Abstract
Background
Hypoxia is often associated with cardiopulmonary diseases, which represent some of the leading causes of mortality worldwide. Long-term hypoxia exposures, whether from disease or environmental condition, can cause cardiomyopathy and lead to heart failure. Indeed, hypoxia-induced heart failure is a hallmark feature of chronic mountain sickness in maladapted populations living at high altitude. In a previously established Drosophila heart model for long-term hypoxia exposure, we found that hypoxia caused heart dysfunction. Calcineurin is known to be critical in cardiac hypertrophy under normoxia, but its role in the heart under hypoxia is poorly understood.
Methods and Results
In the present study, we explore the function of calcineurin, a gene candidate we found downregulated in the Drosophila heart after lifetime and multigenerational hypoxia exposure. We examined the roles of 2 homologs of Calcineurin A, CanA14F, and Pp2B in the Drosophila cardiac response to long-term hypoxia. We found that knockdown of these calcineurin catalytic subunits caused cardiac restriction under normoxia that are further aggravated under hypoxia. Conversely, cardiac overexpression of Pp2B under hypoxia was lethal, suggesting that a hypertrophic signal in the presence of insufficient oxygen supply is deleterious.
Conclusions
Our results suggest a key role for calcineurin in cardiac remodeling during long-term hypoxia with implications for diseases of chronic hypoxia, and it likely contributes to mechanisms underlying these disease states.
Keywords: CanA, cardiac remodeling, cardiomyopathy, chronic ischemic heart disease, chronic mountain sickness, hypertrophy, hypoxia adaptation
Chronic exposure to hypoxia, seen in patients with obstructive sleep apnea, breathing disorders, and pulmonary hypertension, may lead to compromised cardiopulmonary function.1 In mammals, the normal response of the pulmonary circulation to local hypoxia is regional hypertension, which directs blood flow toward better oxygenated regions of the lungs.2,3 However, chronic pulmonary hypertension causes remodeling through a thickening of vascular smooth muscle and can lead to pathological hypertrophy as the heart pumps against increased vascular resistance.4 Untreated cardiac hypertrophy can progress to dilated cardiomyopathy and heart failure.4,5
Pathological cardiac hypertrophy is also a hallmark of chronic mountain sickness, yet is remarkably absent in select, presumably well-adapted, high-altitude populations.6–9 Chronic mountain sickness is characterized by an array of symptoms, and susceptible individuals may have cardiac disease in early adulthood. South American highland populations display a particularly high incidence of chronic mountain sickness, and the incidence of disease was recently correlated with increased expression of the genes ANP32D and SENP1.10 In contrast, EDNRB, a gene associated with well-adapted highland Ethiopians, improved cardiac function during hypoxia in heterozygous knockout mouse models, indicating that gene selection at altitude may confer cardioprotection.11 Despite recent studies establishing a genetic basis underlying high-altitude adaptations, little is known about hypoxia-related mechanisms involved in cardiac remodeling in these populations.12,13
Many pathways are conserved among mammals and Drosophila, including the genetic network orchestrating heart development, as well as the role of hypoxia-inducible factor (HIF-1α) in mediating hypoxia responses.14,15 With its relatively short lifespan and wealth of genetic tools, Drosophila is well suited for genetic and cardiac physiology studies.15–17 The fly model has successfully identified novel genes involved in cardiomyopathies, and detailed cardiac responses can be measured through the use of various imaging techniques.15,17–19 Previously, we established Drosophila heart models for the effects of chronic (lifetime) and multigenerational hypoxia exposure.20 We found that the effects of chronic hypoxia (CH) on heart function are particularly pronounced in flies with HIFα deficiency, consistent with the known roles of HIF in mediating these hypoxia responses in human populations.1,7,20,21 Notably, we also observed significant cardiac alterations in flies selected for survival to multigenerational exposure to hypoxia (hypoxia-selected [HS]).20 HS flies exhibited cardiac restriction compared with normoxia or CH-exposed flies.
Using the Drosophila model to identify new players in mediating cardiac hypoxia responses, we considered calcineurin, known to promote cardiac hypertrophy by activating nuclear factor of activated T-cells.22,23 Transgenic mice expressing activated calcineurin show cardiac hypertrophy, while inhibiting calcineurin ameliorates stress-induced cardiac hypertrophy.24,25 Calcineurin is a calcium/calmodulin-dependent protein phosphatase with 2 subunits, the catalytically active CanA subunit and the regulatory calcium/calmodulin-binding CanB subunit. Drosophila homologs include 3 CanA genes (CanA1, CanA14F, and Pp2B) and 2 CanB genes (CanB and CanB2).26 The CanB subunit promotes Drosophila flight muscle differentiation and regulates catalytic activity of CanA through stabilizing binding of calcium/calmodulin.27 Reducing CanB expression results in cardiomyopathy by impairing cardiomyocyte growth in flies and mice.27–29 CanA14F and Pp2B share 73% to 78% amino acid sequence identity to the human calcineurin A protein and, as neighboring genes on the X chromosome in Drosophila, likely arose by gene duplication in Diptera.26,30 A constitutively active form of the Drosophila Pp2B gene induced cardiac widening in the conical chamber under normoxic conditions, and inhibition of calcineurin in mammalian models is well known to reduce pathological hypertrophy.24,25,31 However, a role for calcineurin in long-term hypoxia exposures, where hypertrophy can be a defining feature of CH-related diseases, has not yet been identified.31,32
Here, we examined flies exposed to CH (3 weeks at 4% O220) and over many generations of hypoxia selection (HS; >250 generations at 4% O233). We found that both hypoxia regimens altered heart function, consistent with our previous findings.20 We then performed cardiac-specific expression profiles and found expression of the calcineurin homologs CanA14F and Pp2B to be highly downregulated in HS hearts and, to a lesser degree, in CH hearts. Heart-specific knockdown of CanA14F or Pp2B in normoxic flies similarly decreased cardiac size, as observed in HS flies. Under hypoxia, CanA14F or Pp2B knockdown resulted in further loss of cardiac performance. Interestingly, gain of Pp2B function, known to increase heart size under normoxia,31 was lethal under CH. This suggests that elevating the activity of a hypertrophy inducer, such as calcineurin A, in a hypoxic environment may cause cardiomyocyte instability, resulting in cardiac death and preventing long-term organismal survival. Our results provide insight into the effects of long-term hypoxia exposure on the heart and define novel roles for calcineurin A in cardiac remodeling because of long-term hypoxia.
Materials and Methods
Microarray and RNA-seq Analysis
Isolated hearts from the HS flies (at 4% O2) and normoxia control (NC) populations (at 21% O2) were used for microarray analysis. Three sets of 50 hearts from 3-week-old males in both HS and NC populations were compared. We also performed RNA-seq, optimized for Drosophila, on 3 sets of 20 hearts from wild-type (w1118) flies under conditions of normoxia or CH (3 weeks 4% O2). Transcriptomics data were filtered based on a minimum fold change of ±1.2, with a corresponding p value <0.05 relative to control lines.
