Abstract
Purpose of review
Hematopoietic stem cells (HSCs) and progenitors are tasked with maintaining hematopoietic homeostasis in the face of numerous insults and challenges, including infection, inflammation and exsanguination. HSCs possess the remarkable ability to reconstitute the entire hematopoietic system of an organism whose own hematopoietic system has been ablated. This ability is exploited routinely in the clinic via HSC transplantation (HSCT). Here, we focus on the physiological and molecular bottlenecks overcome by HSCs during transplantation.
Recent findings
Upon transplantation, HSCs need to encounter a damaged bone marrow (BM) niche, characterized molecularly by increases in oxygen concentrations and an altered cytokine milieu. New mechanisms and pathways have been recently implicated during HSCT, including transplanted HSC-dependent secretion of conditioning molecules that facilitate engraftment and pathways that protect HSCs from perturbed organelle homeostasis.
Summary
Better understanding the molecular processes HSCs employ to withstand the stress of transplant will illuminate novel targets for further improving conditioning regimens and engraftment during HSCT.
Keywords: Hematopoietic Stem Cells, Bone Marrow transplantation, Bone Marrow reconstituting niche, oxidative stress
INTRODUCTION
HSCs are defined functionally by their ability to reconstitute hematopoiesis when transplanted into an organism whose own hematopoietic system has been ablated by irradiation, chemotherapy or disease. To preserve stemness and genome integrity, HSCs are quiescent and, in murine models, rarely divide during an individual’s lifetime unless challenged by insults that stimulate them to proliferate and differentiate (*1). To reconstitute an ablated hematopoietic system, transplanted HSCs must overcome many damaging insults, such as oxidative stress and migration through a bone marrow niche physically damaged by conditioning, resulting in metabolic changes and exit from quiescence in HSCs. Despite all this, HSCT is a clinical success and is employed >50,000 times worldwide each year to treat hematologic disease and cancer patients (2). Unfortunately, still about one-third of autologous or allogeneic transplant recipients will die for reasons other than primary disease relapse, such as infection, organ failure and Graft vs. Host Disease (GvHD) (3). A greater understanding of the molecular bottlenecks that stifle HSC function could illuminate novel therapeutic targets to improve clinical HSCT outcomes. Here, we will focus on reviewing our current understanding of the physical and molecular bottlenecks HSC must overcome during transplant to achieve stable engraftment and hematopoietic reconstitution.
A damaged niche
HSCT patients are usually pre-conditioned by chemotherapy and/or radiotherapy. The HSC BM niche is complex and multifaceted, with adipocytes, endothelial cells, megakaryocytes, heterogeneous stromal cells, macrophages, osteoblasts, and sympathetic nerves all implicated as functional contributors during homeostasis (4, 5). Transplant pre-conditioning disrupts some key components of the HSC BM niche, transforming it from a “BM homeostatic niche (h-Niche)” into what can be thought of as an acute “BM reconstituting niche (r-Niche)”. For example, although osteoblasts appear minimally perturbed by conditioning (4), the sinusoidal vascular network is severely disrupted, becomes leaky, displays changes in morphology and structure, and swells. Genetic alterations of BM vascular endothelium integrity negatively impact HSC function (6**), highlighting the importance of intact vasculature for HSCs. The damaged vasculature takes weeks to recover and donor-derived hematopoietic cells, via the VEGF/VEGFR2 axis, have been implicated as participants in its recovery (4, 7). Gross changes in the levels and locations of cytokines critical to the viability and distribution of HSCs, such as SDF-1 (the Stromal Cell Derived Factor-1; also known as CXC chemokine ligand 12, CXCL12), VEGF, IGF-1, PDGF-BB, and TPO, have also been observed after conditioning (4, 5, 8). Changing CXCL12 levels following irradiation and the break-down of the sinusoidal vasculature results in redistribution of BM megakaryocytes from sinusoidal vessels to the endosteal surface; contributing to the distinct architecture of the r-Niche (4, 5, 9). Indeed, megakaryocytes facilitate BM injury recovery by producing FGF, which also stimulates HSC proliferation via the FGFR and up-regulation of NFκB and CXCR4, (the CXCL12 receptor and a master regulator of HSC trafficking and niche retention) (10). Conditioning regimens are also toxic to the nervous system, especially in children (11). During homeostasis, Schwann cell-derived TGFβ supports the integrity of the HSC pool (12, 13). Damage to BM glia by conditioning could deregulate HSC stores. Conditioning also alters the BM extracellular matrix in ways that functionally feedback on HSCs. Irradiation induces endothelial E-SELECTIN production via NFκB activation and TENASCIN-C production by stromal cells and endothelium (14, 15). Both of these molecules stimulate HSC homing, E-SELECTIN via E-SELECTIN-LIGAND-1 (ESL-1), which is expressed by HSCs, and TENASCIN C via INTEGRIN α9 (16). TENASCIN C/INTEGRIN α9 engagement can trigger HSCs to enter the cell-cycle by up-regulating CyclinD1 and CyclinE1 and down-regulating cyclin-dependent kinase inhibitors (p57(Kip2), p21(Cip1), p16(Ink4a) (14).
During inflammation or vascular damage, adenosine triphosphate (ATP), uridine triphosphate (UTP) and possibly other nucleotides are released into the extracellular environment (17). Most blood cells respond to nucleotides via purinergic P2 receptors (17). P2Y14 is highly expressed by both human and mouse HSPCs (18*, 19). P2y14−/− HSCs display hyper-radiosensitivity due to their inability to regulate ROS levels that accumulate and induce senescence through p38 hyper-activation (19). Moreover, knockdown of P2y14 in murine HSC comprises their engraftment (18*). These data suggest that P2Y14, and potentially additional purinergic receptors, might function as critical sensors of tissue damage by detecting elevated nucleotides in the r-Niche and promoting HSC survival by controlling increasing ROS levels post-transplant.
