.07, β = .27, p < .040), and BSI was marginally predictive of cigarettes per day (b = 1.07, β = .21, p < .090). For all analyses, differential effects (i.e., cross-level interactions) of BSI-18 and acute stress reactivity by day were not observed (ps > .12), suggesting that these effects were consistent across days.
Mediational analyses
We then tested the possibility that the relationship between BSI-18 and stress-induced smoking was mediated by acute stress reactivity. To that end, we conducted bootstrap analyses of the direct and indirect effects of the BSI-18 (5,000 samples). As indicated in Table 1, findings revealed that when including acute stress reactivity in the model, the indirect effect of BSI-18 was significant (b = 0.29, β = .10, p < .05; bootstrapped confidence interval did not contain 0), providing support for statistical mediation. The direct effect of BSI-18 on stress-induced smoking, after we controlled for acute stress reactivity, was not significant (p < .121).
Finally, a similar analysis was conducted to explore the possibility that acute stress reactivity mediated relations between BSI-18 and the number of cigarettes smoked per day, as well as cue- and boredom-induced smoking. In these cases, however, we failed to identify significant indirect effects of BSI-18 (bootstrapped confidence intervals contained 0), suggesting that acute stress reactivity did not mediate effects of BSI on these outcomes.
Discussion
As expected, both elevated levels of general psychological distress and acute psychological distress reactions were predictive of stronger endorsements of smoking in response to stress during a naturalistic quit attempt. Consistent with the hypothesized model, acute psychological distress reactions to the stress induction (acute stress reactivity) mediated the relationship between general distress and this stress-induced smoking, contributing to our understanding of mechanisms underlying the relationship between distress and relapse during smoking cessation. We note that acute stress reactivity also predicted more cigarettes smoked per day during the 14-day diary interval, providing longitudinal evidence for the importance of laboratory-induced acute stress reactivity as a predictor of real-world smoking behavior. General distress, however, was only marginally predictive of cigarettes per day and was not indirectly predictive, either.
Elevated levels of psychological distress, even in the absence of psychopathology, are associated with greater likelihood of smoking initiation as well as with greater difficulty achieving successful cessation (Leventhal & Zvolensky, 2015). In addition, there is a well-established relationship between mood and anxiety psychopathology and smoking. Individuals with mood and anxiety disorders are up to two times more likely to smoke than those with no diagnosis (Gwynn et al., 2008), and having current or past mood or anxiety disorders also decreases the likelihood that a smoker will successfully achieve cessation during a quit attempt (Piper et al., 2010).
In this study, we identified an effect of psychological distress on stress-induced smoking during a quit attempt even in a normative sample in which individuals with either current or past major mental illness were excluded. This effect would likely be more pronounced in a sample including individuals with clinical psychopathology.
Consistent with our hypotheses, over the first 2 weeks of a quit attempt, greater acute stress reactivity in the laboratory predicted increased smoking, as well as stronger endorsements of smoking in response to stress, but not in response to other triggers, such as boredom or smoking cues. Taken together, these results suggest a stress-specific pathway that leads from general distress to acute stress reactivity to stress-induced—but not boredom- or cue-induced—smoking behavior. The mediation model suggests the importance of stress reactivity, or the tendency to react with greater distress to a given stressor. Although acute stress reactivity is a relatively upstream, automatic process, it is amenable to intervention through stress-management training (de Brouwer et al., 2011). It is therefore particularly important to study the role of acute stress reactivity in predicting relapse in high-risk populations of smokers with mood and anxiety psychopathology.
It is interesting to note that although acute stress reactivity was predictive of stress-induced—but not cue- or boredom-induced—smoking, general distress, as measured by the BSI-18, was predictive of all three. These findings suggest that the BSI-18 may be more broadly predictive of smoking episodes that are triggered by a variety of emotional states. Not surprisingly, however, these effects were not mediated by acute stress reactivity.
A strength of the current study was its diverse, community-based sample. However, because participants made a naturalistic quit attempt without any treatment, as anticipated, they were not successful in remaining abstinent for any period of time. Therefore, we were unable to analyze differential effects between smokers who abstained and subsequently relapsed, smokers who successfully maintained abstinence, and smokers who never achieved initial abstinence. Nevertheless, we believe that the results shed light on the role of stress in smoking behavior in the early trajectory of a quit attempt.
Another limitation was the focus on one type of stressor—anticipated painful dental work, whereas social and cognitive stressors were not analyzed. Hormonal reactivity to social stressors, such as the Trier Social Stress Task, has been found to predict outcomes for cigarette smokers and other drug users (Back et al., 2010; Buchmann et al., 2010), but further research is needed to explore relationships between distress and acute reactivity to social stressors.
