Abstract
Germinal centers (GCs) are dynamic microenvironments that form in the secondary lymphoid organs and generate somatically mutated high-affinity antibodies necessary to establish an effective humoral immune response. Tight regulation of GC responses is critical for maintaining self-tolerance. GCs can arise in the absence of purposeful immunization or overt infection (called spontaneous GCs, Spt-GCs). In autoimmune-prone mice and patients with autoimmune disease, aberrant regulation of Spt-GCs is thought to promote the development of somatically mutated pathogenic autoantibodies and the subsequent development of autoimmunity. The mechanisms that control the formation of Spt-GCs and promote systemic autoimmune diseases remain an open question and the focus of ongoing studies. Here, we discuss the most current studies on the role of Spt-GCs in autoimmunity.
Keywords: autoimmunity, spontaneous germinal centers, ectopic germinal centers, self-tolerance
Germinal Centers (GCs)
Germinal centers (GCs) are specialized microarchitectures developed in the secondary lymphoid organs to produce high-affinity antibodies against microbial antigens. Naive B cells initiate a GC reaction by responding to an antigen and obtaining help from CD4+ T cells at the B cell:T cell border that recognize cognate antigen (Figure 1) (1). B cells that obtain CD4+ T cell help seed into the B cell follicle and rapidly proliferate in the presence of Follicular Dendritic Cells (FDCs) to form early GC structures (2). After several days of early GC B cell proliferation, a subset of GC B cells express CXCR4 to migrate toward CXCL12 gradients that are maintained by Reticular Cells (3). The region that contains these rapidly proliferating B cells and Reticular Cells is named the dark zone of the GC, where B cells undergo two major maturation processes of class-switching and somatic hypermutation, as centroblasts. Class-switching is a process by which B cells express a new antibody constant region (IgA, IgE, or IgG) to acquire effector function features that are required to clear a specific type of infection.
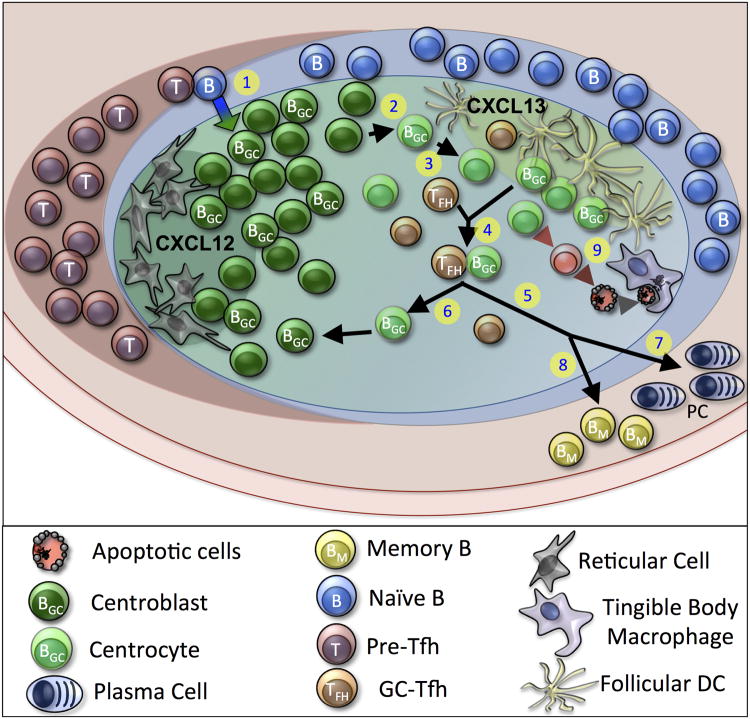
Figure 1. Schematic of the Germinal Center (GC) Reaction.
(1.) Naive B cells initiate a response to antigen and obtain CD4+ T cell help at the B cell:T cell border. (2.) GC B cells rapidly proliferate as centroblasts and interact with Reticular Cells that secrete CXCL12 within the dark zone of the GC. Centroblasts differentiate into centrocytes and follow a CXCL13 gradient that is produced by follicular dendritic cells (FDCs) in the light zone. (3.) Centrocytes compete for limiting antigen within immune complexes that are trapped on the surface of FDCs. (4). Centrocytes with high-affinity BCRs process and present antigen via MHCII to limiting cognate Tfh. In turn, Tfh provide survival signals for the conjugated GC B cell to undego several different fates. (5). GC B cells can remain within the GC and undergo another round of mutation and class switching or (6.) leave the GC and mature to (7.) long-lived plasma cells or (8.) memory B cells that can persist in the periphery for extended periods of time. (9.) Centrocytes that fail to be selected will undergo apoptosis and will be readily cleared from the GC by tingible body macrophages to prevent the buildup of self-antigen.
During somatic hypermutation, B cells use activation induced cytidine deaminase (AID) to induce point mutations in the variable region of the B cell receptor gene to generate a diverse population of GC B cells from the founder B cell clone (4). Due to the random nature of this process, B cells with lower affinity, higher affinity, and novel autoreactivity are generated, necessitating strict selection of the B cells with optimal antigen affinity to maintain tolerance and select for B cells with improved fitness (5, 6). GC B cells then translocate to the light zone of the GC by using CXCR5 to follow a CXCL13 gradient that is maintained by FDCs to recruit GC B cells (centrocytes) and T cells into the light zone for selection (7-9). Within the light zone, GC B cells compete for limiting antigen complexes trapped on the surface of FDCs. B cells which acquire antigens from the FDCs process and present the antigen to follicular helper T (Tfh) cells located in the light zone of GCs in order to receive survival signals for positive selection. Well-regulated selection of GC B cells is vital for maintaining self-tolerance and several immune cell types are utilized in GC B cell selection.
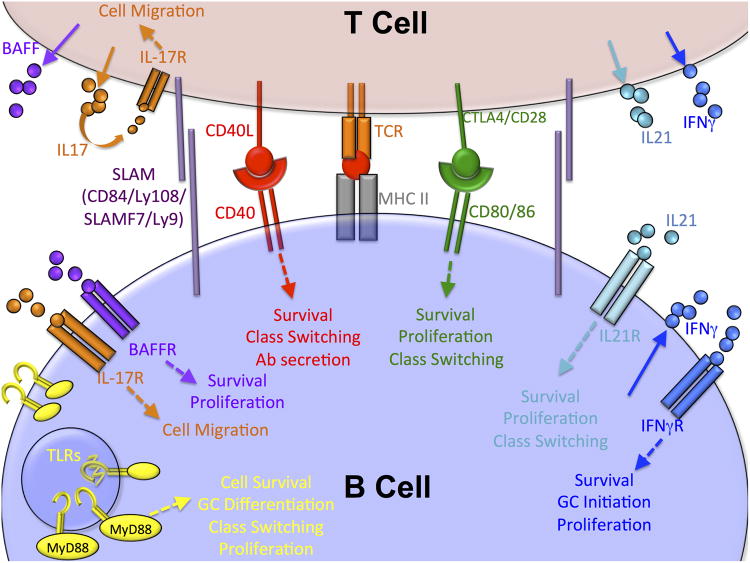
The primary function of T cells within the GC is to support GC stability by costimulation and cytokine signals. To directly regulate GC B cell activity, Tfh utilize a variety of costimulatory molecules including ICOS, SLAM family molecules, CD40L and CTLA4 (10-13). Tfh also secrete cytokines that support GC B cell activity, including BAFF to promote GC B cell survival (14), IL-17 to control B and T cell migration (15), IL-21, IL-4 and IFNγ to drive GC B cell differentiation (16-20) and osteopontin to dampen BCR-mediated apoptosis of GC B cells (21) (Figure 2). Follicular regulatory T cells (Tfr) are a distinct subset of T cells that repress Tfh differentiation to regulate the size of the GC by a Bcl-6/SAP mediated mechanism (22). Tight regulation of costimulatory signaling is required for maintaining B cell tolerance. In the context of autoimmune disease, dysregulation of T cell populations within the GC also can alter GC B cell activation and differentiation by several different mechanisms to break self-tolerance (23).
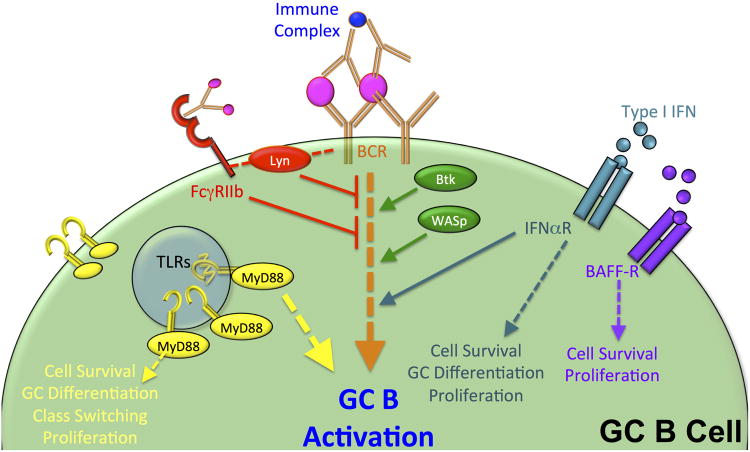
Figure 2. Signaling Pathways that modulate B Cell Receptor (BCR) signaling in Spt-GCs.
Several signaling pathways have been shown to either promote or inhibit BCR signaling in the GC. BCR signaling in the GCs is triggered by BCR interaction with antigen complexes that are captured on the surface of FDCs (Fig 1). Lyn and FcgRIIb (shown in red) have been shown to limit BCR signaling and limit Spt-GC formation. However, TLRs (shown in yellow), Btk (in green), WASp (in green) and CD20 (blue) have been shown to promote BCR signaling pathways and enhance Spt-GC formation. Cytokine signals from FDCs (BAFF) and pDCs (type 1 interferon) also promote BCR signaling as well as GC B cell survival.
Spontaneous Germinal Centers (Spt-GCs)
Recently, with more sensitive techniques and methods for detecting GCs, several labs have discovered that GCs can spontaneously develop (named Spt-GCs) in the absence of overt immunization or detectable adventitious infection (18, 24-28). Spt-GCs are detected in both non-autoimmune and autoimmune strains of mice, but the size and frequency of Spt-GCs are augmented in autoimmune-prone mice, leading to the development of class-switched pathogenic antibodies that drive antibody-mediated autoimmune disease (18, 24, 25, 27, 28). Spt-GCs are reported in several autoimmune diseases in both humans and animal models.
The development of GC structures without an overt immune challenge was first observed in autoimmune-prone (NZB×NZW) F1 (NZB/W) mice and anti-CD8 monoclonal antibody-treated C3H/lpr and C3H/gld mice (26, 29). Furthermore, Mackay and colleagues described the development of “germinal centers in the absence of immunization”, in transgenic mice that overexpress BAFF, to promote systemic autoimmune disease (30). In support of the role of Spt-GCs in promoting autoimmunity, the increased GC sizes correlated with an increased number of autoantibody-producing B cells (25). Also, in type 1 diabetes patients, Spt-GC structures form and specifically recognize self-antigen (31).
Lunzina and colleagues further extended the studies of Spt-GCs to rule out the possibility that Spt-GCs form due to asymptomatic infection. In their study of Spt-GCs, several autoimmune-prone mouse lines (NOD, PN, NZB, NZB/W, MRL lpr/lpr, MRL+/+, and B6 lpr/lpr, and male BXSB mice) developed Spt-GCs while the collected blood tested negative for monitored bacterial, viral, or mycoplasma infections (24). Spt-GCs in these mice required CD40-CD40L interaction to form, and were enlarged in aged mice (24). Mice used in this study were also obtained from several different breeding colony locations to account for local endogenous infections (24).
Although overt immunogenic challenge and adventitious infections have been shown not to be causal factors for Spt-GC formation (24), further studies are required to define the role of endogenous viral populations or retroviral elements in promoting Spt-GCs. In support of the role of endogenous viral infections, transgenic expression of an EBV-encoded CD40 mimic in autoimmune-prone mice promotes a break in tolerance through Spt-GC formation and dysregulation of B cell selection (32). Further, Human Endogenous Retroviruses (HERVs) have been proposed as environmental factors that promote a loss of self-tolerance in genetically susceptible patients (33). HERVs integrate into the genome and promote the initial loss of tolerance by stimulating self-reactive B cells through molecular mimicry (33). In SLE, autoantibodies that are targeted to the 70k/U1 snRNP autoantigen, also exhibit cross-reactivity with a p30 gag protein expressed by a specific class of HERVs (33, 34). As many as 50% of SLE patients express p30 gag-reactive Ab titers while only 3.7% of healthy controls express these antibodies (34), suggesting that further studies into the role of HERVs in persistent Spt-GC responses might provide some insights into this correlation.
At the sites of autoimmune inflammation, transient lymphoid structures (called ectopic lymphoid structures, ESLs) can develop in response to inflammatory cytokine signals (35). ESLs also contain GCs called ectopic germinal centers (e-GCs) that may help generate class-switched and somatically mutated B cell populations at the site of inflammation (35). In autoimmune diseases, ESLs and e-GCs develop in the absence of overt infection to promote chronic relapsing inflammation (35-39). In rheumatoid arthritis (RA) patients, autoantibodies to several self-antigens are observed in correlation with ESLs and AID-expressing e-GCs in inflamed synovial tissue (37, 40). Correspondingly, e-GCs that form in autoimmune-prone mice are phenotypically similar to Spt-GCs in regard to induction, regulation and activity. Overall, genetic susceptibility to autoimmunity is thought to promote the loss of tolerance through Spt-GCs by driving the generation of antibodies with high-affinity to self-antigens. Several studies have implicated the roles of innate sensing, BCR signaling and costimulatory molecules in promoting Spt-GC formation. In humans, these molecules and various downstream signaling components are altered due to genetic mutations, establishing susceptibility that leads to the loss of self-tolerance.
Role of Spt-GCs in several autoimmune diseases
Systemic Lupus Erythematosus (SLE)
Systemic Lupus Erythematosus (SLE) is a progressive and multifaceted autoimmune disease that is characterized by the production of self-reactive antibodies that target nuclear antigens (called anti-nuclear antibodies or ANAs). ANAs are frequently class-switched and somatically mutated, suggesting that they are most likely derived from GCs (25, 41-44). Using tonsil tissues, Cappione and colleagues have shown that negative selection of self-reactive B cell populations in the GC is defective, allowing for self-reactive B cells to survive in the GC (45). In addition, pediatric patients with SLE exhibit increased pre-GC B cells in circulation as compared to healthy controls and e-GC formation has been reported in the thymic tissue of human SLE patients (46, 47). Spt-GCs are observed in several different SLE mouse models, which all develop enlarged Spt-GC structures.
Rheumatoid Arthritis (RA)
Rheumatoid Factor (RF) and anti-citrullinated protein antibodies associated with Rheumatoid Arthritis (RA) are detected in the sera of 50-70% RA patients and class switched autoantibodies targeted against chaperone proteins, nuclear antigens, enzymes, and components of cartilage are also found in the joint tissue (48, 49). Initial report indicated the production of class-switched and high-affinity RF autoantibodies within the synovial tissue of the inflamed joint in humans, suggesting a potential role of e-GC formation at the site of inflammation in this process (50, 51). Later studies by Weyand and Goronzy confirmed the formation of e-GC structures in the synovial tissues of 24% of RA patients, and additional 20% of the RA patients formed B cell:T cell aggregate structures that lacked FDCs (52).In mouse models of RA, several studies have reported both Spt-GC and e-GC formation that contribute to disease progression. Using the KBxN model that expresses a self-antigen-targeted TCR, two separate labs have reported the presence of Spt-GC structures that form within the spleen of these mice (53, 54).
Multiple Sclerosis (MS)
Multiple Sclerosis (MS) is an autoimmune demyelinating disease that specifically targets the central nervous system to cause progressive paralysis. To recapitulate MS in animal models, most animals require some form of immunization with a self-peptide or treatment with a chemical stimulus to develop experimental autoimmune encephalomyelitis (EAE) (55). This EAE model may not recapitulate the spontaneous nature of Spt-GC formation, making studies of the role of Spt-GCs in EAE challenging. However, some groups have characterized Spt-GC formation in animals by analyzing GC structures after the primary B cell response in EAE mice has ended or by using specialized mouse models with mutant B cell receptors (56). Using a mouse model in which B cells and T cells express receptors that are specific for myelin oligodendrocyte glycoprotein, Dang and colleagues found neither e-GCs in the brain tissue nor Spt-GCs in the secondary lymphoid organs in these BCR knock-in mice compared to wild type control mice without the knock-in gene (56). However, a specialized subset of partially activated B cells that are primed to present antigen were found within inflammatory sites (56).
