Abstract
Objective
The principal objective of this investigation was to identify novel cytokine associations with body mass index and Type 2 diabetes.
Methods
Cytokines were profiled from African American women with obesity who donated plasma to the Komen Tissue Bank. Multiplex bead arrays of analytes were used to quantify 88 cytokines and chemokines in association with clinical diagnoses of metabolic health. Regression models were generated after elimination of outliers.
Results
Among women with obesity, Type 2 diabetes was associated with breast adipocyte hypertrophy and with six plasma analytes, including four chemokines: chemokine (C-C motif) ligand 2 (CCL2), chemokine (C-C motif) ligand 16 (CCL16), chemokine (C-X-C motif) ligand 1 (GRO-1) and chemokine (C-X-C motif) ligand 16 (CXCL16); and two growth factors: interleukin-2 and epidermal growth factor. In addition, three analytes were associated with obesity independently of diabetes: interleukin-4, soluble CD40 ligand and chemokine (C-C motif) ligand 3 (CCL3).
Conclusions
Profiling of inflammatory cytokines combined with measures of body mass index may produce a more personalized risk assessment for obesity-associated disease in African American women.
Keywords: Type 2 diabetes, African American women, inflammation, cytokines, women’s health
Introduction
As body mass index (BMI) increases, blood biomarkers of chronic inflammation important for cardiovascular disease, such as C-reactive protein, also increase (1); weight loss usually reduces cytokines. Inflammation positively associates with cardiometabolic risk in numerous studies (2). However, minimal work has surveyed comprehensively the cytokine signatures associated with obesity independent of metabolic disease, cytokine signatures associated with metabolic disease independent of obesity, or joint associations. These biomarkers could help stratify disease risks among patients with different co-morbidities of obesity. Furthermore, these relationships are understudied in African American women, who experience elevated risk for cardiometabolic complications of obesity, including Type 2 diabetes (T2D) and hypertension (3). African American women are expected to experience obesity prevalence of 70% by 2020 (4). For breast cancer patients, co-diagnosis of metabolic syndrome associates with worse prognosis (5). African American women have higher risks of poor-prognosis triple negative cancer and breast cancer mortality than white women (6). We recently reported the first compelling evidence that breast cancer mortality positively associates with T2D in African American women (7).
As the obesity epidemic worsens, the incidence of T2D and obesity-associated cancer is expected to increase. However, obesity is poorly correlated with metabolic disease and imbalanced pro- and anti-inflammatory cytokines. Histologically distinct features called ‘crown like’ structures (CLS) of pro-inflammatory CD68+ macrophages and their product cytokines (8) have been reported in subcutaneous adipose tissue of patients with obesity, and associate with cardiometabolic disease. CLS in breast white adipose tissue (CLS-B) (9) associate with elevated inflammation (10), aromatase (11) and breast cancer risk (12). The limited number of blood-based pro-inflammatory cytokines tested in obesity and T2D, e.g., IL-6 and C-reactive protein, associate with abnormal breast adipocytes (9). Cytokines like IL-6 are functionally significant because they act on cellular signal transduction pathways to drive dangerous changes in breast cancer progression, as we recently showed (13).
Here, we hypothesized that more extensive profiling of cytokines in African American women with T2D and obesity might reveal new signatures associated with metabolic risk. We compared African American women with T2D, with and without obesity and overweight, to African American women without T2D.
Methods
Human Subjects
Boston University Medical Center Institutional Review Board approved procedures in accordance with the Declaration of Helsinki. Non-fasting plasma (as specified in Table S1) and histological sections were selected from volunteers drawn from the general community, who donated specimens to the Susan G. Komen for the Cure® Tissue Bank (KTB) at the Simon Cancer Center (Indiana University). All subjects had BMI >25 kg/m2 and 75% of T2D subjects had co-morbid hypertension. Subjects who were non-diabetic with obesity (ND) by definition did not have T2D and 54% had well-controlled hypertension. Yes/No questionnaire-reported health history was obtained, including T2D, hypertension, hypercholesterolemia, thrombosis, osteoporosis, respiratory disease, stroke, thyroid disease, arthritis and anxiety/depression. Subject characteristics are summarized in Table 1 and demographic, clinical and medication information is provided for each subject in Table S1. Histological, biochemical and molecular methods are described in Supporting Information.