Bioinformatics analysis of gene expression changes was performed using available online tools to describe differential patterns between HS, chronically exposed, and control populations. Gene functional annotation and classification was generated using the Database for Annotation, Visualization and Integrated Discovery bio-informatics module (http://david.abcc.ncifcrf.gov).34,35 Additionally, mapping of Drosophila orthologs to known mammalian metabolic pathways was performed using the Kyoto Encyclopedia of Genes and Genomes array tool (http://www.kegg.jp/kegg/download/keg-tools.html).36 Heat maps were generated from sorted Database for Annotation, Visualization and Integrated Discovery and Kyoto Encyclopedia of Genes and Genomes gene subsets using TIGR’s open source MeV software (http://tm4.org/mev).37
Optical Imaging and Heart Function Analysis
Direct immersion optics were used in conjunction with a digital high-speed camera (120–150 frames/second; Hamamatsu EM-CCD) mounted on a Leica DMLFSA microscope to record 30-second movies of beating hearts, with images captured using HC Image (Hamamatsu Corp.). Cardiac function was analyzed from the high-speed movies using Semiautomatic Optical Heartbeat Analysis software (http://sohasoftware.com), which quantifies diastolic/systolic intervals (SIs), cardiac arrhythmia, diastolic/systolic diameters (DD and SD), and fractional shortening (FS).17,19
Supplementary Methods
Detailed methods for fly stocks, quantitative polymerase chain reaction validation of cardiac gene expression, heart function analyses, and statistical analyses are described in the Data Supplement.
Results
Lifelong and Multigenerational Exposure to Hypoxia Alters Cardiac Performance
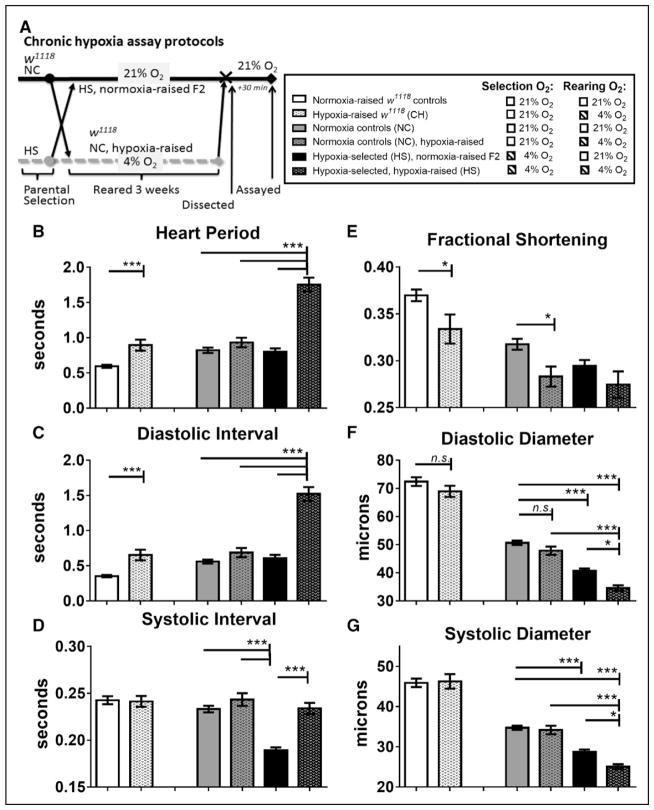
We previously observed that CH exposure of laboratory wild-type flies (w1118) caused significantly altered cardiac performance by 3 weeks in adults, including a slower heartbeat (increased heart period [HP]).20 In addition, flies selected for survival at reduced oxygen levels over many generations (HS) exhibited a similar increase in HP, as well as significant cardiac constriction and decrease in FS compared with both NC populations and laboratory wild-type flies (w1118) after CH conditions (see Data Supplement). We sought to determine which traits were due primarily to adult lifetime CH, as opposed to the effects of hypoxia selection over generations. We conducted a side-by-side comparison of w1118, NC, and HS flies’ cardiac function at normoxia on reoxygenation after 3 weeks CH (Figure 1A). For comparison, we also include our previously published data for w1118 flies (labeled w1118 control and w1118 CH), which exhibit an increased HP after CH, as observed in the hearts of NC hypoxia-raised flies (Figure 1B through 1D).20 Contractility, as measured by FS, was significantly decreased after CH in w1118 and NC flies, but cardiac constriction was relatively slight (Figure 1E through 1G). These results suggest that hearts from both w1118 and NC respond similarly to adult lifelong hypoxia treatment.
Figure 1.
Cardiac response to chronic hypoxia in w1118 flies (CH) compared with hypoxia-selected flies (HS) and their normoxia control (NC) populations, reared under either hypoxia or normoxia. A, Protocol for chronic hypoxia treatment in female w1118 flies and in male HS and NC populations.20 B, Exposure of w1118 flies to chronic hypoxia exposure (CH) significantly increased heart period compared with their w1118 controls; HS flies reared under their chronic hypoxia selection conditions had heart periods significantly longer than either NC or HS population reared under normoxia or hypoxia-reared NC flies. C, Increase in heart period is caused by increases in diastolic intervals. D, Systolic intervals were unchanged after chronic hypoxia in all groups except normoxia-raised HS flies, where systolic interval is significantly decreased compared with NC and HS. E, Fractional shortening is significantly decreased in hearts from both w1118 flies and NC flies exposed to chronic hypoxia. F, Diastolic diameters were unchanged after chronic hypoxia in w1118 and NC, but were significantly reduced in normoxia-raised HS, compared with NC, and in hypoxia-raised HS compared with both NC and hypoxia-raised HS fly. G, Similarly, systolic diameters were unaffected by CH in w1118 or NC lines, but are reduced in normoxia-raised HS compared with NC and in hypoxia-raised HS compared with both NC and hypoxia-raised HS flies. All values are mean ± SEM for hearts (N=75, w1118 controls; N=41, hypoxia-raised w1118 (CH); N=57, NC; N=41, hypoxia-raised NC; N=60, normoxia-raised HS; N=36, HS). Two-way analysis of variance (ANOVA) with Tukey’s multiple comparisons post hoc test; n.s. indicates not significant, *P<0.05, ***P<0.001. Adapted from Zarndt et al20 with permission of the publisher. Copyright© 2015, The American Physiological Society. Authorization for this adaptation has been obtained both from the owner of the copyright in the original work and from the owner of copyright in the translation or adaptation.
To determine whether the cardiac effects in HS flies reversed under normoxia, HS flies were raised for 2 generations at 21% O2 (HS normoxia-raised F2). HS flies raised under hypoxia (4% O2) display much longer HP because of long diastolic interval (Figure 1B and 1C, sixth bars), compared with all the others. The mean HP in the HS flies raised under 21% O2 reverted to levels that were similar to controls (Figure 1B, third and fifth bar). Notably, the SI was significantly reduced in HS hearts exposed to normoxia, compared with NC or w1118 flies (Figure 1D). This suggests that the prolonged HP of HS fly hearts are likely because of a combination of lifetime exposure to hypoxia and heritable genetic changes over many generations under hypoxia selection because CH alone in NC and w1118 flies is insufficient to produce prolonged HP and HS normoxia-raised F2 revert to normal HP.