Autoimmune lymphoproliferative syndrome (ALPS)
Autoimmune lymphoproliferative syndrome (ALPS) is most frequently caused by mutations in the Fas (CD9, Apo-1) gene, which is required for regulation of lymphocyte apoptosis (57, 58). Lymphocyte death via Fas-mediated apoptosis is crucial for maintaining tolerance in the GC. Mouse models with Fas or FasL deficiency develop lupus-like autoimmunity characterized by autoantibody production, lymphadenopathy, splenomegaly and enlarged Spt-GC formation in vivo (24, 59, 60). In addition, overexpression of miR146a, a regulator of the Fas pathway in GC B cells, leads to the development of ALPS-like disease which is associated with enlarged Spt-GC formation, increased autoantibody titers, and evidence of systemic pathological inflammation (61).
Sjogren's Syndrome (SS)
In Sjogren's syndrome (SS), a disease that is characterized by autoimmune inflammation of the salivary and lacrimal glands, the contributions of e-GCs are well characterized (62-65). These GC structures express AID, are regulated by FDC networks, contain BAFF-producing Tfh and produce SS-associated autoantibodies (65-67). Furthermore, in a mouse model of SS, e-GC formation in the salivary gland is also correlated with the development of Spt-GCs in the spleen (68). Current studies regarding the role of e-GCs in SS patients have focused on targeting the chemokines (CXCL1, CXCL13, and CCL21) that regulate the formation of e-GC structures for therapeutic intervention since high expression of these chemokines in patients is associated with progressive disease symptoms (69, 70).
Type 1 Diabetes (T1D)
In autoimmune diabetic disease (Type 1 diabetes, T1D), FDC-containing and AID-expressing e-GCs are formed within the pancreatic islet tissues and contribute to insulitis in non-obese diabetic (NOD) mice (71, 72). Spt-GC formation was first reported by Luzina and colleagues in NOD mouse spleen, but the connection of these two structures to autoimmune disease development was implied (24). Later studies by Wan and colleagues using an insulin-specific B cell model in NOD mice, demonstrated that insulin-binding B cells can form Spt-GCs in multiple lymphoid organs including the draining lymph node, spleen, and non-draining lymph nodes in NOD mice (73). Finally, Spt-GC structures with self-antigen reactive B cells were also observed in the spleens and lymph nodes of human T1D patients (31).
Factors that contribute to Spt-GC formation
The formation and regulation of Spt-GCs is complex. Multiple factors are known to contribute to Spt-GC formation and GC-associated autoimmunity in humans and in mice. These include factors that modulate BCR signaling, alter B:T costimulation/selection, block inhibitory/regulatory receptor function, affect complement-mediated regulation of selection, alter cytokine/chemokine signals and promote self-ligand sensing by pattern recognition receptors.
The Role of B Cell Receptor (BCR) Signaling in Spt-GC formation
A causative link between self-reactive B cell populations and autoimmunity is well established in mouse models and human patients with autoimmune disease (74). Self-reactive B cells are also detected in healthy individuals, but the loss of self-tolerance in autoimmune patients is governed by hyperactive BCR signaling as a result of mutations in BCR signaling molecules (75, 76) (Figure 3). Using a BCR deficient mouse line that expressed the Epstein Barr Virus LMP2A protein, Casola and colleagues reported that BCR signaling strength is a critical contributor to the formation of Spt-GCs (77). LMP2A influences intracellular BCR signaling in the absence of the receptor to control for the effects of antigen specificity. PNA+GL7+CD95+ GC structures in LMP2A expressing mice formed within the Peyer's patches and underwent somatic hypermutation, class switching, relied on T cells for formation and retained a population of FDCs. We and others have shown that BCR specificity for self-antigen is important for the loss of self-tolerance and autoimmunity (25, 78-80).
Figure 3. Signaling Pathways that regulate GC B cell and Tfh selection in Spt-GCs.
After acquiring antigen, B cells present antigen via MHC II to T cells with cognate T cell receptors (TCRs). Several co-stimulatory molecules are involved in B cell activation that leads to GC formation or in the maintenance of GC B cells. Most of the receptors and co-stimulatory molecules that are involved in Spt-GC formation regulate B cell survival, proliferation, and class switching (CD40, CD80/86, IL4R, IL21R, IFNγR, BAFFR, TLRs), but IL-17R controls the migration of Tfh and GC B cells and SLAM family molecules have co-stimulatory functions.
Bruton's Tyrosine Kinase
Bruton's Tyrosine Kinase (Btk) functions through PLC gamma and IKK to regulate B cell development and function (81, 82). Patients that exhibit defects in Btk function develop X-linked agammaglobulinemia (XLA), a disease that is characterized by susceptibility to recurrent bacterial infections due to severely reduced Immunoglobulin formation (83). Mouse models of XID exhibit mutations in Btk that recapitulate the B cell intrinsic phenotypes of the disease (76). Overexpression of Btk using a CD19 promoter increases B cell activation (overexpression of CD80, CD86 and MHCII, and increased calcium flux), B cell susceptibility to Fas-mediated apoptosis, and NF-κB activation (84). Restricting Btk overexpression to B cells is associated with increased Spt-GC and plasma cell formation in the spleen, and this Spt-GC formation requires CD40-CD40L interaction (84, 85). GC structures also fail to form in the lymph nodes of XLA patients (83). In addition, Btk expression in B cells promotes the expansion of Tfh populations in the GC to further support the GC structure (85).
WASp
The Wiskott-Aldrich syndrome protein (WASp) was first identified in association with Wiskott-Aldrich syndrome, an X-linked primary immunodeficiency that is characterized by several autoimmune manifestations including autoimmune cytopenia, arthritis, vasculitis, inflammatory bowel disease and nephropathy (86, 87). Interestingly, WAS patients also exhibit symptoms of primary immunodeficiency including susceptibility to recurrent infections, treatment-resistant eczema and lymphoid malignancies (86). WASp is required for actin polymerization and cytoskeletal organization that allows for lymphocyte migration, immunological synapse formation, regulation of surface receptor expression and intracellular signaling (86, 88). Organism-wide WASp deficiency is associated with higher titers of circulating IgA and IgE, suggesting a B cell intrinsic effect (88).
Several studies have focused on the role of WASp in B cells using different model systems. Overall, WASp in B cells is important for actin cytoskeleton rearrangement, Ca2+ flux after BCR stimulation, B cell development, GC B cell selection and differentiation (88, 89).A reduced number of marginal zone B cells and a proportionally greater number of GC B cells with increased IgM serum antibody titers were observed in mice in which B cells were deficient in WASp (88). Using a bone marrow chimera system, Becker-Herman and colleagues observed increased high-affinity dsDNA-specific IgG titers and enlarged Spt-GC structures when WASp was absent in B cells, suggesting that WASp is a critical negative regulator in B cells that prevents the development of autoimmune disease (75). WASp deficiency in B cells is associated with increased glomerulonephritis and decreased survival of the animals (75). Correspondingly, lentiviral expression of WASp restored B cell development in the spleen and reduced the development of dsDNA-specific IgG2c. (90).
The role of costimulatory molecules in Spt-GC formation
CD40-CD40L
Interaction of CD40 on B cells with the cognate ligand CD40L on Tfh cells is essential for GC differentiation and function (91, 92). Specifically, CD40L-CD40 interaction rescues GC B cells from Fas-mediated apoptosis (91-94), induces GC B cell proliferation (95), initiates immunoglobulin class switching (96), and promotes antibody secretion (97, 98). Both mice and humans that lack functional CD40 or CD40L are incapable of forming antigen induced or Spt-GC structures (96, 99-103). Furthermore, treatment of mice with a CD40L-blocking antibody inhibits GC formation in response to T-dependent antigen immunization and promotes B cell maturation through GC-independent pathways (104, 105). Lunzina and colleagues also confirmed that CD40-CD40L interactions are critical for Spt-GC formation, as anti-CD40L treatment inhibits Spt-GC formation and resultant autoimmunity in mice (24).
CD80 and CD86
CD80/B7-1 and CD86/B7-2 are co-stimulatory molecules that are expressed on the surface of activated antigen presenting cells, including B cells, to interact with CD28 or CTLA-4 on the surface of CD4+ T cells (106, 107). CD80 and CD86 are rapidly upregulated on B cells upon antigen interaction or inflammatory signals to enhance T cell activation and to prevent target T cell anergy (106, 107). In B cells, activation of CD80/CD86 in conjunction with other stimuli induces class switching, B cell proliferation and survival.
Blockade of both CD80 and CD86 in mice by genetic knockout (CD80/CD86-/- mice) or by anti-CD80/86 treatment inhibits GC formation and IgG class switching in both T-dependent antigen-challenged mice and unimmunized control mice (108, 109). Furthermore, CD80/86 deficiency blocked Spt-GC formation in mice that exhibit T cell hyperactivation as a result of a LatY136F mutation (109). Correspondingly, CD28-/- mice also failed to develop Spt-GC structures and exhibit altered class switching (110). With CTLA4 blockade, Tfh differentiation is increased, leading to enlarged Spt-GCs, autoantibody production and autoimmunity development (12). Later studies in B cell intrinsic CD80/CD86-/- mice show formation of GCs with impaired IgG2a responses after influenza challenge (111). Using an HgCl2-induced model of EAE, dual blockade of both CD80 and CD86 significantly reduced GC formation and EAE in Brown Norway Rats (112). Ab-mediated therapeutic blockade of CD80/86 has also protected mice in Ag-induced EAE (113). Blockade of either activation molecule individually was not sufficient to inhibit the loss of self-tolerance (112).
SLAM Family Molecules
Signaling lymphocyte activation molecule (SLAM) family proteins that regulate B cell:T cell interactions, are crucial for maintaining tolerance (25, 114-116). Deletions or point mutations of several Slamf genes have been associated with the development of autoimmune disease in mice and in humans (115-122). In human family-association studies, several single nucleotide polymorphisms (SNPs) in the promoter and coding regions of SLAMF7 and LY9 have been associated with SLE-disease susceptibility (122). Furthermore, defective engagement with SLAMF6 in human T cells is associated with autoimmune disease progression (123).
B6.Sle1b congenic mice, a mouse model for SLE in which the Slamf locus is derived from the lupus-prone NZM2410/NZW strain, develop enlarged Spt-GC structures and accumulate class-switched anti-nuclear antibodies that promote SLE-like autoimmunity (25, 114-116). Enlarged Spt-GC response in B6.Sle1b mice can be reversed and GC tolerance can be restored in B6.Sle1b mice with a B cell-intrinsic overexpression of isoforms of CD84/Slamf5 and Ly108/Slamf6 that are derived from non-autoimmune B6 mice (115), indicating the important roles played by CD84 and Ly108 in maintaining GC tolerance.
The Ly108/slamf6 gene is expressed in three separate isoforms, Ly108.1, Ly108.2 and Ly108-H1, which differentially regulate peripheral B cell and T cell tolerance. B cells that express the Ly108.1 isoform exhibit significantly reduced calcium flux upon BCR engagement and decreased cell death, causing the reduced efficiency of negative selection as compared to the autoimmune resistant isoform (Ly108.2) (116). In addition, Ly108 deficiency in autoimmune-prone mice causes reduced autoAb titers and reduced percentages of effector memory T cells that result in reduced autoimmune disease (117). Finally, overexpression of the Ly108-H1 isoform in T cells or B cells of B6.Sle1b mice reduces autoAb titers, T cell activation, and Spt-GC formation to protect against SLE disease progression (117). These observations reinforce the importance of the SLAM family molecules in regulation of both T cell and B cell-mediated self-tolerance.
The role of Toll-like receptors and Interferon signaling in Spt-GC formation
Toll-Like Receptors
Toll-Like Receptors (TLRs) are a class of pattern recognition receptors (PRRs) that were initially characterized as sensors of molecular patterns found in lipids, proteins, and nucleic acids derived from pathogens. A series of publications utilizing in vitro stimulation assays were the first to demonstrate a functional role for TLRs in self-antigen mediated B cell activation. Specifically, BCR-mediated internalization of self-RNA and self-DNA containing immune complexes (ICs) activated autoimmune rheumatoid factor (RF) transgenic B cells (124-127). Importantly, activation was dependent on synergistic BCR and TLR9 or TLR7 stimulation upon exposure to DNA and RNA-associated autoantigens, respectively. These findings suggested that weaker BCR stimulation in autoimmune B cells confers the need for synergistic TLR signaling to reach an activation threshold that can typically be achieved through the BCR alone when challenged with foreign antigen. Accordingly, a later study documented suboptimal BCR signaling in these B cells and the requirement for BCR and TLR9 synergy to activate a different set of genes than results from the activation of either receptor alone (128).
Since these in vitro studies suggested a potential role for TLRs 7 and 9 in the development of autoimmunity, multiple groups proceeded to study the impact of these receptors in autoimmune mice with a focus on how they control autoantibody production, autoimmune pathology, and cellular activation. Studies in TLR7 and TLR9-deficient MRL lpr/lpr mice demonstrated that TLRs 7 and 9 have opposing roles in SLE development (129, 130). The absence of TLR7 abrogated the production of RNA-specific autoantibodies, impaired pathogenic IgG2a class-switching, and significantly reduced downstream kidney pathology, as a result of reduced immune activation (129). Conversely, the absence of TLR9 enhanced kidney pathology, immune activation, and class-switching (130, 131). Conflicting data in studies on anti-DNA antibody production, IgG2 class-switching, and downstream kidney pathology in several TLR9-deficient autoimmune models including FcγRIIb -/-, Sle1b, WASp, B6.lpr/lpr, pristane-induced lupus, and Nba2 has emerged, indicating the complex role of TLR9 in these processes in different contexts and models (27, 129-136).
Following the generation of TLR7-deficient MRL/lpr mice, a model possessing an extra copy of TLR7 as a result of the translocation of TLR7 from the X to the Y chromosome, was characterized (137, 138). This model, termed Y autoimmune accelerator (Yaa) does not induce overt autoimmunity on a B6 background, but significantly accelerates disease on an autoimmune background (137, 138). Because the Yaa locus contains about 16 genes, including TLR7, further study strived to verify that TLR7 is the major driver of the Yaa phenotype (139, 140). Loss of TLR7 overexpression on both the FcγRIIb-/- and Sle1 autoimmune backgrounds abrogated almost all facets of the Yaa phenotype (139, 140). The generation of transgenic mice overexpressing TLR7 phenocopied Yaa mice, further confirming the predominant role of TLR7 in driving disease (127, 139, 141). These studies demonstrate that TLR7 is a critical driver of disease, whereas TLR9 has a more complex role whereby it appears to have both promoting and regulatory functions in DNA-specific autoantibody production, immune activation, and resultant downstream pathology.
While it was clear that TLR7 is capable of driving disease, the contribution of TLR7 in specific cell-types and the mechanism by which TLR9 regulates disease development remained unclear. Molecular studies had revealed that TLR9 has a greater affinity than TLR7 for the endosomal TLR transport protein, Unc93b1, providing a potential mechanism by which TLR9 can restrain TLR7-induced activation in autoimmune mice (142). In 2010, studies in two different autoimmune models emerged that indicated TLR7-deficiency in TLR9-deficient mice abrogated accelerated pathology observed in these mice (135, 143). Additionally, several experiments suggested that TLR7 and TLR9 may function in a B cell-intrinsic manner to control autoimmune responses (135, 137). In 2012, a comprehensive study emerged that showed a B cell-intrinsic role for TLR7 in driving B and T cell activation and the production of autoantibodies in Sle1.Yaa mice, through eliminating the extra copy of TLR7 specifically in B cells (141). Later in vivo and in vitro studies utilizing self-DNA containing ICs demonstrated that B cell-intrinsic TLR9 control of TLR7 is responsible for inhibiting the development of autoimmunity and B cell activation, establishing the predominant function of TLR7 and TLR9 in controlling B cell responsiveness in autoimmunity (144, 145).
Recent studies have focused on the B cell-intrinsic role of TLRs 7 and 9 in mediating lupus-like autoimmune responses. A potential role in GC responses was apparent from an earlier study that showed increased Tfh marker expression on CD4 T cells in autoimmune Yaa mice (138). Accordingly, the impact of TLR7- and TLR9-deficiency in non-autoimmune mice and two different autoimmune mouse models, Sle1b and WASp, was comprehensively assessed (27, 136, 141). In addition to impacts on cellular activation, TLR7 was found to promote Spt-GC responses on both non-autoimmune and autoimmune backgrounds in a B cell-intrinsic manner (27, 136). Interestingly, in the same studies, TLR9 was found to negatively regulate Spt-GC responses in a B cell-intrinsic manner (27, 136). These studies suggest that the GC represents a major site whereby B cell-intrinsic TLR signaling drives SLE.
