Abstract
Objective
To characterize the adoption of antiobesity pharmacotherapies, as compared with that of the newest antidiabetes pharmacotherapy, subtype 2 sodium-glucose transport protein inhibitors (SGLT2s), among prescribers in the United States.
Methods
A retrospective analysis of 2012 to 2015 data extracted from the IMS Health National Prescription Audit™ and Xponent™ assessed adoption rates of antiobesity pharmacotherapies and SGLT2s.
Results
The number of dispensed antidiabetes prescriptions was 15 times the number of dispensed antiobesity prescriptions. The antiobesity market share was: 74.0% phentermine, 18.6% new antiobesity pharmacotherapies. The mean increase in prescriptions/month were: 25,259 for SGLT2s, 5,154 for new antiobesity pharmacotherapies, and 2,718 for phentermine. Medical specialties prescribing the majority of the analysis medications were Family Medicine/General Practice and Internal Medicine. Endocrinology had the highest prevalence of prescribers of any subspecialty.
Conclusions
The adoption rate of SGLT2s was nearly exponential, while the adoption rate of new antiobesity pharmacotherapies was linear. Considering the relative prevalence of obesity to diabetes and that obesity is a major cause of diabetes, these results are paradoxical and suggest systematic barriers against the prescribing of antiobesity pharmacotherapies. The under-prescribing of antiobesity pharmacotherapies is widely acknowledged, but this is the first prescription data of these new medications to demonstrate its extent in the United States.
Introduction
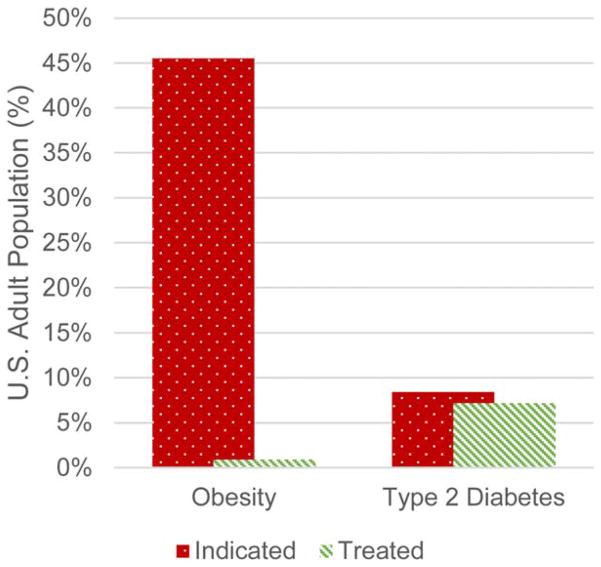
In the United States, more than two thirds (69%) of adults are overweight, more than one third have obesity (35%), and 6.4% have extreme obesity (1). Obesity is a major risk factor for a constellation of diseases including type 2 diabetes and cardiovascular disease (2). Antiobesity pharmacotherapy is indicated as an adjunct to reduced-calorie diet and increased physical activity in adults with a body mass index ≥30 or ≥27 kg/m2 with hypertension, type 2 diabetes, or dyslipidemia. Just under half (46%) of adults in the United States fit the criteria for use of antiobesity pharmacotherapy, but only 2% of those receive such treatment (3,4). This is in sharp contrast to the 8.4% of adults in the United States diagnosed with diabetes (5), with 86% of those receiving antidiabetes pharmacotherapy (6) (Figure 1).
Figure 1.
Prevalence of obesity and diabetes and pharmacotherapy utilization of antiobesity pharmacotherapies and antidiabetes pharmacotherapies. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
It has taken the past 20 years for the world’s leading public health and medical organizations to recognize obesity as a disease. Beginning with the Institute of Medicine in 1995, which then recognized obesity as a disease and recommended use of pharmacotherapy in treatment of the disease (7). In 1997, the World Health Organization followed suit (8), continuing with the National Institutes of Health in 1998 (9) and in 2000 (10), the American College of Physicians in 2005 (11), the American Association of Clinical Endocrinologists and the American College of Endocrinologists in 2012 (12), and the American Medical Association in 2013 (13). In June 2013, the American Heart Association, the American College of Cardiology, and The Obesity Society issued joint guidelines for the management of overweight and obesity in adults (14). In February 2015, The Endocrine Society issued clinical practice guidelines on the pharmacological management of obesity (15). The declaration by each organization included the recommendation of considering antiobesity pharmacotherapy as an adjunct to behavioral management in appropriate individuals.