Cognitive stressors may commonly occur in smokers’ daily lives (e.g., completing a difficult work project) but have received less attention. One study found that persistence on the Paced Auditory Serial Addition Test was not correlated with smoking relapse, although acute stress reactivity was not analyzed (Brown et al., 2009). In addition, stress may be only one of many precipitants of smoking during a quit attempt. Further research is warranted to study the effects of other smoking precipitants as well (e.g., cue exposures, boredom). In addition, results relied on retrospective reports made during diary entries an average of approximately 2 hours after the onset of a smoking event. As with any delayed report, there is the possibility of recall bias. Future studies using ecological momentary assessment may provide a more bias-free approach. Finally, our assessment of acute stress relied on a brief visual analog scale and did not include physiological measures of acute stress reactivity. Such measures in future studies would provide convergent evidence for mediation effects.
The importance of reactivity to laboratory stimuli as a predictor of real-world outcomes has been a source of controversy over the past several years. For example, in one recent study, cigarette craving in response to laboratory cues was found not to correlate with similar events in near-real time using ecological momentary assessment (Shiffman et al., 2015). In contrast, the current results demonstrate that acute laboratory stress responses prospectively predict both the number of cigarettes smoked during a quit attempt and stronger endorsements of stress-induced smoking. Similarly, Calhoun et al. (2007) found that acute stress reactivity in the laboratory was predictive of shorter time to relapse among smokers. It is possible that stress responses in the laboratory are more robustly related to the effects of stress in the natural environment, whereas laboratory smoking cue exposures are less concordant with responses to real-world cues. The present study provides additional longitudinal evidence that laboratory models of stress are useful to understand the role of stress in real-world smoking behavior.
The association between acute stress reactivity and stress-induced smoking during a quit attempt highlights the importance of targeting stress management in interventions for smoking cessation. In our mediation model, acute stress reactivity accounted for the association between elevated basal levels of distress and stress-induced smoking, indicating that stress management may be a particularly important intervention target for smokers with general mood and anxiety symptoms. This study contributes to the evidence supporting the predictive power of laboratory stress reactivity, and further research should continue to use this efficient paradigm while testing its real-world applications.
Footnotes
This work was supported by National Institutes of Health Grants R34 DA031327 and R21AA020955 (to Joel Erblich, principal investigator).
References
- Al’Absi M., Hatsukami D., Davis G. L. Attenuated adrenocorticotropic responses to psychological stress are associated with early smoking relapse. Psychopharmacology. 2005;181:107–117. doi: 10.1007/s00213-005-2225-3. doi:10.1007/s00213-005-2225-3. [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. 4th ed. Washington, DC: Author; 1994. Diagnostic and statistical manual of mental disorders. [Google Scholar]
- Anderson S. M., Brunzell D. H. Low dose nicotine and antagonism of β2 subunit containing nicotinic acetylcholine receptors have similar effects on affective behavior in mice. PLoS ONE. 2012;7(11):e48665. doi: 10.1371/journal.pone.0048665. doi:10.1371/journal.pone.0048665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andreu Y., Galdón M. J., Dura E., Ferrando M., Murgui S., García A., Ibáñez E. Psychometric properties of the Brief Symptoms Inventory-18 (BSI-18) in a Spanish sample of outpatients with psychiatric disorders. Psicothema. 2008;20:844–850. [PubMed] [Google Scholar]
