Abstract
This CIBMTR report describes the use of hematopoietic stem cell transplantation (HSCT) in 4408 pediatric patients with cancer undergoing allogeneic (allo) and 3076 undergoing autologous (auto) HSCT in the United States between 2008 and 2014. In both settings, there was a greater proportion of males (n=4327; 57%), children<10 years of age (n=4412; 59%), Caucasians (n=5787; 77%) and children with a performance score ≥ 90% at HSCT (n=6187; 83%). Leukemia was the most common indication for an allo-transplant (n=4170; 94%), and among these, acute lymphoblastic leukemia (ALL) in second complete remission (n=829; 20%) and acute myeloid leukemia in first complete remission (n=800; 19%) were the most common. The most frequently used donor relation, stem cell sources and HLA match were unrelated donor (n=2933; 67%), bone marrow (n=2378; 54%), matched at 8 out of 8 HLA antigens (n=1098; 37%) respectively. Most allo-transplants used myeloablative conditioning (n=4070; 92%) and calcineurin inhibitors and methotrexate (n=2245; 51%) for acute graft versus host disease prophylaxis. Neuroblastoma was the most common primary neoplasm for an auto-transplant (n=1338; 44%). Tandem auto-transplants for neuroblastoma declined after 2012 (40% in 2011, 25% in 2012, 8% in 2014) whereas tandem auto-transplants have increased for brain tumors (57% in 2008, 77% in 2014). Allo-transplants from relatives other than HLA-identical siblings doubled between 2008 and 2014 (3% in 2008 and 6% in 2014). These trends will be monitored in future reports of transplant practices in the US.
Keywords: Hematopoietic stem cell transplantation, Pediatric cancers
Introduction
Hematopoietic stem cell transplant (HSCT) is an established and widely accepted treatment for a variety of malignant and non-malignant disorders.1 Indications and approaches to pediatric HSCT differ significantly from adults in several aspects including underlying diagnoses, associated co-morbidities and stem cell sources. HSCT trends over time, in both adult and pediatric patients have been reported by different groups but a focused view on dedicated pediatric- specific HSCT trends is lacking.2-4
Information on transplant activity is important and relevant for patients, donors, physicians, healthcare providers and regulatory authorities. Establishing the overall trend and activity of HSCT within the pediatric population may lead to improved understanding of the prevalent clinical practices, serve to identify areas of health care and resource disparities and lead to future prospective studies. Lastly, these may also help to predict future trends within the field and help allocate resources to improve patient outcomes.3
This report describes the activity in pediatric HSCT for cancers in the United States between 2008-2014 using data from the Center for International Blood and Marrow Transplant Research (CIBMTR).
Methods
The data reported here comprises all first HSCTs performed in the United States between 2008 and 2014 in children ≤ 18 years of age, as reported to the CIBMTR. The CIBMTR is a voluntary working group of greater than 500 transplant centers worldwide that contribute detailed data on consecutive HSCTs to the Statistical Center located at the Medical College of Wisconsin in Milwaukee and at the National Marrow Donor Program (NMDP) Coordinating Center in Minneapolis. Data quality is ensured by on-site audits of participating centers. All studies conducted by the CIBMTR are performed in compliance with all applicable federal regulations pertaining to the protection of human research participants.
With the establishment of the Stem Cell Transplant Outcome Database (SCTOD) in 2007, all allogeneic transplants (allo-HSCT) performed in the United States are required to be registered with the CIBMTR. Reporting for autologous HSCTs to CIBMTR however, is voluntary.
The data reported here summarizes HSCT characteristics based on patient age, diagnosis, type of transplant (autologous versus allogeneic), type of donor (related versus unrelated), intensity of conditioning regimen, Human Leukocyte Antigen (HLA) match (matched versus mismatched) and prevalent graft-versus-host disease (GVHD) prophylaxis.
Disease status for pediatric acute lymphoblastic leukemia (ALL) and acute myeloid leukemia (AML) were assigned as first (CR1), second (CR2), or third (CR3) complete remission. Tandem transplants within the autologous HSCT (auto-HSCT) group were defined as planned multiple auto-HSCTs in the same patient.
The definition of myeloablative, reduced intensity and non-myeloablative conditioning regimens were based on intent of the conditioning regimen as reported by the treating center to the CIBMTR. Since the intensity of conditioning regimens were determined by the treating physicians and not by the CIBMTR, no clear definitions of the intensity of conditioning regimens can be outlined on this study, but it is assumed that the commonly accepted guidelines were followed.5,6
Results
Allogeneic HSCT
Data on 4408 pediatric allo-HSCT patients from 119 centers are described (Table 1). Median age of patients at the time of HSCT was 10 years (range <1-18) and approximately 3% of patients (n=119) were under 1 year of age. Fifty eight percent (n=2542) of all patients comprised of males. Fourteen percent of patients (n=621) were assigned a performance score< 90 at the time of transplant. Seventy-seven percent (n= 3422) of patients were Caucasians. Fifty-four percent (n=2378) of patients received bone marrow, 28% (n=1247) received umbilical cord blood (UCB) and 18% (n= 783) received peripheral blood stem cells (PBSC) as their stem cell source. Within the UCB group, 28% of patients (n=349) received a double UCB transplant. Sixty seven percent of donors were unrelated (n=2933) while 33 % (n=1475) were related donors.
Table 1.