As noted previously, hypoxia-reared HS flies are smaller in overall size than HS normoxia-raised F2, NC, or w1118 control flies.20,33,38 However, the smaller HS heart size was not simply because of an overall reduction in fly size because the diameters were still significantly smaller for HS flies after normalizing to abdominal segment and tibia lengths.20 In this study, hearts from HS flies exhibited reduced DD and SD, and the diameters remained constricted even when raised for 2 generations at normoxia (Figure 1E through 1G, fifth and sixth bars). Previously, we concluded that the smaller heart size was not simply because of a reduction in the overall size of the fly because the average diameters were still significantly smaller for HS flies after normalizing the heart diameters to abdominal segment and tibia lengths in normoxia- and hypoxia-raised HS and NC.20 Taken together, this suggests that cardiac restriction in HS flies persist across generations, regardless of normoxic- or hypoxic-rearing condition.
Cardiac-Specific Gene Expression of HS- and CH-Exposed Flies
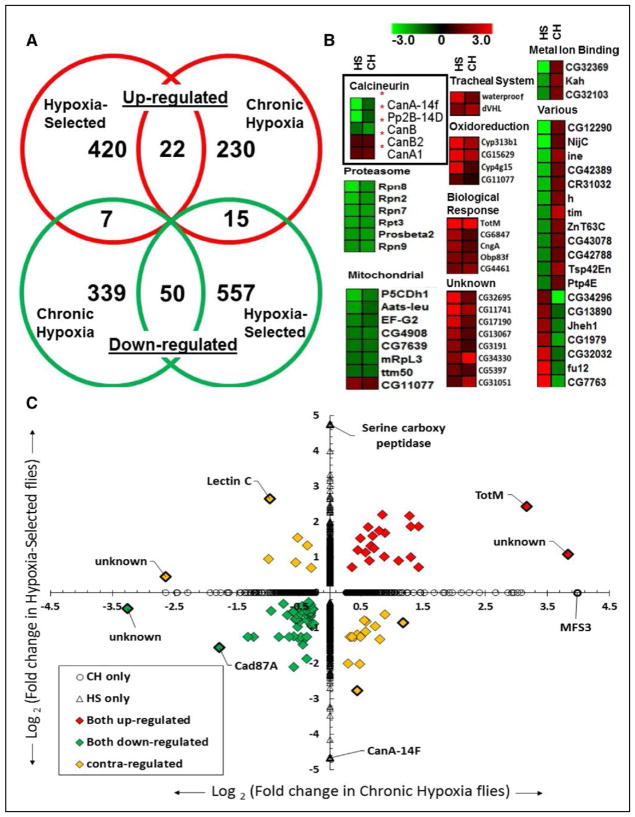
Although cardiac size in HS flies (raised at either 21% or 4%) is significantly reduced, this is not observed in CH flies (w1118 or NC; Figure 1E through 1G), suggesting that the cardiac restriction is the result of multigenerational hypoxia exposure rather than CH alone. To identify potential genetic mechanisms underlying HS cardiac restriction, we performed microarray analysis on isolated hearts from NC and HS lines using transcriptome arrays covering 13 061 total predicted genes from the Drosophila melanogaster genome (see Materials and Methods). Differential gene expression was defined as ±1.2-fold change in expression relative to control hearts with concomitant p values of <0.05. We identified a total of 449 upregulated genes and 622 downregulated genes in hearts from HS flies compared with NC (Figure 2A; Table I in the Data Supplement).
Figure 2.
Cardiac-specific differential gene expression in hypoxia-selected populations and in w1118 flies exposed to chronic hypoxia. Tran-scriptome analysis of RNA from isolated Drosophila hearts containing both myocardial and pericardial cells. A, Venn diagram depicting the number of genes differentially expressed in common or uniquely in hypoxia-selected flies (HS) compared with chronic hypoxia-exposed w1118 flies (CH) under 4% O2. B, Heat maps for groups of upregulated, downregulated, or contraregulated genes between HS and CH populations. The various categories include the 22 contraregulated genes between HS and CH populations. C, Log2-fold change in cardiac expression between HS vs CH flies with >1.2-fold upregulated (red), downregulated (green), or contraregulated genes and with P<0.05.
We also determined differential gene expression in hearts of CH flies relative to w1118 controls (see Materials and Methods). We identified 267 upregulated and 396 downregulated genes in CH hearts (Table II in the Data Supplement). Comparative meta-analysis of HS and CH expression profiles identified 22 commonly upregulated and 50 commonly down-regulated genes (Figure 2A; Table III in the Data Supplement). Notably, 22 genes were contraregulated between HS and CH populations. Next, we generated heat maps of differentially expressed gene subsets based on Kyoto Encyclopedia of Genes and Genomes metabolic and Database for Annotation, Visualization and Integrated Discovery gene ontology cluster analysis, which identified genes in categories related to cardiac remodeling (Figure 2B). We also generated a volcano plot of significant log2-fold changes (Figure IA in the Data Supplement), which were subsequently cross-compared between CH and HS expression data (Figure 2C).
CH flies and HS flies did exhibit differential gene expression relative to controls that were unique to each condition. CH flies showed activation of gene subsets enriched in non-coding RNA processing, extracellular matrix formation (especially collagens), and tracheal branching, while exhibiting suppression of ribosomal, sarcomeric, mitochondrial, and proteosomal genes. (Figure IB in the Data Supplement). Conversely, HS flies exhibited enhanced gene expression of cytochrome P450, endopeptidases, and immune response genes, while showing suppression of c-Jun N-terminal kinases cascade and sarcomeric and stress response genes (Figure IC in the Data Supplement).
Long-term hypoxia exposure causes a well-established shift from oxidative phosphorylation toward glycolysis and triacylglyceride synthesis in a variety of organisms and tissues.32,39,40 Our cardiac gene expression analysis corroborated these findings, especially in HS hearts; genes favoring glycolysis and triglyceride synthesis were upregulated, whereas oxidative phosphorylation components were downregulated (Figure 2B; Figure II in the Data Supplement). Interestingly, CH hearts showed increased expression of lactate dehydrogenase, suggesting a shift toward anaerobic glucose oxidation (Figure II in the Data Supplement).
Next, we examined expression levels of known cardiac hypertrophy regulatory transcription factors GATA binding protein 4 and Mef2 (myocyte enhancer factor-2). The GATA binding protein 4 ortholog, pannier (pnr), was statistically unchanged in HS (−1.4-fold; P=0.4) and CH (+1.4-fold; P=0.09) flies. Interestingly, Mef2 was statistically increased in HS (+4.0-fold; P=0.003) but not in CH (−1.1-fold; P=0.005; Tables I and II in the Data Supplement). Importantly, we also found that several members of the calcineurin gene family were differentially expressed in hearts of both CH and HS flies (Figure 2B). CanB2 expression was upregulated as was CanA1, although CanA1 expression is much less abundant in the heart compared with other calcineurin genes.41 Relatedly, quantitative polymerase chain reaction experiments validate CanB, a known CanB2 repressor, was down-regulated and CanB2, a known suppressor of CanA ortholog Pp2B, was upregulated after CH (Figure IIIA and IIIB in the Data Supplement).27 Pp2B, an enhancer of CanA14F expression, was downregulated along with CanA14F (Figure 2B; Figure IIIA and IIIB in the Data Supplement).41 CanA14F was overall the most downregulated gene in HS hearts (25-fold; Figure 2C; Table I in the Data Supplement). Given calcineurin’s known role in hypertrophy and metabolism,23,31,32 we chose to focus on the putative effector targets within the cal-cineurin network, CanA14F and Pp2B, for further studies. We hypothesized that cardiac downregulation of calcineurin, as observed with long-term hypoxia exposure, results in cardiac restriction under normoxic conditions in hypoxia-naïve flies.