- Back S. E., Hartwell K., DeSantis S. M., Saladin M., McRae-Clark A. L., Price K. L., Brady K. T. Reactivity to laboratory stress provocation predicts relapse to cocaine. Drug and Alcohol Dependence. 2010;106:21–27. doi: 10.1016/j.drugalcdep.2009.07.016. doi:10.1016/j.drugalcdep.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biener L., Abrams D. B. The Contemplation Ladder: Validation of a measure of readiness to consider smoking cessation. Health Psychology. 1991;10:360–365. doi: 10.1037//0278-6133.10.5.360. doi:10.1037/0278-6133.10.5.360. [DOI] [PubMed] [Google Scholar]
- Bond A., Lader M. The use of analogue scales in rating subjective feelings. British Journal of Medical Psychology. 1974;47:211–218. doi:10.1111/j.2044-8341.1974.tb02285.x. [Google Scholar]
- Boyko E. J., Trone D. W., Peterson A. V, Jacobson I. G., Littman A. J., Maynard C., Bricker J. B. Longitudinal investigation of smoking initiation and relapse among younger and older US military personnel. American Journal of Public Health. 2015;105:1220–1229. doi: 10.2105/AJPH.2014.302538. doi:10.2105/AJPH.2014.302538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breslau N., Kilbey M. M., Andreski P. Nicotine withdrawal symptoms and psychiatric disorders: Findings from an epidemiologic study of young adults. American Journal of Psychiatry. 1992;149:464–469. doi: 10.1176/ajp.149.4.464. doi:10.1176/ajp.149.4.464. [DOI] [PubMed] [Google Scholar]
- Brown R. A., Lejuez C. W., Strong D. R., Kahler C. W., Zvolensky M. J., Carpenter L. L., Price L. H. A prospective examination of distress tolerance and early smoking lapse in adult self-quitters. Nicotine & Tobacco Research. 2009;11:493–502. doi: 10.1093/ntr/ntp041. doi:10.1093/ntr/ntp041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchmann A. F., Laucht M., Schmid B., Wiedemann K., Mann K., Zimmermann U. S. Cigarette craving increases after a psychosocial stress test and is related to cortisol stress response but not to dependence scores in daily smokers. Journal of Psychopharmacology. 2010;24:247–255. doi: 10.1177/0269881108095716. doi:10.1177/0269881108095716. [DOI] [PubMed] [Google Scholar]
- Calhoun P. S., Dennis M. F., Beckham J. C. Emotional reactivity to trauma stimuli and duration of past smoking cessation attempts in smokers with posttraumatic stress disorder. Experimental and Clinical Psychopharmacology. 2007;15:256–263. doi: 10.1037/1064-1297.15.3.256. doi:10.1037/1064-1297.15.3.256. [DOI] [PubMed] [Google Scholar]
- Cella D. F., Perry S. W. Reliability and concurrent validity of three visual-analogue mood scales. Psychological Reports. 1986;59:827–833. doi: 10.2466/pr0.1986.59.2.827. doi:10.2466/pr0.1986.59.2.827. [DOI] [PubMed] [Google Scholar]
- Cohen S., Lichtenstein E. Perceived stress, quitting smoking, and smoking relapse. Health Psychology. 1990;9:466–478. doi: 10.1037//0278-6133.9.4.466. doi:10.1037/0278-6133.9.4.466. [DOI] [PubMed] [Google Scholar]
- Colamussi L., Bovbjerg D. H., Erblich J. Stress- and cue-induced cigarette craving: Effects of a family history of smoking. Drug and Alcohol Dependence. 2007;88:251–258. doi: 10.1016/j.drugalcdep.2006.11.006. doi:10.1016/j.drugalcdep.2006.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper J., Borland R., McKee S. A., Yong H. H., Dugué P. A. Depression motivates quit attempts but predicts relapse: Differential findings for gender from the International Tobacco Control Study. Addiction. 2016;111:1438–1447. doi: 10.1111/add.13290. doi:10.1111/add.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. C., Zautra A. J., Wolf L. D., Tennen H., Yeung E. W. Mindfulness and cognitive-behavioral interventions for chronic pain: Differential effects on daily pain reactivity and stress reactivity. Journal of Consulting and Clinical Psychology. 2015;83:24–35. doi: 10.1037/a0038200. doi:10.1037/a0038200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- de Brouwer S. J. M., Kraaimaat F. W., Sweep F. C. J., Donders R. T., Eijsbouts A., van Koulil S., Evers A. W. M. Psychophysiological responses to stress after stress management training in patients with rheumatoid arthritis. PLoS ONE. 2011;6(12):e27432. doi: 10.1371/journal.pone.0027432. doi:10.1371/journal.pone.0027432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derogatis L. R. San Antonio, TX: Pearson; 2001. Brief symptom inventory (BSI) 18: Administration, scoring, and procedures manual. [Google Scholar]
- Erblich J., Bovbjerg D. H. In vivo versus imaginal smoking cue exposures: Is seeing believing? Experimental and Clinical Psychopharmacology. 2004;12:208–215. doi: 10.1037/1064-1297.12.3.208. doi:10.1037/1064-1297.12.3.208. [DOI] [PubMed] [Google Scholar]