The most commonly prescribed antiobesity pharmacotherapies in the United States are: phentermine, naltrexone +bupropion, lorcaserin, and topiramate +phentermine. Phentermine was FDA approved in 1959 for short-term treatment of obesity (3 months). Topiramate + phentermine and lorcaserin were FDA approved in 2012 and naltrexone +bupropion in 2014. Topiramate +phentermine, lorcaserin, and naltrexone +bupropion (new antiobesity pharmacotherapies) were the first medications in 13 years to receive FDA approval for long-term management of obesity in adults. The newest class of antidiabetes pharmacotherapy, subtype 2 sodium-glucose transport protein inhibitors (SGLT2s), served as comparators in this analysis due to their similarly timed commercial availability as the new antiobesity pharmacotherapies and their midrange placement in the American Association of Clinical Endocrinologists/American College of Endocrinologists Glycemic Control Algorithm (16). The SGLT2s include canagliflozin, dapagliflozin, and empagliflozin and were FDA approved to treat adults with type 2 diabetes mellitus in 2013 and 2014.
Methods
This study was approved, with the requirement of informed consent waived, by the Weill Cornell Medical College Institutional Review Board. This is a retrospective analysis, 2012–2015, of deidentified extracted data from the IMS Health National Prescription Audit™ and Xponent™ databases. IMS Health is the industry standard source of national prescription activity for all pharmaceutical products in the United States. The National Prescription Audit is a database of all dispensed prescription information from 38,939 U.S. pharmacies (retail, long-term care, and mail service), which represents 70% of the dispensed outpatient prescription volume in the United States (17). The un-captured prescription volume is projected to 100% by proprietary IMS Health methodologies. Xponent contains demographic and dispensed prescription information for U.S. prescribers. Prescriber demographic information is obtained by IMS Health from the American Medical Association’s Physician Professional Data, which are based on clinician self-selected responses.
Wholesale acquisition costs for each medication were obtained from the Wolters Kluwer Clinical Drug Information application, Medi-Span Price Rx™. Wholesale acquisition costs are the manufacturer’s list price for their drug to wholesalers or direct purchasers in the United States.
Total numbers of active physicians, active physicians per specialty, and active physicians per state were obtained from the Association of American Medical Colleges’ 2014 Physician Specialty Data Book (18) and State Physician Workforce Data Book 2013 (19). Both publications source their data from the same source as IMS Health, the American Medical Association’s Physician Masterfile, which is informed by the American Medical Association’s Census of Physicians and National Graduate Medical Education Census.
For this analysis, medical specialties in Xponent were grouped according to the groupings defined in the Association of American Medical Colleges’ publications (e.g., Endocrinology, Diabetes & Metabolism, and Diabetes were group together as Endocrinology). Prescriber specialties were further grouped into physicians and non-physicians (e.g., nurse practitioner, physician assistant, chiropractor, dentist, podiatrist). The continental states were grouped into regions according to the U.S. Census Bureau (20).
This analysis assessed the adoption rate of antiobesity pharmacotherapies and SGLT2s by evaluating the change in mean prescriptions per month over the analysis period using univariate linear regressions. Three separate univariate linear regressions were performed to model prescriptions dispensed over time for new antiobesity pharmacotherapies, SGLT2s, and phentermine, respectively. Time was divided into monthly intervals for each pharmacotherapy group, starting from the month of inception for the new pharmacotherapy groups, until August 2015 (i.e., September 2012 to August 2015 for new antiobesity pharmacotherapies, April 2013 to August 2015 for SGLT2s, and January 2012 to August 2015 for phentermine). Estimates along with 95% confidence intervals (CI) for the mean change in prescriptions dispensed per 1-month increase in calendar time, for each of the three pharmacotherapy groups, were obtained from the regressions and used for descriptive purposes.
For each pharmacotherapy group, the ratio of new to continuing dispensed prescription volumes was descriptively compared. Prescriber groups were compared by descriptive proportions according to prescription volumes, medical specialty, geographic region, and prescriber-drug overlap.
Results
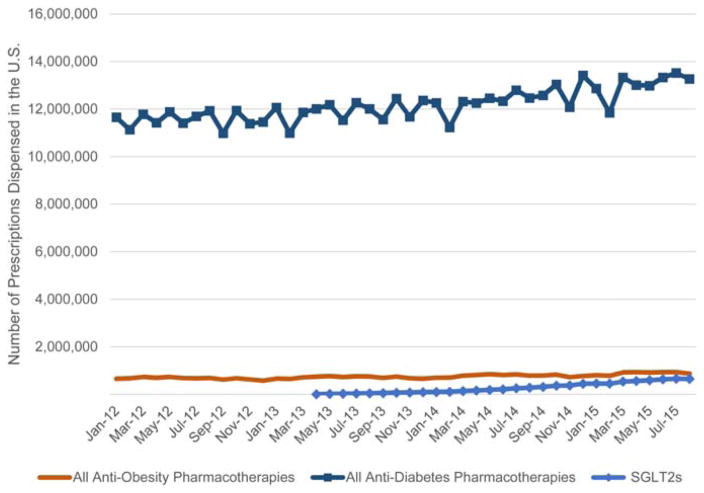
As of August 2015, the number of dispensed antidiabetes prescriptions, excluding insulin, was 15 times the number of dispensed antiobesity prescriptions. SGLT2s comprised 4.9% of the antidiabetes pharmacotherapy market share, which was equivalent to three-quarters of all dispensed antiobesity prescriptions (Figure 2).