Type I IFN (T1IFN)
The type 1 interferon (T1IFN) family is a large group of diverse signaling molecules (IFN-α, -β, -ε, -κ and -ω) that all bind to the same primary signaling receptor (IFNαR1). Despite the identical primary receptor binding specificity, each member of the T1IFN family elicits distinct signaling cascades as a result of co-receptor availability (IFNαR2a, IFNαR2b, or IFNαR2c) (146) and binding affinity for the receptor complex (147). Type I IFN is heavily associated with the onset and progression of human SLE, evidenced by a characteristic interferon signature (148). In human SLE, a T1IFN signature is closely correlated with autoantibody seropositivity and elevated levels of T1IFN can be observed in patient sera 2 years prior to SLE diagnosis (149-151). pDCs are believed to be the major producers of type I IFN, triggered by the sensing of RNA and DNA-containing immune complexes which stimulate endosomal TLRs (152). Depletion of pDCs in the mouse models of SLE prevents the development of Spt-GCs and autoimmunity, indicating the contribution of pDC function to Spt-GC formation and subsequent disease manifestations (152, 153).
In addition to pDCs outside the GC microenvironment, FDCs within the GC have also been proposed as producers of Type I interferon through a TLR-dependent mechanism, albeit not experimentally proven (154). In B cells, T1IFN treatment induces heightened response to BCR stimulation, proliferation and resistance to Fas-mediated apoptosis in vitro (155-158). Conversely, B cells deficient in IFNαR1 signaling exhibit defective class switching, reduced expression of activation markers and MHCII (148, 156, 159, 160). Spt-GC responses are reduced in BXD2 autoimmune mice deficient in IFNαR1(161). Surprisingly, the Rawlings group finds no major effects of IFNαR deficiency on autoimmune GC response using the Wiskot-Aldrich syndrome (WAS) chimera model of B cell-driven autoimmunity (20), suggesting that the function of T1 IFN in Spt-GC regulation can vary between autoimmune models. These conflicting results in animal models closely reflect similar challenges that are reported in human clinical trials showing differential outcomes among SLE patients for T1IFN-blocking therapies (162). Further studies are warranted to delineate the factors that determine the role of T1IFN in Spt-GC dysregulation.
Type II IFN
Like Type I interferon, the role of Type II interferon in autoimmune disease manifestation is well documented (163). In both human patients and mouse model of SLE, the concentration of circulating IFNγ can be an indicator of SLE disease onset and severity (150, 163-165). The role of IFNγ, however, is not consistent among all autoimmune diseases. In MS, blockade of IFNγ signaling in humans worsens MS disease onset (166), but blockade of IFNγ signaling has been shown to reduce autoantibody production and resultant autoimmunity in multiple mouse models of SLE (18, 20, 167-172).
Decrease in mRNA decay of ifng transcripts in T cells is shown to be associated with follicular T helper (Tfh) cell accumulation, increased Spt-GC formation and SLE-like autoimmunity in Roquinsan/san mice (19). Also, IFNγR1 deficiency in B cells abolishes Spt-GC formation and associated autoantibody production in two distinct mouse models of SLE (18, 20). IFNγR-mediated Spt-GC development requires STAT1 signaling (18). Jackson and colleagues confirmed the role of the JAK-STAT pathway by using JAK inhibitors to prevent in vitro Spt-GC differentiation in human B cells (20). Interestingly, IFNγR1-deficient mice are able to form GCs upon immunization with a foreign antigen, indicating that the role of IFNγR1 is limited to the Spt-GC response (18).
Cytokine/Chemokine Signaling in Spt-GCs
B-cell activating factor (BAFF)
The B-cell activating factor (BAFF) plays a critical role in primary B cell development (30, 173). BAFF is involved in the survival of plasmablasts (174) and GC B cells (14). BAFF also increases proliferation upon BCR crosslinking (175). In addition, treatment of mice with a BAFF decoy receptor ablated the formation of splenic GCs and IgG1 production in mice after immunization, suggesting that BAFF is crucial for the maintenance of GC response (176, 177). In support of these findings, mice with reduced BAFF-receptor signaling formed GCs but failed to sustain these GCs upon immunization (178, 179). In SLE-prone mice, BAFF-blocking therapy did not ablate Spt-GC formation, but reduced the number of autoantibody producing cells (180). Interestingly, BAFF overexpression promotes enlarged GC formation in the Peyer's patches of the gut in the absence of immunization (30).
IL-21
The role of IL-21R signaling in initiating the GC response is well established (16, 181, 182). IL-21 is produced by both Th17 and Tfh cells and supports GC B cell survival and proliferation (15, 16, 183, 184). In B cells, IL-21R signaling activates STAT3 to induce the expression of Bcl-6, a master regulator of the GC response (182, 185). Mice deficient in IL-21, IL-21R, and B cell conditional deletion of STAT3 all exhibit hindered class-switched antibody responses to T-dependent immunization, suggesting a defect in induced GC formation (16, 181, 182, 185). However, treatment of IL-21R-/- mice with RNA-loaded VLPs can drive GC and VLP-specific Ab responses, suggesting that IL-21R signaling can regulate BCR, but not RNA-sensing innate signaling (181).
In autoimmune prone MRL.lpr/lpr and BXD2 mice, IL-21R deficiency causes an absence of Spt-GC formation and inhibits the autoantibody- associated cutaneous SLE disease, kidney nephritis and plasma cell formation (186, 187). Our unpublished data also show absolute requirement of IL21R signaling in Spt-GC formation in B6.Sle1b mouse model of SLE (P.P.D and Z.S.M.R). In SLE-prone BXSB.Yaa mice, IL-21 expression is increased and promotes IgG1, IgG2b and IgG3 class switching (188), suggesting the role of IL21 signaling in Spt-GC formation. In humans, several polymorphisms in the IL21R locus are associated with the onset of MS and SLE (189-191), and elevated concentrations of IL-21 in the sera of RA patients correlated with autoantibody production and disease severity scores (192).
Chemokines
The migration of B cells through GC structures is crucial for the separation of rapidly proliferating GC B cells that are undergoing class switching and somatic hypermutation in the dark zone from GC B cells that are competing for survival signals during selection in the light zone. In GCs, this separation is largely mediated by chemokines that regulate the movement of GC B cells in response to different chemokine stimuli (193).
CXC and CCR chemokine receptors (CXCR4, CXCR5, CCR6, CCR7, CCR9, CCR10, S1PR1, S1PR3, CNR2, and GPR183) are G-protein-coupled receptors (GPCRs) that utilize regulators of G protein signaling (RGS) proteins to modulate the magnitude of the intracellular signaling cascade (193). Several RGS proteins are expressed in B cells and modulate their migratory responses (15, 194-198). Blocking all RGS function via mutation in Gai2, a guanine exchange subunit that interacts with RGSs, causes the accumulation of poorly organized splenic structures that resemble Spt-GCs (195). Rgs1-/- mice exhibit enhanced B cell migration in response to CXCL12 and CXCL13, which results in enhanced Spt-GC, as well as immunized GC, responses (197). Also, Rgs13-/- mice exhibited greater formation of Peyer's patches and unrestricted enlargement of GC formation after immunization (194). Other studies of RGSs reported increased expression of Rgs2, Rgs9, and Rgs10, and decreased expression of Rgs14, Rgs18 and Rgs19 proteins in GCs (199), but the function of the entire repertoire of RGSs in the maintenance of GC is not yet fully defined. As evidenced by previous studies, multiple RGSs are likely to play a crucial role in regulating Spt-GC formation and in modulating autoimmune disease development. Future studies will likely define the role of this class of proteins in greater detail.
IL-17
IL-17R signaling has been shown to control the migratory capacity of both GC B cells and Tfh cells within the GC (200, 201). The production of IL-17 was initially observed in CD4+ T cells within the GC (15), and later studies by the Mountz lab further defined these IL-17 producing cells as Tfh (200). These IL-17 producing Tfh did not produce IL-21, indicating that two distinct subsets of Tfh contribute to the regulation of the GC (200). In Tfh, IL17R signaling upregulates the expression of Rgs16 to block chemotaxis of Tfh out of the GC microenvironment (200). In GC B cells, a similar pathway utilizes Rgs16 and Rgs13 to block GC B cell migration to the dark zone in response to CXCL12 (15, 201). Overexpression of IL-17 via adenoviral transduction increases GC size and T:B cell interactions whereas antibody-mediated blockade of IL-17Ra signaling inhibits Spt-GC formation (15). Furthermore, deficiency of RORγT, which is classically thought to be a master regulator of Th17 differentiation had pronounced effects on the ratio of Tfh to Tfr in GCs, and in turn, on Spt-GC formation (210). Mice deficient in RORgT have increased serum levels of BAFF, IL-21 and RORγT-independent production of IL-17 by Tfh (202). The role of IL-17 in Spt-GC formation and regulation appears to be context dependent, as Spt-GC formation in TLR7 overexpression model of SLE does not require IL-17 (10), while it contributes to Spt-GC formation and autoimmune responses in SLE-prone BXD2 mice (200).
Inhibitory Receptor Signaling and Apoptotic Cell Clearance in GC Regulation
The currently accepted model for the initiation of autoimmunity involves synergy between genetic susceptibility and a triggering event that induces the accumulation of apoptotic cells that activate autoreactive B and T cells (203-205). Tingible body macrophages located within the GC are primarily responsible for the clearance of GC B cells undergoing apoptosis as a consequence of negative selection. Subsequent secondary necrosis due to inefficient clearance results in immunogenic rather than tolerogenic responses by activating multiple cell types in the GC (203).
Apoptotic cell clearance in the GC
Tingible body macrophages clear apoptotic cells within GCs by Mer Tyrosine Kinase (MerTK) and Mfge8-integrin-mediated clearance mechanisms (205-207). MerTK dually functions to clear apoptotic cells and signal through a receptor tyrosine kinase domain to induce the production of anti-inflammatory cytokines and dampen TLR and inflammatory cytokine responses (208). We have shown that Mer-deficient mice exhibit enhanced GC responses and B cell and T cell activation that promotes the development of autoimmunity (205, 206). Hanayama et al. first showed that Mfge8-deficient mice exhibit deficient apoptotic cell clearance in GCs, leading to enhanced Spt-GC responses in aged mice (207). Kranich et al. later showed FDCs, rather than tingible body macrophages, are the major producers of Mfge8, which is trapped on the surface by tingible body macrophages (209). In human SLE patients, genetic polymorphisms are present in MerTK adaptor molecules, Gas6 and protein S, and in Mfge8 (210). Further, apoptotic cell accumulation has been observed in GCs within lymph nodes derived from SLE patients and phagocytic cells derived from lupus patients exhibit impaired clearance of apoptotic cells and their debris (211, 212).
Fc Receptors
Fc receptors bind to the Fc portion of antibodies that are complexed with antigens, cell debris and/or opsonized pathogens to enact activating or inhibitory signals within a recipient immune cell (213). Although several Fc receptors are found on the surface of immune cells in humans and mice, most of these receptors are found on the surface of myeloid cells and few are found on the surface of lymphocytes (213, 214). As these receptors can be both activating and inhibitory, several studies have linked human susceptibility to autoimmune disease with dysregulated activity of Fcg receptors (28, 213-217). Correspondingly, several FcγRs have been the focus of novel targeted therapeutics for the treatment of autoimmune diseases (214).
FcγRIIb is a low-affinity inhibitory Fc receptor that is found on the surface of B cells that negatively regulates signaling through the B cell receptor (28, 218). In humans, two different mutations in the transmembrane region of FcgRIIb are correlated with susceptibility to SLE (217, 219-224). The FcγRIIb-232T mutation exhibits high disease association among European-origin SLE patients (225), with a higher prevalence of expression among African, African-American, and southeast Asian populations with SLE (5-11% of patients) (217, 219-221, 223, 224, 226). FcγRIIb-232T-expressing human B cells exhibit defective inhibitory functions, allowing for microclustering of BCRs and resultant cellular activation, causing hyperactivity in B cells (218). In FcγRIIb-/- mice, Spt-GC and Tfh responses are increased, causing greater SLE-associated disease pathology (increased ANA titers and kidney pathology) (28). These elevated Spt-GC, Tfh and severe autoimmune responses in FcγRIIb-/- mice have recently been shown to be mediated by FcγRIIb deficiency and lupus-associated SLAM family genes derived from the 129 strain (28).
Lyn
Lyn is a Src family tyrosine kinase that can both activate and inhibit BCR signaling cascades in B cells (227-229). The activating functions of Lyn can be compensated by other Src family kinases within the same signaling cascade (227), whereas Lyn plays non redundant role in mediating signaling in the inhibitory feedback pathways, including recruitment of FcγRIIb signaling in B cells (228, 230). Thus, B cells that lack Lyn exhibit BCR signaling hyperactivity leading to increased proliferative responses, activation marker expression, and capacity for antigen presentation (229, 230). Lyn deficiency in B cells also promotes anti-nuclear antibody formation, antibody-mediated kidney pathology and elevated levels of pro-inflammatory cytokines that promote SLE-like disease (229, 231). The autoimmune phenotypes including Spt-GC responses in Lyn-/- mice are mediated by MyD88 signaling in B cells (229, 231, 232). Interestingly, conditional deletion of Lyn in B cells under the control of CD79Cre resulted in a significant reduction in Spt-GC formation, rather than expansion (232). Even though bone marrow B cell development is not affected by conditional deletion of Lyn, these mice have significantly reduced number of mature B cells, suggesting that fewer cells are available to enter the GC reactions (229). Further studies using Cre mice (i.e., CD23Cre) that will delete Lyn at the mature B cell stage may elucidate stage-specific role of Lyn in Spt-GC development.
Complement Receptors and Proteins
Complement proteins and receptors are an integral component of the selection process of B cells within the GC. Complement receptors (CRs) on the surface of FDCs aid in trapping immune complexes on the surface of FDCs in limiting quantities to promote competition-mediated selection of B cells with high affinity BCRs. SLE patients carry mutations and SLE-associated SNPs in both Complement Receptor 1 (CR1) and Complement Receptor 2 (CR2) genes that contribute to disease (233-235). Deficiencies of CR1 and CR2 on FDCs cause deficiencies in GC maintenance, indicated by reduced efficiency of the IgG response to immunization (236).
CR2 can bind to many ligands including complement proteins (including C4 and C1q), CD23, EBV surface proteins, and T1IFN (237-243). The interaction of CR2 with these ligands has been shown to affect B cell development in the GC by regulating class switching, apoptosis, and B:T cell contact to regulate plasma cell formation (243-245). In mouse models of SLE, CR2 deficiency in B6.lpr mice contributes to increased autoantibody formation and renal disease, however MRL/Lpr mice with CR2 deficiency expressed increased autoantibodies without renal disease (246-248).
Over 75% of SLE patients are deficient in C1q and C4 proteins (249). Also, C1q and C4 knockout mice produce autoantibodies and develop autoimmune kidney disease (154, 246, 250, 251). In 564Igi mice, which contain a BCR knock-in that targets nuclear antigens, C4 deficiency results in an enlarged Spt-GC phenotype without antinuclear IgG accumulation (79, 252). However, in MRL.lpr/lpr mice, C4 deficiency exacerbates IgG autoantibody production and autoimmune disease (246). Thus, the role of complement proteins in promoting Spt-GC formation and autoimmune disease is dependent on specific genetic factors that have not yet been elucidated.
Conclusions
The role of Spt-GCs in several different autoimmune diseases is well established in the literature by multiple research groups. Intriguingly, several studies have identified factors and signaling pathways that are vital for Spt-GC responses, but are not required for the GC responses induced (iGC) by foreign antigens, suggesting that distinct signaling mechanisms may control autoimmune Spt-GC and induced GC responses. Delineating distinct cell-intrinsic pathways that promote Spt-GC versus iGC responses could uncover signaling molecules that can inform the development of targeted therapeutics for autoimmune disease, limiting the use of non-specific immunosuppression based therapies. As we have described above, several different pathways have been shown to regulate Spt-GC formation and activity by controlling GC initiation at the B cell:T cell border, by controlling GC selection through the regulation of FDC and Tfh function or by altering cell migration in the GC structure by cytokine and chemokine signaling. Although many of these pathways have been identified, few have been fully characterized, necessitating future work to investigate these pathways in greater detail while continuing to identify novel factors that regulate Spt-GCs.
Acknowledgments
This work was supported by the National Institutes of Health grant RO1A1091670 to Z.S.M.R and F31AI122608 to P.P.D.