- Erblich J., Lerman C., Self D. W., Diaz G. A., Bovbjerg D. H. Effects of dopamine D2 receptor (DRD2) and transporter (SLC6A3) polymorphisms on smoking cue-induced cigarette craving among African-American smokers. Molecular Psychiatry. 2005;10:407–414. doi: 10.1038/sj.mp.4001588. doi:10.1038/sj.mp.4001588. [DOI] [PubMed] [Google Scholar]
- Erblich J., Michalowski A. Impulsivity moderates the relationship between previous quit failure and cue-induced craving. Addictive Behaviors. 2015;51:7–11. doi: 10.1016/j.addbeh.2015.06.044. doi:10.1016/j.addbeh.2015.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Falba T., Teng H. M., Sindelar J. L., Gallo W.T. The effect of involuntary job loss on smoking intensity and relapse. Addiction. 2005;100:1330–1339. doi: 10.1111/j.1360-0443.2005.01150.x. doi:10.1111/j.1360-0443.2005.01150.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferguson S. G., Shiffman S. Effect of high-dose nicotine patch on craving and negative affect leading up to lapse episodes. Psychopharmacology. 2014;231:2595–2602. doi: 10.1007/s00213-013-3429-6. doi:10.1007/s00213-013-3429-6. [DOI] [PubMed] [Google Scholar]
- Gwynn R. C., McQuistion H. L., McVeigh K. H., Garg R. K., Frieden T. R., Thorpe L. E. Prevalence, diagnosis, and treatment of depression and generalized anxiety disorder in a diverse urban community. Psychiatric Services. 2008;59:641–647. doi: 10.1176/ps.2008.59.6.641. doi:10.1176/ps.2008.59.6.641. [DOI] [PubMed] [Google Scholar]
- Hayes A. F. New York, NY: Guilford Press; 2013. Introduction to mediation, moderation, and conditional process analysis. [Google Scholar]
- Heatherton T. F., Kozlowski L. T., Frecker R. C., Fagerström K. O. The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. doi:10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hughes J. R. Motivating and helping smokers to stop smoking. Journal of General Internal Medicine. 2003;18:1053–1057. doi: 10.1111/j.1525-1497.2003.20640.x. doi:10.1111/j.1525-1497.2003.20640.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kowalkowski M. A., Goltz H. H., Petersen N. J., Amiel G. E., Lerner S. P., Latini D. M. Educational opportunities in bladder cancer: Increasing cystoscopic adherence and the availability of smoking-cessation programs. Journal of Cancer Education. 2014;29:739–745. doi: 10.1007/s13187-014-0649-3. doi:10.1007/s13187-014-0649-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krystal J. H., Seibyl J. P., Price L. H., Woods S. W, Heninger G. R., Aghajanian G. K., Charney D. S. m-Chlorophenylpiperazine effects in neuroleptic-free schizophrenic patients. Evidence implicating serotonergic systems in the positive symptoms of schizophrenia. Archives of General Psychiatry. 1993;50:624–635. doi: 10.1001/archpsyc.1993.01820200034004. doi:10.1001/archpsyc.1993.01820200034004. [DOI] [PubMed] [Google Scholar]
- Leventhal A. M., Zvolensky M. J. Anxiety, depression, and cigarette smoking: A transdiagnostic vulnerability framework to understanding emotion-smoking comorbidity. Psychological Bulletin. 2015;141:176–212. doi: 10.1037/bul0000003. doi:10.1037/bul0000003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linares Scott T. J., Heil S. H., Higgins S. T., Badger G. J., Bernstein I. M. Depressive symptoms predict smoking status among pregnant women. Addictive Behaviors. 2009;34:705–708. doi: 10.1016/j.addbeh.2009.04.003. doi:10.1016/j.addbeh.2009.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martín-García E., Barbano M. F., Galeote L., Maldonado R. New operant model of nicotine-seeking behaviour in mice. International Journal of Neuropsychopharmacology. 2009;12:343–356. doi: 10.1017/S1461145708009279. doi:10.1017/S1461145708009279. [DOI] [PubMed] [Google Scholar]
- Notley C., Blyth A., Craig J., Edwards A., Holland R. Postpartum smoking relapse—a thematic synthesis of qualitative studies. Addiction. 2015;110:1712–1723. doi: 10.1111/add.13062. doi:10.1111/add.13062. [DOI] [PubMed] [Google Scholar]
- Perkins K. A. Does smoking cue-induced craving tell us anything important about nicotine dependence? Addiction. 2009;104:1610–1616. doi: 10.1111/j.1360-0443.2009.02550.x. doi:10.1111/j.1360-0443.2009.02550.x. [DOI] [PubMed] [Google Scholar]