Figure 2.
Volumes of dispensed prescriptions of all antiobesity pharmacotherapies, all antidiabetes pharmacotherapies (excluding insulin), and SGLT2s. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
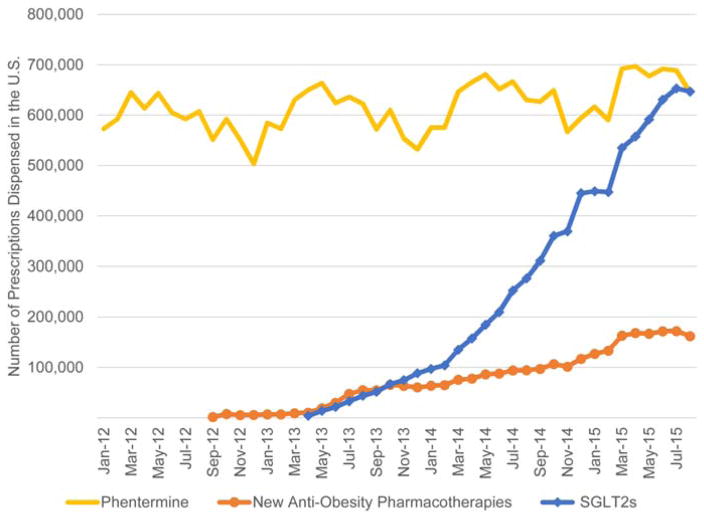
The antiobesity pharmacotherapy market share was: 74.0% phentermine and 18.6% new antiobesity pharmacotherapies (Figure 3). The mean increases in prescriptions per month were: 25,259 for SGLT2s (95% CI 23,133–27,383 P <0.0001), 5,154 for new antiobesity pharmacotherapies (95% CI 4,800–5,507 P <0.0001), and 2,718 for phentermine (95% CI 1,345–4,089 P = 0.0003) (Figure 3, Table 1). As of August 2015, for each new prescription dispensed there were 5.4 continuing prescriptions dispensed for phentermine, 4.6 for SGLT2s, and 1.4 for new antiobesity pharmacotherapies.
Figure 3.
Volumes of dispensed prescriptions of phentermine, new antiobesity pharmacotherapies, and SGLT2s. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
TABLE 1.
Output from separate univariate linear regressions to estimate mean change in prescriptions per month
| Estimate | Std. error | 95% CI | t value | P (>|t|) | ||
|---|---|---|---|---|---|---|
| SGLT2s | Intercept | −109,595.00 | 17,788.00 | (−146,093.58 to −73,097.20) | −6.16 | |
| Month | 25,259.00 | 1,036.00 | (23,133.65 to 27,383.66) | 24.39 | <0.0001 | |
| New antiobesity | Intercept | −18,225.60 | 3,689.90 | (−25,724.34 to −10,726.84) | −4.94 | |
| Month | 5,153.90 | 173.90 | (4,800.51 to 5,507.37) | 29.64 | <0.0001 | |
| Phentermine | Intercept | 568,472.80 | 14,325.70 | (539,359.45 to 597,586.08) | 39.68 | |
| Month | 2,717.60 | 675.20 | (1,345.47 to 4,089.79) | 4.03 | 0.0003 |
Of the 900,000 prescribers in the IMS Health Xponent database, from 2012 to 2015, 173,882 (19%) prescribed phentermine, 104,612 (12%) prescribed a new antiobesity pharmacotherapy, and 102,002 (11%) prescribed an SGLT2. Of the 829,962 active physicians in the United States, 129,414 (16%) prescribed phentermine, 79,624 (10%) prescribed a new antiobesity pharmacotherapy, and 70,898 (9%) prescribed an SGLT2 (Table 2).
TABLE 2.