These studies were supported by grant number AI091670 to Z.S.M.R and AI122608 to P.P.D. from the NIH
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Cyster JG. B cell follicles and antigen encounters of the third kind. Nat Immunol. 2010;11:989–996. doi: 10.1038/ni.1946. [DOI] [PubMed] [Google Scholar]
- 2.De Silva NS, Klein U. Dynamics of B cells in germinal centres. Nat Rev Immunol. 2015;15:137–148. doi: 10.1038/nri3804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Rodda LB, Bannard O, Ludewig B, Nagasawa T, Cyster JG. Phenotypic and Morphological Properties of Germinal Center Dark Zone Cxcl12-Expressing Reticular Cells. J Immunol. 2015;195:4781–4791. doi: 10.4049/jimmunol.1501191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gatto D, Brink R. The germinal center reaction. J Allergy Clin Immunol. 2010;126:898–907. doi: 10.1016/j.jaci.2010.09.007. quiz 908-899. [DOI] [PubMed] [Google Scholar]
- 5.Detanico T, St Clair JB, Aviszus K, Kirchenbaum G, Guo W, Wysocki LJ. Somatic mutagenesis in autoimmunity. Autoimmunity. 2013;46:102–114. doi: 10.3109/08916934.2012.757597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schroeder K, Herrmann M, Winkler TH. The role of somatic hypermutation in the generation of pathogenic antibodies in SLE. Autoimmunity. 2013;46:121–127. doi: 10.3109/08916934.2012.748751. [DOI] [PubMed] [Google Scholar]
- 7.Schaerli P, Willimann K, Lang AB, Lipp M, Loetscher P, Moser B. CXC chemokine receptor 5 expression defines follicular homing T cells with B cell helper function. J Exp Med. 2000;192:1553–1562. doi: 10.1084/jem.192.11.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cyster JG, Ansel KM, Reif K, Ekland EH, Hyman PL, Tang HL, Luther SA, Ngo VN. Follicular stromal cells and lymphocyte homing to follicles. Immunol Rev. 2000;176:181–193. doi: 10.1034/j.1600-065x.2000.00618.x. [DOI] [PubMed] [Google Scholar]
- 9.Allen CD, Ansel KM, Low C, Lesley R, Tamamura H, Fujii N, Cyster JG. Germinal center dark and light zone organization is mediated by CXCR4 and CXCR5. Nat Immunol. 2004;5:943–952. doi: 10.1038/ni1100. [DOI] [PubMed] [Google Scholar]
- 10.Walsh ER, Pisitkun P, Voynova E, Deane JA, Scott BL, Caspi RR, Bolland S. Dual signaling by innate and adaptive immune receptors is required for TLR7-induced B-cell-mediated autoimmunity. Proc Natl Acad Sci U S A. 2012;109:16276–16281. doi: 10.1073/pnas.1209372109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yusuf I, Kageyama R, Monticelli L, Johnston RJ, Ditoro D, Hansen K, Barnett B, Crotty S. Germinal center T follicular helper cell IL-4 production is dependent on signaling lymphocytic activation molecule receptor (CD150) J Immunol. 2010;185:190–202. doi: 10.4049/jimmunol.0903505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang H, Geng J, Wen X, Bi E, Kossenkov AV, Wolf AI, Tas J, Choi YS, Takata H, Day TJ, Chang LY, Sprout SL, Becker EK, Willen J, Tian L, Wang X, Xiao C, Jiang P, Crotty S, Victora GD, Showe LC, Tucker HO, Erikson J, Hu H. The transcription factor Foxp1 is a critical negative regulator of the differentiation of follicular helper T cells. Nat Immunol. 2014;15:667–675. doi: 10.1038/ni.2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wang CJ, Heuts F, Ovcinnikovs V, Wardzinski L, Bowers C, Schmidt EM, Kogimtzis A, Kenefeck R, Sansom DM, Walker LS. CTLA-4 controls follicular helper T-cell differentiation by regulating the strength of CD28 engagement. Proc Natl Acad Sci U S A. 2015;112:524–529. doi: 10.1073/pnas.1414576112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Goenka R, Matthews AH, Zhang B, O'Neill PJ, Scholz JL, Migone TS, Leonard WJ, Stohl W, Hershberg U, Cancro MP. Local BLyS production by T follicular cells mediates retention of high affinity B cells during affinity maturation. J Exp Med. 2014;211:45–56. doi: 10.1084/jem.20130505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hsu HC, Yang P, Wang J, Wu Q, Myers R, Chen J, Yi J, Guentert T, Tousson A, Stanus AL, Le TV, Lorenz RG, Xu H, Kolls JK, Carter RH, Chaplin DD, Williams RW, Mountz JD. Interleukin 17-producing T helper cells and interleukin 17 orchestrate autoreactive germinal center development in autoimmune BXD2 mice. Nat Immunol. 2008;9:166–175. doi: 10.1038/ni1552. [DOI] [PubMed] [Google Scholar]
- 16.Zotos D, Coquet JM, Zhang Y, Light A, D'Costa K, Kallies A, Corcoran LM, Godfrey DI, Toellner KM, Smyth MJ, Nutt SL, Tarlinton DM. IL-21 regulates germinal center B cell differentiation and proliferation through a B cell-intrinsic mechanism. J Exp Med. 2010;207:365–378. doi: 10.1084/jem.20091777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ding Y, Li J, Yang P, Luo B, Wu Q, Zajac AJ, Wildner O, Hsu HC, Mountz JD. Interleukin-21 promotes germinal center reaction by skewing the follicular regulatory T cell to follicular helper T cell balance in autoimmune BXD2 mice. Arthritis Rheumatol. 2014;66:2601–2612. doi: 10.1002/art.38735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Domeier PP, Chodisetti SB, Soni C, Schell SL, Elias MJ, Wong EB, Cooper TK, Kitamura D, Rahman ZS. IFN-γ receptor and STAT1 signaling in B cells are central to spontaneous germinal center formation and autoimmunity. J Exp Med. 2016;213:715–732. doi: 10.1084/jem.20151722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lee SK, Silva DG, Martin JL, Pratama A, Hu X, Chang PP, Walters G, Vinuesa CG. Interferon-γ excess leads to pathogenic accumulation of follicular helper T cells and germinal centers. Immunity. 2012;37:880–892. doi: 10.1016/j.immuni.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 20.Jackson SW, Jacobs HM, Arkatkar T, Dam EM, Scharping NE, Kolhatkar NS, Hou B, Buckner JH, Rawlings DJ. B cell IFN-γ receptor signaling promotes autoimmune germinal centers via cell-intrinsic induction of BCL-6. J Exp Med. 2016;213:733–750. doi: 10.1084/jem.20151724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Tahir S, Fukushima Y, Sakamoto K, Sato K, Fujita H, Inoue J, Uede T, Hamazaki Y, Hattori M, Minato N. A CD153+CD4+ T follicular cell population with cell-senescence features plays a crucial role in lupus pathogenesis via osteopontin production. J Immunol. 2015;194:5725–5735. doi: 10.4049/jimmunol.1500319. [DOI] [PubMed] [Google Scholar]
- 22.Linterman MA, Pierson W, Lee SK, Kallies A, Kawamoto S, Rayner TF, Srivastava M, Divekar DP, Beaton L, Hogan JJ, Fagarasan S, Liston A, Smith KG, Vinuesa CG. Foxp3+ follicular regulatory T cells control the germinal center response. Nat Med. 2011;17:975–982. doi: 10.1038/nm.2425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vinuesa CG, Sanz I, Cook MC. Dysregulation of germinal centres in autoimmune disease. Nat Rev Immunol. 2009;9:845–857. doi: 10.1038/nri2637. [DOI] [PubMed] [Google Scholar]
- 24.Luzina IG, Atamas SP, Storrer CE, daSilva LC, Kelsoe G, Papadimitriou JC, Handwerger BS. Spontaneous formation of germinal centers in autoimmune mice. J Leukoc Biol. 2001;70:578–584. [PubMed] [Google Scholar]
- 25.Wong EB, Khan TN, Mohan C, Rahman ZS. The Lupus-Prone NZM2410/NZW Strain-Derived Sle1b Sublocus Alters the Germinal Center Checkpoint in Female Mice in a B Cell-Intrinsic Manner. J Immunol. 2012;189:5667–5681. doi: 10.4049/jimmunol.1201661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Giese T, Davidson WF. Chronic treatment of C3H-lpr/lpr and C3H-gld/gld mice with anti-CD8 monoclonal antibody prevents the accumulation of double negative T cells but not autoantibody production. J Immunol. 1994;152:2000–2010. [PubMed] [Google Scholar]
- 27.Soni C, Wong EB, Domeier PP, Khan TN, Satoh T, Akira S, Rahman ZS. B cell-intrinsic TLR7 signaling is essential for the development of spontaneous germinal centers. J Immunol. 2014;193:4400–4414. doi: 10.4049/jimmunol.1401720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Soni C, Domeier PP, Wong EB, Shwetank, Khan TN, Elias MJ, Schell SL, Lukacher AE, Cooper TK, Rahman ZS. Distinct and synergistic roles of FcγRIIB deficiency and 129 strain-derived SLAM family proteins in the development of spontaneous germinal centers and autoimmunity. J Autoimmun. 2015;63:31–46. doi: 10.1016/j.jaut.2015.06.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ermak TH, Steger HJ, Wofsy D. Treatment of murine lupus with monoclonal antibody to L3T4. II. Effects on immunohistopathology of thymus, spleen, and lymph node. Lab Invest. 1989;61:447–456. [PubMed] [Google Scholar]
- 30.Mackay F, Woodcock SA, Lawton P, Ambrose C, Baetscher M, Schneider P, Tschopp J, Browning JL. Mice transgenic for BAFF develop lymphocytic disorders along with autoimmune manifestations. J Exp Med. 1999;190:1697–1710. doi: 10.1084/jem.190.11.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bogdani M. Thinking Outside the Cell: A Key Role for Hyaluronan in the Pathogenesis of Human Type 1 Diabetes. Diabetes. 2016;65:2105–2114. doi: 10.2337/db15-1750. [DOI] [PubMed] [Google Scholar]
- 32.Peters AL, Stunz LL, Meyerholz DK, Mohan C, Bishop GA. Latent membrane protein 1, the EBV-encoded oncogenic mimic of CD40, accelerates autoimmunity in B6.Sle1 mice. J Immunol. 2010;185:4053–4062. doi: 10.4049/jimmunol.0904065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Tugnet N, Rylance P, Roden D, Trela M, Nelson P. Human Endogenous Retroviruses (HERVs) and Autoimmune Rheumatic Disease: Is There a Link? Open Rheumatol J. 2013;7:13–21. doi: 10.2174/1874312901307010013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Perl A, Colombo E, Dai H, Agarwal R, Mark KA, Banki K, Poiesz BJ, Phillips PE, Hoch SO, Reveille JD. Antibody reactivity to the HRES-1 endogenous retroviral element identifies a subset of patients with systemic lupus erythematosus and overlap syndromes. Correlation with antinuclear antibodies and HLA class II alleles. Arthritis Rheum. 1995;38:1660–1671. doi: 10.1002/art.1780381119. [DOI] [PubMed] [Google Scholar]
- 35.Pitzalis C, Jones GW, Bombardieri M, Jones SA. Ectopic lymphoid-like structures in infection, cancer and autoimmunity. Nat Rev Immunol. 2014;14:447–462. doi: 10.1038/nri3700. [DOI] [PubMed] [Google Scholar]
- 36.Cañete JD, Celis R, Moll C, Izquierdo E, Marsal S, Sanmartí R, Palacín A, Lora D, de la Cruz J, Pablos JL. Clinical significance of synovial lymphoid neogenesis and its reversal after anti-tumour necrosis factor alpha therapy in rheumatoid arthritis. Ann Rheum Dis. 2009;68:751–756. doi: 10.1136/ard.2008.089284. [DOI] [PubMed] [Google Scholar]
- 37.Humby F, Bombardieri M, Manzo A, Kelly S, Blades MC, Kirkham B, Spencer J, Pitzalis C. Ectopic lymphoid structures support ongoing production of class-switched autoantibodies in rheumatoid synovium. PLoS Med. 2009;6:e1. doi: 10.1371/journal.pmed.0060001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Perros F, Dorfmüller P, Montani D, Hammad H, Waelput W, Girerd B, Raymond N, Mercier O, Mussot S, Cohen-Kaminsky S, Humbert M, Lambrecht BN. Pulmonary lymphoid neogenesis in idiopathic pulmonary arterial hypertension. Am J Respir Crit Care Med. 2012;185:311–321. doi: 10.1164/rccm.201105-0927OC. [DOI] [PubMed] [Google Scholar]
- 39.Hill ME, Shiono H, Newsom-Davis J, Willcox N. The myasthenia gravis thymus: a rare source of human autoantibody-secreting plasma cells for testing potential therapeutics. J Neuroimmunol. 2008;201-202:50–56. doi: 10.1016/j.jneuroim.2008.06.027. [DOI] [PubMed] [Google Scholar]
- 40.Cantaert T, Kolln J, Timmer T, van der Pouw Kraan TC, Vandooren B, Thurlings RM, Cañete JD, Catrina AI, Out T, Verweij CL, Zhang Y, Tak PP, Baeten D. B lymphocyte autoimmunity in rheumatoid synovitis is independent of ectopic lymphoid neogenesis. J Immunol. 2008;181:785–794. doi: 10.4049/jimmunol.181.1.785. [DOI] [PubMed] [Google Scholar]
- 41.Kim SJ, Zou YR, Goldstein J, Reizis B, Diamond B. Tolerogenic function of Blimp-1 in dendritic cells. J Exp Med. 2011;208:2193–2199. doi: 10.1084/jem.20110658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Tiller T, Kofer J, Kreschel C, Busse CE, Riebel S, Wickert S, Oden F, Mertes MM, Ehlers M, Wardemann H. Development of self-reactive germinal center B cells and plasma cells in autoimmune Fc gammaRIIB-deficient mice. J Exp Med. 2010;207:2767–2778. doi: 10.1084/jem.20100171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wellmann U, Letz M, Herrmann M, Angermüller S, Kalden JR, Winkler TH. The evolution of human anti-double-stranded DNA autoantibodies. Proc Natl Acad Sci U S A. 2005;102:9258–9263. doi: 10.1073/pnas.0500132102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Diamond B, Katz JB, Paul E, Aranow C, Lustgarten D, Scharff MD. The role of somatic mutation in the pathogenic anti-DNA response. Annu Rev Immunol. 1992;10:731–757. doi: 10.1146/annurev.iy.10.040192.003503. [DOI] [PubMed] [Google Scholar]
- 45.Cappione A, Anolik JH, Pugh-Bernard A, Barnard J, Dutcher P, Silverman G, Sanz I. Germinal center exclusion of autoreactive B cells is defective in human systemic lupus erythematosus. J Clin Invest. 2005;115:3205–3216. doi: 10.1172/JCI24179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Arce E, Jackson DG, Gill MA, Bennett LB, Banchereau J, Pascual V. Increased frequency of pre-germinal center B cells and plasma cell precursors in the blood of children with systemic lupus erythematosus. J Immunol. 2001;167:2361–2369. doi: 10.4049/jimmunol.167.4.2361. [DOI] [PubMed] [Google Scholar]
- 47.Levinson AI, Wheatley LM. The thymus and the pathogenesis of myasthenia gravis. Clin Immunol Immunopathol. 1996;78:1–5. doi: 10.1006/clin.1996.0001. [DOI] [PubMed] [Google Scholar]
- 48.Song YW, Kang EH. Autoantibodies in rheumatoid arthritis: rheumatoid factors and anticitrullinated protein antibodies. QJM. 2010;103:139–146. doi: 10.1093/qjmed/hcp165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Nell-Duxneuner V, Machold K, Stamm T, Eberl G, Heinzl H, Hoefler E, Smolen JS, Steiner G. Autoantibody profiling in patients with very early rheumatoid arthritis: a follow-up study. Ann Rheum Dis. 2010;69:169–174. doi: 10.1136/ard.2008.100677. [DOI] [PubMed] [Google Scholar]
- 50.Jones V, Taylor PC, Jacoby RK, Wallington TB. Synovial synthesis of rheumatoid factors and immune complex constituents in early arthritis. Ann Rheum Dis. 1984;43:235–239. doi: 10.1136/ard.43.2.235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Schröder AE, Greiner A, Seyfert C, Berek C. Differentiation of B cells in the nonlymphoid tissue of the synovial membrane of patients with rheumatoid arthritis. Proc Natl Acad Sci U S A. 1996;93:221–225. doi: 10.1073/pnas.93.1.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Weyand CM, Goronzy JJ. Ectopic germinal center formation in rheumatoid synovitis. Ann N Y Acad Sci. 2003;987:140–149. doi: 10.1111/j.1749-6632.2003.tb06042.x. [DOI] [PubMed] [Google Scholar]