Cardiac Response to CH With Knockdown of Calcineurins in Myocardial and Pericardial Cells
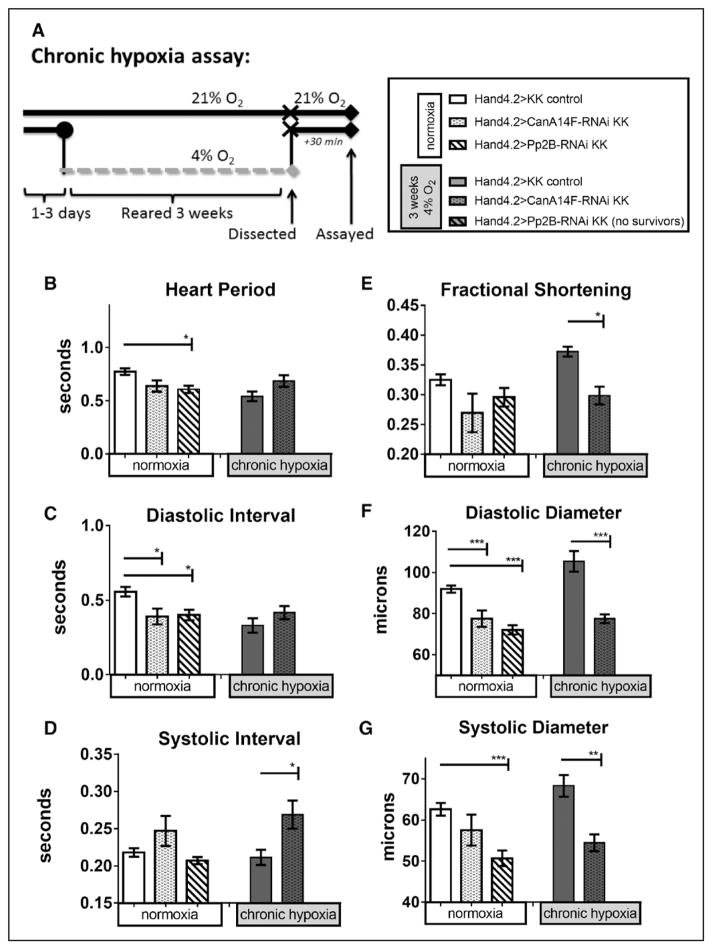
We used cardiac-specific CanA14F and Pp2B RNAi knockdown to determine their potential roles in cardiac remodeling under normoxia and CH (see Figure 3A for assay protocol). Knockdown was spatially limited to the cardiomyocytes and pericardial cells using the Hand4.2-Gal4 driver and validated for reduction in cardiac mRNA expression levels using quantitative polymerase chain reaction (Figure IIIC and IIID in the Data Supplement; see also Zarndt et al20). Hand4.2>CanA14F-RNAi, but not Hand4.2>Pp2B-RNAi, survived to 3 weeks CH. Under normoxia conditions, both cardiac-specific Pp2B-RNAi and CanA14F-RNAi knockdown hearts exhibit a decrease in HP, although not significantly in CanA14F, primarily attributed to a decreased diastolic interval (DI; Figure 3B and 3C). After CH, HP and DI did not change on cardiac CanA14F knockdown relative to hypoxia control flies (Figure 3B and 3C). Interestingly, the SI increased after CH in CanA14F knockdown flies (Figure 3D), possibly because of a compromise in the heart’s ability to contract and relax efficiently. Under normoxia, both CanA14F and Pp2B knockdown hearts exhibited cardiac constriction via decreased DD and SD, although CanA14F did not reach significance (Figure 3F and 3G). However, no appreciable alterations in FS were observed under normoxia (Figure 3E). After CH, cardiac-specific CanA14F-RNAi knockdown hearts exhibited dramatically reduced DD and SD, with a concomitant decrease in FS (Figure 3E through 3G).
Figure 3.
Effect of chronic hypoxia on heart function in hearts with myocardial and pericardial knockdown of calcineurins. A, left, Protocol for chronic hypoxia assay. B–G, Heart function data under normoxia (left bars) or CH exposure (right bars) per genotypes indicated in figure key (A, right): progeny of CanA14F-RNAi and Pp2B-RNAi crossed to a Hand4.2-Gal4 driver causes knockdown (KD) specifically in pericardial and myocardial cells during development through adulthood. Hearts were dissected and assayed under 21% O2 after 3 weeks at 21% or 4% O2 (as in Zarndt et al20). Note that cardiac Pp2B-RNAi KD flies under CH did not survive to 3 weeks. B, Heart period (HP) was reduced on cardiac Pp2B-RNAi KD in normoxia (condition: F=14.24, P=0.0002; genotype: F=8.089, P=0.0004; interaction=2.7, P=0.07). CanA14F KD had a similar put less pronounced effect. Under hypoxia, CanA14F KD had no significant effect. C, Similarly, diastolic intervals (DI) were reduced on cardiac CanA14F-RNAi and Pp2B-RNAi KD under normoxia, but not under CH (DI condition: F=13, P=0.0004; genotype: F=3.8, P=0.025; interaction =2.1, P=0.13). D, Systolic intervals (SI) were significantly elevated only in cardiac CanA14F KD flies after CH compared with controls under hypoxia (SI condition: F=0.64, P=0.43; genotype: F=7.8, P=0.0006; interaction =1.2, P=0.31). E, Fractional shortening (FS) was not significantly changed on cardiac calcineurin KD in normoxic flies. However, under hypoxia controls, CanA14F-RNAi KD in the heart results in reduced FS after CH (condition: F=0.53, P=0.47; genotype: F=20, P<0.0001; interaction=9.0, P=0.0002). F, Diastolic diameters were decreased significantly with both cardiac CanA14F-RNAi and PP2B-RNAi KD at normoxia, as well as with CanA14F KD under CH (condition: F=7.3, P=0.0009; genotype: F=14, P=0.0003; interaction=7.3, P=0.0009). G, Systolic diameters were similarly decreased with CanA14F and Pp2B KD at normoxia, as well as CanA14F KD under CH (condition: F=11, P=0.0009; genotype: F=1.4, P=0.26; interaction=F=3.0, P=0.055). All values are mean ± SEM (normoxia: N=37 KK control, N=33 CanA14F-RNAi, N=32 Pp2B-RNAi; chronic hypoxia: N=43 KK control, N=20 CanA14F-RNAi, N=20 Pp2B-RNAi). Two-way analysis of variance (ANOVA) and Sidak or Dunn multiple comparisons tests; *<0.05, **P<0.01, ***P<0.001. See also Materials and Methods.