These studies and others establish that the r-Niche is physically and molecularly distinct from the h-Niche (20). Moreover, they suggest that the recovery of the r-Niche, and successful HSC engraftment, depends, in part, on transplanted hematopoietic cells, including HSPCs.
Functional HSC must home to the reconstituting niche (r-Niche)
To effectively reconstitute hematopoiesis, transplanted HSCs must both find their way to the BM and stably establish themselves within the r-Niche. HSC BM homing following transplant is rapid (hours to 1–2 days) and requires rolling, anchorage to the BM sinusoids, trans-endothelial migration and stable interaction with niche components (8, 21). CXCL12 is critically required for HSC migration from the fetal liver to the BM during embryogenesis (22). Antibody blocking of CXCR4 (by AMD-3100) or elevated CXCL12 plasma levels can mobilize HSPCs from the BM to the periphery (13, 23). CXCL12 is critical for BM HSC homing during transplant, where it is expressed by osteoblasts and endothelium (4, 24, 25). CXCL12 levels increase in the r-Niche after conditioning, which attracts HSCs and facilitates their stable engraftment (4, 8). Since many BM proteinases, such as Matrix Metalloproteinases 2/9 (MMP2 and MMP9), can cleave CXCL12 and negatively affect its activity as a chemo-attractant, it would be of interest to study their levels in a r-Niche. Modulating the activity of these proteinases could benefit current HSCT protocols (21, 26). Other chemo-attractants have also been implicated in directing the migration and mobilization of HSCs, including CCL2, CCL5, CXCL10, IL-8, SCF, LTD4, sphingosin-1-phosphate and ceramide-1 phosphate (21, 26–29).
Notably, conditioning by irradiation or cyclophosphamide results in elevated cleavage of COMPLEMENT COMPONENT3 (C3) into C3a and iC3b in PB and BM (30). HSC express C3aR and CR3 (also known as CD11b/CD18 or Mac-1), which are receptors for C3 cleavage fragments (30). C3a sensitizes human and mouse HSCs to CXCL12 gradients by promoting CXCR4 incorporation into membrane lipid rafts, while iC3b deposited on damaged BM stroma increases HSC adhesion to niche components via interaction with CR3 (30). Importantly, C3−/− mice are more sensitive to G-CSF mobilization (30). Although these mice have normal steady-state hematopoiesis, they display a delay in hematopoietic recovery when subjected to sub-lethal irradiation or HSC transplantation. C3−/− HSCs function normally when transplanted into C3+/+ recipients, indicating that this phenotype results from a defect in the BM niche (30). Thus, cleaved C3 is important for BM regeneration and reconstitution following conditioning and may be an essential component of the r-Niche (30).
Both mouse and human HSC have also been shown to express multiple molecules that critically regulate their ability to physically engage r-Niche cells (such as the sinusoidal endothelium). For example, blocking or genetic loss of integrins, such as α4β1, α9β1, and α4β7, inhibit robust HSC engraftment (21, 31, 32). HSC rolling on the endothelium, necessary for extravasation into the BM space, is regulated by interactions with endothelial P and L-SECTININs (33). Antibody blocking of CD44 also blocks HSC engraftment (34). More recently, CYTOHESIN-1 and JAM-B/JAM-C interactions have also been implicated in HSC niche engagement (35, 36), as have factors that likely regulate to the ability to HSCs to physically move through the vasculature and BM, such as ARHGEF5, a Rho guanine nucleotide exchange factor important for podosome formation (37). Not surprisingly, knockdown of Arhgef5 in murine HSPCs significantly impairs their transplantation (18*). Further, conditioning regimens can impact how HSC physically engage the r-Niche. For example, BM endothelium up-regulates MAdCAM-1, an α4β1 ligand, in response to total body irradiation (31). Blocking MAdCAM-1 in this context is highly detrimental to HSC engraftment (31).
HSC-directed niche conditioning facilitates engraftment
Evidence is accumulating that transplanted HSCs can themselves act on the r-Niche in ways that promote their own engraftment. For example, knockdown of secreted factors or molecules that regulate the biogenesis of secreted factors (e.g. Fstl1, Crispld1) in mouse HSPCs reduces their transplantation (18*). Although the mechanisms here are unknown, FSTL1 is a negative regulator BMP signaling that has been implicated in vasculature remodeling and ischemic cardiomyocyte regeneration (38, 39). HSCs express putative “niche-conditioning” molecules implicated in HSC quiescence, such as RNase ANGIOGENIN, IL-8 and EMBIGEN (40**). HSCs also express ESL-1, a regulator of HSC proliferative homeostasis via repression of TGFβ production by HSCs and other hematopoietic cells. ESL-1-deficient HSCs produce more TGFβ, which conditions the niche to induce quiescence in wild type co-transplanted HSCs (41*). Degrading enzymes, such as MMPs, are important for efficient HSC homing (8). Up-regulation of MMP-2, MMP-9 and MT1-MMP facilitates HSCT and BM homing by promoting extravasation via degradation of extra-cellular matrix components, enhancing migration towards CXCL12, and by releasing soluble Kit ligand from BM stroma (42). As mentioned, transplanted hematopoietic cells promote vasculature recovery after severe irradiation (43). Conversely, transplanted hematopoietic cells can also negatively influence r-Niche recovery: donor-derived angiopoietin-1 (ANGPT-1) slows vasculature recovery after irradiation, likely by negatively regulating endothelial cell proliferation (7). Thus, a better understanding of the reciprocal interactions between incoming HSCs and the r-Niche could illuminate novel strategies for improving engraftment.
Oxidative stress and changing metabolic needs
Steady-state HSCs are largely quiescent, depend on glycolysis for energy production, and, consequently, display low levels of radical oxygen species (ROS) (44-*,46). The h-Niche is irrigated with a heterogeneous network of arterioles, which carry oxygenated blood and are most abundant near the endosteum, and sinusoids, which carry less oxygenated blood and are abundant in the central BM (20, 47–49). This creates an oxygen gradient in the BM, from ≈4% O2 near the endosteum to ≈2% in the central marrow (50, 51). However, the distribution of HSCs throughout the BM suggests that HSC maintain a hypoxic profile regardless of their location and external O2 tension (24, 47, 48, 50–52). Although it is formally possible that super-low O2 levels might exist in tight regions proximal to HSCs (50).