- 53.Hou L, Block KE, Huang H. Artesunate abolishes germinal center B cells and inhibits autoimmune arthritis. PLoS One. 2014;9:e104762. doi: 10.1371/journal.pone.0104762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Victoratos P, Kollias G. Induction of autoantibody-mediated spontaneous arthritis critically depends on follicular dendritic cells. Immunity. 2009;30:130–142. doi: 10.1016/j.immuni.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 55.McCarthy DP, Richards MH, Miller SD. Mouse models of multiple sclerosis: experimental autoimmune encephalomyelitis and Theiler's virus-induced demyelinating disease. Methods Mol Biol. 2012;900:381–401. doi: 10.1007/978-1-60761-720-4_19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Dang AK, Tesfagiorgis Y, Jain RW, Craig HC, Kerfoot SM. Meningeal Infiltration of the Spinal Cord by Non-Classically Activated B Cells is Associated with Chronic Disease Course in a Spontaneous B Cell-Dependent Model of CNS Autoimmune Disease. Front Immunol. 2015;6:470. doi: 10.3389/fimmu.2015.00470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Krammer PH. CD95's deadly mission in the immune system. Nature. 2000;407:789–795. doi: 10.1038/35037728. [DOI] [PubMed] [Google Scholar]
- 58.Martin DA, Zheng L, Siegel RM, Huang B, Fisher GH, Wang J, Jackson CE, Puck JM, Dale J, Straus SE, Peter ME, Krammer PH, Fesik S, Lenardo MJ. Defective CD95/APO-1/Fas signal complex formation in the human autoimmune lymphoproliferative syndrome, type Ia. Proc Natl Acad Sci U S A. 1999;96:4552–4557. doi: 10.1073/pnas.96.8.4552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Takahashi T, Tanaka M, Brannan CI, Jenkins NA, Copeland NG, Suda T, Nagata S. Generalized lymphoproliferative disease in mice, caused by a point mutation in the Fas ligand. Cell. 1994;76:969–976. doi: 10.1016/0092-8674(94)90375-1. [DOI] [PubMed] [Google Scholar]
- 60.Watanabe-Fukunaga R, Brannan CI, Copeland NG, Jenkins NA, Nagata S. Lymphoproliferation disorder in mice explained by defects in Fas antigen that mediates apoptosis. Nature. 1992;356:314–317. doi: 10.1038/356314a0. [DOI] [PubMed] [Google Scholar]
- 61.Guo Q, Zhang J, Li J, Zou L, Xie Z, Fu X, Jiang S, Chen G, Jia Q, Li F, Wan Y, Wu Y. Forced miR-146a expression causes autoimmune lymphoproliferative syndrome in mice via downregulation of Fas in germinal center B cells. Blood. 2013;121:4875–4883. doi: 10.1182/blood-2012-08-452425. [DOI] [PubMed] [Google Scholar]
- 62.Risselada AP, Looije MF, Kruize AA, Bijlsma JW, van Roon JA. The role of ectopic germinal centers in the immunopathology of primary Sjögren's syndrome: a systematic review. Semin Arthritis Rheum. 2013;42:368–376. doi: 10.1016/j.semarthrit.2012.07.003. [DOI] [PubMed] [Google Scholar]
- 63.Reksten TR, Jonsson MV, Szyszko EA, Brun JG, Jonsson R, Brokstad KA. Cytokine and autoantibody profiling related to histopathological features in primary Sjogren's syndrome. Rheumatology (Oxford) 2009;48:1102–1106. doi: 10.1093/rheumatology/kep149. [DOI] [PubMed] [Google Scholar]
- 64.Le Pottier L, Devauchelle V, Fautrel A, Daridon C, Saraux A, Youinou P, Pers JO. Ectopic germinal centers are rare in Sjogren's syndrome salivary glands and do not exclude autoreactive B cells. J Immunol. 2009;182:3540–3547. doi: 10.4049/jimmunol.0803588. [DOI] [PubMed] [Google Scholar]
- 65.Salomonsson S, Jonsson MV, Skarstein K, Brokstad KA, Hjelmström P, Wahren-Herlenius M, Jonsson R. Cellular basis of ectopic germinal center formation and autoantibody production in the target organ of patients with Sjögren's syndrome. Arthritis Rheum. 2003;48:3187–3201. doi: 10.1002/art.11311. [DOI] [PubMed] [Google Scholar]
- 66.Bombardieri M, Barone F, Humby F, Kelly S, McGurk M, Morgan P, Challacombe S, De Vita S, Valesini G, Spencer J, Pitzalis C. Activation-induced cytidine deaminase expression in follicular dendritic cell networks and interfollicular large B cells supports functionality of ectopic lymphoid neogenesis in autoimmune sialoadenitis and MALT lymphoma in Sjögren's syndrome. J Immunol. 2007;179:4929–4938. doi: 10.4049/jimmunol.179.7.4929. [DOI] [PubMed] [Google Scholar]
- 67.Szodoray P, Jonsson R. The BAFF/APRIL system in systemic autoimmune diseases with a special emphasis on Sjögren's syndrome. Scand J Immunol. 2005;62:421–428. doi: 10.1111/j.1365-3083.2005.01688.x. [DOI] [PubMed] [Google Scholar]
- 68.Karnell JL, Mahmoud TI, Herbst R, Ettinger R. Discerning the kinetics of autoimmune manifestations in a model of Sjögren's syndrome. Mol Immunol. 2014;62:277–282. doi: 10.1016/j.molimm.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 69.Barone F, Bombardieri M, Manzo A, Blades MC, Morgan PR, Challacombe SJ, Valesini G, Pitzalis C. Association of CXCL13 and CCL21 expression with the progressive organization of lymphoid-like structures in Sjögren's syndrome. Arthritis Rheum. 2005;52:1773–1784. doi: 10.1002/art.21062. [DOI] [PubMed] [Google Scholar]
- 70.Hansen A, Reiter K, Ziprian T, Jacobi A, Hoffmann A, Gosemann M, Scholze J, Lipsky PE, Dörner T. Dysregulation of chemokine receptor expression and function by B cells of patients with primary Sjögren's syndrome. Arthritis Rheum. 2005;52:2109–2119. doi: 10.1002/art.21129. [DOI] [PubMed] [Google Scholar]
- 71.Astorri E, Bombardieri M, Gabba S, Peakman M, Pozzilli P, Pitzalis C. Evolution of ectopic lymphoid neogenesis and in situ autoantibody production in autoimmune nonobese diabetic mice: cellular and molecular characterization of tertiary lymphoid structures in pancreatic islets. J Immunol. 2010;185:3359–3368. doi: 10.4049/jimmunol.1001836. [DOI] [PubMed] [Google Scholar]
- 72.Lesage S, Hartley SB, Akkaraju S, Wilson J, Townsend M, Goodnow CC. Failure to censor forbidden clones of CD4 T cells in autoimmune diabetes. J Exp Med. 2002;196:1175–1188. doi: 10.1084/jem.20020735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Wan X, Thomas JW, Unanue ER. Class-switched anti-insulin antibodies originate from unconventional antigen presentation in multiple lymphoid sites. J Exp Med. 2016;213:967–978. doi: 10.1084/jem.20151869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Suurmond J, Calise J, Malkiel S, Diamond B. DNA-reactive B cells in lupus. Curr Opin Immunol. 2016;43:1–7. doi: 10.1016/j.coi.2016.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Becker-Herman S, Meyer-Bahlburg A, Schwartz MA, Jackson SW, Hudkins KL, Liu C, Sather BD, Khim S, Liggitt D, Song W, Silverman GJ, Alpers CE, Rawlings DJ. WASp-deficient B cells play a critical, cell-intrinsic role in triggering autoimmunity. J Exp Med. 2011;208:2033–2042. doi: 10.1084/jem.20110200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Rawlings DJ, Witte ON. Bruton's tyrosine kinase is a key regulator in B-cell development. Immunol Rev. 1994;138:105–119. doi: 10.1111/j.1600-065x.1994.tb00849.x. [DOI] [PubMed] [Google Scholar]
- 77.Casola S, Otipoby KL, Alimzhanov M, Humme S, Uyttersprot N, Kutok JL, Carroll MC, Rajewsky K. B cell receptor signal strength determines B cell fate. Nat Immunol. 2004;5:317–327. doi: 10.1038/ni1036. [DOI] [PubMed] [Google Scholar]
- 78.Vuyyuru R, Mohan C, Manser T, Rahman ZS. The lupus susceptibility locus Sle1 breaches peripheral B cell tolerance at the antibody-forming cell and germinal center checkpoints. J Immunol. 2009;183:5716–5727. doi: 10.4049/jimmunol.0804215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Berland R, Fernandez L, Kari E, Han JH, Lomakin I, Akira S, Wortis HH, Kearney JF, Ucci AA, Imanishi-Kari T. Toll-like receptor 7-dependent loss of B cell tolerance in pathogenic autoantibody knockin mice. Immunity. 2006;25:429–440. doi: 10.1016/j.immuni.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 80.Gaudin E, Hao Y, Rosado MM, Chaby R, Girard R, Freitas AA. Positive selection of B cells expressing low densities of self-reactive BCRs. J Exp Med. 2004;199:843–853. doi: 10.1084/jem.20030955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Petro JB, Rahman SM, Ballard DW, Khan WN. Bruton's tyrosine kinase is required for activation of IkappaB kinase and nuclear factor kappaB in response to B cell receptor engagement. J Exp Med. 2000;191:1745–1754. doi: 10.1084/jem.191.10.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Maas A, Hendriks RW. Role of Bruton's tyrosine kinase in B cell development. Dev Immunol. 2001;8:171–181. doi: 10.1155/2001/28962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Conley ME. B cells in patients with X-linked agammaglobulinemia. J Immunol. 1985;134:3070–3074. [PubMed] [Google Scholar]
- 84.Kil LP, de Bruijn MJ, van Nimwegen M, Corneth OB, van Hamburg JP, Dingjan GM, Thaiss F, Rimmelzwaan GF, Elewaut D, Delsing D, van Loo PF, Hendriks RW. Btk levels set the threshold for B-cell activation and negative selection of autoreactive B cells in mice. Blood. 2012;119:3744–3756. doi: 10.1182/blood-2011-12-397919. [DOI] [PubMed] [Google Scholar]
- 85.Corneth OB, de Bruijn MJ, Rip J, Asmawidjaja PS, Kil LP, Hendriks RW. Enhanced Expression of Bruton's Tyrosine Kinase in B Cells Drives Systemic Autoimmunity by Disrupting T Cell Homeostasis. J Immunol. 2016;197:58–67. doi: 10.4049/jimmunol.1600208. [DOI] [PubMed] [Google Scholar]
- 86.Gennery AR. The sting of WASP deficiency: autoimmunity exposed. Blood. 2016;127:173–175. doi: 10.1182/blood-2015-10-677237. [DOI] [PubMed] [Google Scholar]
- 87.Dupuis-Girod S, Medioni J, Haddad E, Quartier P, Cavazzana-Calvo M, Le Deist F, de Saint Basile G, Delaunay J, Schwarz K, Casanova JL, Blanche S, Fischer A. Autoimmunity in Wiskott-Aldrich syndrome: risk factors, clinical features, and outcome in a single-center cohort of 55 patients. Pediatrics. 2003;111:e622–627. doi: 10.1542/peds.111.5.e622. [DOI] [PubMed] [Google Scholar]
- 88.Recher M, Burns SO, de la Fuente MA, Volpi S, Dahlberg C, Walter JE, Moffitt K, Mathew D, Honke N, Lang PA, Patrizi L, Falet H, Keszei M, Mizui M, Csizmadia E, Candotti F, Nadeau K, Bouma G, Delmonte OM, Frugoni F, Fomin AB, Buchbinder D, Lundequist EM, Massaad MJ, Tsokos GC, Hartwig J, Manis J, Terhorst C, Geha RS, Snapper S, Lang KS, Malley R, Westerberg L, Thrasher AJ, Notarangelo LD. B cell-intrinsic deficiency of the Wiskott-Aldrich syndrome protein (WASp) causes severe abnormalities of the peripheral B-cell compartment in mice. Blood. 2012;119:2819–2828. doi: 10.1182/blood-2011-09-379412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Meyer-Bahlburg A, Becker-Herman S, Humblet-Baron S, Khim S, Weber M, Bouma G, Thrasher AJ, Batista FD, Rawlings DJ. Wiskott-Aldrich syndrome protein deficiency in B cells results in impaired peripheral homeostasis. Blood. 2008;112:4158–4169. doi: 10.1182/blood-2008-02-140814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Astrakhan A, Sather BD, Ryu BY, Khim S, Singh S, Humblet-Baron S, Ochs HD, Miao CH, Rawlings DJ. Ubiquitous high-level gene expression in hematopoietic lineages provides effective lentiviral gene therapy of murine Wiskott-Aldrich syndrome. Blood. 2012;119:4395–4407. doi: 10.1182/blood-2011-03-340711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Koopman G, Keehnen RM, Lindhout E, Zhou DF, de Groot C, Pals ST. Germinal center B cells rescued from apoptosis by CD40 ligation or attachment to follicular dendritic cells, but not by engagement of surface immunoglobulin or adhesion receptors, become resistant to CD95-induced apoptosis. Eur J Immunol. 1997;27:1–7. doi: 10.1002/eji.1830270102. [DOI] [PubMed] [Google Scholar]
- 92.Foy TM, Laman JD, Ledbetter JA, Aruffo A, Claassen E, Noelle RJ. gp39-CD40 interactions are essential for germinal center formation and the development of B cell memory. J Exp Med. 1994;180:157–163. doi: 10.1084/jem.180.1.157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Liu YJ, Joshua DE, Williams GT, Smith CA, Gordon J, MacLennan IC. Mechanism of antigen-driven selection in germinal centres. Nature. 1989;342:929–931. doi: 10.1038/342929a0. [DOI] [PubMed] [Google Scholar]
- 94.Fang W, Nath KA, Mackey MF, Noelle RJ, Mueller DL, Behrens TW. CD40 inhibits B cell apoptosis by upregulating bcl-xL expression and blocking oxidant accumulation. Am J Physiol. 1997;272:C950–956. doi: 10.1152/ajpcell.1997.272.3.C950. [DOI] [PubMed] [Google Scholar]
- 95.Lane P, Traunecker A, Hubele S, Inui S, Lanzavecchia A, Gray D. Activated human T cells express a ligand for the human B cell-associated antigen CD40 which participates in T cell-dependent activation of B lymphocytes. Eur J Immunol. 1992;22:2573–2578. doi: 10.1002/eji.1830221016. [DOI] [PubMed] [Google Scholar]
- 96.Fuleihan R, Ramesh N, Geha RS. Role of CD40-CD40-ligand interaction in Ig-isotype switching. Curr Opin Immunol. 1993;5:963–967. doi: 10.1016/0952-7915(93)90113-7. [DOI] [PubMed] [Google Scholar]
- 97.Rousset F, Garcia E, Banchereau J. Cytokine-induced proliferation and immunoglobulin production of human B lymphocytes triggered through their CD40 antigen. J Exp Med. 1991;173:705–710. doi: 10.1084/jem.173.3.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Grabstein KH, Maliszewski CR, Shanebeck K, Sato TA, Spriggs MK, Fanslow WC, Armitage RJ. The regulation of T cell-dependent antibody formation in vitro by CD40 ligand and IL-2. J Immunol. 1993;150:3141–3147. [PubMed] [Google Scholar]
- 99.Xu J, Foy TM, Laman JD, Elliott EA, Dunn JJ, Waldschmidt TJ, Elsemore J, Noelle RJ, Flavell RA. Mice deficient for the CD40 ligand. Immunity. 1994;1:423–431. doi: 10.1016/1074-7613(94)90073-6. [DOI] [PubMed] [Google Scholar]
- 100.DiSanto JP, Bonnefoy JY, Gauchat JF, Fischer A, de Saint Basile G. CD40 ligand mutations in x-linked immunodeficiency with hyper-IgM. Nature. 1993;361:541–543. doi: 10.1038/361541a0. [DOI] [PubMed] [Google Scholar]
- 101.Kawabe T, Naka T, Yoshida K, Tanaka T, Fujiwara H, Suematsu S, Yoshida N, Kishimoto T, Kikutani H. The immune responses in CD40-deficient mice: impaired immunoglobulin class switching and germinal center formation. Immunity. 1994;1:167–178. doi: 10.1016/1074-7613(94)90095-7. [DOI] [PubMed] [Google Scholar]