Table 1.
Characteristics of African American female subjects with obesity
| ND | T2D | P | |
|---|---|---|---|
| No. | 11 | 28 | - |
| Age, y | |||
| Mean (SD)a | 57.1 (7.2) | 58.9 (7.7) | 0.5 |
| BMIa, kg/m2 | |||
| Mean (SD) | 30.4 (4.0) | 36.5 (7.4) | 0.002 |
| Medicationb | |||
| Metformin | 0 | 12 (43%) | 0.048 |
| Anti-inflammatory (oral) | 5 (45%) | 8 (29%) | 0.506 |
| Anti-hypertensive | 4 (36%) | 22 (79%) | 0.368 |
| Lipid-lowering | 2 (18%) | 16 (57%) | 0.191 |
| Hypertensiond | 6 (54%) | 21 (75%) | 0.776 |
A: p-value (P) computed from t-test. B: p-value from Fisher exact test
Cytokine profiling
Plasma from KTB donors was snap-frozen, shipped in cryogenic vials and maintained at −80°C until assay. Plasma underwent fewer than two freeze thaw cycles before measurements. Cytokines and chemokines were determined using magnetic bead assays (EMD Millipore, human cytokine/chemokine panels I, II, III and IGF1&2; 23-plex, 25-plex and 41-plex systems) with a miniaturization drop array and washer system (Curiox Biosystems, Inc), with quantitation on a Luminex Magpix instrument using xPONENT 4.2 software to fit standard curves (Luminex).
Adipose tissue analyses
Adipocyte area reflects metabolic function; larger adipocytes are typically insulin resistant and metabolically abnormal. However, range of metabolic health has not been previously correlated with breast adipocyte area. Individual adipocytes (≥100 cells/subject) were traced by Image J, areas were recorded for four fields of view and histograms of cell size distribution were generated. ND subjects (n=11) were compared T2D subjects (n=28). To assess CLS-B frequency, 42 samples of hematoxylin and eosin stained breast adipose tissue from ND and T2D subjects were examined with blinding to metabolic status and the number of CLS/section for each subject enumerated. Adipocyte-associated CD68 was confirmed by mouse monoclonal anti-human CD68 (PG-M1, Dako), with biotinylated goat anti-mouse IgG (Dako) as secondary.
Statistical analysis
Data are shown as means ± SEM unless indicated. Cytokine data were log-transformed, outliers (absolute values > 3 standard deviations) were removed; data in duplicate were analyzed using mixed effect regression models for repeated measures. Models were adjusted by BMI and all medications. A search strategy was implemented to remove non-significant confounders from the fully adjusted model, based on likelihood ratio test. Results in Table 2 list the cytokine specific adjustment. Statistical significance was based on p-value < 0.05. Enumerated CLS were compared between T2D and ND using regression for repeated measures, adjusting for age and BMI. Analyses were conducted in R v3.
Table 2.
Cytokine associations
| A. Cytokines associated with T2D/ND statusa | |||
|---|---|---|---|
|
| |||
| Cytokine | Regression Coefficient | Standard Error | P value |
| IL-2 | 0.205 | 0.088 | 0.020b |
| EGF | −0.546 | 0.248 | 0.028 |
| CXCL16 | 0.179 | 0.088 | 0.041 |
| MCP-1/CCL2 | 0.311 | 0.156 | 0.046 |
| CCL16/HCC-4 | 0.314 | 0.136 | 0.020 |
| GRO-1 | 0.790 | 0.356 | 0.027c |
|
| |||
| B. Cytokines associated with BMI independently of T2Dd | |||
|
| |||
| IL-4 | 0.510 | 0.249 | 0.040 |
| sCD40L | 1.142 | 0.410 | 0.005 |
| MIP-1α/CCL3 | 3.127 | 1.311 | 0.017e |
Regression coefficient (log-fold change comparing T2D versus ND), standard error and P value to test whether the regression coefficient is different from 0.