Prescriber groups, compared by descriptive proportions according to prescription volumes, specialty, region, and prescriber-drug overlap
| Group | Phentermine
|
New antiobesity pharmacotherapies
|
SGLT2s
|
||||||
|---|---|---|---|---|---|---|---|---|---|
| A | B | C | A | B | C | A | B | C | |
| Prescribers in Xponent™ | 19% | 100% | 100% | 12% | 100% | 100% | 11% | 100% | 100% |
| Active physicians in the U.S. | 16% | 74% | 81% | 10% | 76% | 86% | 9% | 70% | 83% |
| Primary specialty | |||||||||
| Family Medicine/General Practice | 48% | 30% | 42% | 32% | 34% | 34% | 32% | 34% | 33% |
| Internal Medicine | 27% | 18% | 19% | 21% | 22% | 27% | 22% | 24% | 25% |
| Endocrinology | 37% | 1% | 2% | 47% | 3% | 13% | 64% | 4% | 22% |
| Obstetrics/Gynecology | 25% | 6% | 7% | 12% | 5% | 4% | |||
| Psychiatry | 10% | 2% | 1% | 4% | 2% | 1% | |||
| Emergency Medicine | 8% | 2% | 1% | 2% | 1% | <1% | 2% | 1% | <1% |
| General Surgery | 11% | 2% | 2% | 4% | 1% | 1% | |||
| Cardiovascular Diseases | 7% | 1% | 1% | 5% | 1% | <1% | |||
| Internal Medicine/Pediatrics | 14% | 1% | <1% | ||||||
| Geriatric Medicine | 13% | 1% | <1% | ||||||
| Pediatrics | 4% | 1% | 1% | ||||||
| Nephrology | 4% | <1% | <1% | ||||||
| Unavailable | N/A | 2% | <1% | N/A | 2% | <1% | N/A | 10% | 3% |
| Physicians by region | |||||||||
| South | 20% | 43% | 46% | 13% | 44% | 46% | 11% | 42% | 41% |
| Midwest | 16% | 22% | 16% | 10% | 21% | 16% | 9% | 22% | 16% |
| West | 14% | 20% | 10% | 8% | 17% | 11% | 6% | 16% | 13% |
| Northeast | 10% | 14% | 7% | 7% | 17% | 12% | 7% | 19% | 12% |
| Pacific | 12% | 1% | <1% | 7% | <1% | <1% | 8% | 1% | <1% |
| Unavailable | N/A | <1% | <1% | N/A | <1% | <1% | N/A | <1% | <1% |
| Prescriber-drug overlap | |||||||||
| Phentermine | 69% | 57% | |||||||
| New antiobesity pharmacotherapies | 41% | 51% | |||||||
| SGLT2s | 33% | 50% | |||||||
Prescriber-drug overlap represents the percentage of prescribers of the column drug category who also prescribed the row drug category.
A: % of group who prescribed drug(s) = drug prescribers from group/active physicians in group. B: % of drug prescribers from group = drug prescribers from group/all prescribers of drug(s). C: % of drug prescriptions in the U.S. from group = drug prescriptions from group/all prescriptions of drug(s). Specialties reported represent the top eight most frequent prescribing specialties per drug category.
The Medicine subspecialty with the highest prevalence of prescribers of all drug categories is Endocrinology (n = 6,519), with 37% (n = 2,388) having prescribed phentermine, 47% (n = 3,040) having prescribed a new antiobesity pharmacotherapy, and 64% (n = 4,180) having prescribed an SGLT2 (Table 2). Further, endocrinologists prescribed the new antiobesity pharmacotherapies and SGLT2s at a higher rate than any other specialty (Table 2).
Medical specialties prescribing the majority of each of the antiobesity pharmacotherapies and the SGLT2s were Family Medicine/General Practice and Internal Medicine (Table 2). The top medical specialties common to all three drug categories are: Family Medicine/General Practice, Internal Medicine, Endocrinology, and Emergency Medicine. The top medical specialties common to the antiobesity drug categories, but not SGLT2s are: Obstetrics/Gynecology, Psychiatry, and General Surgery. The top medical specialties prescribing SGLT2s, but neither antiobesity drug category are: Internal Medicine/Pediatrics, Geriatric Medicine, and Nephrology. The Cardiovascular Diseases specialty is common among the top medical specialties prescribing new antiobesity pharmacotherapies and SGLT2s, but not phentermine. Pediatrics is among the top medical specialties prescribing phentermine, but neither of the other drug categories.
The geographic region with the highest prevalence of physician prescribers (Table 2), highest percent of physician prescribers, and highest percent of dispensed prescriptions in each drug category is the South, followed by the Midwest (Table 2).
The highest percentage of overlap-prescribing are of new antiobesity pharmacotherapy prescribers: 69% have also prescribed phentermine, while 41% of phentermine prescribers have also prescribed a new antiobesity pharmacotherapy. Of SGLT2 prescribers, 57% have also prescribed phentermine, while the inverse represents the lowest percentage overlap: 33% of phentermine prescribers have prescribed an SGLT2. Fifty percent of prescribers of a new antiobesity pharmacotherapy have prescribed an SGLT2, while 51% of prescribers of an SGLT2 have prescribed a new antiobesity pharmacotherapy (Table 2).
The wholesale acquisition costs of a 30-day supply of the new antiobesity pharmacotherapies are $199.50, with the exception of the two lower dose strengths of topiramate +phentermine, which are $170.65 and $165.20. The average wholesale acquisition cost of generic phentermine is $37.14. The wholesale acquisition costs of the SGLT2s range from $342.82 to $342.94.
Discussion
The adoption rate of SGLT2s was nearly exponential, while the adoption rate of new antiobesity pharmacotherapies was linear. Echoing the findings of Xia et al., the geographic distribution of dispensed antiobesity pharmacotherapies (4) and SGLT2s match the geographic prevalence of obesity (21) and diabetes (22). The specialties that are expected to treat obesity and diabetes (Family Medicine/General Practice, Internal Medicine, and Endocrinology) are the top prescribing medical specialties. That endocrinologists prescribe the new antiobesity pharmacotherapies and SGLT2s in the highest proportion of any Medicine subspecialty reflects that they are operating within their area of expertise. Endocrinologists, however, only comprise 0.8% of active physicians in the United States, which is not sufficient to significantly impact the obesity epidemic (18).