Demographics and transplant characteristics in pediatric patients undergoing allogeneic and autologous transplant between 2008 and 2014.
| Allogeneic HSCT N (%) | Autologous HSCT N (%) | |
|---|---|---|
| Number of patients | 4408 | 3076 |
| Number of centers | 119 | 113 |
| Median age at transplant (range), years | 10 (<1-18) | 5 (<1-18) |
| < 1 year | 119 (3) | 84 (3) |
| 1-10 years | 2253 (51) | 2159 (70) |
| 11-18 years | 2036 (46) | 833 (27) |
| Gender | ||
| Male | 2542 (58) | 1785 (58) |
| Female | 1866 (42) | 1291 (42) |
| Lansky performance score at transplant | ||
| ≥90 | 3722 (84) | 2465 (80) |
| <90 | 621 (14) | 507 (16) |
| Missing | 65 (1) | 104 (3) |
| Race | ||
| Caucasian/White | 3422 (78) | 2365 (77) |
| Black | 471 (11) | 428 (14) |
| Asian/Hawaiian/Pacific Islander | 252 (6) | 144 (5) |
| Native American/American Indian | 43 (<1) | 16 (<1) |
| Unknown/Declined | 220 (5) | 123 (4) |
| Ethnicity | ||
| Hispanic | 1169 (27) | 563 (18) |
| Non- Hispanic | 3180 (72) | 2446 (80) |
| Missing | 59 (1) | 67 (2) |
| Treatment protocol | ||
| Single Allogeneic | 4390 (99) | N/A |
| Tandem Autologous-Allogeneic | 18 (<1) | N/A |
| Single Autologous | N/A | 2359 (77) |
| Tandem Autologous-Autologous | N/A | 717 (23) |
| Stem cell source | ||
| Bone marrow | 2378 (54) | 49 (2) |
| Peripheral blood | 783 (18) | 3024 (98) |
| Umbilical cord blood | 1247 (28) | 3 (<1) |
| Donor (allogeneic HSCT only) | ||
| HLA-identical sibling^ | 1152 (26)^ | N/A |
| Other relative | 323 (7) | |
| Unrelated donor | 2933 (67) | |
| HLA Match (other relative donor) | ||
| Matched | 90 (28) | |
| Mismatched | 230 (71) | |
| Unknown | 3(1) | |
| HLA Match (unrelated donor) | ||
| Peripheral blood or bone marrow matched 8/8 | 1098 (37) | |
| Peripheral blood or bone marrow mismatched (<8/8) | 493 (17) | |
| Peripheral blood or bone marrow matching unknown | 135 (5) | |
| Umbilical cord blood match matched (6/6) | 168 (5) | |
| Umbilical cord blood mismatched (5/6) | 570 (20) | |
| Umbilical cord blood mismatched (<5/6) | 352 (12) | |
| Missing umbilical cord data | 117 (4) | |
| Conditioning intensity (as reported by center, allogeneic HSCT only) | ||
| Myeloablative | 4070 (92) | N/A |
| Reduced intensity/Non-myeloablative | 302 (7) | |
| Missing | 36 (<1) | |
| Total body irradiation containing conditioning | ||
| No | 1350 (31) | 2511 (82) |
| Yes | 2725 (62) | 23 (<1) |
| Missing | 333 (8) | 542 (18) |
| Total body irradiation in acute lymphoid leukemia | ||
| No | 117 (6) | |
| Yes | 1752 (92) | |
| Missing | 28 (2) | |
| Total body irradiation in acute myeloid leukemia | ||
| No | 796 (52) | |
| Yes | 544 (35) | |
| Missing | 193 (13) | |
| GVHD prophylaxis (allogeneic HSCT only) | ||
| Ex-vivo T-cell depletion | 122 (3) | N/A |
| CD34+ selection | 146 (3) | |
| Post-transplant Cyclophosphamide | 28 (<1) | |
| Calcineurin inhibitor alone (either tacrolimus or cyclosporine) | 178 (4) | |
| Calcineurin inhibitor + Mycophenolate mofetil | 1377 (31) | |
| Calcineurin inhibitor + Methotrexate | 2245 (51) | |
| Calcineurin inhibitor + other | 234 (5) | |
| Other(s)* | 52 (1) | |
| Missing | 26 (<1) | |
| Median follow-up of survivors (range), months | 37 (<1-88) | 35 (1-84) |
Syngeneic HSCTs n=9
MTX ± other (not CNI) (n=43), MMF ± other (not CNI) (n=9)
Forty one percent (n=1207) of unrelated donors received umbilical cord blood and of these patients, 47% (n=570) were HLA matched at 5 of 6 antigens, followed by 29% (n=352), who were HLA matched at <5/6 antigens. Of the patients who received either unrelated bone marrow or peripheral blood stem cells, 37% (n=1098) were HLA matched at 8 out of 8 antigens, followed by 17% (n=493) who were HLA mismatched. In the related donor transplant group, 21% (n=323) were reported as other relative donors. Of these other relative donors, 71% (n=230) were HLA mismatched while 28% (n=90) were HLA matched at 8 of 8 antigens. Seventy nine percent (n=1152) of related donors were HLA identical sibling donors.
Ninety-two percent of patients (n=4070) received a myeloablative conditioning (MAC) regimen, of which 67 %(n=2725) included total body irradiation (TBI). A combination of a calcineurin inhibitor with methotrexate (51%, n=2245) was the predominant GVHD prophylaxis regimen followed by calcineurin inhibitor with mycophenolate mofetil (31%, n=1377). Post-transplant cyclophosphamide as acute GVHD prophylaxis was used in <1% of allo-HSCTs. Ex-vivo T-cell depletion (n=122) and CD34+ selection (n=146) was performed in a total of 6% of patients.
The utilization of different intensities of conditioning regimens, donor type/relation and stem cell sources from 2008-2014 are shown in Figure 1A, 1B and 1C. The overall predominance of myeloablative conditioning regimens (∼90%) and unrelated donors (∼40%) have remained fairly stable over the last 7 years. There was a modest decline in the use of umbilical cord blood (32% in 2008 and 23% in 2014) while the use of bone marrow as a stem cell source has remained relatively stable (50% in 2008 and 58% in 2014). The use of double umbilical cord blood has also remained stable over the last 7 years (25% in 2008 and 23% in 2014). Within donor relation, despite small numbers, it was observed that other relative donors have doubled (3% in 2008 and 6% in 2014) (Figure 1B).