- Piferi R. L., Kline K. A., Younger J., Lawler K. A. An alternative approach for achieving cardiovascular baseline: Viewing an aquatic video. International Journal of Psychophysiology. 2000;37:207–217. doi: 10.1016/s0167-8760(00)00102-1. doi:10.1016/S0167-8760(00)00102-1. [DOI] [PubMed] [Google Scholar]
- Piper M. E., Smith S. S., Schlam T. R., Fleming M. F., Bittrich A. A., Brown J. L., Baker T. B. Psychiatric disorders in smokers seeking treatment for tobacco dependence: Relations with tobacco dependence and cessation. Journal of Consulting and Clinical Psychology. 2010;78:13–23. doi: 10.1037/a0018065. doi:10.1037/a0018065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saladin M. E., Wray J. M., Carpenter M. J., McClure E. A., LaRowe S. D., Upadhyaya H. P., Gray K. M. Menstrual cycle phase effects in the gender dimorphic stress cue reactivity of smokers. Nicotine & Tobacco Research. 2015;17:607–611. doi: 10.1093/ntr/ntu203. doi:10.1093/ntr/ntu203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiftman S., Ferguson S. G., Gwaltney C. J., Balabanis M. H., Shadel W. G. Reduction of abstinence-induced withdrawal and craving using high-dose nicotine replacement therapy. Psychopharmacology. 2006;184:637–644. doi: 10.1007/s00213-005-0184-3. doi:10.1007/s00213-005-0184-3. [DOI] [PubMed] [Google Scholar]
- Shiffman S., Li X., Dunbar M. S., Tindle H. A., Scholl S. M., Ferguson S. G. Does laboratory cue reactivity correlate with real-world craving and smoking responses to cues? Drug and Alcohol Dependence. 2015;155:163–169. doi: 10.1016/j.drugalcdep.2015.07.673. doi:10.1016/j.drugalcdep.2015.07.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shuter J., Bernstein S. L., Moadel A. B. Cigarette smoking behaviors and beliefs in persons living with HIV/AIDS. American Journal of Health Behavior. 2012;36:75–85. doi: 10.5993/ajhb.36.1.8. doi:10.5993/AJHB.36.1.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha R. Modeling stress and drug craving in the laboratory: Implications for addiction treatment development. Addiction Biology. 2009;14:84–98. doi: 10.1111/j.1369-1600.2008.00134.x. doi:10.1111/j.1369-1600.2008.00134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- U.S. Department of Health and Human Services. Rockville, MD: Office of the Surgeon General; 2014. The Health Consequences of Smoking—50 Years of Progress: A Report of the Surgeon General, 2014. Retrieved from https://www.surgeongeneral.gov/library/reports/50-years-of-progress. [Google Scholar]
- Wen K. Y., Miller S. M., Roussi P., Belton T. D., Baman J., Kilby L., Hernandez E. A content analysis of self-reported barriers and facilitators to preventing postpartum smoking relapse among a sample of current and former smokers in an underserved population. Health Education Research. 2015;30:140–151. doi: 10.1093/her/cyu048. doi:10.1093/her/cyu048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wright S. W., Lindsell C. J., Hinckley W. R., Williams A., Holland C., Lewis C. H., Heimburger G. High fidelity medical simulation in the difficult environment of a helicopter: Feasibility, self-efficacy and cost. BMC Medical Education. 2006;6:49. doi: 10.1186/1472-6920-6-49. doi:10.1186/1472-6920-6-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvolensky M. J., Bakhshaie J., Sheffer C., Perez A., Goodwin R. D. Major depressive disorder and smoking relapse among adults in the United States: A 10-year, prospective investigation. Psychiatry Research. 2015;226:73–77. doi: 10.1016/j.psychres.2014.11.064. doi:10.1016/j.psychres.2014.11.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zvolensky M. J., Gibson L. E., Vujanovic A. A., Gregor K., Bernstein A., Kahler C., Feldner M. T. Impact of posttraumatic stress disorder on early smoking lapse and relapse during a self-guided quit attempt among community-recruited daily smokers. Nicotine & Tobacco Research. 2008;10:1415–1427. doi: 10.1080/14622200802238951. doi:10.1080/14622200802238951. [DOI] [PubMed] [Google Scholar]
- Zvolensky M. J., Johnson K. A., Leyro T. M., Hogan J., Tursi L. Quit-attempt history: Relation to current levels of emotional vulnerability among adult cigarette users. Journal of Studies on Alcohol and Drugs. 2009;70:551–554. doi: 10.15288/jsad.2009.70.551. doi:10.15288/jsad.2009.70.551. [DOI] [PubMed] [Google Scholar]