The similar proportions of physicians prescribing both antiobesity pharmacotherapies and SGLT2s indicate that there is an awareness among prescribers of antiobesity pharmacotherapies and a comfort with using them. The disparity in dispensed prescription volumes and in the ratio of new prescriptions to continuing prescriptions between antiobesity pharmacotherapies and SGLT2s indicate that there are barriers to antiobesity pharmacotherapy initiation and long-term adherence. This is likely the result of multiple factors stemming from the delayed recognition of obesity as a disease by leading public health and medical organizations.
An analysis of the National Ambulatory Medical Care Survey and the Behavioral Risk Factor Surveillance System indicated that less than half of adults with obesity are being advised to lose weight by healthcare professionals (23). Cited barriers include physician, patient, and medical system factors: lack of reimbursement, limited time during office visits, lack of training in counseling, competing demands, low confidence in the ability to treat and change patient behaviors, limited resources, the perception that patients are not motivated, and a paucity of proven and effective interventions to treat obesity (23).
The disparate study drug discontinuation rates in clinical trials of antiobesity pharmacotherapies [33% for phentermine (24), 38% for topiramate +phentermine (25), 44% for lorcaserin (26), 46% for naltrexone +bupropion (27)] and SGLT2s [12% for canagliflozin (28), 14% for dapagliflozin (29), 9% for empagliflozin (30)] illustrate long-term adherence barriers with antiobesity pharmacotherapy. The most common reasons for discontinuation were: non-compliance, lost to follow-up, lack of efficacy, and adverse events.
Out-of-pocket cost to patients is a likely a significant barrier to antiobesity pharmacotherapy initiation and long-term adherence. While new antiobesity pharmacotherapy wholesale acquisition costs are somewhat lower than that of SGLT2s, the lack of insurance coverage makes antiobesity pharmacotherapy far less affordable for most Americans. Antiobesity pharmacotherapies are either excluded from insurance formularies, thus limiting to those with the means of self-pay, or require prior authorization (4), thus requiring significantly more physician and office staff time to prescribe. This is in contrast to SGLT2s, which are included in insurance formularies and, depending on the plan, are categorized as low as Tier 1, i.e., having the lowest copay available (31,32). As of 2013, only 14 state Medicaid programs provided coverage for at least one antiobesity pharmacotherapy and all required prior authorization. Antiobesity pharmacotherapy is specifically excluded from Medicare Part D (4).
Effectiveness expectations for antiobesity pharmacotherapy by patients and physicians may exceed the clinically meaningful level, which is considered ≥5% weight loss (13), and may be a long-term adherence barrier. For a drug to receive FDA approval for the management of obesity, it must meet at least one of the following efficacy criteria: (1) ≥5% difference in mean 1-year weight loss between active-treated and placebo groups or (2) ≥35% subjects in the active-treated group lose ≥5% of body weight after 1 year. In clinical trials, mean body weight change observed in those completing the trials hovers just above the clinically meaningful threshold: −7% after 6 months with phentermine (24); −10% on the low dose and −12% on the high dose after 1 year with topiramate +phentermine (25); −8% after 1 year with lorcaserin (26); and −8% after 1 year with naltrexone +bupropion (27). Comparatively, the mean 6-month glycated hemoglobin (HbA1c) reductions and body weight changes observed in the SGLT2 clinical trials were: −1.16% Hba1c and −3.3% body weight with canagliflozin (28), −0.89% HbA1c and −4.3% body weight with dapagliflozin (29), −0.78% HbA1c and −2.48% body weight with empagliflozin (30). Achieving 5% to 10% weight loss improves insulin sensitivity and β-cell function (33) and is associated with an increased likelihood of achieving a −0.5% reduction in HbA1c in overweight adults with type 2 diabetes (odds ratio 3.52) (34). The efficacy on glycemic control in overweight adults with type 2 diabetes has been studied as a secondary end point in clinical trials of two of the antiobesity pharmacotherapies. In those who completed the trials, HbA1c reductions and body weight changes observed were: −1.1% HbA1c and −5.8% body weight after 1 year with lorcaserin (35) and −0.4% HbA1c and −9.0% body weight after 2 years with topiramate + phentermine (36).