Transplant pre-conditioning, and the resulting cell damage, elevates BM O2 levels (51). Transplanted HSPCs distribute throughout this landscape of elevated oxygen (51). Under low oxygen, HIF-1α is active and transcriptionally promotes glycolysis. As O2 levels rise, HIF-1α is targeted for degradation, promoting a shift in HSC metabolism from glycolysis to oxidative phosphorylation (OXPHOS), which further increases internal ROS levels (44, 45). Increased ROS impairs HSC self-renewal, quiescence, and promotes their mobilization from the bone marrow (53, 54). Indeed, purified ROSLow HSCs display superior repopulating activity relative to ROSHigh HSCs (44). HSCs also become exposed to supra-physiologic levels of oxygen during their isolation and handling prior to transplant (55**). Inhibition of ROS accumulation by anti-oxidants such as N-acetyl-L-cysteine (NAC) or via manipulation of signaling pathways linked to ROS accumulation (p38 MAPK, the miR-212/132 cluster (Mirc19) or SIRT3 overexpression) rescues HSC function and transplantation (54, 56–58). Thus, HSC must engage molecular pathways to resolve these insults in order to achieve stable engraftment and hematopoietic repopulation. Indeed, a failure to transition from glycolysis into OXPHOS blocks HSC differentiation (59, **60), indicating that is required for effective hematopoietic reconstitution. Further, constitutive mTOR activation, a master sensor of cellular metabolic needs, is detrimental to HSC quiescence and serial repopulation, which appears to be in part explained by elevated ROS (61, 62). Moreover, the PML-PPARδ pathway for fatty acid oxidation regulates asymmetric versus symmetric HSC division and disruption of this pathway leads HSC exhaustion (53).
SIRT1, a deacetylase that globally coordinates metabolic changes in response to nutrient levels, is also required for optimal HSC transplantation (63). SIRT1 functions upstream of FOXO3 in HSCs to modulate changing intracellular ROS levels (**60, 63). Indeed, FOXO factors are well known critical regulators of HSC ROS, both during homeostasis and transplantation (**60, 64). While FoxO-deficient bone marrow lacks long-term engraftment, in vivo treatment with NAC reverts this phenotype (65). Similarly, Foxa3−/− HSCs display high ROS post-transplant and compromised serial transplantation (18*). A SIRT1-related enzyme, SIRT3, is also required for optimal HSC transplantation, but only in aged HSC (58). SIRT3 regulates the acetylation of mitochondrial proteins (65). Thus, to effectively repopulate, transplanted HSCs must affect a balance between their shifting metabolic needs and the detrimental effects of elevated ROS on their self-renewal and differentiation.
Epigenetic regulators
Recently, several studies have illuminated single cell heterogeneity within the HSC pool and suggested a model where HSC function is transplantable and imprinted by specific epigenetic patterns, such as DNA methylation, hydroxymethylation, and histone modification (66, *67, 68). Transplantation may perturb the HSC epigenetic landscape. For example, as HSCs switch from glycolysis to OXPHOS during transplant, increasing α-ketoglutarate and decreasing succinate may activate histone demethylases and trigger differentiation (65). The compromised differentiation potential, enhanced self-renewal, and sensitization to apoptosis of HSC deficient in Dnmt1 or Dnmt3a demonstrates that DNA methylation levels can significantly impact HSC function (69, 70). Active DNA de-methylation also perturbs HSC transplantation. TET family proteins hydroxylate 5-methylcytosine to 5-hydroxymethylcytosine, which is then deaminated by AID/APOBEC proteins before processed into cytosine by BER glycosylases. Tet2−/− mice display increased HSC numbers and increased HSC repopulating activity (71). In contrast, Aid−/− mice display expansion of myeloid cells and anemia due to reduced erythroid progenitors, but display normal HSC self-renewal (72). Histone modification patterns also appear important for HSC transplantation. Loss of EED, which methylates H3K27 as part of the Polycomb Repressive Complex 2, results in HSC exhaustion (66, 73). In contrast, Ezh2 overexpression perpetuates HSC serial transplantation (74). HSCs also display bivalent domains that contain H3K4me3 and H3K27me3 (75). To what extent the epigenetic landscape of HSC is altered by transplantation remains an open question.
Transplant challenges organelle homeostasis
Recent data suggests that transplanted HSCs must cope with perturbations in organelle homeostasis. For example, the essential autophagy gene, Atg7, is required for HSC repopulation (76). Further, as transplanted HSC exit quiescence and increase oxidative phosphorylation, mitochondria numbers increase (44, 55**, 77). This activates mitochondrial pathways regulating oxidative stress, such as SIRT1, a deacetylase that targets FOXO transcription factors in HSCs (**60). Loss of SIRT7, a regulator of the mitochondrial unfolded protein response, result in reduced repopulating activity, reflecting the dependence of transplanted HSCs on this pathway (**78).
Oxidative stress, accumulating mis-folded proteins, or calcium disequilibrium can induce endoplasmic reticulum (ER) stress (79). Glucose-regulated protein 78 (GPR78), an ER chaperone, regulates and inactivates multiple ER stress sensors (80). Gpr78-deficient mice show a loss in HSCs (81). The ER unfolded protein response (UPR) is resolved by inhibition of translation, activation of ubiquitin-dependent degradation of mis-folded proteins or by increased ER biogenesis (79). Indeed, overexpression of ERDJ4 (a canonical UPR chaperone) in human HSCs enhances their repopulating activity, indicating that ERDJ4 protects against transplant-induced ER stress (**82). Further, human HSPCs display higher expression of PERK pathway members and decreased expression of IRE1, suggesting that HSCs depend on this ER stress response pathway (**82).