Figure 1.
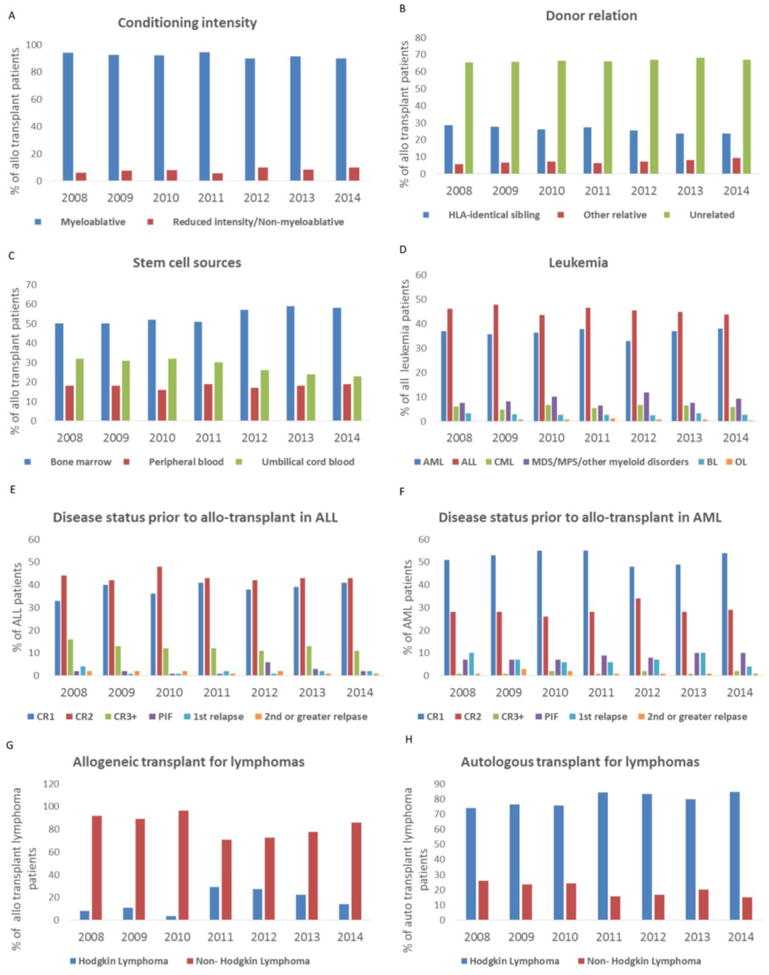
1A. Conditioning intensity for all allogeneic transplants between 2008 and 2014. The percentage of allogeneic transplant patients who received either myeloablative or a reduced intensity or non/myeloablative conditioning regimen are shown.
1B. Donor relation in allogeneic HSCT between 2008 and 2014. The percentage of all allogeneic transplants that received either an HLA-identical sibling donors, other relative donors or unrelated donors are shown.
1C. Stem cell sources in allogeneic HSCT between 2008 and 2014. The percentage of all allogeneic transplants that received either a bone marrow, peripheral blood stem cell or umbilical cord blood are shown.
1D. Allogeneic transplant for various leukemia subtypes between 2008 and 2014. The percentage of all leukemia patients that received an allogeneic transplant that were acute myeloid leukemia (AML), acute lymphoid leukemia (ALL), chronic myeloid leukemia (CML), myelodysplastic syndrome/myeloproliferative neoplasms or other myeloid disorders (MDS/MPN), bi-phenotypic leukemia (BL) or other leukemia (OL) are shown.
1E. Disease status prior to an allogeneic transplant in acute lymphoid leukemia (ALL) is shown. Percentage of ALL patients transplanted in first complete remission (CR1), second complete remission (CR2), third complete remission (CR3), primary induction failure (PIF), first relapse and second or greater relapse is shown.
1F. Disease status prior to an allogeneic transplant in acute myeloid leukemia (AML) is shown. Percentage of AML patients transplanted in first complete remission (CR1), second complete remission (CR2), third complete remission (CR3), primary induction failure (PIF), first relapse and second or greater relapse is shown
1G. Percentage of patients with lymphoma who received an allogeneic transplant between 2008 and 2014.
1H. Percentage of patients with lymphoma who received an autologous transplant between 2008 and 2014.
Leukemia
Leukemia was the most common indication for an allo-HSCT (n= 4170, 95%). The number of transplants for various subtypes of leukemia, such as ALL, AML, chronic myeloid leukemia (CML), and bi-phenotypic leukemia have been stable over the past 7 years (Figure 1D). The number of patients transplanted for ALL (n=1896, 46%) was higher than AML (n=1516, 36%) (Table 2). The number of transplants for myelodysplastic syndrome (MDS) and myeloproliferative neoplasms (MPN) have also remained stable over the past 7 years (Figure 1D). TBI was part of the conditioning regimen in 92% (n=1752) of patients with ALL and 35% (n=544) of patients with AML (Table 1).
Table 2.