- 102.Castigli E, Alt FW, Davidson L, Bottaro A, Mizoguchi E, Bhan AK, Geha RS. CD40-deficient mice generated by recombination-activating gene-2-deficient blastocyst complementation. Proc Natl Acad Sci U S A. 1994;91:12135–12139. doi: 10.1073/pnas.91.25.12135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Renshaw BR, Fanslow WC, Armitage RJ, Campbell KA, Liggitt D, Wright B, Davison BL, Maliszewski CR. Humoral immune responses in CD40 ligand-deficient mice. J Exp Med. 1994;180:1889–1900. doi: 10.1084/jem.180.5.1889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.William J, Euler C, Christensen S, Shlomchik MJ. Evolution of autoantibody responses via somatic hypermutation outside of germinal centers. Science. 2002;297:2066–2070. doi: 10.1126/science.1073924. [DOI] [PubMed] [Google Scholar]
- 105.Weller S, Faili A, Garcia C, Braun MC, Le Deist F, de Saint Basile FG, Hermine GO, Fischer A, Reynaud CA, Weill JC. CD40-CD40L independent Ig gene hypermutation suggests a second B cell diversification pathway in humans. Proc Natl Acad Sci U S A. 2001;98:1166–1170. doi: 10.1073/pnas.98.3.1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 107.Shahinian A, Pfeffer K, Lee KP, Kündig TM, Kishihara K, Wakeham A, Kawai K, Ohashi PS, Thompson CB, Mak TW. Differential T cell costimulatory requirements in CD28-deficient mice. Science. 1993;261:609–612. doi: 10.1126/science.7688139. [DOI] [PubMed] [Google Scholar]
- 108.Borriello F, Sethna MP, Boyd SD, Schweitzer AN, Tivol EA, Jacoby D, Strom TB, Simpson EM, Freeman GJ, Sharpe AH. B7-1 and B7-2 have overlapping, critical roles in immunoglobulin class switching and germinal center formation. Immunity. 1997;6:303–313. doi: 10.1016/s1074-7613(00)80333-7. [DOI] [PubMed] [Google Scholar]
- 109.Chevrier S, Genton C, Malissen B, Malissen M, Acha-Orbea H. Dominant Role of CD80-CD86 Over CD40 and ICOSL in the Massive Polyclonal B Cell Activation Mediated by LAT(Y136F) CD4(+) T Cells. Front Immunol. 2012;3:27. doi: 10.3389/fimmu.2012.00027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ferguson SE, Han S, Kelsoe G, Thompson CB. CD28 is required for germinal center formation. J Immunol. 1996;156:4576–4581. [PubMed] [Google Scholar]
- 111.Rau FC, Dieter J, Luo Z, Priest SO, Baumgarth N. B7-1/2 (CD80/CD86) direct signaling to B cells enhances IgG secretion. J Immunol. 2009;183:7661–7671. doi: 10.4049/jimmunol.0803783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.MacPhee IA, Turner DR, Yagita H, Oliveira DB. CD80(B7.1) and CD86(B7.2) do not have distinct roles in setting the Th1/Th2 balance in autoimmunity in rats. Scand J Immunol. 2001;54:486–494. doi: 10.1046/j.1365-3083.2001.00998.x. [DOI] [PubMed] [Google Scholar]
- 113.Miller SD, Vanderlugt CL, Lenschow DJ, Pope JG, Karandikar NJ, Dal Canto MC, Bluestone JA. Blockade of CD28/B7-1 interaction prevents epitope spreading and clinical relapses of murine EAE. Immunity. 1995;3:739–745. doi: 10.1016/1074-7613(95)90063-2. [DOI] [PubMed] [Google Scholar]
- 114.Wandstrat AE, Nguyen C, Limaye N, Chan AY, Subramanian S, Tian XH, Yim YS, Pertsemlidis A, Garner HR, Morel L, Wakeland EK. Association of extensive polymorphisms in the SLAM/CD2 gene cluster with murine lupus. Immunity. 2004;21:769–780. doi: 10.1016/j.immuni.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 115.Wong EB, Soni C, Chan AY, Domeier PP, Shwetank, Abraham T, Limaye N, Khan TN, Elias MJ, Chodisetti SB, Wakeland EK, Rahman ZS. B cell-intrinsic CD84 and Ly108 maintain germinal center B cell tolerance. J Immunol. 2015;194:4130–4143. doi: 10.4049/jimmunol.1403023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kumar KR, Li L, Yan M, Bhaskarabhatla M, Mobley AB, Nguyen C, Mooney JM, Schatzle JD, Wakeland EK, Mohan C. Regulation of B cell tolerance by the lupus susceptibility gene Ly108. Science. 2006;312:1665–1669. doi: 10.1126/science.1125893. [DOI] [PubMed] [Google Scholar]
- 117.Keszei M, Detre C, Rietdijk ST, Muñoz P, Romero X, Berger SB, Calpe S, Liao G, Castro W, Julien A, Wu YY, Shin DM, Sancho J, Zubiaur M, Morse HC, Morel L, Engel P, Wang N, Terhorst C. A novel isoform of the Ly108 gene ameliorates murine lupus. J Exp Med. 2011;208:811–822. doi: 10.1084/jem.20101653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Keszei M, Latchman YE, Vanguri VK, Brown DR, Detre C, Morra M, Arancibia-Carcamo CV, Arancibia CV, Paul E, Calpe S, Castro W, Wang N, Terhorst C, Sharpe AH. Auto-antibody production and glomerulonephritis in congenic Slamf1-/- and Slamf2-/- [B6.129] but not in Slamf1-/- and Slamf2-/- [BALB/c.129] mice. Int Immunol. 2011;23:149–158. doi: 10.1093/intimm/dxq465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Koh AE, Njoroge SW, Feliu M, Cook A, Selig MK, Latchman YE, Sharpe AH, Colvin RB, Paul E. The SLAM family member CD48 (Slamf2) protects lupus-prone mice from autoimmune nephritis. J Autoimmun. 2011;37:48–57. doi: 10.1016/j.jaut.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.de Salort J, Cuenca M, Terhorst C, Engel P, Romero X. Ly9 (CD229) Cell-Surface Receptor is Crucial for the Development of Spontaneous Autoantibody Production to Nuclear Antigens. Front Immunol. 2013;4:225. doi: 10.3389/fimmu.2013.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Suzuki A, Yamada R, Kochi Y, Sawada T, Okada Y, Matsuda K, Kamatani Y, Mori M, Shimane K, Hirabayashi Y, Takahashi A, Tsunoda T, Miyatake A, Kubo M, Kamatani N, Nakamura Y, Yamamoto K. Functional SNPs in CD244 increase the risk of rheumatoid arthritis in a Japanese population. Nat Genet. 2008;40:1224–1229. doi: 10.1038/ng.205. [DOI] [PubMed] [Google Scholar]
- 122.Cunninghame Graham DS, Vyse TJ, Fortin PR, Montpetit A, Cai YC, Lim S, McKenzie T, Farwell L, Rhodes B, Chad L, Hudson TJ, Sharpe A, Terhorst C, Greenwood CM, Wither J, Rioux JD C. G. Investigators. Association of LY9 in UK and Canadian SLE families. Genes Immun. 2008;9:93–102. doi: 10.1038/sj.gene.6364453. [DOI] [PubMed] [Google Scholar]
- 123.Chatterjee M, Kis-Toth K, Thai TH, Terhorst C, Tsokos GC. SLAMF6-driven co-stimulation of human peripheral T cells is defective in SLE T cells. Autoimmunity. 2011;44:211–218. doi: 10.3109/08916934.2010.530627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Leadbetter EA, Rifkin IR, Hohlbaum AM, Beaudette BC, Shlomchik MJ, Marshak-Rothstein A. Chromatin-IgG complexes activate B cells by dual engagement of IgM and Toll-like receptors. Nature. 2002;416:603–607. doi: 10.1038/416603a. [DOI] [PubMed] [Google Scholar]
- 125.Viglianti GA, Lau CM, Hanley TM, Miko BA, Shlomchik MJ, Marshak-Rothstein A. Activation of autoreactive B cells by CpG dsDNA. Immunity. 2003;19:837–847. doi: 10.1016/s1074-7613(03)00323-6. [DOI] [PubMed] [Google Scholar]
- 126.Lau CM, Broughton C, Tabor AS, Akira S, Flavell RA, Mamula MJ, Christensen SR, Shlomchik MJ, Viglianti GA, Rifkin IR, Marshak-Rothstein A. RNA-associated autoantigens activate B cells by combined B cell antigen receptor/Toll-like receptor 7 engagement. J Exp Med. 2005;202:1171–1177. doi: 10.1084/jem.20050630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Avalos AM, Busconi L, Marshak-Rothstein A. Regulation of autoreactive B cell responses to endogenous TLR ligands. Autoimmunity. 2010;43:76–83. doi: 10.3109/08916930903374618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Busconi L, Bauer JW, Tumang JR, Laws A, Perkins-Mesires K, Tabor AS, Lau C, Corley RB, Rothstein TL, Lund FE, Behrens TW, Marshak-Rothstein A. Functional outcome of B cell activation by chromatin immune complex engagement of the B cell receptor and TLR9. J Immunol. 2007;179:7397–7405. doi: 10.4049/jimmunol.179.11.7397. [DOI] [PubMed] [Google Scholar]
- 129.Christensen SR, Shupe J, Nickerson K, Kashgarian M, Flavell RA, Shlomchik MJ. Toll-like receptor 7 and TLR9 dictate autoantibody specificity and have opposing inflammatory and regulatory roles in a murine model of lupus. Immunity. 2006;25:417–428. doi: 10.1016/j.immuni.2006.07.013. [DOI] [PubMed] [Google Scholar]
- 130.Wu X, Peng SL. Toll-like receptor 9 signaling protects against murine lupus. Arthritis Rheum. 2006;54:336–342. doi: 10.1002/art.21553. [DOI] [PubMed] [Google Scholar]
- 131.Christensen SR, Kashgarian M, Alexopoulou L, Flavell RA, Akira S, Shlomchik MJ. Toll-like receptor 9 controls anti-DNA autoantibody production in murine lupus. J Exp Med. 2005;202:321–331. doi: 10.1084/jem.20050338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Lartigue A, Courville P, Auquit I, François A, Arnoult C, Tron F, Gilbert D, Musette P. Role of TLR9 in anti-nucleosome and anti-DNA antibody production in lpr mutation-induced murine lupus. J Immunol. 2006;177:1349–1354. doi: 10.4049/jimmunol.177.2.1349. [DOI] [PubMed] [Google Scholar]
- 133.Ehlers M, Fukuyama H, McGaha TL, Aderem A, Ravetch JV. TLR9/MyD88 signaling is required for class switching to pathogenic IgG2a and 2b autoantibodies in SLE. J Exp Med. 2006;203:553–561. doi: 10.1084/jem.20052438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Summers SA, Hoi A, Steinmetz OM, O'Sullivan KM, Ooi JD, Odobasic D, Akira S, Kitching AR, Holdsworth SR. TLR9 and TLR4 are required for the development of autoimmunity and lupus nephritis in pristane nephropathy. J Autoimmun. 2010;35:291–298. doi: 10.1016/j.jaut.2010.05.004. [DOI] [PubMed] [Google Scholar]
- 135.Santiago-Raber ML, Dunand-Sauthier I, Wu T, Li QZ, Uematsu S, Akira S, Reith W, Mohan C, Kotzin BL, Izui S. Critical role of TLR7 in the acceleration of systemic lupus erythematosus in TLR9-deficient mice. J Autoimmun. 2010;34:339–348. doi: 10.1016/j.jaut.2009.11.001. [DOI] [PubMed] [Google Scholar]
- 136.Jackson SW, Scharping NE, Kolhatkar NS, Khim S, Schwartz MA, Li QZ, Hudkins KL, Alpers CE, Liggitt D, Rawlings DJ. Opposing impact of B cell-intrinsic TLR7 and TLR9 signals on autoantibody repertoire and systemic inflammation. J Immunol. 2014;192:4525–4532. doi: 10.4049/jimmunol.1400098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Pisitkun P, Deane JA, Difilippantonio MJ, Tarasenko T, Satterthwaite AB, Bolland S. Autoreactive B cell responses to RNA-related antigens due to TLR7 gene duplication. Science. 2006;312:1669–1672. doi: 10.1126/science.1124978. [DOI] [PubMed] [Google Scholar]
- 138.Subramanian S, Tus K, Li QZ, Wang A, Tian XH, Zhou J, Liang C, Bartov G, McDaniel LD, Zhou XJ, Schultz RA, Wakeland EK. A Tlr7 translocation accelerates systemic autoimmunity in murine lupus. Proc Natl Acad Sci U S A. 2006;103:9970–9975. doi: 10.1073/pnas.0603912103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Deane JA, Pisitkun P, Barrett RS, Feigenbaum L, Town T, Ward JM, Flavell RA, Bolland S. Control of toll-like receptor 7 expression is essential to restrict autoimmunity and dendritic cell proliferation. Immunity. 2007;27:801–810. doi: 10.1016/j.immuni.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Fairhurst AM, Hwang SH, Wang A, Tian XH, Boudreaux C, Zhou XJ, Casco J, Li QZ, Connolly JE, Wakeland EK. Yaa autoimmune phenotypes are conferred by overexpression of TLR7. Eur J Immunol. 2008;38:1971–1978. doi: 10.1002/eji.200838138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Hwang SH, Lee H, Yamamoto M, Jones LA, Dayalan J, Hopkins R, Zhou XJ, Yarovinsky F, Connolly JE, Curotto de Lafaille MA, Wakeland EK, Fairhurst AM. B cell TLR7 expression drives anti-RNA autoantibody production and exacerbates disease in systemic lupus erythematosus-prone mice. J Immunol. 2012;189:5786–5796. doi: 10.4049/jimmunol.1202195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Fukui R, Saitoh S, Matsumoto F, Kozuka-Hata H, Oyama M, Tabeta K, Beutler B, Miyake K. Unc93B1 biases Toll-like receptor responses to nucleic acid in dendritic cells toward DNA- but against RNA-sensing. J Exp Med. 2009;206:1339–1350. doi: 10.1084/jem.20082316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Nickerson KM, Christensen SR, Shupe J, Kashgarian M, Kim D, Elkon K, Shlomchik MJ. TLR9 regulates TLR7- and MyD88-dependent autoantibody production and disease in a murine model of lupus. J Immunol. 2010;184:1840–1848. doi: 10.4049/jimmunol.0902592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Desnues B, Macedo AB, Roussel-Queval A, Bonnardel J, Henri S, Demaria O, Alexopoulou L. TLR8 on dendritic cells and TLR9 on B cells restrain TLR7-mediated spontaneous autoimmunity in C57BL/6 mice. Proc Natl Acad Sci U S A. 2014;111:1497–1502. doi: 10.1073/pnas.1314121111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Nündel K, Green NM, Shaffer AL, Moody KL, Busto P, Eilat D, Miyake K, Oropallo MA, Cancro MP, Marshak-Rothstein A. Cell-intrinsic expression of TLR9 in autoreactive B cells constrains BCR/TLR7-dependent responses. J Immunol. 2015;194:2504–2512. doi: 10.4049/jimmunol.1402425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Hardy MP, Owczarek CM, Trajanovska S, Liu X, Kola I, Hertzog PJ. The soluble murine type I interferon receptor Ifnar-2 is present in serum, is independently regulated, and has both agonistic and antagonistic properties. Blood. 2001;97:473–482. doi: 10.1182/blood.v97.2.473. [DOI] [PubMed] [Google Scholar]
- 147.Piehler J, Thomas C, Garcia KC, Schreiber G. Structural and dynamic determinants of type I interferon receptor assembly and their functional interpretation. Immunol Rev. 2012;250:317–334. doi: 10.1111/imr.12001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Kiefer K, Oropallo MA, Cancro MP, Marshak-Rothstein A. Role of type I interferons in the activation of autoreactive B cells. Immunol Cell Biol. 2012;90:498–504. doi: 10.1038/icb.2012.10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Wilson LE, Widman D, Dikman SH, Gorevic PD. Autoimmune disease complicating antiviral therapy for hepatitis C virus infection. Semin Arthritis Rheum. 2002;32:163–173. doi: 10.1053/sarh.2002.37277. [DOI] [PubMed] [Google Scholar]