Adjusted for anti-inflammatory use
Adjusted for anti-hypertensive use
Regression coefficient (log-fold change for a change of 1 of log(BMI)), standard error and P value to test whether the regression coefficient is different from 0.
Adjusted for metformin and lipid-lowering medication
Abbreviations: IL-2, Interleukin 2; EGF, epidermal growth factor; CXCL16, C-X-C motif chemokine ligand 16; MCP-1/CCL2, C-C motif chemokine ligand 1; CCL16/HCC-4, C-C motif chemokine ligand 16; GRO-1/CXCL1, C-X-C motif chemokine ligand 1; IL-4, Interleukin 4; sCD40L, soluble CD40 ligand; MIP-1α/CCL3, C-C motif chemokine ligand 3.
Results
Descriptive Statistics
All subjects with overweight (BMI 25.0 – 29.9 kg/m2) or obesity (BMI ≥ 30 kg/m2) (Table 1) were classified into one of two groups, T2D or ND: 39 post-menopausal, self-reported African American women without cancer (age range 44–71 years). Medication history was available for 34/39 subjects. ND subjects had mean BMI of 30.4 kg/m2 (SD 4.0) and lacked T2D or metabolic syndrome. T2D subjects had mean BMI of 36.5 kg/m2 (SD 7.4) and 21/28 (75%) had co-morbid hypertension, based upon self-report or medications. Donors were not fasted, thus plasma adipokines were not assayed, nor were values available for homeostatic model assessment of insulin resistance (HOMA-IR).
Histological assessment of breast AT inflammation
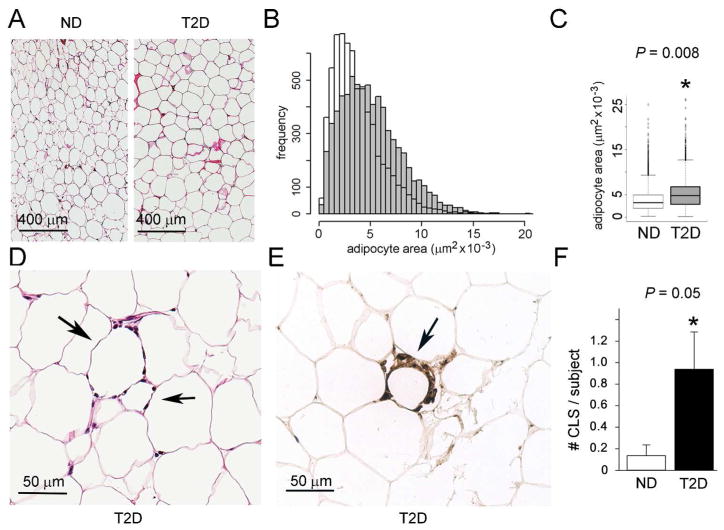
Adipocyte dysfunction, including hypertrophy (14) is known to associate with tissue inflammation, such as CLS (9). Furthermore, breast adipocyte size associates with systemic inflammation, insulin resistance, and increased aromatase (10–12). We expected that T2D subjects would have larger adipocytes with CLS-B present, compared to ND, consistent with blood biomarkers. Representative histological sections are shown (Figure 1A). The area distribution for T2D subjects (Figure 1B, grey) is right-shifted compared to ND (Figure 1B, white), indicating that adipocytes in T2D tissue tend to be larger and less healthy than ND. Mean adipocyte area (Figure 1C) was significantly larger in T2D than ND subjects (p=0.008). Representative CLS-B is shown (Figure 1D, E). CLS-B frequency was higher in T2D (p=0.05) (Figure 1F).
Figure 1. Adipocyte area and CLS frequency in breast adipose tissue of ND and T2D subjects with obesity.