Conclusion
Classic pre-transplant conditioning triggers a plethora of cellular responses that cumulatively select for the small population of bone marrow cells we know as transplantable HSCs. These cellular responses likely exist to counteract environmental insults to the hematopoietic system (e.g. infection, exsanguination, starvation, etc…) by allowing HSC division and activation while safeguarding genome integrity and stemness. Although the molecular mechanisms behind many of these responses have been recently illuminated, we are still far from fully understanding HSC engraftment. Indeed, numerous novel alternative conditioning methods reveal that HSCT does not depend on complete bone marrow ablation (e.g. dietary Valine depletion, anti-c-Kit or CD45 antibodies, CD45-saporin, inhibition of stromal cell heparan sulfate synthesis and E-SELECTIN antagonists (**83,84). These new methods hold the promise of alleviating the non-hematopoietic toxicity associated with classic conditioning regimens, which will be especially important for children and young adults. Further, each likely imposes unique molecular pressures on transplanted HSCs. Further study of these alternative conditioning regimens will yield new insight into additional molecular bottlenecks that stifle transplanted HSC.
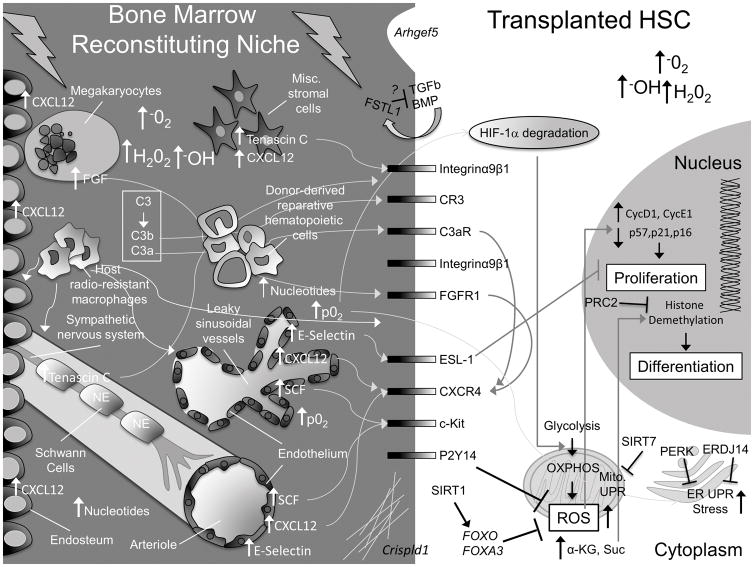
Figure 1. Summary of molecular alterations driven by classical pre-transplant conditioning regimens in HSCs and the bone marrow niche.
Here, we present a schematic to highlight some of the gross physical and molecular changes that occur in the bone marrow niche and within HSCs. For simplicity, not every known cellular component of the niche is pictured. In the niche, C3 is cleaved to C3b and C3a, which interact with HSC CR3 and C3aR receptors and stimulate homing by increasing, among other things, CXCR4. Megakaryocytes, which are attracted to the endosteum from sinusoidal vessels by increasing endosteal-CXCL12, also upregulate CXCR4 on HSCs via increased secretion of FGF. Schwann cells and stromal cells release TENASCIN C, which stimulates HSC migration and adhesion. Endothelial cells upregulate E-SELECTIN, CXCL12 and SCF. Sinusoidal vessels are damaged and leaky, resulting in an increase in O2 partial pressure (p02) and BM ROS levels. This contributes to H1F-1α degradation in HSCs, promoting their transition from glycolysis to oxidative respiration (OXPHOS), which further increases intracellular ROS levels. FOXOs, FOXA3, and signaling downstream of P2Y14 help HSCs cope with rising ROS levels. SIRT1 activates FOXOs. SIRT7 inhibits the increase in the mitochondrial unfolded protein response (UPR). Increased ROS stimulates HSC division and an ER-UPR. PERK and ERDJ14 counteract this effect in transplanted HSCs. Free nucleotide levels rise in the BM and are sensed by purinergic receptors, like P2Y14, which regulates ROS. Increasing intracellular α-Ketoglutarate (α-KG) promotes HSC differentiation via Histone demethylation. PRC2 complex counteracts this effect by promoting Histone methylation. Transplanted HSCs condition the reconstituting niche by secreting FSTL1 and extracellular matrix components (via Crispld1) and (very likely) additional factors (e.g. IL-8). Transplanted hematopoietic cells facilitate recovery of the conditioned niche. Figure Key: the bone marrow space is depicted on a dark gray background, the HSC intracellular space is light gray, and the HSC nucleus is dark gray. Major cell types are labeled in white font, major changes in the bone marrow space are labeled in white font, and major changes in the HSC are labeled in black font.
KEY POINTS.
Transplant conditioning regimens induce dramatic changes in the HSC bone marrow niche.
Transplanted HSCs are subject to significant metabolic changes, perturbed organelle homeostasis, and elevated ROS levels.
HSCs autonomously condition their new home to facilitate engraftment.
Acknowledgments
We thank the McKinney-Freeman laboratory and Department of Hematology at St. Jude Children’s Research Hospital (SJCRH) for critical discussions and reading of the manuscript.
Financial support and sponsorship
This work was supported by the American Society of Hematology (S.M.-F.), the Hartwell Foundation (S.M.-F.), the NIDDK (K01DK080846 and R01DK104028, S.M.-F.), the American Lebanese Syrian Associated Charities (S.M.-F.).
Footnotes
Conflicts of interest.
Authors declare no conflicts of interest.