- 150.Munroe ME, Lu R, Zhao YD, Fife DA, Robertson JM, Guthridge JM, Niewold TB, Tsokos GC, Keith MP, Harley JB, James JA. Altered type II interferon precedes autoantibody accrual and elevated type I interferon activity prior to systemic lupus erythematosus classification. Ann Rheum Dis. 2016 doi: 10.1136/annrheumdis-2015-208140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Bennett L, Palucka AK, Arce E, Cantrell V, Borvak J, Banchereau J, Pascual V. Interferon and granulopoiesis signatures in systemic lupus erythematosus blood. J Exp Med. 2003;197:711–723. doi: 10.1084/jem.20021553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Sisirak V, Ganguly D, Lewis KL, Couillault C, Tanaka L, Bolland S, D'Agati V, Elkon KB, Reizis B. Genetic evidence for the role of plasmacytoid dendritic cells in systemic lupus erythematosus. J Exp Med. 2014;211:1969–1976. doi: 10.1084/jem.20132522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Rowland SL, Riggs JM, Gilfillan S, Bugatti M, Vermi W, Kolbeck R, Unanue ER, Sanjuan MA, Colonna M. Early, transient depletion of plasmacytoid dendritic cells ameliorates autoimmunity in a lupus model. J Exp Med. 2014;211:1977–1991. doi: 10.1084/jem.20132620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Heesters BA, Das A, Chatterjee P, Carroll MC. Do follicular dendritic cells regulate lupus-specific B cells? Mol Immunol. 2014;62:283–288. doi: 10.1016/j.molimm.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Mathian A, Gallegos M, Pascual V, Banchereau J, Koutouzov S. Interferon-α induces unabated production of short-lived plasma cells in pre-autoimmune lupus-prone (NZB×NZW)F1 mice but not in BALB/c mice. Eur J Immunol. 2011;41:863–872. doi: 10.1002/eji.201040649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Braun D, Caramalho I, Demengeot J. IFN-alpha/beta enhances BCR-dependent B cell responses. Int Immunol. 2002;14:411–419. doi: 10.1093/intimm/14.4.411. [DOI] [PubMed] [Google Scholar]
- 157.Gujer C, Sandgren KJ, Douagi I, Adams WC, Sundling C, Smed-Sörensen A, Seder RA, Karlsson Hedestam GB, Loré K. IFN-α produced by human plasmacytoid dendritic cells enhances T cell-dependent naïve B cell differentiation. J Leukoc Biol. 2011;89:811–821. doi: 10.1189/jlb.0810460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Badr G, Saad H, Waly H, Hassan K, Abdel-Tawab H, Alhazza IM, Ahmed EA. Type I interferon (IFN-alpha/beta) rescues B-lymphocytes from apoptosis via PI3Kdelta/Akt, Rho-A, NFkappaB and Bcl-2/Bcl(XL) Cell Immunol. 2010;263:31–40. doi: 10.1016/j.cellimm.2010.02.012. [DOI] [PubMed] [Google Scholar]
- 159.Moisini I, Huang W, Bethunaickan R, Sahu R, Ricketts PG, Akerman M, Marion T, Lesser M, Davidson A. The Yaa locus and IFN-α fine-tune germinal center B cell selection in murine systemic lupus erythematosus. J Immunol. 2012;189:4305–4312. doi: 10.4049/jimmunol.1200745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Swanson CL, Wilson TJ, Strauch P, Colonna M, Pelanda R, Torres RM. Type I IFN enhances follicular B cell contribution to the T cell-independent antibody response. J Exp Med. 2010;207:1485–1500. doi: 10.1084/jem.20092695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 161.Wang JH, Li J, Wu Q, Yang P, Pawar RD, Xie S, Timares L, Raman C, Chaplin DD, Lu L, Mountz JD, Hsu HC. Marginal zone precursor B cells as cellular agents for type I IFN-promoted antigen transport in autoimmunity. J Immunol. 2010;184:442–451. doi: 10.4049/jimmunol.0900870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lauwerys BR, Hachulla E, Spertini F, Lazaro E, Jorgensen C, Mariette X, Haelterman E, Grouard-Vogel G, Fanget B, Dhellin O, Vandepapelière P, Houssiau FA. Down-regulation of interferon signature in systemic lupus erythematosus patients by active immunization with interferon α-kinoid. Arthritis Rheum. 2013;65:447–456. doi: 10.1002/art.37785. [DOI] [PubMed] [Google Scholar]
- 163.Pollard KM, Cauvi DM, Toomey CB, Morris KV, Kono DH. Interferon-γ and systemic autoimmunity. Discov Med. 2013;16:123–131. [PMC free article] [PubMed] [Google Scholar]
- 164.Csiszár A, Nagy G, Gergely P, Pozsonyi T, Pócsik E. Increased interferon-gamma (IFN-gamma), IL-10 and decreased IL-4 mRNA expression in peripheral blood mononuclear cells (PBMC) from patients with systemic lupus erythematosus (SLE) Clin Exp Immunol. 2000;122:464–470. doi: 10.1046/j.1365-2249.2000.01369.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Harigai M, Kawamoto M, Hara M, Kubota T, Kamatani N, Miyasaka N. Excessive production of IFN-gamma in patients with systemic lupus erythematosus and its contribution to induction of B lymphocyte stimulator/B cell-activating factor/TNF ligand superfamily-13B. J Immunol. 2008;181:2211–2219. doi: 10.4049/jimmunol.181.3.2211. [DOI] [PubMed] [Google Scholar]
- 166.Panitch HS, Hirsch RL, Haley AS, Johnson KP. Exacerbations of multiple sclerosis in patients treated with gamma interferon. Lancet. 1987;1:893–895. doi: 10.1016/s0140-6736(87)92863-7. [DOI] [PubMed] [Google Scholar]
- 167.Jacob CO, van der Meide PH, McDevitt HO. In vivo treatment of (NZB X NZW)F1 lupus-like nephritis with monoclonal antibody to gamma interferon. J Exp Med. 1987;166:798–803. doi: 10.1084/jem.166.3.798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Ozmen L, Roman D, Fountoulakis M, Schmid G, Ryffel B, Garotta G. Experimental therapy of systemic lupus erythematosus: the treatment of NZB/W mice with mouse soluble interferon-gamma receptor inhibits the onset of glomerulonephritis. Eur J Immunol. 1995;25:6–12. doi: 10.1002/eji.1830250103. [DOI] [PubMed] [Google Scholar]
- 169.Balomenos D, Rumold R, Theofilopoulos AN. Interferon-gamma is required for lupus-like disease and lymphoaccumulation in MRL-lpr mice. J Clin Invest. 1998;101:364–371. doi: 10.1172/JCI750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Haas C, Ryffel B, Le Hir M. IFN-gamma receptor deletion prevents autoantibody production and glomerulonephritis in lupus-prone (NZB × NZW)F1 mice. J Immunol. 1998;160:3713–3718. [PubMed] [Google Scholar]
- 171.Schwarting A, Wada T, Kinoshita K, Tesch G, Kelley VR. IFN-gamma receptor signaling is essential for the initiation, acceleration, and destruction of autoimmune kidney disease in MRL-Fas(lpr) mice. J Immunol. 1998;161:494–503. [PubMed] [Google Scholar]
- 172.Lawson BR, Prud'homme GJ, Chang Y, Gardner HA, Kuan J, Kono DH, Theofilopoulos AN. Treatment of murine lupus with cDNA encoding IFN-gammaR/Fc. J Clin Invest. 2000;106:207–215. doi: 10.1172/JCI10167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Batten M, Groom J, Cachero TG, Qian F, Schneider P, Tschopp J, Browning JL, Mackay F. BAFF mediates survival of peripheral immature B lymphocytes. J Exp Med. 2000;192:1453–1466. doi: 10.1084/jem.192.10.1453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Balázs M, Martin F, Zhou T, Kearney J. Blood dendritic cells interact with splenic marginal zone B cells to initiate T-independent immune responses. Immunity. 2002;17:341–352. doi: 10.1016/s1074-7613(02)00389-8. [DOI] [PubMed] [Google Scholar]
- 175.Schneider P, MacKay F, Steiner V, Hofmann K, Bodmer JL, Holler N, Ambrose C, Lawton P, Bixler S, Acha-Orbea H, Valmori D, Romero P, Werner-Favre C, Zubler RH, Browning JL, Tschopp J. BAFF, a novel ligand of the tumor necrosis factor family, stimulates B cell growth. J Exp Med. 1999;189:1747–1756. doi: 10.1084/jem.189.11.1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Yan M, Marsters SA, Grewal IS, Wang H, Ashkenazi A, Dixit VM. Identification of a receptor for BLyS demonstrates a crucial role in humoral immunity. Nat Immunol. 2000;1:37–41. doi: 10.1038/76889. [DOI] [PubMed] [Google Scholar]
- 177.Vora KA, Wang LC, Rao SP, Liu ZY, Majeau GR, Cutler AH, Hochman PS, Scott ML, Kalled SL. Cutting edge: germinal centers formed in the absence of B cell-activating factor belonging to the TNF family exhibit impaired maturation and function. J Immunol. 2003;171:547–551. doi: 10.4049/jimmunol.171.2.547. [DOI] [PubMed] [Google Scholar]
- 178.Rahman ZS, Rao SP, Kalled SL, Manser T. Normal induction but attenuated progression of germinal center responses in BAFF and BAFF-R signaling-deficient mice. J Exp Med. 2003;198:1157–1169. doi: 10.1084/jem.20030495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Schiemann B, Gommerman JL, Vora K, Cachero TG, Shulga-Morskaya S, Dobles M, Frew E, Scott ML. An essential role for BAFF in the normal development of B cells through a BCMA-independent pathway. Science. 2001;293:2111–2114. doi: 10.1126/science.1061964. [DOI] [PubMed] [Google Scholar]
- 180.Ramanujam M, Wang X, Huang W, Schiffer L, Grimaldi C, Akkerman A, Diamond B, Madaio MP, Davidson A. Mechanism of action of transmembrane activator and calcium modulator ligand interactor-Ig in murine systemic lupus erythematosus. J Immunol. 2004;173:3524–3534. doi: 10.4049/jimmunol.173.5.3524. [DOI] [PubMed] [Google Scholar]
- 181.Bessa J, Kopf M, Bachmann MF. Cutting edge: IL-21 and TLR signaling regulate germinal center responses in a B cell-intrinsic manner. J Immunol. 2010;184:4615–4619. doi: 10.4049/jimmunol.0903949. [DOI] [PubMed] [Google Scholar]
- 182.Linterman MA, Beaton L, Yu D, Ramiscal RR, Srivastava M, Hogan JJ, Verma NK, Smyth MJ, Rigby RJ, Vinuesa CG. IL-21 acts directly on B cells to regulate Bcl-6 expression and germinal center responses. J Exp Med. 2010;207:353–363. doi: 10.1084/jem.20091738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Nurieva RI, Chung Y, Hwang D, Yang XO, Kang HS, Ma L, Wang YH, Watowich SS, Jetten AM, Tian Q, Dong C. Generation of T follicular helper cells is mediated by interleukin-21 but independent of T helper 1, 2, or 17 cell lineages. Immunity. 2008;29:138–149. doi: 10.1016/j.immuni.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Vogelzang A, McGuire HM, Yu D, Sprent J, Mackay CR, King C. A fundamental role for interleukin-21 in the generation of T follicular helper cells. Immunity. 2008;29:127–137. doi: 10.1016/j.immuni.2008.06.001. [DOI] [PubMed] [Google Scholar]
- 185.Fornek JL, Tygrett LT, Waldschmidt TJ, Poli V, Rickert RC, Kansas GS. Critical role for Stat3 in T-dependent terminal differentiation of IgG B cells. Blood. 2006;107:1085–1091. doi: 10.1182/blood-2005-07-2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 186.Rankin AL, Guay H, Herber D, Bertino SA, Duzanski TA, Carrier Y, Keegan S, Senices M, Stedman N, Ryan M, Bloom L, Medley Q, Collins M, Nickerson-Nutter C, Craft J, Young D, Dunussi-Joannopoulos K. IL-21 receptor is required for the systemic accumulation of activated B and T lymphocytes in MRL/MpJ-Fas(lpr/lpr)/J mice. J Immunol. 2012;188:1656–1667. doi: 10.4049/jimmunol.1003871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 187.Ding BB, Bi E, Chen H, Yu JJ, Ye BH. IL-21 and CD40L synergistically promote plasma cell differentiation through upregulation of Blimp-1 in human B cells. J Immunol. 2013;190:1827–1836. doi: 10.4049/jimmunol.1201678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.McPhee CG, Bubier JA, Sproule TJ, Park G, Steinbuck MP, Schott WH, Christianson GJ, Morse HC, Roopenian DC. IL-21 is a double-edged sword in the systemic lupus erythematosus-like disease of BXSB.Yaa mice. J Immunol. 2013;191:4581–4588. doi: 10.4049/jimmunol.1300439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Nohra R, Beyeen AD, Guo JP, Khademi M, Sundqvist E, Hedreul MT, Sellebjerg F, Smestad C, Oturai AB, Harbo HF, Wallström E, Hillert J, Alfredsson L, Kockum I, Jagodic M, Lorentzen J, Olsson T. RGMA and IL21R show association with experimental inflammation and multiple sclerosis. Genes Immun. 2010;11:279–293. doi: 10.1038/gene.2009.111. [DOI] [PubMed] [Google Scholar]
- 190.Webb R, Merrill JT, Kelly JA, Sestak A, Kaufman KM, Langefeld CD, Ziegler J, Kimberly RP, Edberg JC, Ramsey-Goldman R, Petri M, Reveille JD, Alarcón GS, Vilá LM, Alarcón-Riquelme ME, James JA, Gilkeson GS, Jacob CO, Moser KL, Gaffney PM, Vyse TJ, Nath SK, Lipsky P, Harley JB, Sawalha AH. A polymorphism within IL21R confers risk for systemic lupus erythematosus. Arthritis Rheum. 2009;60:2402–2407. doi: 10.1002/art.24658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Sawalha AH, Kaufman KM, Kelly JA, Adler AJ, Aberle T, Kilpatrick J, Wakeland EK, Li QZ, Wandstrat AE, Karp DR, James JA, Merrill JT, Lipsky P, Harley JB. Genetic association of interleukin-21 polymorphisms with systemic lupus erythematosus. Ann Rheum Dis. 2008;67:458–461. doi: 10.1136/ard.2007.075424. [DOI] [PubMed] [Google Scholar]
- 192.Liu R, Wu Q, Su D, Che N, Chen H, Geng L, Chen J, Chen W, Li X, Sun L. A regulatory effect of IL-21 on T follicular helper-like cell and B cell in rheumatoid arthritis. Arthritis Res Ther. 2012;14:R255. doi: 10.1186/ar4100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Mountz JD, Wang JH, Xie S, Hsu HC. Cytokine regulation of B-cell migratory behavior favors formation of germinal centers in autoimmune disease. Discov Med. 2011;11:76–85. [PMC free article] [PubMed] [Google Scholar]
- 194.Hwang IY, Hwang KS, Park C, Harrison KA, Kehrl JH. Rgs13 constrains early B cell responses and limits germinal center sizes. PLoS One. 2013;8:e60139. doi: 10.1371/journal.pone.0060139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Hwang IY, Park C, Harrison K, Boularan C, Galés C, Kehrl JH. An essential role for RGS protein/Gαi2 interactions in B lymphocyte-directed cell migration and trafficking. J Immunol. 2015;194:2128–2139. doi: 10.4049/jimmunol.1401952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 196.Estes JD, Thacker TC, Hampton DL, Kell SA, Keele BF, Palenske EA, Druey KM, Burton GF. Follicular dendritic cell regulation of CXCR4-mediated germinal center CD4 T cell migration. J Immunol. 2004;173:6169–6178. doi: 10.4049/jimmunol.173.10.6169. [DOI] [PubMed] [Google Scholar]
- 197.Moratz C, Hayman JR, Gu H, Kehrl JH. Abnormal B-cell responses to chemokines, disturbed plasma cell localization, and distorted immune tissue architecture in Rgs1-/- mice. Mol Cell Biol. 2004;24:5767–5775. doi: 10.1128/MCB.24.13.5767-5775.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Shi GX, Harrison K, Wilson GL, Moratz C, Kehrl JH. RGS13 regulates germinal center B lymphocytes responsiveness to CXC chemokine ligand (CXCL)12 and CXCL13. J Immunol. 2002;169:2507–2515. doi: 10.4049/jimmunol.169.5.2507. [DOI] [PubMed] [Google Scholar]
- 199.Boularan C, Kehrl JH. Implications of non-canonical G-protein signaling for the immune system. Cell Signal. 2014;26:1269–1282. doi: 10.1016/j.cellsig.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Ding Y, Li J, Wu Q, Yang P, Luo B, Xie S, Druey KM, Zajac AJ, Hsu HC, Mountz JD. IL-17RA is essential for optimal localization of follicular Th cells in the germinal center light zone to promote autoantibody-producing B cells. J Immunol. 2013;191:1614–1624. doi: 10.4049/jimmunol.1300479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Xie S, Li J, Wang JH, Wu Q, Yang P, Hsu HC, Smythies LE, Mountz JD. IL-17 activates the canonical NF-kappaB signaling pathway in autoimmune B cells of BXD2 mice to upregulate the expression of regulators of G-protein signaling 16. J Immunol. 2010;184:2289–2296. doi: 10.4049/jimmunol.0903133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Wichner K, Stauss D, Kampfrath B, Krüger K, Müller G, Rehm A, Lipp M, Höpken UE. Dysregulated development of IL-17- and IL-21-expressing follicular helper T cells and increased germinal center formation in the absence of RORγt. FASEB J. 2016;30:761–774. doi: 10.1096/fj.15-274001. [DOI] [PubMed] [Google Scholar]