Cardiac Response to CH With knockdown of Calcineurins in Myocardial Cells
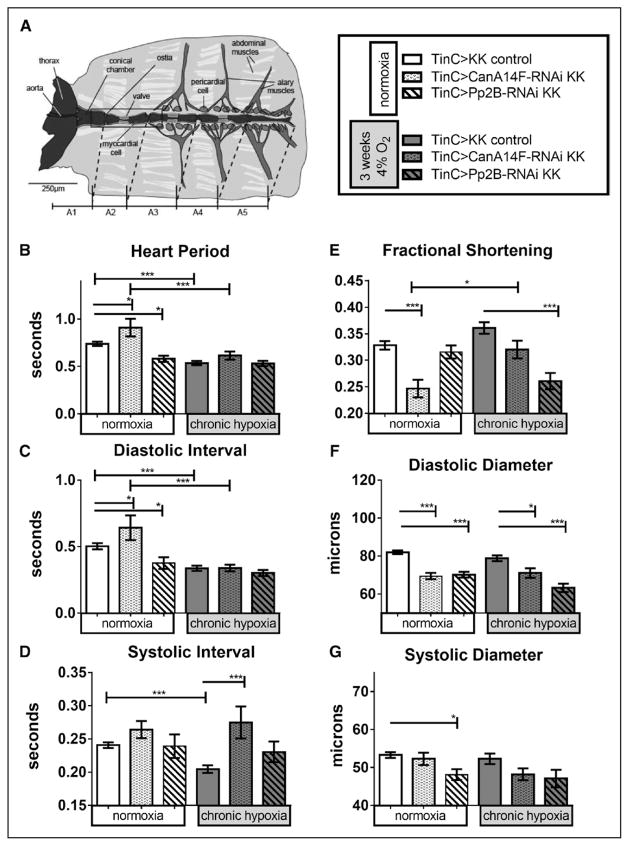
To determine whether altered cardiac function was because of calcineurin knockdown specifically in cardiomyocytes, we repeated these experiments with the myocardial-specific TinCΔ4-Gal4 driver (TinC; Figure 4). Unlike knockdown in both myocardial and pericardial cells, Pp2B and CanA14F knockdown only in the myocardium survived to 3 weeks CH (Figures 3 and 4).
Figure 4.
Effect of chronic hypoxia on heart function in hearts with myocardial-specific knockdown of calcineurins. A, left, Diagram of Drosophila heart tube within dissected abdominal preparation (image courtesy of Dr. Georg Vogler). TinC-Gal4 drives expression specifically in myocardial cells (central tube), excluding the ostia (inflow valves). Hand4.2-Gal4-driven expression includes the ostia, as well as the pericardial cells. B–G, Heart function data under normoxia (left bars) or CH exposure (right bars) per genotypes indicated in figure key (A, right): progeny of CanA14F-RNAi and Pp2B-RNAi crossed to a TinC-Gal4 driver causes knockdown (KD) specifically in myocardial cells during embryonic development and in pupal stages through adulthood. Hearts were dissected and assayed under 21% O2 after 3 weeks at 21% or 4% O2 (as in Zarndt et al20). B, Heart period (HP) was elevated in CanA14F-RNAi but lowered in Pp2B-RNAi flies under normoxic conditions. After 3 weeks CH, HP was reduced for control and did not changed further on calcineurin KD (condition: F=14, P=0.0002; genotype: F=8.1, P=0.0004; interaction =2.7, P=0.070). C, Diastolic intervals changed similar to HP (condition: F=13, P=0.0004; genotype: F=3.8, P=0.025; interaction: F=2.1, P=0.13). D, Systolic intervals (SI) did not change on myocardial calcineurin KD under normoxia but were reduced in control flies after CH. Calcineurin KD in the myocardium under CH increased the systolic interval, similar to the Hand4.2-Gal4 data in Figure 3D (condition: F=0.64, P=0.43; genotype: F=7.8, P=0.0006; interaction: F=1.2, P=0.31). E, Fractional shortening (FS) was significantly reduced in myocardial CanA14F-RNAi KD, compared with controls, at normoxia and compared with CanA14F KD under CH. Myocardial Pp2B KD exhibited reduced FS on CH, but not under normoxia (condition: F=0.53, P=0.47; genotype: F=20, P<0.0001; interaction: F=9.0, P=0.0002). F, Diastolic diameters were decreased significantly with CanA14F-RNAi and Pp2B-RNAi KD under normoxia and CH (condition: F=7.3, P=0.0009; genotype: F=14, P=0.0003; interaction: F=7.3, P=0.0009). F, Systolic diameters were decreased, significantly only with Pp2B-RNAi KD at normoxia (condition: F=11, P=0.0009; genotype: F=1.4, P=0.26; interaction: F=3.0, P=0.055). All values are mean ± SEM (normoxia: N=32 KK control, N=30 CanA14F-RNAi, N=38 Pp2B-RNAi; chronic hypoxia: N=28 KK control, N=22 CanA14F-RNAi, N=20 Pp2B-RNAi). Data analysis: Kruskal–Wallis test and Dunn multiple comparisons post hoc test and 2-way analysis of variance (ANOVA) and Tukey’s multiple comparisons post hoc test; n.s.=not significant, *P<0.05, **P<0.01, ***P<0.001.
Although we observed some changes in HP, DI, and SI with myocardial knockdown of CanA14F and Pp2B under normoxia, CH did not cause much of a further change in HP and DI relative to hypoxic controls (Figure 4A through 4C). After CH exposure, however, SI was significantly increased with myocardial CanA14F knockdown (Figure 4D), similar to knockdown in both myocardial and pericardial cells (Figure 3D). This is consistent with the idea that cardiac deficiency of calcineurin, particularly in the myocardium, compromises contractility when combined with CH.
TinC-driven knockdown of CanA14F and Pp2B knockdown caused a reduction in DD, SD (Pp2B knockdown only), and FS (CanA14F only) under normoxia (Figure 4E through 4G). This is similar to the findings with Hand4.2-Gal4 (Figure 3E through 3G). On exposure to CH, heart size parameters were not reduced more than under normoxia (Figure 4E through 4G), except that Pp2B knockdown flies show significantly reduced FS with CH because of decreased DD. Similar phenotypes were observed using the Hand4.2 driver, where CanA14F and Pp2B knockdown caused cardiac constriction in normoxia and CH exposure (Figure 3E through 3G).
To address whether the lethality observed with the Hand4.2 driver is caused by a Pp2B deficiency in the pericardial cells, we knocked down Pp2B with the pericardial-specific sticks and stones driver.42 We found that cardiac function was unaltered under pericardial knockdown of Pp2B, and there was no noticeable effect on survival under normoxia or hypoxia (Figure IV in the Data Supplement).
These data suggest that cardiac expression of both Pp2B and CanA14F play important roles in cardiac size and function under both normoxia and CH conditions. Specifically, a minimal level of Pp2B in the myocardium seems to be essential for adult survival under long-term hypoxia exposure and likely plays a critical role in cardiac adaptation to hypoxia. Additionally, cardiac knockdown of CanA14F under hypoxia seems to further aggravate heart function, including contractility, similar to the effects of reduced HIF1α under hypoxia.20 These data are particularly interesting in the context of HS, where basal expression of these genes is substantially suppressed and might underlie the observed multigenerational cardiac restriction, even after 2 generations of normoxia (Figure 1F and 1G, fifth bars).