References and recommended reading
Papers of remarkable interest are highlighted as:
* Of special interest
** Of outstanding interest
- *1.Bernitz JM, Kim HS, MacArthur B, Sieburg H, Moore K. Hematopoietic Stem Cells Count and Remember Self-Renewal Divisions. Cell. 2016;167(5):1296–309. e10. doi: 10.1016/j.cell.2016.10.022. Epub 2016/11/15. This study describes a population of dormant HSCs that persist throughout adulthood and also presents evidence that HSCs retain memory of their proliferative history and that long term HSCs are exceptionally rare in the aged HSC pool. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gratwohl A, Baldomero H, Aljurf M, Pasquini MC, Bouzas LF, Yoshimi A, et al. Hematopoietic stem cell transplantation: a global perspective. JAMA. 2010;303(16):1617–24. doi: 10.1001/jama.2010.491. Epub 2010/04/29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Current Uses and Outcomes of Hematopoietic Cell Transplantation (HCT): CIBMTR Summary Slides, [database on the Internet]. 2016.
- 4.Sasine JP, Yeo KT, Chute JP. Concise Review: Paracrine Functions of Vascular Niche Cells in Regulating Hematopoietic Stem Cell Fate. Stem Cells Transl Med. 2016 doi: 10.5966/sctm.2016-0254. Epub 2016/09/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Olson TS, Caselli A, Otsuru S, Hofmann TJ, Williams R, Paolucci P, et al. Megakaryocytes promote murine osteoblastic HSC niche expansion and stem cell engraftment after radioablative conditioning. Blood. 2013;121(26):5238–49. doi: 10.1182/blood-2012-10-463414. Epub 2013/05/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **6.Itkin T, Gur-Cohen S, Spencer JA, Schajnovitz A, Ramasamy SK, Kusumbe AP, et al. Distinct bone marrow blood vessels differentially regulate haematopoiesis. Nature. 2016;532(7599):323–8. doi: 10.1038/nature17624. Epub 2016/04/14. This study describes how differences in oxygen permeability among bone marrow vessels regulate HSC migration and differentiation due to ROS levels. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhou BO, Ding L, Morrison SJ. Hematopoietic stem and progenitor cells regulate the regeneration of their niche by secreting Angiopoietin-1. Elife. 2015;4:e05521. doi: 10.7554/eLife.05521. Epub 2015/03/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lapidot T, Dar A, Kollet O. How do stem cells find their way home? Blood. 2005;106(6):1901–10. doi: 10.1182/blood-2005-04-1417. Epub 2005/05/14. [DOI] [PubMed] [Google Scholar]
- 9.Niswander LM, Fegan KH, Kingsley PD, McGrath KE, Palis J. SDF-1 dynamically mediates megakaryocyte niche occupancy and thrombopoiesis at steady state and following radiation injury. Blood. 2014;124(2):277–86. doi: 10.1182/blood-2014-01-547638. Epub 2014/04/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhao M, Ross JT, Itkin T, Perry JM, Venkatraman A, Haug JS, et al. FGF signaling facilitates postinjury recovery of mouse hematopoietic system. Blood. 2012;120(9):1831–42. doi: 10.1182/blood-2011-11-393991. Epub 2012/07/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Moore IM. Central nervous system toxicity of cancer therapy in children. J Pediatr Oncol Nurs. 1995;12(4):203–10. doi: 10.1177/104345429501200405. discussion 11. Epub 1995/10/01. [DOI] [PubMed] [Google Scholar]
- 12.Yamazaki S, Ema H, Karlsson G, Yamaguchi T, Miyoshi H, Shioda S, et al. Nonmyelinating Schwann cells maintain hematopoietic stem cell hibernation in the bone marrow niche. Cell. 2011;147(5):1146–58. doi: 10.1016/j.cell.2011.09.053. Epub 2011/11/29. [DOI] [PubMed] [Google Scholar]
- 13.Birbrair A, Frenette PS. Niche heterogeneity in the bone marrow. Ann N Y Acad Sci. 2016;1370(1):82–96. doi: 10.1111/nyas.13016. Epub 2016/03/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakamura-Ishizu A, Okuno Y, Omatsu Y, Okabe K, Morimoto J, Uede T, et al. Extracellular matrix protein tenascin-C is required in the bone marrow microenvironment primed for hematopoietic regeneration. Blood. 2012;119(23):5429–37. doi: 10.1182/blood-2011-11-393645. Epub 2012/05/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gattazzo F, Urciuolo A, Bonaldo P. Extracellular matrix: a dynamic microenvironment for stem cell niche. Biochim Biophys Acta. 2014;1840(8):2506–19. doi: 10.1016/j.bbagen.2014.01.010. Epub 2014/01/15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sreeramkumar V, Leiva M, Stadtmann A, Pitaval C, Ortega-Rodriguez I, Wild MK, et al. Coordinated and unique functions of the E-selectin ligand ESL-1 during inflammatory and hematopoietic recruitment in mice. Blood. 2013;122(24):3993–4001. doi: 10.1182/blood-2013-07-514497. Epub 2013/10/10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Di Virgilio F, Chiozzi P, Ferrari D, Falzoni S, Sanz JM, Morelli A, et al. Nucleotide receptors: an emerging family of regulatory molecules in blood cells. Blood. 2001;97(3):587–600. doi: 10.1182/blood.v97.3.587. Epub 2001/02/07. [DOI] [PubMed] [Google Scholar]