- 203.Rahman ZS. Impaired clearance of apoptotic cells in germinal centers: implications for loss of B cell tolerance and induction of autoimmunity. Immunol Res. 2011;51:125–133. doi: 10.1007/s12026-011-8248-4. [DOI] [PubMed] [Google Scholar]
- 204.Wakeland EK, Liu K, Graham RR, Behrens TW. Delineating the genetic basis of systemic lupus erythematosus. Immunity. 2001;15:397–408. doi: 10.1016/s1074-7613(01)00201-1. [DOI] [PubMed] [Google Scholar]
- 205.Khan TN, Wong EB, Soni C, Rahman ZS. Prolonged Apoptotic Cell Accumulation in Germinal Centers of Mer-Deficient Mice Causes Elevated B Cell and CD4+ Th Cell Responses Leading to Autoantibody Production. J Immunol. 2013 doi: 10.4049/jimmunol.1200824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Rahman ZS, Shao WH, Khan TN, Zhen Y, Cohen PL. Impaired apoptotic cell clearance in the germinal center by Mer-deficient tingible body macrophages leads to enhanced antibody-forming cell and germinal center responses. J Immunol. 2010;185:5859–5868. doi: 10.4049/jimmunol.1001187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Hanayama R, Tanaka M, Miyasaka K, Aozasa K, Koike M, Uchiyama Y, Nagata S. Autoimmune disease and impaired uptake of apoptotic cells in MFG-E8-deficient mice. Science. 2004;304:1147–1150. doi: 10.1126/science.1094359. [DOI] [PubMed] [Google Scholar]
- 208.Rothlin CV, Ghosh S, Zuniga EI, Oldstone MB, Lemke G. TAM receptors are pleiotropic inhibitors of the innate immune response. Cell. 2007;131:1124–1136. doi: 10.1016/j.cell.2007.10.034. [DOI] [PubMed] [Google Scholar]
- 209.Kranich J, Krautler NJ, Heinen E, Polymenidou M, Bridel C, Schildknecht A, Huber C, Kosco-Vilbois MH, Zinkernagel R, Miele G, Aguzzi A. Follicular dendritic cells control engulfment of apoptotic bodies by secreting Mfge8. J Exp Med. 2008;205:1293–1302. doi: 10.1084/jem.20071019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Hu CY, Wu CS, Tsai HF, Chang SK, Tsai WI, Hsu PN. Genetic polymorphism in milk fat globule-EGF factor 8 (MFG-E8) is associated with systemic lupus erythematosus in human. Lupus. 2009;18:676–681. doi: 10.1177/0961203309103027. [DOI] [PubMed] [Google Scholar]
- 211.Herrmann M, Voll RE, Zoller OM, Hagenhofer M, Ponner BB, Kalden JR. Impaired phagocytosis of apoptotic cell material by monocyte-derived macrophages from patients with systemic lupus erythematosus. Arthritis Rheum. 1998;41:1241–1250. doi: 10.1002/1529-0131(199807)41:7<1241::AID-ART15>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 212.Baumann I, Kolowos W, Voll RE, Manger B, Gaipl U, Neuhuber WL, Kirchner T, Kalden JR, Herrmann M. Impaired uptake of apoptotic cells into tingible body macrophages in germinal centers of patients with systemic lupus erythematosus. Arthritis Rheum. 2002;46:191–201. doi: 10.1002/1529-0131(200201)46:1<191::AID-ART10027>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 213.Nimmerjahn F, Ravetch JV. Fcgamma receptors as regulators of immune responses. Nat Rev Immunol. 2008;8:34–47. doi: 10.1038/nri2206. [DOI] [PubMed] [Google Scholar]
- 214.Yu X, Lazarus AH. Targeting FcγRs to treat antibody-dependent autoimmunity. Autoimmun Rev. 2016;15:510–512. doi: 10.1016/j.autrev.2016.02.006. [DOI] [PubMed] [Google Scholar]
- 215.Ben Mkaddem S, Hayem G, Jönsson F, Rossato E, Boedec E, Boussetta T, El Benna J, Launay P, Goujon JM, Benhamou M, Bruhns P, Monteiro RC. Shifting FcγRIIA-ITAM from activation to inhibitory configuration ameliorates arthritis. J Clin Invest. 2014;124:3945–3959. doi: 10.1172/JCI74572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Clavel C, Nogueira L, Laurent L, Iobagiu C, Vincent C, Sebbag M, Serre G. Induction of macrophage secretion of tumor necrosis factor alpha through Fcgamma receptor IIa engagement by rheumatoid arthritis-specific autoantibodies to citrullinated proteins complexed with fibrinogen. Arthritis Rheum. 2008;58:678–688. doi: 10.1002/art.23284. [DOI] [PubMed] [Google Scholar]
- 217.Kyogoku C, Dijstelbloem HM, Tsuchiya N, Hatta Y, Kato H, Yamaguchi A, Fukazawa T, Jansen MD, Hashimoto H, van de Winkel JG, Kallenberg CG, Tokunaga K. Fcgamma receptor gene polymorphisms in Japanese patients with systemic lupus erythematosus: contribution of FCGR2B to genetic susceptibility. Arthritis Rheum. 2002;46:1242–1254. doi: 10.1002/art.10257. [DOI] [PubMed] [Google Scholar]
- 218.Xu L, Li G, Wang J, Fan Y, Wan Z, Zhang S, Shaheen S, Li J, Wang L, Yue C, Zhao Y, Wang F, Brzostowski J, Chen YH, Zheng W, Liu W. Through an ITIM-independent mechanism the FcγRIIB blocks B cell activation by disrupting the colocalized microclustering of the B cell receptor and CD19. J Immunol. 2014;192:5179–5191. doi: 10.4049/jimmunol.1400101. [DOI] [PubMed] [Google Scholar]
- 219.Nimmerjahn F, Ravetch JV. FcγRs in health and disease. Curr Top Microbiol Immunol. 2011;350:105–125. doi: 10.1007/82_2010_86. [DOI] [PubMed] [Google Scholar]
- 220.Willcocks LC, Carr EJ, Niederer HA, Rayner TF, Williams TN, Yang W, Scott JA, Urban BC, Peshu N, Vyse TJ, Lau YL, Lyons PA, Smith KG. A defunctioning polymorphism in FCGR2B is associated with protection against malaria but susceptibility to systemic lupus erythematosus. Proc Natl Acad Sci U S A. 2010;107:7881–7885. doi: 10.1073/pnas.0915133107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Li X, Wu J, Carter RH, Edberg JC, Su K, Cooper GS, Kimberly RP. A novel polymorphism in the Fcgamma receptor IIB (CD32B) transmembrane region alters receptor signaling. Arthritis Rheum. 2003;48:3242–3252. doi: 10.1002/art.11313. [DOI] [PubMed] [Google Scholar]
- 222.Clatworthy MR, Willcocks L, Urban B, Langhorne J, Williams TN, Peshu N, Watkins NA, Floto RA, Smith KG. Systemic lupus erythematosus-associated defects in the inhibitory receptor FcgammaRIIb reduce susceptibility to malaria. Proc Natl Acad Sci U S A. 2007;104:7169–7174. doi: 10.1073/pnas.0608889104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Siriboonrit U, Tsuchiya N, Sirikong M, Kyogoku C, Bejrachandra S, Suthipinittharm P, Luangtrakool K, Srinak D, Thongpradit R, Fujiwara K, Chandanayingyong D, Tokunaga K. Association of Fcgamma receptor IIb and IIIb polymorphisms with susceptibility to systemic lupus erythematosus in Thais. Tissue Antigens. 2003;61:374–383. doi: 10.1034/j.1399-0039.2003.00047.x. [DOI] [PubMed] [Google Scholar]
- 224.Chen JY, Wang CM, Wu JM, Ho HH, Luo SF. Association of rheumatoid factor production with FcgammaRIIIa polymorphism in Taiwanese rheumatoid arthritis. Clin Exp Immunol. 2006;144:10–16. doi: 10.1111/j.1365-2249.2006.03021.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 225.Smith KG, Clatworthy MR. FcgammaRIIB in autoimmunity and infection: evolutionary and therapeutic implications. Nat Rev Immunol. 2010;10:328–343. doi: 10.1038/nri2762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 226.Khoury SJ, Gallon L, Chen W, Betres K, Russell ME, Hancock WW, Carpenter CB, Sayegh MH, Weiner HL. Mechanisms of acquired thymic tolerance in experimental autoimmune encephalomyelitis: thymic dendritic-enriched cells induce specific peripheral T cell unresponsiveness in vivo. J Exp Med. 1995;182:357–366. doi: 10.1084/jem.182.2.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Saijo K, Schmedt C, Su IH, Karasuyama H, Lowell CA, Reth M, Adachi T, Patke A, Santana A, Tarakhovsky A. Essential role of Src-family protein tyrosine kinases in NF-kappaB activation during B cell development. Nat Immunol. 2003;4:274–279. doi: 10.1038/ni893. [DOI] [PubMed] [Google Scholar]
- 228.Ohashi PS, DeFranco AL. Making and breaking tolerance. Curr Opin Immunol. 2002;14:744–759. doi: 10.1016/s0952-7915(02)00406-5. [DOI] [PubMed] [Google Scholar]
- 229.Lamagna C, Scapini P, van Ziffle JA, DeFranco AL, Lowell CA. Hyperactivated MyD88 signaling in dendritic cells, through specific deletion of Lyn kinase, causes severe autoimmunity and inflammation. Proc Natl Acad Sci U S A. 2013;110:E3311–3320. doi: 10.1073/pnas.1300617110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Gross AJ, Proekt I, De Franco AL. Elevated BCR signaling and decreased survival of Lyn-deficient transitional and follicular B cells. Eur J Immunol. 2011;41:3645–3655. doi: 10.1002/eji.201141708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Silver KL, Crockford TL, Bouriez-Jones T, Milling S, Lambe T, Cornall RJ. MyD88-dependent autoimmune disease in Lyn-deficient mice. Eur J Immunol. 2007;37:2734–2743. doi: 10.1002/eji.200737293. [DOI] [PubMed] [Google Scholar]
- 232.Hua Z, Gross AJ, Lamagna C, Ramos-Hernández N, Scapini P, Ji M, Shao H, Lowell CA, Hou B, DeFranco AL. Requirement for MyD88 signaling in B cells and dendritic cells for germinal center anti-nuclear antibody production in Lyn-deficient mice. J Immunol. 2014;192:875–885. doi: 10.4049/jimmunol.1300683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 233.Levy E, Ambrus J, Kahl L, Molina H, Tung K, Holers VM. T lymphocyte expression of complement receptor 2 (CR2/CD21): a role in adhesive cell-cell interactions and dysregulation in a patient with systemic lupus erythematosus (SLE) Clin Exp Immunol. 1992;90:235–244. doi: 10.1111/j.1365-2249.1992.tb07935.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Wilson JG, Murphy EE, Wong WW, Klickstein LB, Weis JH, Fearon DT. Identification of a restriction fragment length polymorphism by a CR1 cDNA that correlates with the number of CR1 on erythrocytes. J Exp Med. 1986;164:50–59. doi: 10.1084/jem.164.1.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Douglas KB, Windels DC, Zhao J, Gadeliya AV, Wu H, Kaufman KM, Harley JB, Merrill J, Kimberly RP, Alarcón GS, Brown EE, Edberg JC, Ramsey-Goldman R, Petri M, Reveille JD, Vilá LM, Gaffney PM, James JA, Moser KL, Alarcón-Riquelme ME, Vyse TJ, Gilkeson GS, Jacob CO, Ziegler JT, Langefeld CD, Ulgiati D, Tsao BP, Boackle SA. Complement receptor 2 polymorphisms associated with systemic lupus erythematosus modulate alternative splicing. Genes Immun. 2009;10:457–469. doi: 10.1038/gene.2009.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 236.Fang Y, Xu C, Fu YX, Holers VM, Molina H. Expression of complement receptors 1 and 2 on follicular dendritic cells is necessary for the generation of a strong antigen-specific IgG response. J Immunol. 1998;160:5273–5279. [PubMed] [Google Scholar]
- 237.Iida K, Nadler L, Nussenzweig V. Identification of the membrane receptor for the complement fragment C3d by means of a monoclonal antibody. J Exp Med. 1983;158:1021–1033. doi: 10.1084/jem.158.4.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 238.Weis JJ, Tedder TF, Fearon DT. Identification of a 145,000 Mr membrane protein as the C3d receptor (CR2) of human B lymphocytes. Proc Natl Acad Sci U S A. 1984;81:881–885. doi: 10.1073/pnas.81.3.881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Nemerow GR, Houghten RA, Moore MD, Cooper NR. Identification of an epitope in the major envelope protein of Epstein-Barr virus that mediates viral binding to the B lymphocyte EBV receptor (CR2) Cell. 1989;56:369–377. doi: 10.1016/0092-8674(89)90240-7. [DOI] [PubMed] [Google Scholar]
- 240.Aubry JP, Pochon S, Graber P, Jansen KU, Bonnefoy JY. CD21 is a ligand for CD23 and regulates IgE production. Nature. 1992;358:505–507. doi: 10.1038/358505a0. [DOI] [PubMed] [Google Scholar]
- 241.Aubry JP, Pochon S, Gauchat JF, Nueda-Marin A, Holers VM, Graber P, Siegfried C, Bonnefoy JY. CD23 interacts with a new functional extracytoplasmic domain involving N-linked oligosaccharides on CD21. J Immunol. 1994;152:5806–5813. [PubMed] [Google Scholar]
- 242.Delcayre AX, Salas F, Mathur S, Kovats K, Lotz M, Lernhardt W. Epstein Barr virus/complement C3d receptor is an interferon alpha receptor. EMBO J. 1991;10:919–926. doi: 10.1002/j.1460-2075.1991.tb08025.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Holers VM. Complement receptors and the shaping of the natural antibody repertoire. Springer Semin Immunopathol. 2005;26:405–423. doi: 10.1007/s00281-004-0186-y. [DOI] [PubMed] [Google Scholar]
- 244.Bonnefoy JY, Henchoz S, Hardie D, Holder MJ, Gordon J. A subset of anti-CD21 antibodies promote the rescue of germinal center B cells from apoptosis. Eur J Immunol. 1993;23:969–972. doi: 10.1002/eji.1830230432. [DOI] [PubMed] [Google Scholar]
- 245.Grosjean I, Lachaux A, Bella C, Aubry JP, Bonnefoy JY, Kaiserlian D. CD23/CD21 interaction is required for presentation of soluble protein antigen by lymphoblastoid B cell lines to specific CD4+ T cell clones. Eur J Immunol. 1994;24:2982–2986. doi: 10.1002/eji.1830241209. [DOI] [PubMed] [Google Scholar]
- 246.Prodeus AP, Goerg S, Shen LM, Pozdnyakova OO, Chu L, Alicot EM, Goodnow CC, Carroll MC. A critical role for complement in maintenance of self-tolerance. Immunity. 1998;9:721–731. doi: 10.1016/s1074-7613(00)80669-x. [DOI] [PubMed] [Google Scholar]
- 247.Wu X, Jiang N, Deppong C, Singh J, Dolecki G, Mao D, Morel L, Molina HD. A role for the Cr2 gene in modifying autoantibody production in systemic lupus erythematosus. J Immunol. 2002;169:1587–1592. doi: 10.4049/jimmunol.169.3.1587. [DOI] [PubMed] [Google Scholar]
- 248.Boackle SA, Culhane KK, Brown JM, Haas M, Bao L, Quigg RJ, Holers VM. CR1/CR2 deficiency alters IgG3 autoantibody production and IgA glomerular deposition in the MRL/lpr model of SLE. Autoimmunity. 2004;37:111–123. doi: 10.1080/08916930410001685063. [DOI] [PubMed] [Google Scholar]
- 249.Walport MJ. Complement and systemic lupus erythematosus. Arthritis Res. 2002;4(3):S279–293. doi: 10.1186/ar586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Botto M. C1q knock-out mice for the study of complement deficiency in autoimmune disease. Exp Clin Immunogenet. 1998;15:231–234. doi: 10.1159/000019076. [DOI] [PubMed] [Google Scholar]
- 251.Pickering MC, Botto M, Taylor PR, Lachmann PJ, Walport MJ. Systemic lupus erythematosus, complement deficiency, and apoptosis. Adv Immunol. 2000;76:227–324. doi: 10.1016/s0065-2776(01)76021-x. [DOI] [PubMed] [Google Scholar]
- 252.Chatterjee P, Agyemang AF, Alimzhanov MB, Degn S, Tsiftsoglou SA, Alicot E, Jones SA, Ma M, Carroll MC. Complement C4 maintains peripheral B-cell tolerance in a myeloid cell dependent manner. Eur J Immunol. 2013;43:2441–2450. doi: 10.1002/eji.201343412. [DOI] [PMC free article] [PubMed] [Google Scholar]