(A) Micrographs of representative, fixed, hematoxylin and eosin (H&E)-stained paraffin sections of breast adipose tissue from ND and T2D subjects. Magnification was the same for both sections. Scale bar = 400 μm. (B) Overlay plot of histograms of cell area distributions, generated from measurements of cell area in a histological section. (C) Mean area of adipocytes in breast adipose tissue from ND and T2D groups (N = 39 total; 11 were ND and 28 were T2D). The median number of cells measured was 221, with comparison by Student’s t-test. (P = 0.008 after adjusting for age and BMI) (D) Representative micrograph of H&E-stained breast adipose tissue showing apparent ‘crown-like’ structures, CLS (arrows). (E) Representative micrograph of anti-CD68 stain confirms CLS (Ref 8) within breast adipose tissue of T2D subject. Scale bar = 50 μm. (F) Numbers of CLS in breast adipose tissue sections of 11 ND and 28 T2D subjects with two sections per subject examined. The plot shows the mean for each group (P = 0.05 by Student’s t-test). (*, P ≤ 0.05)
Plasma cytokine biomarkers associated with metabolic phenotype
Although insulin resistance associates with local and systemic elevations of pro-inflammatory cytokines (15), studies of plasma cytokines in adults with obesity have focused on limited subsets of analytes and not comprehensively surveyed plasma with multiplex technologies. We therefore systematically quantified a total of 88 cytokines/chemokines to reveal novel associations with T2D. Six cytokines associated with T2D (Table 2A; Figure S1): four were elevated in T2D patients and two in ND patients. Known pro-inflammatory molecules, TNF-α and IL-6, were not significantly different between T2D and ND. In addition, three cytokines were associated with BMI, independently of T2D (Table 2B and Figure S2). The analysis suggests that the true association among these cytokines is with BMI and the association with T2D is indirect.
Discussion
Vulnerable populations, such as African American women with obesity, who experience elevated risk from obesity-driven co-morbidities such as breast cancer, could benefit from more personalized profiling of immunometabolic biomarkers. Some studies in white women (12) suggest that histological markers of inflammation in breast (16) might help identify at-risk patients, if blood-based cytokines reflect breast risk factors (9). However, this approach has not been extended to African American women and profiles of cytokines have been limited. Precision medicine approaches to risk management should consider traditionally underserved patients to address needful populations. Non-invasive clinical measures and blood biomarkers should correlate with breast adipose tissue biomarkers for breast cancer incidence or progression. Our ability to identify significant differences in cytokines and chemokines among individuals drawn from a random, non-clinical population of subjects recruited at diverse sites across the country has value for traditionally underserved subjects, who might have difficulty with a fasting protocol or reluctance to travel to a hospital for a blood draw for research purposes. We have previously shown that estimates of cancer risk drawn from non-fasting populations are likely underestimates of the true effect of abnormal metabolism (17).
Chronic, unresolved inflammation in obesity has previously identified chemotactic and adipokine signaling molecules (18). Different subtypes of obesity can reveal blood biomarkers for stratifying cardiometabolic risk (19,20). Although a novel group of 7 cytokines and chemokines associates with T2D, 81 of the other analytes tested (Table S2) are not significantly correlated with metabolic disease, simplifying the task of developing personalized medicine profiles for high risk patients. The biomarkers described here can now be examined for utility as prognostic tools and to develop drug targets. CCL11, sCD40L and IL-4 are previously implicated in obesity; our analysis suggests that sCD40L and IL-4 are associated with BMI and T2D mediates the effect. Evidence links chemotactic factors such as CXCL16 to breast cancer aggressiveness (21), but functional studies in obesity are needed.
Breast adipocyte hypertrophy and inflammation correlate in white women with elevated aromatase expression and breast cancer risk (10–12). These tissue specific features associate with clinical diagnoses such as T2D in obesity and blood biomarkers (1). We show here in African American women that T2D associates with a rightward shift in adipocyte size distribution, elevated CLS-B and novel blood biomarkers. We are unable to evaluate risks for progression of T2D or cancer, due to the cross-sectional design, or to obtain measures of regional adiposity to correlate with cytokine and metabolic data. Interventions and recommendations for bariatric surgery that are tailored to patients’ inflammatory blood profile could be an important tool for risk assessment in obesity, but this step awaits investigation.