- *18.Holmfeldt P, Ganuza M, Marathe H, He B, Hall T, Kang G, et al. Functional screen identifies regulators of murine hematopoietic stem cell repopulation. J Exp Med. 2016;213(3):433–49. doi: 10.1084/jem.20150806. Epub 2016/02/18. This screen uncovers 17 new molecular regulators implicated in bone marrow transplantation, revealing new mechanisms in HSCT, including HSC-dependent niche conditioning. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cho J, Yusuf R, Kook S, Attar E, Lee D, Park B, et al. Purinergic P2Y(1)(4) receptor modulates stress-induced hematopoietic stem/progenitor cell senescence. J Clin Invest. 2014;124(7):3159–71. doi: 10.1172/JCI61636. Epub 2014/06/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lassailly F, Foster K, Lopez-Onieva L, Currie E, Bonnet D. Multimodal imaging reveals structural and functional heterogeneity in different bone marrow compartments: functional implications on hematopoietic stem cells. Blood. 2013;122(10):1730–40. doi: 10.1182/blood-2012-11-467498. Epub 2013/07/03. [DOI] [PubMed] [Google Scholar]
- 21.Sahin AO, Buitenhuis M. Molecular mechanisms underlying adhesion and migration of hematopoietic stem cells. Cell Adh Migr. 2012;6(1):39–48. doi: 10.4161/cam.18975. Epub 2012/06/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Glass TJ, Lund TC, Patrinostro X, Tolar J, Bowman TV, Zon LI, et al. Stromal cell-derived factor-1 and hematopoietic cell homing in an adult zebrafish model of hematopoietic cell transplantation. Blood. 2011;118(3):766–74. doi: 10.1182/blood-2011-01-328476. Epub 2011/05/31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mendelson A, Frenette PS. Hematopoietic stem cell niche maintenance during homeostasis and regeneration. Nat Med. 2014;20(8):833–46. doi: 10.1038/nm.3647. Epub 2014/08/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ding L, Morrison SJ. Haematopoietic stem cells and early lymphoid progenitors occupy distinct bone marrow niches. Nature. 2013;495(7440):231–5. doi: 10.1038/nature11885. Epub 2013/02/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greenbaum A, Hsu YM, Day RB, Schuettpelz LG, Christopher MJ, Borgerding JN, et al. CXCL12 in early mesenchymal progenitors is required for haematopoietic stem-cell maintenance. Nature. 2013;495(7440):227–30. doi: 10.1038/nature11926. Epub 2013/02/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ratajczak MZ, Suszynska M. Emerging Strategies to Enhance Homing and Engraftment of Hematopoietic Stem Cells. Stem Cell Rev. 2016;12(1):121–8. doi: 10.1007/s12015-015-9625-5. Epub 2015/09/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim CH, Wu W, Wysoczynski M, Abdel-Latif A, Sunkara M, Morris A, et al. Conditioning for hematopoietic transplantation activates the complement cascade and induces a proteolytic environment in bone marrow: a novel role for bioactive lipids and soluble C5b-C9 as homing factors. Leukemia. 2012;26(1):106–16. doi: 10.1038/leu.2011.185. Epub 2011/07/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.iak M, Abdelbaset-Ismail A, Suszynska M, Abdel-Latif A, Ratajczak J, Ratajczak MZ. Novel evidence that the mannan-binding lectin pathway of complement activation plays a pivotal role in triggering mobilization of hematopoietic stem/progenitor cells by activation of both the complement and coagulation cascades. Leukemia. 2017;31(1):262–5. doi: 10.1038/leu.2016.278. Epub 2016/11/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ratajczak MZ, Kim CH, Wojakowski W, Janowska-Wieczorek A, Kucia M, Ratajczak J. Innate immunity as orchestrator of stem cell mobilization. Leukemia. 2010;24(10):1667–75. doi: 10.1038/leu.2010.162. Epub 2010/08/13. [DOI] [PubMed] [Google Scholar]
- 30.Reca RWM, Yan J, Lambris JD, Ratajczak MZ. Current Topics in Complement. Advances in Experimental Medicine and Biology. In: JDL, editor. Advances in Experimental Medicine and Biology. Boston, MA: Springer; 2006. [Google Scholar]
- 31.Murakami JL, Xu B, Franco CB, Hu X, Galli SJ, Weissman IL, et al. Evidence that beta7 Integrin Regulates Hematopoietic Stem Cell Homing and Engraftment Through Interaction with MAdCAM-1. Stem Cells Dev. 2016;25(1):18–26. doi: 10.1089/scd.2014.0551. Epub 2015/10/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Grassinger J, Haylock DN, Storan MJ, Haines GO, Williams B, Whitty GA, et al. Thrombin-cleaved osteopontin regulates hemopoietic stem and progenitor cell functions through interactions with alpha9beta1 and alpha4beta1 integrins. Blood. 2009;114(1):49–59. doi: 10.1182/blood-2009-01-197988. Epub 2009/05/07. [DOI] [PubMed] [Google Scholar]
- 33.Frenette PS, Subbarao S, Mazo IB, von Andrian UH, Wagner DD. Endothelial selectins and vascular cell adhesion molecule-1 promote hematopoietic progenitor homing to bone marrow. Proc Natl Acad Sci U S A. 1998;95(24):14423–8. doi: 10.1073/pnas.95.24.14423. Epub 1998/11/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Avigdor A, Goichberg P, Shivtiel S, Dar A, Peled A, Samira S, et al. CD44 and hyaluronic acid cooperate with SDF-1 in the trafficking of human CD34+ stem/progenitor cells to bone marrow. Blood. 2004;103(8):2981–9. doi: 10.1182/blood-2003-10-3611. Epub 2004/04/09. [DOI] [PubMed] [Google Scholar]
- 35.Rak J, Foster K, Potrzebowska K, Safaee Talkhoncheh M, Miharada N, Komorowska K, et al. Cytohesin 1 regulates homing and engraftment of human hematopoietic stem- and progenitor cells. Blood. 2016 doi: 10.1182/blood-2016-06-720649. Epub 2016/12/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Arcangeli ML, Bardin F, Frontera V, Bidaut G, Obrados E, Adams RH, et al. Function of Jam-B/Jam-C interaction in homing and mobilization of human and mouse hematopoietic stem and progenitor cells. Stem Cells. 2014;32(4):1043–54. doi: 10.1002/stem.1624. Epub 2013/12/21. [DOI] [PubMed] [Google Scholar]
- 37.Kuroiwa M, Oneyama C, Nada S, Okada M. The guanine nucleotide exchange factor Arhgef5 plays crucial roles in Src-induced podosome formation. J Cell Sci. 2011;124(Pt 10):1726–38. doi: 10.1242/jcs.080291. Epub 2011/04/29. [DOI] [PubMed] [Google Scholar]