Pediatric allogeneic and autologous transplants described by donor and stem cell source.
| Allogeneic (Allo) | Autologous (Auto) | Total | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Related Donors | Unrelated Donors | ||||||||||||||||
| HLA id sibling | Other relative | Total | Total | Allo | Auto | Total Allo + Auto | |||||||||||
| Indication for transplant | BM* | PB^ | CB# | BM | PB | CB | BM | PB | CB | BM | PB | CB | |||||
| Total | 957 | 165 | 30 | 185 | 128 | 10 | 1475 | 1236 | 490 | 1207 | 2933 | 49 | 3024 | 3 | 4408 | 3076 | 7484 |
| Leukemia | 910 | 135 | 29 | 168 | 117 | 10 | 1369 | 1177 | 455 | 1169 | 2801 | 2 | 16 | 0 | 4170 | 18 | 4188 |
| AML& | 323 | 46 | 11 | 56 | 39 | 3 | 478 | 413 | 178 | 447 | 1038 | 2 | 15 | 0 | 1516 | 17 | 1533 |
| ALL@ | 456 | 57 | 15 | 85 | 58 | 3 | 674 | 493 | 203 | 526 | 1222 | 0 | 1 | 0 | 1896 | 1 | 1897 |
| CML+ | 45 | 10 | 2 | 8 | 2 | 1 | 68 | 97 | 20 | 65 | 182 | 0 | 0 | 0 | 250 | 0 | 250 |
| Bi-phenotypic leukemia | 25 | 8 | 0 | 7 | 8 | 0 | 48 | 25 | 8 | 37 | 70 | 0 | 0 | 0 | 118 | 0 | 118 |
| MPN%/MDS$ | 56 | 13 | 1 | 12 | 8 | 3 | 93 | 139 | 45 | 86 | 270 | 0 | 0 | 0 | 363 | 0 | 363 |
| Other leukemias | 5 | 1 | 0 | 0 | 2 | 0 | 8 | 10 | 1 | 8 | 19 | 0 | 0 | 0 | 27 | 0 | 27 |
| Lymphoma | 44 | 25 | 1 | 13 | 9 | 0 | 92 | 57 | 32 | 36 | 125 | 11 | 495 | 0 | 217 | 506 | 723 |
| Hodgkin lymphoma | 5 | 5 | 0 | 4 | 2 | 0 | 16 | 11 | 6 | 0 | 17 | 5 | 396 | 0 | 33 | 401 | 434 |
| Non-Hodgkin lymphoma | 39 | 20 | 1 | 9 | 7 | 0 | 76 | 46 | 26 | 36 | 108 | 6 | 99 | 0 | 184 | 105 | 289 |
| Plasma cell disorder | 0 | 1 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 0 | 5 | 0 | 2 | 5 | 7 |
| Solid tumors | 3 | 4 | 0 | 4 | 2 | 0 | 13 | 1 | 3 | 2 | 6 | 36 | 2508 | 3 | 19 | 2547 | 2566 |
| Medulloblastoma | 1 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 11 | 377 | 1 | 2 | 389 | 391 |
| Other CNS tumors | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 13 | 395 | 1 | 0 | 409 | 409 |
| Neuroblastoma | 1 | 2 | 0 | 1 | 0 | 0 | 4 | 0 | 0 | 1 | 1 | 4 | 1333 | 1 | 5 | 1338 | 1343 |
| Ewing family tumors | 1 | 1 | 0 | 0 | 2 | 0 | 4 | 0 | 2 | 1 | 3 | 2 | 127 | 0 | 7 | 129 | 136 |
| Retinoblastoma | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 3 | 32 | 0 | 0 | 35 | 35 |
| Germ cell tumors | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 68 | 0 | 0 | 68 | 68 |
| Wilms tumor | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | 68 | 0 | 0 | 70 | 70 |
| Gonadal tumors | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 44 | 0 | 0 | 45 | 45 |
| Rhabdomyo sarcoma | 0 | 1 | 0 | 2 | 0 | 0 | 3 | 0 | 1 | 0 | 1 | 0 | 17 | 0 | 4 | 17 | 21 |
| Other solid tumors | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 47 | 0 | 1 | 47 | 48 |
Acute myeloid leukemia,
Acute lymphoblastic leukemia,
Chronic myeloid leukemia,
Myeloproliferative neoplasm,
Myelodysplastic syndrome,
Bone marrow,
Peripheral blood stem cells,
Cord blood
Within pediatric AML, 98.8% of patients (n=1516) received an allo-HSCT. Of these patients, 29.4% received unrelated UCB (n=447), 27.2% received unrelated bone marrow (n=413) and 21.3% of patients (n=323) received bone marrow from a matched sibling donor (Table 2). Within pediatric ALL, 99.9% of patients received an allo-HSCT. Of these patients, 27.7 % (n=526) received an unrelated UCB, 26% (n=493) received unrelated bone marrow and 24% of patients (n=456) received bone marrow from a matched sibling donor (Table 2). Therefore, within pediatric AML and ALL, the use of an unrelated UCB was comparable to bone marrow from either a sibling or unrelated donor. Unrelated UCB use was also comparable to marrow utilization from either a sibling or unrelated donor in pediatric bi-phenotypic leukemia, and MDS/MPN (Table 2).
Trends of disease status prior to transplant for pediatric ALL and AML are shown in Figure 1E and 1F. Transplant in CR1 remains the most common indication in AML (n=800, 52% of all AML transplants) while transplant in CR2 is most common in ALL (n=829, 43.7% ALL transplants) over the last 7 years. Trends for transplant in CR2, CR3, and in relapse remain unchanged over the last 7 years for AML. Similarly, trends for transplant in CR1, primary induction failure, and in relapse remain unchanged in pediatric ALL over the last 7 years. A small decrease in transplants in CR3 in pediatric ALL (16% in 2008 and 11% in 2014) was observed.