A previous study reported that excess calcineurin activity causes cardiac hypertrophy in Drosophila,31 similar to observations in the mouse heart.22–25,31 We wondered if CH exposure could counteract calcineurin-induced cardiac hypertrophy. Remarkably, overexpressing Pp2B in the heart using either Hand4.2 or TinC drivers resulted in lethality under CH conditions. Thus, cardiac overexpression of calcineurin under CH is deleterious, resulting in death of the organism. This outcome may be because of a high oxygen demand on initiation of the calcineurin-evoked hypertrophic response, which cannot be met because of hypoxia, likely resulting in metabolic and, thus, functional cardiac collapse that is lethal in the long term.
Discussion
In humans, multigenerational adaptation to CH leads to either enhanced cardiac function or dysfunction in a population- and genotype-specific manner.4–6,13,43 Our laboratory model of HS flies exhibited significant changes in most cardiac parameters measured, compared with changes observed in normoxia controls (NC and w1118) and when raised under a matched duration of CH (Figure 1).20 Remarkably, the reduction in cardiac diameters in HS flies remained when reared for 2 generations under normoxia and when corrected for body size (21% O2; Figure 1F and 1G, fifth bars). Reduced organismal size under HS itself is likely adaptive for survival under hypoxia because of reduced oxygen availability and, hence, reduced metabolic output.44 Notably, we do not see significant cardiac restriction when rearing control populations (NC and w1118) under lifelong CH, suggesting that cardiac restriction in HS flies is a heritable trait. Across genotypes, HP was increased after hypoxia exposure. Significantly, HS flies raised in normoxia for 2 generations have a HP comparable to NCs, suggesting that some chronotropic effects are reversible.
Our comparison of transcriptomes of isolated hearts from HS and CH flies exhibit shared regulation of genes in mitochondrial and proteosomal families (downregulation), and tracheal, oxidoreduction, and immune response (upregulation), consistent with observations in hearts of other model organisms,13,45,46 thus, indicating that the fly heart displays evolutionarily conserved cardiac responses to hypoxia. Because CanA14F and Pp2B were downregulated in both HS and CH hearts, we tested their role in regulating the fly’s heart function during CH. We found that knockdown of CanA14F or Pp2B caused cardiac restriction under normoxia or hypoxia, similar to HS flies with multigenerational selection. Further, calcineurin knockdown combined with hypoxia caused cardiac restriction and reduced FS. Given that flies with strong knockdown of Pp2B in both pericardial and myocardial cells survive under normoxia but not under CH, it is likely that the Pp2B gene also plays an important role in the cardiac response to hypoxia stress. Further, myocardial-specific knockdown of either calcineurin produced similar changes as with knockdown in both pericardial and myocardial cells, suggesting that at least part of this response is heart-autonomous.
In human populations, hearts from well-adapted Tibetans exhibit increased myocardial glucose uptake and lower cardiac phosphocreatine-to-ATP ratios, even when these high-altitude natives live for many years at low altitude.5,6,13,43 We suggest that calcineurins are involved in regulating this process, based on our findings and their known roles promoting the metabolic shift toward glycolysis during pathological car-diomyopathies.23,32 Moreover, active calcineurin stabilizes the HIF and, thus, favors a shift toward glycolysis,1,23 a shift which is also observed in whole HS flies.39,40,47 Notably, our Kyoto Encyclopedia of Genes and Genomes analysis of HS cardiac gene expression also noted a similar shift in HS fly hearts.
Human populations that are well adapted to multigenerational hypoxia, such as Tibetan Sherpas, show beneficial cardiac adaptations to their native high altitudes, including reduced rates of pathological right ventricular hypertrophy, increased ability to raise maximal heart rate and cardiac output at altitude, and increased myocardial glucose uptake.6,7 However, other high-altitude populations exhibit signs of cardiac disease, including cardiomyopathies and pathological hypertrophy because of multigenerational exposure to hypoxia.5,48 Recent evidence from high-altitude human and model organisms, including flies exposed to hypoxia, indicates shared genetic and physiological adaptations based on selection for long-term hypoxia survival.49 Intriguingly, one of the few genomic regions found to be under positive selection in both HS flies and in multiple, well-adapted human populations contains SPRY, the fly homolog sprouty, a proposed negative regulator of epidermal growth factor that impacts calcineurin function, although no mechanism had been suggested for this gene in long-term hypoxia adaptation.49,50 Interestingly, the tracheal branching Gene Ontology classification, which contains epidermal growth factor, is modified, suggesting that hypoxia does exert a general effect on growth-related biological processes.
We conclude that calcineurin signaling is a critical factor during long-term hypoxia exposure of the Drosophila, as well as the mammalian heart. It is well established that chronic elevation of intracellular calcium in muscle cells stabilizes calmodulin binding to calcineurin B, thus, promoting activation of calcineurin A25,28,29,31,32,50 (see Figure IIIB in the Data Supplement). Calcineurin serves many functions in the cell, including dephosphorylation of nuclear factor of activated T-cells (in vertebrates), which allows translocation into the nucleus and activation of transcription factors, including GATA binding protein 4 and MEF2, to induce hypertrophic genes. A reduction in calcineurin signaling because of long-term hypoxia exposure would then reduce induction of hypertrophic genes, which in turn leads to the cardiac restriction phenotype we observe in HS and CanA14F/Pp2B knockdown hearts. We propose that given calcineurins role in cardiac hypertrophy, modulation of calcineurin may also play a role in the adaptation to long-term hypoxia.
In summary, we examined heart function and transcriptional changes in Drosophila populations selected through chronic and multigenerational exposure to severe hypoxic conditions. Several genetic changes were identified in the hearts of HS flies that include downregulation of calcineurin and oxidative phosphorylation genes and upregulation of glycolysis. While multiple pathways undoubtedly contribute to cardiac remodeling observed in HS flies, we demonstrate that modulation of 2 Drosophila homologs of calcineurin A, CanA14F and Pp2B, are important in causing restriction in the Drosophila heart during prolonged, especially multigenerational, hypoxia exposure. Cardiac remodeling identified in Drosophila may also play a crucial role in mammalian cardiac adaptation or the progression toward disease during long-term hypoxia exposures.
Supplementary Material
CLINICAL PERSPECTIVE.
Chronic exposure to hypoxia is associated with compromised cardiopulmonary function in humans, as seen in patients with obstructive sleep apnea and pulmonary hypertension, as well as in individuals living for prolonged periods at high altitude. In this study, we used the laboratory fruit fly model,Drosophila, to compare the gene expression profiles of hearts from populations exposed to multigenerational or lifelong hypoxia. Many pathways are conserved between humans and Drosophila, including those orchestrating heart development and hypoxia responses; thus, we aimed to find conserved genes altered during long-term hypoxia exposures, which may be relevant to human cardiac disease progression or adaptation to hypoxia. We identified calcineurin as a gene significantly downregulated during both multigenerational and life-long chronic hypoxia exposures inDrosophila hearts. Using genetic tools available in Drosophila, we found calcineurin downregulation to cause cardiac restriction in both hypoxia-naïve flies and those exposed to chronic hypoxia, mimicking previous observations in flies adapted over many generations to survive extreme hypoxia. We postulate that downregulation of calcineurin causes cardiac restriction during long-term hypoxia exposures in Drosophila, and modulation of calcineurin could play a crucial role in mammalian cardiac adaptation to high altitude or progression toward disease during prolonged hypoxia. Genetic or pharmacological inhibition of calcineurin may be a useful means for reducing cardiac hypertrophy during long-term exposures to chronic hypoxia in humans.