- 38.Geng Y, Dong Y, Yu M, Zhang L, Yan X, Sun J, et al. Follistatin-like 1 (Fstl1) is a bone morphogenetic protein (BMP) 4 signaling antagonist in controlling mouse lung development. Proc Natl Acad Sci U S A. 2011;108(17):7058–63. doi: 10.1073/pnas.1007293108. Epub 2011/04/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Wei K, Serpooshan V, Hurtado C, Diez-Cunado M, Zhao M, Maruyama S, et al. Epicardial FSTL1 reconstitution regenerates the adult mammalian heart. Nature. 2015;525(7570):479–85. doi: 10.1038/nature15372. Epub 2015/09/17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **40.Silberstein L, Goncalves KA, Kharchenko PV, Turcotte R, Kfoury Y, Mercier F, et al. Proximity-Based Differential Single-Cell Analysis of the Niche to Identify Stem/Progenitor Cell Regulators. Cell Stem Cell. 2016;19(4):530–43. doi: 10.1016/j.stem.2016.07.004. Epub 2016/08/16. This study analyzes for the first time at a single cell level the expression pattern of mesenchymal cells in the BM niche and compared them according to the distance from their distance from the HSC in order to identify factors implicated in preserving HSC stemness. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *41.Leiva M, Quintana JA, Ligos JM, Hidalgo A. Haematopoietic ESL-1 enables stem cell proliferation in the bone marrow by limiting TGFbeta availability. Nat Commun. 2016;7:10222. doi: 10.1038/ncomms10222. Epub 2016/01/09. This work is an excellent example of donor cell niche conditioning driven by transplanted ESL-1−/−HSC aberrent TGFβ production. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shirvaikar N, Marquez-Curtis LA, Janowska-Wieczorek A. Hematopoietic Stem Cell Mobilization and Homing after Transplantation: The Role of MMP-2, MMP-9, and MT1-MMP. Biochem Res Int. 2012;2012:685267. doi: 10.1155/2012/685267. Epub 2012/04/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hooper AT, Butler JM, Nolan DJ, Kranz A, Iida K, Kobayashi M, et al. Engraftment and reconstitution of hematopoiesis is dependent on VEGFR2-mediated regeneration of sinusoidal endothelial cells. Cell Stem Cell. 2009;4(3):263–74. doi: 10.1016/j.stem.2009.01.006. Epub 2009/03/07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simsek T, Kocabas F, Zheng J, Deberardinis RJ, Mahmoud AI, Olson EN, et al. The distinct metabolic profile of hematopoietic stem cells reflects their location in a hypoxic niche. Cell Stem Cell. 2010;7(3):380–90. doi: 10.1016/j.stem.2010.07.011. Epub 2010/09/02. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Takubo K, Nagamatsu G, Kobayashi CI, Nakamura-Ishizu A, Kobayashi H, Ikeda E, et al. Regulation of glycolysis by Pdk functions as a metabolic checkpoint for cell cycle quiescence in hematopoietic stem cells. Cell Stem Cell. 2013;12(1):49–61. doi: 10.1016/j.stem.2012.10.011. Epub 2013/01/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *46.Maryanovich M, Zaltsman Y, Ruggiero A, Goldman A, Shachnai L, Zaidman SL, et al. An MTCH2 pathway repressing mitochondria metabolism regulates haematopoietic stem cell fate. Nat Commun. 2015;6:7901. doi: 10.1038/ncomms8901. Epub 2015/07/30. This study reports that the mitochondrial carrier homologue 2 (MTCH2) protein functions downstream of BID and ATM in to regulate metabolism and HSC homeostasis. [DOI] [PubMed] [Google Scholar]
- 47.Nombela-Arrieta C, Pivarnik G, Winkel B, Canty KJ, Harley B, Mahoney JE, et al. Quantitative imaging of haematopoietic stem and progenitor cell localization and hypoxic status in the bone marrow microenvironment. Nat Cell Biol. 2013;15(5):533–43. doi: 10.1038/ncb2730. Epub 2013/04/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kunisaki Y, Bruns I, Scheiermann C, Ahmed J, Pinho S, Zhang D, et al. Arteriolar niches maintain haematopoietic stem cell quiescence. Nature. 2013;502(7473):637–43. doi: 10.1038/nature12612. Epub 2013/10/11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Kusumbe AP, Ramasamy SK, Adams RH. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature. 2014;507(7492):323–8. doi: 10.1038/nature13145. Epub 2014/03/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nombela-Arrieta C, Silberstein LE. The science behind the hypoxic niche of hematopoietic stem and progenitors. Hematology Am Soc Hematol Educ Program. 2014;2014(1):542–7. doi: 10.1182/asheducation-2014.1.542. Epub 2015/02/20. [DOI] [PubMed] [Google Scholar]
- 51.Spencer JA, Ferraro F, Roussakis E, Klein A, Wu J, Runnels JM, et al. Direct measurement of local oxygen concentration in the bone marrow of live animals. Nature. 2014;508(7495):269–73. doi: 10.1038/nature13034. Epub 2014/03/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ding L, Saunders TL, Enikolopov G, Morrison SJ. Endothelial and perivascular cells maintain haematopoietic stem cells. Nature. 2012;481(7382):457–62. doi: 10.1038/nature10783. Epub 2012/01/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ito K, Carracedo A, Weiss D, Arai F, Ala U, Avigan DE, et al. A PML-PPAR-delta pathway for fatty acid oxidation regulates hematopoietic stem cell maintenance. Nat Med. 2012;18(9):1350–8. doi: 10.1038/nm.2882. Epub 2012/08/21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ito K, Hirao A, Arai F, Takubo K, Matsuoka S, Miyamoto K, et al. Reactive oxygen species act through p38 MAPK to limit the lifespan of hematopoietic stem cells. Nat Med. 2006;12(4):446–51. doi: 10.1038/nm1388. Epub 2006/03/28. [DOI] [PubMed] [Google Scholar]