The reluctance of prescribers to adopt antiobesity pharmacotherapy until the results of long-term outcome trials are known may be based on the history of antiobesity pharmacotherapies being removed from the market by the FDA due to post-approval safety concerns, i.e., fenfluramine, rimonabant, and sibutramine (37). Cardiovascular outcome trials of lorcaserin and naltrexone +bupropion are ongoing and in the planning stages for topiramate +phentermine. Results of these trials will be the first to show the effect of antiobesity pharmacotherapy on hard end points and, if positive, will likely lead to an increase in their adoption. The cardiovascular outcome trial of empagliflozin showed that patients who took empagliflozin had a lower rate of cardiovascular events and mortality, as compared with those taking placebo (38). The cardiovascular outcome trials of canagliflozin and dapagliflozin are ongoing.
An analysis of the National Health and Nutrition Examination Survey reported that the weight loss strategy most associated with a self-reported body weight loss of ≥10% in the prior year, among adults with obesity, was antiobesity pharmacotherapy (odds ratio 2.05). Yet the analysis also indicated that antiobesity pharmacotherapy was reported as the least utilized weight loss strategy (3.5%) among adults with obesity who had attempted to lose weight in the prior year (39).
The fact that phentermine, which was FDA approved in 1959 for short-term use and with little clinical trial data, remains the most frequently prescribed antiobesity pharmacotherapy, by a large margin, is likely due to its low cost and its familiarity among patients and prescribers. Phentermine’s high proportion of continuing prescriptions to new prescriptions indicates that it is often being prescribed off-label, i.e., for longer than 3 months (15,40). Ironically, the very low continuing prescription to new prescription ratio of new antiobesity pharmacotherapies, which are approved for long-term use, indicate that they are being used for the short term. The cyclical nadirs in the volume of dispensed phentermine prescriptions occurring each December, followed immediately by a dramatic uptick, are likely explained by the New Year’s resolution phenomenon, indicating that phentermine prescribing, and ostensibly all antiobesity pharmacotherapy prescribing, is patient driven.
Conclusion
This analysis captures the early phase adoption and prescribing patterns of the first medications in 13 years to receive FDA approval for long-term management of obesity. Considering the relative prevalence of obesity to diabetes and that obesity is a major cause of diabetes, these results are paradoxical, suggest systematic barriers against the prescribing of antiobesity pharmacotherapies, and highlight an unmet need. The under-prescribing of antiobesity pharmacotherapies is widely acknowledged, but this is the first prescription data on these new medications to demonstrate its extent in the United States.
Acknowledgments
Funding agencies: The statistical analysis portion of this work was supported by grant UL1TR000457 of the Clinical and Translational Science Center at Weill Cornell Medical College. This work was supported by material donated by VIVUS, Inc.
We gratefully acknowledge the contributions of Michael Chen, MBA, for facilitating this project from its inception and George Thomas, Jr., MD, for providing guidance and clinical insight. We also especially acknowledge the statistical guidance of Paul Christos, DrPH, MS. The statements, findings, conclusions, views, and opinions contained and expressed in this abstract are based in part on data obtained under license from the following IMS Health Incorporated information services: National Prescription Audit™ and Xponent™, 2012–2015. All Rights Reserved. The statements, findings, conclusions, views, and opinions contained and expressed herein are not necessarily those of IMS Health Incorporated or any of its affiliated or subsidiary entities.
Footnotes
Disclosure: SR was an employee of VIVUS, Inc., which manufactures topiramate +phentermine, during the data collection, extraction, analysis, and writing of this manuscript. He is currently an employee of Vallytics Global, LLP, San Jose, California. LJA is on the advisory board of Eisai, Gelesis, GI Dynamics, Jamieson Labs, Janssen, JOVIA Health, Novo Nordisk A/S, Pfizer, Takeda, and VIVUS; is a shareholder of Gelesis, Jamieson Labs, Myos Corp., and Zafgen; is on the board of directors of Jamieson Labs and Myos Corp.; and receives research funding from Aspire Bariatrics and Eisai. The other authors declared no conflict of interest.
Author contributions: Study concept and design: CET, APS, LJA. Acquisition, analysis, or interpretation of data: All authors. Drafting of the manuscript: CET. Critical revision of the manuscript: All authors. Statistical analysis: EAM, CET. Obtained funding: CET, LJA. Administrative, technical, or material support: CET, SR. Study supervision: CET, LJA. For the purposes of this analysis, then VIVUS, Inc., employee and manuscript coauthor SR extracted and deidentified the study-specified data from the IMS Health National Prescription Audit™ and Xponent™ and the Wolters Kluwer Medi-Span Price Rx™ and provided it to the other coauthors at no cost. Other than SR’s authorship contributions, the funding agencies had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; or decision to submit the manuscript for publication.