Conclusion
Combining profiling of inflammatory cytokines with measures of BMI may lead to a more personalized risk assessment for African American women with obesity.
Supplementary Material
What is already known?
Chronic inflammation in obesity is associated with both Type 2 diabetes and breast cancer risk.
African American women experience higher risk for triple negative breast cancer, worse outcomes and higher risk for metabolic disease compared to white women.
Plasma cytokine biomarkers reflect breast adipose tissue biomarkers of inflammation and stratify risk for breast cancer.
What does this study add?
African American women have not been the focus of a cohort study to determine associations between local and systemic inflammation and Type 2 diabetes in obesity.
Not many groups have studied inflammation in non-diabetic adults with obesity, and those that have only examine traditional markers like IL-6, TNF-α and C-reactive protein. We comprehensively profile plasma cytokines to develop an unbiased signature of inflammation associated with obesity and Type 2 diabetes in a non-clinical population.
We report novel relationships of cytokines associated with Type 2 diabetes independently of obesity, and with obesity independently of Type 2 diabetes.
Acknowledgments
Funding: NIH CA058420, CA182898, CA128006, DK090455
We thank M. Au of Boston University Medical Center’s Analytical Core Facility and M. Lian of Curiox Biosystems, Inc. for expert technical assistance with the multiplex with drop array assays. Samples from the Susan G. Komen for the Cure® Tissue Bank at the Indiana University Simon Cancer Center were used in this study; we are grateful to J. Henry for invaluable help to obtain these samples and annotated data, and for discussion about Komen Tissue Bank subjects. We thank KTB stakeholders, including Indiana University where samples were collected, the donors and their families, whose help and participation made this work possible. This work was conducted in response to a call for research from the National Cancer Institute to ‘Bridge the Gap’ between basic, clinical and population science (PAR-13–081).
Footnotes
Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. The authors declare no conflict of interest
Author contributions: GVD, supervised study, provided funding, wrote manuscript; PS, performed biostatistical analyses; AHT, KJS, FLL, performed assays; GA, JRP, provided essential comments and feedback.
References
- 1.Howe LR, Subbaramaiah K, Hudis CA, Dannenberg AJ. Molecular pathways: adipose inflammation as a mediator of obesity-associated cancer. Clin Cancer Res. 2013;19:6074–6083. doi: 10.1158/1078-0432.CCR-12-2603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Willerson JT, Ridker PM. Inflammation as a cardiovascular risk factor. Circulation. 2004;109(21 Suppl 1):II2–10. doi: 10.1161/01.CIR.0000129535.04194.38. [DOI] [PubMed] [Google Scholar]
- 3.Hajjar I, Kotchen TA. Trends in prevalence, awareness, treatment, and control of hypertension in the United States, 1988–2000. JAMA. 2003;290:199–206. doi: 10.1001/jama.290.2.199. [DOI] [PubMed] [Google Scholar]
- 4.Beydoun MA, Wang Y. Gender-ethnic disparity in BMI and waist circumference distribution shifts in US adults. Obesity (Silver Spring) 2009;17:169–176. doi: 10.1038/oby.2008.492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Berrino F, Villarini A, Traina A, et al. Metabolic syndrome and breast cancer prognosis. Breast Cancer Res Treat. 2014;147:159–165. doi: 10.1007/s10549-014-3076-6. [DOI] [PubMed] [Google Scholar]
- 6.DeSantis CE, Fedewa SA, Goding Sauer A, et al. Breast cancer statistics, 2015: Convergence of incidence rates between black and white women. CA Cancer J Clin. 2016;66:31–42. doi: 10.3322/caac.21320. [DOI] [PubMed] [Google Scholar]