Lymphoma
Approximately 5% of all allogeneic HSCTs (n=217) were performed for an underlying diagnosis of a lymphoma. Non-Hodgkin lymphoma (NHL) was the most common indication for an allo- HSCT among these disorders (85%, n=184). Allo-HSCT for Hodgkin lymphoma demonstrated a decline in 2010, followed by an increase in 2011 and then a slow decline again (Figure 1G). Allogeneic HSCT for NHL also demonstrated a decline in 2011 followed by an increase annually thereafter. Similar to pediatric leukemia, unrelated bone marrow (n=46) and unrelated UCB (n=36) were comparable to sibling marrow transplants (n=39) in pediatric allo-HSCTs for NHL (Table 2).
Autologous HSCT
Data on 3076 pediatric auto-HSCT patients from 113 centers are shown in Table 1. Median age of patients at the time of HSCT was 5 years (range<1-18 years) and 70% (n=2159) were between 1-10 years of age. Males comprised 58% (n=1785) of all autologous HSCT patients. Sixteen percent (n=507) of patients reported a performance score of <90 at the time of transplant. Similar to allo-HSCT, 77% (n=2365) of patients were Caucasian. Of the auto-HSCTs, 23% (n=717) were tandem HSCTs. PBSC were used in 98% (n=3024) of all auto-HSCTs.
Neuroblastoma was the predominant indication for single auto-HSCT (n=1338; 43.4%) followed by medulloblastoma (n=389; 12.6%), with overall percentages remaining stable from 2008 to 2014 (Figure 2A). The use of tandem auto-HSCT for neuroblastoma has declined since 2012 (40% in 2011, 25% in 2012, 8% in 2014) while it has increased for central nervous system tumors from 2008 to 2014 (57% in 2008, 77% in 2014) (Figure 2B). Auto-HSCT activity for Ewing's family of tumors was low and variable over the last 7 years (e.g. 9% of auto-HSCTs in 2008-2009 and 4% in 2013). Lymphoma was the indication for transplant in 16% (n=506) of auto-HSCTs from 2008 to 2014. Within this group, Hodgkin lymphoma was the most common indication (79%, n=401). Auto-HSCT for Hodgkin lymphoma shows increased activity over last seven years (74% in 2008 and 84% in 2014). In contrast, activity in auto-HSCT for NHL shows a decline from 2008 to 2014 (26% in 2008 and 15% in 2014) (Fig 1H).
Figure 2.
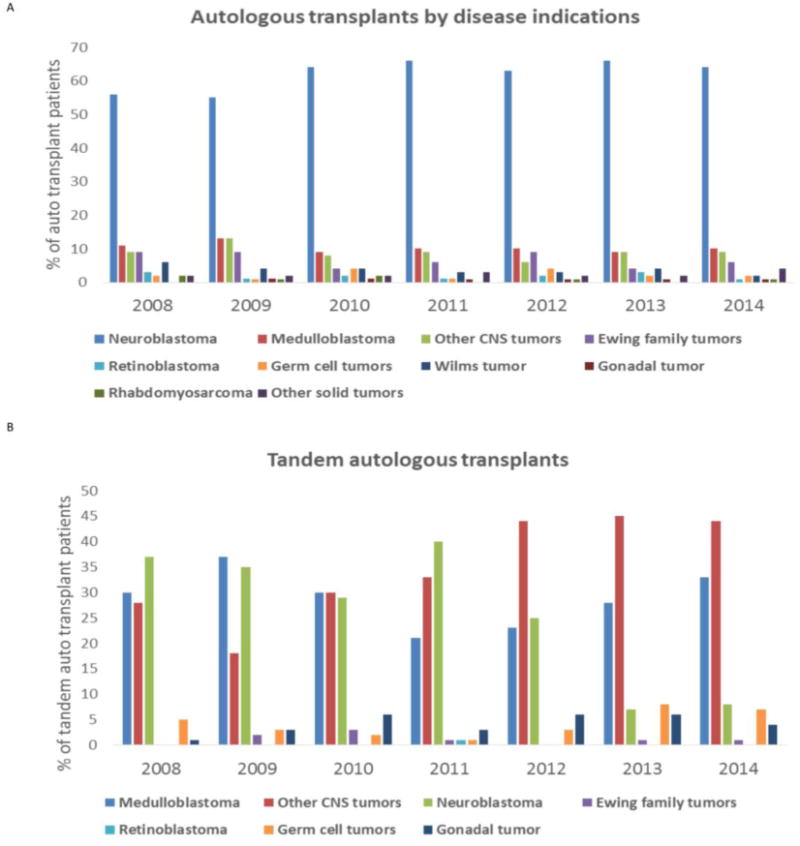
2A. Percentage of patients with various solid tumors who underwent an autologous transplant between 2008 and 2014. Percentage of all autologous transplant patients with an underlying diagnosis of a neuroblastoma, medulloblastoma, other central nervous system (CNS) tumors, Ewing family tumors, retinoblastoma, germ cell tumors, Wilms tumor, gonadal tumors, rhabdomyosarcoma and other solid tumors.
2B. Percentage of patients with various solid tumors who received tandem autologous HSCTs between 2008 and 2014. Percentage of all tandem autologous transplant patients with an underlying diagnosis of medulloblastoma, other central nervous system (CNS) tumors, neuroblastoma, Ewing family tumors, retinoblastoma, germ cell tumors and gonadal tumors.
Discussion
This pediatric focused report from the CIBMTR highlights contemporary trends in allogeneic and autologous HSCT use for pediatric cancers in the United States. Mandatory reporting ensures that data capture by the Stem Cell Therapeutic Outcomes Database (SCTOD) for allo-HSCT is a complete and reliable representation of current practices.