Acknowledgments
We thank Drs Dan Zhou and Gabriel G. Haddad for providing samples from their hypoxia-selected Drosophila populations for cardiac function and heart transcriptome analysis. We also thank Zhouhui (Zoe) Gan and Alexander Zambon for participating in the early phases of cardiac transcriptome analysis, and student interns Christina Lin and Alizée Blanchin for their cheerful assistance and maintenance of fly crosses.
Sources of Funding
This work was supported by fellowships to Dr Zarndt (Frontiers in Innovation Scholars Program/National Institutes of Health [NIH]) and Dr Walls (National Research Service Award/NIH), by a grant from the American Heart Association to Dr Ocorr (SDG), and grants from NIH to Dr Bodmer (R01 HL54732, P01 HL098053, and P01 AG033456).
Footnotes
The Data Supplement is available at http://circgenetics.ahajournals.org/lookup/suppl/doi:10.1161/CIRCGENETICS.117.001706/-/DC1.
Disclosures
None.
References
- 1.Prabhakar NR, Semenza GL. Adaptive and maladaptive cardiorespiratory responses to continuous and intermittent hypoxia mediated by hypoxia-inducible factors 1 and 2. Physiol Rev. 2012;92:967–1003. doi: 10.1152/physrev.00030.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Monge C, León-Velarde F. Physiological adaptation to high altitude: oxygen transport in mammals and birds. Physiol Rev. 1991;71:1135–1172. doi: 10.1152/physrev.1991.71.4.1135. [DOI] [PubMed] [Google Scholar]
- 3.Ramirez JM, Folkow LP, Blix AS. Hypoxia tolerance in mammals and birds: from the wilderness to the clinic. Annu Rev Physiol. 2007;69:113–143. doi: 10.1146/annurev.physiol.69.031905.163111. [DOI] [PubMed] [Google Scholar]
- 4.Penaloza D, Arias-Stella J. The heart and pulmonary circulation at high altitudes: healthy highlanders and chronic mountain sickness. Circulation. 2007;115:1132–1146. doi: 10.1161/CIRCULATIONAHA.106.624544. [DOI] [PubMed] [Google Scholar]
- 5.Lankford HV, Swenson ER. Dilated hearts at high altitude: words from on high. High Alt Med Biol. 2014;15:511–519. doi: 10.1089/ham.2014.1047. [DOI] [PubMed] [Google Scholar]
- 6.Gilbert-Kawai ET, Milledge JS, Grocott MP, Martin DS. King of the mountains: Tibetan and Sherpa physiological adaptations for life at high altitude. Physiology. 2014;29:388–402. doi: 10.1152/physiol.00018.2014. [DOI] [PubMed] [Google Scholar]
- 7.Lorenzo FR, Huff C, Myllymäki M, Olenchock B, Swierczek S, Tashi T, et al. A genetic mechanism for Tibetan high-altitude adaptation. Nat Genet. 2014;46:951–956. doi: 10.1038/ng.3067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sahota IS, Panwar NS. Prevalence of chronic mountain sickness in high altitude districts of Himachal Pradesh. Indian J Occup Environ Med. 2013;17:94–100. doi: 10.4103/0019-5278.130839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Udpa N, Ronen R, Zhou D, Liang J, Stobdan T, Appenzeller O, et al. Whole genome sequencing of Ethiopian highlanders reveals conserved hypoxia tolerance genes. Genome Biol. 2014;15:R36. doi: 10.1186/gb-2014-15-2-r36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cole AM, Petousi N, Cavalleri GL, Robbins PA. Genetic variation in SENP1 and ANP32D as predictors of chronic mountain sickness. High Alt Med Biol. 2014;15:497–499. doi: 10.1089/ham.2014.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stobdan T, Zhou D, Ao-Ieong E, Ortiz D, Ronen R, Hartley I, et al. Endothelin receptor B, a candidate gene from human studies at high altitude, improves cardiac tolerance to hypoxia in genetically engineered heterozygote mice. Proc Natl Acad Sci USA. 2015;112:10425–10430. doi: 10.1073/pnas.1507486112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eichstaedt CA, Antão T, Pagani L, Cardona A, Kivisild T, Mormina M. The Andean adaptive toolkit to counteract high altitude maladaptation: genome-wide and phenotypic analysis of the Collas. PLoS One. 2014;9:e93314. doi: 10.1371/journal.pone.0093314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Frisancho AR. Developmental functional adaptation to high altitude: review. Am J Hum Biol. 2013;25:151–168. doi: 10.1002/ajhb.22367. [DOI] [PubMed] [Google Scholar]
- 14.Lavista-Llanos S, Centanin L, Irisarri M, Russo DM, Gleadle JM, Boc-ca SN, et al. Control of the hypoxic response in Drosophila melanogaster by the basic helix-loop-helix PAS protein similar. Mol Cell Biol. 2002;22:6842–6853. doi: 10.1128/MCB.22.19.6842-6853.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Vogler G, Bodmer R, Akasaka T. A Drosophila model for congenital heart disease. Drug Discovery Today: Disease Models. 2009;6:47–54. doi: 10.1016/j.ddmod.2009.11.002. [DOI] [Google Scholar]
- 16.Mohr SE, Hu Y, Kim K, Housden BE, Perrimon N. Resources for functional genomics studies in Drosophila melanogaster. Genetics. 2014;197:1–18. doi: 10.1534/genetics.113.154344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ocorr K, Vogler G, Bodmer R. Methods to assess Drosophila heart development, function and aging. Methods. 2014;68:265–272. doi: 10.1016/j.ymeth.2014.03.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Dekanty A, Romero NM, Bertolin AP, Thomas MG, Leishman CC, Perez-Perri JI, et al. Drosophila genome-wide RNAi screen identifies multiple regulators of HIF-dependent transcription in hypoxia. PLoS Genet. 2010;6:e1000994. doi: 10.1371/journal.pgen.1000994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ocorr K, Perrin L, Lim HY, Qian L, Wu X, Bodmer R. Genetic control of heart function and aging in Drosophila. Trends Cardiovasc Med. 2007;17:177–182. doi: 10.1016/j.tcm.2007.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zarndt R, Piloto S, Powell FL, Haddad GG, Bodmer R, Ocorr K. Cardiac responses to hypoxia and reoxygenation in Drosophila. Am J Physiol Regul Integr Comp Physiol. 2015;309:R1347–R1357. doi: 10.1152/ajpregu.00164.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Semenza GL. Hypoxia-inducible factor 1 and cardiovascular disease. Annu Rev Physiol. 2014;76:39–56. doi: 10.1146/annurev-physiol-021113-170322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Molkentin JD, Lu JR, Antos CL, Markham B, Richardson J, Robbins J, et al. A calcineurin-dependent transcriptional pathway for cardiac hypertrophy. Cell. 1998;93:215–228. doi: 10.1016/s0092-8674(00)81573-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wilkins BJ, Dai YS, Bueno OF, Parsons SA, Xu J, Plank DM, et al. Calcineurin/coupling participates in pathological, but not physiological, cardiac hypertrophy. Circ Res. 2004;94:110–118. doi: 10.1161/01.RES.0000109415.17511.18. [DOI] [PubMed] [Google Scholar]