References
- 1.Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of childhood and adult obesity in the United States, 2011–2012. JAMA. 2014;311:806–814. doi: 10.1001/jama.2014.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eckel RH, Grundy SM, Zimmet PZ. The metabolic syndrome. Lancet. 2005;365:1415–1428. doi: 10.1016/S0140-6736(05)66378-7. [DOI] [PubMed] [Google Scholar]
- 3.Samaranayake NR, Ong KL, Leung RY, Cheung BM. Management of obesity in the NHANES, 2007–2008. Ann Epidemiol. 2012;22:349–353. doi: 10.1016/j.annepidem.2012.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Xia Y, Kelton CM, Guo JJ, Bian B, Heaton PC. Treatment of obesity: Pharmacotherapy trends in the United States from 1999 to 2010. Obesity (Silver Spring) 2015;23:1721–1728. doi: 10.1002/oby.21136. [DOI] [PubMed] [Google Scholar]
- 5.National Center for Health Statistics. Health, United States, 2014. Hyattsville, MD: U.S. Department of Health and Human Services; 2015. p. 165. [Google Scholar]
- 6.Centers for Disease Control and Prevention. National Diabetes Statistics Report: Estimates of Diabetes and its Burden in the United States, 2014. Atlanta, GA: U.S. Department of Health and Human Services; 2014. p. 5. [Google Scholar]
- 7.Institute of Medicine. Weighing the Options: Criteria for Evaluating Weight-Management Programs. Washington, DC: National Academy of Sciences Press; 1995. [PubMed] [Google Scholar]
- 8.World Health Organization. Obesity: Preventing and Managing the Global Epidemic - Report of a WHO Consultation on Obesity. Geneva: WHO; 1997. [PubMed] [Google Scholar]
- 9.National Institutes of Health. Clinical Guidelines on the Identification, Evaluation, and Treatment of Overweight and Obesity in Adults–The Evidence Report. 1998. NIH Publication 98-4083. [Google Scholar]
- 10.National Institutes of Health. The Practical Guide: Identification, Evaluation and Treatment of Overweight and Obesity in Adults. 2000. NIH Publication 00-4084. [Google Scholar]
- 11.Snow V, Barry P, Fitterman N, Qaseem A, Weiss K Clinical Efficacy Assessment Subcommittee of the American College of Physicians. Pharmacologic and surgical management of obesity in primary care: a clinical practice guideline from the American College of Physicians. Ann Intern Med. 2005;142:525–531. doi: 10.7326/0003-4819-142-7-200504050-00011. [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez-Campoy JM, St Jeor ST, Castorino K, et al. Clinical practice guidelines for healthy eating for the prevention and treatment of metabolic and endocrine diseases in adults: cosponsored by the American Association of Clinical Endocrinologists/the American College of Endocrinology and the Obesity Society. Endocr Pract. 2013;19:1–82. doi: 10.4158/EP13155.GL. [DOI] [PubMed] [Google Scholar]
- 13.American Medical Association. Recognition of Obesity as a Disease. American Medical Association; 2013. Policy H-440.842. [Google Scholar]
- 14.Jensen MD, Ryan DH, Donato KA, et al. Guidelines (2013) for managing overweight and obesity in adults. Obesity. 2014;22(S2):S1–S410. doi: 10.1002/oby.20819. [DOI] [PubMed] [Google Scholar]
- 15.Apovian CM, Aronne LJ, Bessesen DH, et al. Pharmacological management of obesity: an endocrine society clinical practice guideline. J Clin Endocrinol Metab. 2015;100:342–362. doi: 10.1210/jc.2014-3415. [DOI] [PubMed] [Google Scholar]
- 16.American Association of Clinical Endocrinologists. AACE comprehensive diabetes management algorithm. Endocr Pract. 2013;19:327–336. doi: 10.4158/endp.19.2.a38267720403k242. [DOI] [PubMed] [Google Scholar]
- 17.IMS Institute for Healthcare Informatics. HSRN Data Brief: National Prescription Audit. Collegeville, PA: IMS Health; 2011. [Google Scholar]
- 18.Association of American Medical Colleges Center for Workforce Studies. 2014 Physician Specialty Data Book. Washington, DC: Association of American Medical Colleges; 2014. [Google Scholar]
- 19.Association of American Medical Colleges Center for Workforce Studies. 2013 State Physician Workforce Data Book. Washington, DC: Association of American Medical Colleges; 2013. [Google Scholar]
- 20.United States Census Bureau. [Accessed February 16, 2016];Census regions and divisions of the United States [on the Internet] http://www2.census.gov/geo/pdfs/maps-data/maps/reference/us_regdiv.pdf. Updated July 28, 2015.
- 21.National Center for Chronic Disease Prevention and Health Promotion. Obesity prevalence maps [Web page] [Accessed October 20, 2015];Centers for Disease Control and Prevention website. www.cdc.gov/obesity/data/prevalence-maps.html. Updated September 11, 2015.
- 22.Division of Diabetes Translation. Maps of trends in diagnosed diabetes. [Access October 20, 2015];Centers for Disease Control and Prevention website. 2016 Apr; [on the Internet] www.cdc.gov/diabetes/statistics/slides/maps_diabetes_trends.pdf. Updated January 2015.