In the US, unrelated donor transplants have surpassed transplants using sibling donors for malignancies. Increase in unrelated donor transplants in the US is likely a testament to an expanding unrelated donor registry, better HLA matching, improved supportive care for HSCT and comparable outcomes of related and matched unrelated donors.7 Increasing unrelated donor transplants could also be a reflection of smaller families, which lowers the likelihood of finding a sibling donor. The majority of children undergoing an allogeneic HSCT for cancers were Caucasians. Although this is not surprising given that the majority of the US population is comprised of Caucasians, it could perhaps be a reflection of the limited NMDP donor pool for non-Caucasians and an underlying health care disparity in the minority groups.8
Mismatched unrelated donor stem cell sources (<8 out of 8 HLA matched bone marrow or peripheral blood and <6/6 HLA matched umbilical cord blood; n=1415) are being utilized comparably to fully matched unrelated donor stem cell sources (8 of 8 HLA matched bone marrow or peripheral blood stem cells and 6 out of 6 HLA matched cord blood; n=1266). This is likely due to tolerance of HLA mismatched donors in high-risk pediatric malignancies along with improved cord blood banking and supportive care measures for patients. There is also a potential for an improved graft-versus-leukemia effect due to the increased tolerance for HLA mismatch.
Percentage of other relative donors has doubled in 2014 compared to 2008; although the overall numbers of these remain small. The majority of other relative donors were HLA mismatched. As detailed information regarding the degree of HLA match for this specific subgroup was beyond the scope of this report, it is difficult to comment with certainty if these other relative donors were being utilized for haplo-identical transplants.9 The 2013 EBMT survey reports an increasing use of haplo-identical donor transplants in Europe.10 It is possible that, especially with novel strategies such as post-HSCT cyclophosphamide and TCRαβ T-cell depletion,11 haplo-identical HSCTs may increase in the United States as well in the future. This trend will need to be observed closely in future reports.
The preference for bone marrow and UCB grafts over PBSCs for allo-HSCT in pediatric setting remain steady, likely due to the known higher risk of chronic GVHD associated with PBSC grafts. 12 Unrelated UCB grafts are still being used comparably to bone marrow grafts in pediatric AML, ALL, bi-phenotypic leukemia and MPN/MDS. This is likely related to increased and improved inventories of the cord blood banks and faster availability of this product, which is an important consideration during HSCT for malignancies. Use of double UCB grafts have remained stable over the last 7 years, however it is possible that its use will decline in subsequent years following the results of the BMT Clinical Trials Network/Children's Oncology Group study which did not demonstrate any survival advantage after a double UCB transplant in hematological malignancies 13 as well as the increasing use of stem cell expansion modalities and haplo-identical donor transplants. Ultimately, the overall cost of procuring double cord units and the consideration of improved stem cell doses in double cord blood units in individual instances may continue to influence this parameter and our supposition will need to be evaluated in future observations over years.
Interestingly, within pediatric leukemia, TBI was employed in 35% of AML patients despite lack of evidence of a distinct advantage of using TBI in children with AML in CR1 or beyond.14-16 We anticipate that in future observations, the use of TBI in AML will decline in the face of existing evidence which demonstrates a lack of superiority of TBI over busulfan based regimens. Transplant trends for pediatric ALL in CR2 and AML in CR1, the two most common indications, remain unchanged over the last 7 years. The stable rates of transplant for children with AML in CR1 during 2008-2014 probably reflects the guidelines in Children's Oncology Group (COG) AAML0531 (2006-2010) and the current COG study AAML1031 (2011- present) where low risk patients with AML in CR1 are treated with chemotherapy and transplant is deferred until CR2. The overall numbers of HSCTs for CML, MDS/MPN/other myeloid disorders, bi-phenotypic leukemia and other leukemia have also remained stable over 2008-2014. A slight decline in transplants for ALL in CR3 is likely explained either by advances in prognostic factors such as minimal residual disease monitoring and referral in CR2 if patients demonstrate MRD positivity despite late relapse17 or by an increasing use of chimeric-antigen-receptor (CAR)-T cells18 and/or novel biologic therapies19 over the past few years.
We recognize that open clinical trials during the time of our data collection might have influenced several observations made in this report. Examples of such trials include the Bone Marrow Transplant – Clinical Trials Network protocol 0501 which was a phase III randomized multi- institutional clinical trial of single versus double umbilical cord blood transplantation in children with hematological malignancies using fludarabine, cyclophosphamide and TBI as the conditioning regimen. The specifications of this trial could have contributed to the use of TBI in pediatric AML and the completion of this trial in 2012 could account for the decline in UCB use over time in all allogeneic transplants. We believe that it would be prudent to view the results of our study through the lens of ongoing open clinical trials during the time of our data reporting, which could have a significant influence on some of the trends observed.
Within lymphomas, allo-HSCTs for NHL are predominant and stable over the past 7 years. There was an increase in allo-HSCTs for Hodgkin lymphoma in 2011 followed by a slow decline over the next few years. In 2010, a retrospective report demonstrated a survival advantage for patients who receive a RIC allo-HSCT for Hodgkin lymphoma after a relapse following an auto-HSCT20, likely lending enthusiasm to this approach. However, overall number of patients receiving an allo-HSCT for Hodgkin lymphoma remains small, and therefore this trend should be interpreted with caution.
High dose chemotherapy and auto-HSCT is still the standard of care for patients with Hodgkin lymphoma who relapse with non-localized disease after frontline therapy and this is reflected by the predominance of auto-HSCTs for Hodgkin lymphoma within the lymphoma group. Neuroblastoma continues to be the predominant indication for auto-HSCTs over the past 7 years. Tandem transplants for neuroblastoma however demonstrated a decline 2012 onwards. A likely reason for this transient decline in tandem transplants for neuroblastoma could be due to the increased use of immune based therapies such as anti-GD2 antibodies.21 This decline will need to be evaluated in future observations, since the most recent randomized controlled trial conducted by the Children's Oncology Group demonstrated an improved event free survival with tandem transplants for neuroblastoma.22 Tandem transplants are increasing for CNS tumors, consistent with data that has shown improved survival with this approach.23, 24
We anticipate that this observed increase in tandem transplants in children with CNS tumors may change in the future with the start of the randomized clinical trial Head Start 4, which will randomize high risk CNS tumor patients to receive either one or three autologous transplants. Transplant for Ewing's sarcoma has remained somewhat controversial with conflicting reports regarding its efficacy. European data and limited retrospective reports demonstrate improved outcomes with auto-HSCT in patients with Ewing sarcoma with high risk features.25, 26 No clear trend was observed in our report with regards to auto-HSCT for Ewing's family tumors and this will also require closer and longer term follow up.