- 24.Sussman MA, Lim HW, Gude N, Taigen T, Olson EN, Robbins J, et al. Prevention of cardiac hypertrophy in mice by calcineurin inhibition. Science. 1998;281:1690–1693. doi: 10.1126/science.281.5383.1690. [DOI] [PubMed] [Google Scholar]
- 25.Taigen T, De Windt LJ, Lim HW, Molkentin JD. Targeted inhibition of calcineurin prevents agonist-induced cardiomyocyte hypertrophy. Proc Natl Acad Sci USA. 2000;97:1196–1201. doi: 10.1073/pnas.97.3.1196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Miskei M, Ádám C, Kovács L, Karányi Z, Dombrádi V. Molecular evolution of phosphoprotein phosphatases in Drosophila. PLoS One. 2011;6:e22218. doi: 10.1371/journal.pone.0022218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gajewski K, Wang J, Molkentin JD, Chen EH, Olson EN, Schulz RA. Requirement of the calcineurin subunit gene canB2 for indirect flight muscle formation in Drosophila. Proc Natl Acad Sci USA. 2003;100:1040–1045. doi: 10.1073/pnas.0337662100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Maillet M, Davis J, Auger-Messier M, York A, Osinska H, Piquereau J, et al. Heart-specific deletion of CnB1 reveals multiple mechanisms whereby calcineurin regulates cardiac growth and function. J Biol Chem. 2010;285:6716–6724. doi: 10.1074/jbc.M109.056143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schaeffer PJ, Desantiago J, Yang J, Flagg TP, Kovacs A, Weinheimer CJ, et al. Impaired contractile function and calcium handling in hearts of cardiac-specific calcineurin b1-deficient mice. Am J Physiol Heart Circ Physiol. 2009;297:H1263–H1273. doi: 10.1152/ajpheart.00152.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brown L, Chen MX, Cohen PT. Identification of a cDNA encoding a Drosophila calcium/calmodulin regulated protein phosphatase, which has its most abundant expression in the early embryo. FEBS Lett. 1994;339:124–128. doi: 10.1016/0014-5793(94)80398-6. [DOI] [PubMed] [Google Scholar]
- 31.Lee TE, Yu L, Wolf MJ, Rockman HA. Galactokinase is a novel modifier of calcineurin-induced cardiomyopathy in Drosophila. Genetics. 2014;198:591–603. doi: 10.1534/genetics.114.166777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wilkins BJ, Molkentin JD. Calcineurin and cardiac hypertrophy: where have we been? Where are we going? J Physiol. 2002;541(pt 1):1–8. doi: 10.1113/jphysiol.2002.017129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Zhou D, Xue J, Chen J, Morcillo P, Lambert JD, White KP, et al. Experimental selection for Drosophila survival in extremely low O(2) environment. PLoS One. 2007;2:e490. doi: 10.1371/journal.pone.0000490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang da W, Sherman BT, Lempicki RA. Systematic and integrative analysis of large gene lists using DAVID bioinformatics resources. Nat Protoc. 2009;4:44–57. doi: 10.1038/nprot.2008.211. [DOI] [PubMed] [Google Scholar]
- 35.Huang da W, Sherman BT, Lempicki RA. Bioinformatics enrichment tools: paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009;37:1–13. doi: 10.1093/nar/gkn923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kanehisa M, Bork P. Bioinformatics in the post-sequence era. Nat Genet. 2003;33(suppl):305–310. doi: 10.1038/ng1109. [DOI] [PubMed] [Google Scholar]
- 37.Saeed AI, Sharov V, White J, Li J, Liang W, Bhagabati N, et al. TM4: a free, open-source system for microarray data management and analysis. Biotechniques. 2003;34:374–378. doi: 10.2144/03342mt01. [DOI] [PubMed] [Google Scholar]
- 38.Zhou D, Udpa N, Gersten M, Visk DW, Bashir A, Xue J, et al. Experimental selection of hypoxia-tolerant Drosophila melanogaster. Proc Natl Acad Sci USA. 2011;108:2349–2354. doi: 10.1073/pnas.1010643108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Feala JD, Coquin L, Zhou D, Haddad GG, Paternostro G, McCulloch AD. Metabolism as means for hypoxia adaptation: metabolic profiling and flux balance analysis. BMC Syst Biol. 2009;3:91. doi: 10.1186/1752-0509-3-91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou D, Xue J, Lai JC, Schork NJ, White KP, Haddad GG. Mechanisms underlying hypoxia tolerance in Drosophila melanogaster: hairy as a metabolic switch. PLoS Genet. 2008;4:e1000221. doi: 10.1371/journal.pgen.1000221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gramates LS, Marygold SJ, Santos GD, Urbano JM, Antonazzo G, Matthews BB, et al. the FlyBase Consortium. FlyBase at 25: looking to the future. Nucleic Acids Res. 2017;45(D1):D663–D671. doi: 10.1093/nar/gkw1016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lee S, Bao H, Ishikawa Z, Wang W, Lim HY. Cardiomyocyte regulation of systemic lipid metabolism by the apolipoprotein B-containing lipoproteins in Drosophila. PLoS Genet. 2017;13:e1006555. doi: 10.1371/journal.pgen.1006555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Mellor A, Boos C, Holdsworth D, Begley J, Hall D, Lumley A, et al. Cardiac biomarkers at high altitude. High Alt Med Biol. 2014;15:452–458. doi: 10.1089/ham.2014.1035. [DOI] [PubMed] [Google Scholar]
- 44.Klok CJ, Harrison JF. Atmospheric hypoxia limits selection for large body size in insects. PLoS One. 2009;4:e3876. doi: 10.1371/journal.pone.0003876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Li M, Zhao C. Study on Tibetan chicken embryonic adaptability to chronic hypoxia by revealing differential gene expression in heart tissue. Sci China C Life Sci. 2009;52:284–295. doi: 10.1007/s11427-009-0005-8. [DOI] [PubMed] [Google Scholar]
- 46.Marques IJ, Leito JT, Spaink HP, Testerink J, Jaspers RT, Witte F, et al. Transcriptome analysis of the response to chronic constant hypoxia in zebrafish hearts. J Comp Physiol B. 2008;178:77–92. doi: 10.1007/s00360-007-0201-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhou D, Haddad GG. Genetic analysis of hypoxia tolerance and susceptibility in Drosophila and humans. Annu Rev Genomics Hum Genet. 2013;14:25–43. doi: 10.1146/annurev-genom-091212-153439. [DOI] [PubMed] [Google Scholar]
- 48.Zhou D, Udpa N, Ronen R, Stobdan T, Liang J, Appenzeller O, et al. Whole-genome sequencing uncovers the genetic basis of chronic mountain sickness in Andean highlanders. Am J Hum Genet. 2013;93:452–462. doi: 10.1016/j.ajhg.2013.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jha AR, Zhou D, Brown CD, Kreitman M, Haddad GG, White KP. Shared genetic signals of hypoxia adaptation in Drosophila and in high-altitude human populations. Mol Biol Evol. 2016;33:501–517. doi: 10.1093/molbev/msv248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sullivan KM, Rubin GM. The Ca(2+)-calmodulin-activated protein phosphatase calcineurin negatively regulates EGF receptor signaling in Drosophila development. Genetics. 2002;161:183–193. doi: 10.1093/genetics/161.1.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.