- 23.Kushner RF. Tackling obesity: is primary care up to the challenge? Arch Intern Med. 2010;170:121–123. doi: 10.1001/archinternmed.2009.479. [DOI] [PubMed] [Google Scholar]
- 24.Aronne LJ, Wadden TA, Peterson C, Winslow D, Odeh S, Gadde KM. Evaluation of phentermine and topiramate versus phentermine/topiramate extended-release in obese adults. Obesity (Silver Spring) 2013;21:2163–2171. doi: 10.1002/oby.20584. [DOI] [PubMed] [Google Scholar]
- 25.Gadde KM, Allison DB, Ryan DH, et al. Effects of low-dose, controlled-release, phentermine plus topiramate combination on weight and associated comorbidities in overweight and obese adults (CONQUER): a randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377:1341–1352. doi: 10.1016/S0140-6736(11)60205-5. [DOI] [PubMed] [Google Scholar]
- 26.Aronne L, Shanahan W, Fain R, et al. Safety and efficacy of lorcaserin: a combined analysis of the BLOOM and BLOSSOM trials. Postgrad Med. 2014;126:7–18. doi: 10.3810/pgm.2014.10.2817. [DOI] [PubMed] [Google Scholar]
- 27.Apovian CM, Aronne L, Rubino D, et al. A randomized, phase 3 trial of naltrexone SR/bupropion SR on weight and obesity-related risk factors (COR-II) Obesity (Silver Spring) 2013;21:935–943. doi: 10.1002/oby.20309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stenlof K, Cefalu WT, Kim KA, et al. Efficacy and safety of canagliflozin monotherapy in subjects with type 2 diabetes mellitus inadequately controlled with diet and exercise. Diabetes Obes Metab. 2013;15:372–382. doi: 10.1111/dom.12054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ferrannini E, Ramos SJ, Salsali A, Tang W, List JF. Dapagliflozin monotherapy in type 2 diabetic patients with inadequate glycemic control by diet and exercise: a randomized, double-blind, placebo-controlled, phase 3 trial. Diabetes Care. 2010;33:2217–2224. doi: 10.2337/dc10-0612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Roden M, Weng J, Eilbracht J, et al. Empagliflozin monotherapy with sitagliptin as an active comparator in patients with type 2 diabetes: a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Diabetes Endocrinol. 2013;1:208–219. doi: 10.1016/S2213-8587(13)70084-6. [DOI] [PubMed] [Google Scholar]
- 31.Farxiga for US Healthcare Professionals. Formulary finder [Web page] [Accessed February 15, 2016];AstraZeneca website. https://www.farxiga-hcp.com/access-savings/formulary-finder.html.
- 32.Invokana for US Healthcare Professionals. Patient affordability and access map [Web page] [Accessed February 15, 2016];Janssen website. http://www.invokanahcp.com/formulary-map#. Updated February 08, 2016.
- 33.Magkos F, Fraterrigo G, Yoshino J, et al. Effects of moderate and subsequent progressive weight loss on metabolic function and adipose tissue biology in humans with obesity. Cell Metab. 2016;23:591–601. doi: 10.1016/j.cmet.2016.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wing RR, Lang W, Wadden TA, et al. Benefits of modest weight loss in improving cardiovascular risk factors in overweight and obese individuals with type 2 diabetes. Diabetes Care. 2011;34:1481–1486. doi: 10.2337/dc10-2415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.O’Neil PM, Smith SR, Weissman NJ, et al. Randomized placebo-controlled clinical trial of lorcaserin for weight loss in type 2 diabetes mellitus: the BLOOM-DM study. Obesity (Silver Spring) 2012;20:1426–1436. doi: 10.1038/oby.2012.66. [DOI] [PubMed] [Google Scholar]
- 36.Garvey WT, Ryan DH, Look M, et al. Two-year sustained weight loss and metabolic benefits with controlled-release phentermine/topiramate in obese and overweight adults (SEQUEL): a randomized, placebo-controlled, phase 3 extension study. Am J Clin Nutr. 2012;95:297–308. doi: 10.3945/ajcn.111.024927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Block JP, Choudhry NK, Carpenter DP, et al. Time series analyses of the effect of FDA communications on use of prescription weight loss medications. Obesity (Silver Spring) 2014;22:943–949. doi: 10.1002/oby.20596. [DOI] [PubMed] [Google Scholar]
- 38.Zinman B, Wanner C, Lachin JM, et al. Empagliflozin, cardiovascular outcomes, and mortality in type 2 diabetes. N Engl J Med. 2015;373:2117–2128. doi: 10.1056/NEJMoa1504720. [DOI] [PubMed] [Google Scholar]
- 39.Nicklas JM, Huskey KW, Davis RB, Wee CC. Successful weight loss among obese US adults. Am J Prev Med. 2012;42:481–485. doi: 10.1016/j.amepre.2012.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Hendricks EJ, Rothman RB, Greenway FL. How physician obesity specialists use drugs to treat obesity. Obesity (Silver Spring) 2009;17:1730–1735. doi: 10.1038/oby.2009.69. [DOI] [PubMed] [Google Scholar]