This study has several limitations. Despite the presence of the SCTOD, the data capture is dependent on available reporting from treating centers, especially as reporting on autologous HSCT data to the CIBMTR is voluntary. This report focusses on trends reflecting changes in practice and does not include information pertaining to degree of HLA matching for other relative donors, rates of overall and event-free survival and rates of complications such as acute and chronic GVHD. Despite large overall number of patients, several sub-categories including transplants for ALL in CR3, other family donor transplants and allo-HSCT for Hodgkin lymphoma remain small.
In conclusion, while most pediatric activities in HSCT have remained stable over the last 7 years, several important evolving trends have become apparent. We observed a recent decline in tandem transplants for neuroblastoma and an increase in tandem transplants for central nervous system tumors. We speculate that tandem transplants for neuroblastoma might re-emerge in subsequent years in the United States based on recent clinical trial results. We also speculate that tandem transplants may decline for children with CNS tumors during the enrollment of Head Start 4 and the results of this trial may influence this trend in future observations. We see a small decrease in ALL transplants in CR3 and speculate this to be a result of better prognostic markers and novel cellular and biologic therapies that have emerged recently. We also note the minor increase in the use of other family donors, despite very small numbers and will need to follow this trend over time. Most current publications related to increasing haplo-identical transplants, and decline in the use of double cord blood transplants will likely change this landscape and should be monitored closely in future reports. Similarly, we expect further paradigm changes in the overall HSCT trends for cancer especially with increasing use of cellular therapy and targeted immunotherapies for solid tumors.
Highlights.
In patients with leukemia, acute lymphoblastic leukemia in second complete remission was the most common indication for allogeneic transplantation
Neuroblastoma was the predominant indication for single autologous transplant
Use of tandem autologous transplants has increased for central nervous system tumors while it has declined for neuroblastoma
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Copelan EA. Hematopoietic stem-cell transplantation. The New England journal of medicine. 2006;354(17):1813–1826. doi: 10.1056/NEJMra052638. [DOI] [PubMed] [Google Scholar]
- 2.Pasquini MC, Wang Z, Horowitz MM, Gale RP. 2010 report from the Center for International Blood and Marrow Transplant Research (CIBMTR): current uses and outcomes of hematopoietic cell transplants for blood and bone marrow disorders. Clinical transplants. 2010:87–105. [PubMed] [Google Scholar]
- 3.Passweg JR, Baldomero H, Peters C, Gaspar HB, Cesaro S, Dreger P, et al. Hematopoietic SCT in Europe: data and trends in 2012 with special consideration of pediatric transplantation. Bone marrow transplantation. 2014;49(6):744–750. doi: 10.1038/bmt.2014.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baldomero H, Gratwohl M, Gratwohl A, Tichelli A, Niederwieser D, Madrigal A, et al. The EBMT activity survey 2009: trends over the past 5 years. Bone marrow transplantation. 2011;46(4):485–501. doi: 10.1038/bmt.2011.11. [DOI] [PubMed] [Google Scholar]
- 5.Bacigalupo A, Ballen K, Rizzo D, Giralt S, Lazarus H, Ho V, et al. Defining the intensity of conditioning regimens: working definitions. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2009;15(12):1628–1633. doi: 10.1016/j.bbmt.2009.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Giralt S, Ballen K, Rizzo D, Bacigalupo A, Horowitz M, Pasquini M, et al. Reduced-intensity conditioning regimen workshop: defining the dose spectrum. Report of a workshop convened by the center for international blood and marrow transplant research. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2009;15(3):367–369. doi: 10.1016/j.bbmt.2008.12.497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yakoub-Agha I. Transplantations from HLA-identical siblings versus 10/10 HLA-matched unrelated donors. Seminars in hematology. 2016;53(2):74–76. doi: 10.1053/j.seminhematol.2016.01.013. [DOI] [PubMed] [Google Scholar]
- 8.Majhail NS, Nayyar S, Santibanez ME, Murphy EA, Denzen EM. Racial disparities in hematopoietic cell transplantation in the United States. Bone marrow transplantation. 2012;47(11):1385–1390. doi: 10.1038/bmt.2011.214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schultz KR. Haploidentical transplantation in children. Blood. 2010;115(17):3420–3421. doi: 10.1182/blood-2010-01-263657. [DOI] [PubMed] [Google Scholar]
- 10.Passweg JR, Baldomero H, Bader P, Bonini C, Cesaro S, Dreger P, et al. Hematopoietic SCT in Europe 2013: recent trends in the use of alternative donors showing more haploidentical donors but fewer cord blood transplants. Bone marrow transplantation. 2015;50(4):476–482. doi: 10.1038/bmt.2014.312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maschan M, Shelikhova L, Ilushina M, Kurnikova E, Boyakova E, Balashov D, et al. TCR-alpha/beta and CD19 depletion and treosulfan-based conditioning regimen in unrelated and haploidentical transplantation in children with acute myeloid leukemia. Bone marrow transplantation. 2016;51(5):668–674. doi: 10.1038/bmt.2015.343. [DOI] [PubMed] [Google Scholar]