- 7.Charlot M, Castro-Webb N, Bethea TN, Bertrand K, Boggs DA, Denis GV, Adams-Campbell LL, Rosenberg L, Palmer JR. Diabetes and breast cancer mortality in Black women. Cancer Causes Control. 2017;28:61–67. doi: 10.1007/s10552-016-0837-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Apovian CM, Bigornia S, Mott M, et al. Adipose macrophage infiltration is associated with insulin resistance and vascular endothelial dysfunction in obese subjects. Arterioscler Thromb Vasc Biol. 2008;28:1654–1659. doi: 10.1161/ATVBAHA.108.170316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Iyengar NM, Zhou XK, Gucalp A, et al. Systemic correlates of white adipose tissue inflammation in early-stage breast cancer. Clin Cancer Res. 2016;22:2283–2289. doi: 10.1158/1078-0432.CCR-15-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Morris PG, Zhou XK, Gucalp A, Giri DD, Harbus M, Falcone DJ, Vahdat LT, Awad MI, Krasne MD, Subbaramaiah K, Morrow M, Hudis CA, Dannenberg A. Obesity and menopausal status as determinants of procarcinogenic breast inflammation. J Clin Oncol. 2014;32(26_suppl):40. [Google Scholar]
- 11.Subbaramaiah K, Morris PG, Zhou XK, Morrow M, Du B, Giri D, Kopelovich L, Hudis CA, Dannenberg AJ. Increased levels of COX-2 and prostaglandin E2 contribute to elevated aromatase expression in inflamed breast tissue of obese women. Cancer Discov. 2012;2(4):356–65. doi: 10.1158/2159-8290.CD-11-0241. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 12.Morris PG, Hudis CA, Giri D, et al. Inflammation and increased aromatase expression occur in the breast tissue of obese women with breast cancer. Cancer Prev Res (Phila) 2011;4:1021–1029. doi: 10.1158/1940-6207.CAPR-11-0110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Andrieu G, Tran AH, Strissel KJ, Denis GV. BRD4 Regulates breast cancer dissemination through Jagged1/Notch1 signaling. Cancer Res. 2016;76:6555–6567. doi: 10.1158/0008-5472.CAN-16-0559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Klöting N, Blüher M. Adipocyte dysfunction, inflammation and metabolic syndrome. Rev Endocr Metab Disord. 2014;15:277–287. doi: 10.1007/s11154-014-9301-0. [DOI] [PubMed] [Google Scholar]
- 15.Monteiro R, Azevedo I. Chronic inflammation in obesity and the metabolic syndrome. Mediators Inflamm. 2010;2010 doi: 10.1155/2010/289645. pii: 289645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sun X, Casbas-Hernandez P, Bigelow C, et al. Normal breast tissue of obese women is enriched for macrophage markers and macrophage-associated gene expression. Breast Can Res Treat. 2012;131:1003–1012. doi: 10.1007/s10549-011-1789-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Moore LL, Chadid S, Singer MR, Kreger BE, Denis GV. Metabolic health reduces risk of obesity-related cancer in Framingham Study adults. The Framingham Study. Cancer Epidemiol Biomarkers Prev. 2014;23:2057–2065. doi: 10.1158/1055-9965.EPI-14-0240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Phillips CM, Perry IJ. Does inflammation determine metabolic health status in obese and nonobese adults? J Clin Endocrinol Metab. 2013;98:E1610–E1619. doi: 10.1210/jc.2013-2038. [DOI] [PubMed] [Google Scholar]
- 19.Ip B, Cilfone NA, Belkina AC, et al. Th17 cytokines differentiate obesity from obesity-associated type 2 diabetes and promote TNFα production. Obesity (Silver Spring) 2016;24:102–112. doi: 10.1002/oby.21243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Beek L, Lips MA, Visser A, et al. Increased systemic and adipose tissue inflammation differentiates obese women with T2DM from obese women with normal glucose tolerance. Metabolism. 2014;63:492–501. doi: 10.1016/j.metabol.2013.12.002. [DOI] [PubMed] [Google Scholar]
- 21.Allaoui R, Bergenfelz C, Mohlin S, et al. Cancer-associated fibroblast-secreted CXCL16 attacts monocytes to promote stroma activation in triple-negative breast cancers. Nature Commun. 2016;7:13050. doi: 10.1038/ncomms13050. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.