- 12.Flowers ME, Parker PM, Johnston LJ, Matos AV, Storer B, Bensinger WI, et al. Comparison of chronic graft-versus-host disease after transplantation of peripheral blood stem cells versus bone marrow in allogeneic recipients: long-term follow-up of a randomized trial. Blood. 2002;100(2):415–419. doi: 10.1182/blood-2002-01-0011. [DOI] [PubMed] [Google Scholar]
- 13.Wagner JE, E M, Carter SL, Haut PR, Peres E, Schultz KR, Thompson J, *, Wall DA, MD Kurtzberg J. No survival advantage after double umbilical cord blood (UCB) compared to single UCB transplant in children with hematological malignancy: Results of the Blood and Marrow Transplant Clinical Trials Network (BMT CTN 0501) randomized trial. ASH Annual Meeting 2012; Atlanta, Georgia. [Google Scholar]
- 14.de Berranger E, Cousien A, Petit A, Peffault de Latour R, Galambrun C, Bertrand Y, et al. Impact on long-term OS of conditioning regimen in allogeneic BMT for children with AML in first CR: TBI+CY versus BU+CY: a report from the Societe Francaise de Greffe de Moelle et de Therapie Cellulaire. Bone marrow transplantation. 2014;49(3):382–388. doi: 10.1038/bmt.2013.185. [DOI] [PubMed] [Google Scholar]
- 15.Gupta V, Lazarus HM, Keating A. Myeloablative conditioning regimens for AML allografts: 30 years later. Bone marrow transplantation. 2003;32(10):969–978. doi: 10.1038/sj.bmt.1704285. [DOI] [PubMed] [Google Scholar]
- 16.Sisler IY, Koehler E, Koyama T, Domm JA, Ryan R, Levine JE, et al. Impact of conditioning regimen in allogeneic hematopoetic stem cell transplantation for children with acute myelogenous leukemia beyond first complete remission: a pediatric blood and marrow transplant consortium (PBMTC) study. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2009;15(12):1620–1627. doi: 10.1016/j.bbmt.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 17.Mehta PA, Davies SM. Allogeneic transplantation for childhood ALL. Bone marrow transplantation. 2008;41(2):133–139. doi: 10.1038/sj.bmt.1705914. [DOI] [PubMed] [Google Scholar]
- 18.Lee DW, Kochenderfer JN, Stetler-Stevenson M, Cui YK, Delbrook C, Feldman SA, et al. T cells expressing CD19 chimeric antigen receptors for acute lymphoblastic leukaemia in children and young adults: a phase 1 dose-escalation trial. Lancet. 2015;385(9967):517–528. doi: 10.1016/S0140-6736(14)61403-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gerhard Zugmaier, H R, Locatelli Franco, Rizzari Carmelo, Trippett Tanya M, Borkhardt Arndt, et al. A Phase 1/2 Study Of Blinatumomab In Pediatric Patients With Relapsed/Refractory B-Cell Precursor Acute Lymphoblastic Leukemia. Blood. 2013;122(70) [Google Scholar]
- 20.Sarina B, Castagna L, Farina L, Patriarca F, Benedetti F, Carella AM, et al. Allogeneic transplantation improves the overall and progression-free survival of Hodgkin lymphoma patients relapsing after autologous transplantation: a retrospective study based on the time of HLA typing and donor availability. Blood. 2010;115(18):3671–3677. doi: 10.1182/blood-2009-12-253856. [DOI] [PubMed] [Google Scholar]
- 21.Kushner BH, Ostrovnaya I, Cheung IY, Kuk D, Modak S, Kramer K, et al. Lack of survival advantage with autologous stem-cell transplantation in high-risk neuroblastoma consolidated by anti-GD2 immunotherapy and isotretinoin. Oncotarget. 2016;7(4):4155–4166. doi: 10.18632/oncotarget.6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Park JRKS, London WB, Naranjo A, Cohn SL, Hogarty MD, et al. A phase III randomized clinical trial (RCT) of tandem myeloablative autologous stem cell transplant (ASCT) using peripheral blood stem cell (PBSC) as consolidation therapy for high-risk neuroblastoma (HR-NB): A Children's Oncology Group (COG) study. Journal of clinical oncology: official journal of the American Society of Clinical Oncology. 2016;34(suppl) abstr LBA3. [Google Scholar]
- 23.Sung KW, Lim DH, Lee SH, Yoo KH, Koo HH, Kim JH, et al. Tandem high-dose chemotherapy and auto-SCT for malignant brain tumors in children under 3 years of age. Bone marrow transplantation. 2013;48(7):932–938. doi: 10.1038/bmt.2012.263. [DOI] [PubMed] [Google Scholar]
- 24.Sung KW, Lim do H, Son MH, Lee SH, Yoo KH, Koo HH, et al. Reduced-dose craniospinal radiotherapy followed by tandem high-dose chemotherapy and autologous stem cell transplantation in patients with high-risk medulloblastoma. Neuro-oncology. 2013;15(3):352–359. doi: 10.1093/neuonc/nos304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ljungman P, Bregni M, Brune M, Cornelissen J, de Witte T, Dini G, et al. Allogeneic and autologous transplantation for haematological diseases, solid tumours and immune disorders: current practice in Europe 2009. Bone marrow transplantation. 2010;45(2):219–234. doi: 10.1038/bmt.2009.141. [DOI] [PubMed] [Google Scholar]
- 26.Fraser CJ, Weigel BJ, Perentesis JP, Dusenbery KE, DeFor TE, Baker KS, et al. Autologous stem cell transplantation for high-risk Ewing's sarcoma and other pediatric solid tumors. Bone marrow transplantation. 2006;37(2):175–181. doi: 10.1038/sj.bmt.1705224. e-pub ahead of print 2005/11/08. [DOI] [PubMed] [Google Scholar]