- **55.Mantel CR, O’Leary HA, Chitteti BR, Huang X, Cooper S, Hangoc G, et al. Enhancing Hematopoietic Stem Cell Transplantation Efficacy by Mitigating Oxygen Shock. Cell. 2015;161(7):1553–65. doi: 10.1016/j.cell.2015.04.054. Epub 2015/06/16. This work reveals the damaging consequences of manipulating HSCs in ambient air to HSC long-term engraftment and illuminates pharmacological intervections that counter this effect. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Mehta A, Zhao JL, Sinha N, Marinov GK, Mann M, Kowalczyk MS, et al. The MicroRNA-132 and MicroRNA-212 Cluster Regulates Hematopoietic Stem Cell Maintenance and Survival with Age by Buffering FOXO3 Expression. Immunity. 2015;42(6):1021–32. doi: 10.1016/j.immuni.2015.05.017. Epub 2015/06/18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hu L, Cheng H, Gao Y, Shi M, Liu Y, Hu Z, et al. Antioxidant N-acetyl-L-cysteine increases engraftment of human hematopoietic stem cells in immune-deficient mice. Blood. 2014;124(20):e45–8. doi: 10.1182/blood-2014-03-559369. Epub 2014/10/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brown K, Xie S, Qiu X, Mohrin M, Shin J, Liu Y, et al. SIRT3 reverses aging-associated degeneration. Cell Rep. 2013;3(2):319–27. doi: 10.1016/j.celrep.2013.01.005. Epub 2013/02/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Yu WM, Liu X, Shen J, Jovanovic O, Pohl EE, Gerson SL, et al. Metabolic regulation by the mitochondrial phosphatase PTPMT1 is required for hematopoietic stem cell differentiation. Cell Stem Cell. 2013;12(1):62–74. doi: 10.1016/j.stem.2012.11.022. Epub 2013/01/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **60.Rimmele P, Liang R, Bigarella CL, Kocabas F, Xie J, Serasinghe MN, et al. Mitochondrial metabolism in hematopoietic stem cells requires functional FOXO3. EMBO Rep. 2015;16(9):1164–76. doi: 10.15252/embr.201439704. Epub 2015/07/26. This study reveals a novel role for Foxo3 in HSC as a regulator of mitochondrial metabolism that is independent of its role in regulating ROS levels in HSCs. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Qian P, He XC, Paulson A, Li Z, Tao F, Perry JM, et al. The Dlk1-Gtl2 Locus Preserves LT-HSC Function by Inhibiting the PI3K-mTOR Pathway to Restrict Mitochondrial Metabolism. Cell Stem Cell. 2016;18(2):214–28. doi: 10.1016/j.stem.2015.11.001. Epub 2015/12/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chen C, Liu Y, Liu R, Ikenoue T, Guan KL, Liu Y, et al. TSC-mTOR maintains quiescence and function of hematopoietic stem cells by repressing mitochondrial biogenesis and reactive oxygen species. J Exp Med. 2008;205(10):2397–408. doi: 10.1084/jem.20081297. Epub 2008/09/24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Rimmele P, Bigarella CL, Liang R, Izac B, Dieguez-Gonzalez R, Barbet G, et al. Aging-like phenotype and defective lineage specification in SIRT1-deleted hematopoietic stem and progenitor cells. Stem Cell Reports. 2014;3(1):44–59. doi: 10.1016/j.stemcr.2014.04.015. Epub 2014/07/30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Ludin A, Gur-Cohen S, Golan K, Kaufmann KB, Itkin T, Medaglia C, et al. Reactive oxygen species regulate hematopoietic stem cell self-renewal, migration and development, as well as their bone marrow microenvironment. Antioxid Redox Signal. 2014;21(11):1605–19. doi: 10.1089/ars.2014.5941. Epub 2014/04/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Chandel NS, Jasper H, Ho TT, Passegue E. Metabolic regulation of stem cell function in tissue homeostasis and organismal ageing. Nat Cell Biol. 2016;18(8):823–32. doi: 10.1038/ncb3385. Epub 2016/07/19. [DOI] [PubMed] [Google Scholar]
- 66.Beerman I, Rossi DJ. Epigenetic Control of Stem Cell Potential during Homeostasis, Aging, and Disease. Cell Stem Cell. 2015;16(6):613–25. doi: 10.1016/j.stem.2015.05.009. Epub 2015/06/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *67.Yu VW, Yusuf RZ, Oki T, Wu J, Saez B, Wang X, et al. Epigenetic Memory Underlies Cell-Autonomous Heterogeneous Behavior of Hematopoietic Stem Cells. Cell. 2016;167(5):1310–22. e17. doi: 10.1016/j.cell.2016.10.045. Epub 2016/11/20. Color marking and transplantation studies reveal that HSC function and lineage potential is epigenetically imprinted and preserved following transplantation. [DOI] [PubMed] [Google Scholar]
- 68.Wilson NK, Kent DG, Buettner F, Shehata M, Macaulay IC, Calero-Nieto FJ, et al. Combined Single-Cell Functional and Gene Expression Analysis Resolves Heterogeneity within Stem Cell Populations. Cell Stem Cell. 2015;16(6):712–24. doi: 10.1016/j.stem.2015.04.004. Epub 2015/05/26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Trowbridge JJ, Snow JW, Kim J, Orkin SH. DNA methyltransferase 1 is essential for and uniquely regulates hematopoietic stem and progenitor cells. Cell Stem Cell. 2009;5(4):442–9. doi: 10.1016/j.stem.2009.08.016. Epub 2009/10/03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Challen GA, Sun D, Jeong M, Luo M, Jelinek J, Berg JS, et al. Dnmt3a is essential for hematopoietic stem cell differentiation. Nat Genet. 2011;44(1):23–31. doi: 10.1038/ng.1009. Epub 2011/12/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Moran-Crusio K, Reavie L, Shih A, Abdel-Wahab O, Ndiaye-Lobry D, Lobry C, et al. Tet2 loss leads to increased hematopoietic stem cell self-renewal and myeloid transformation. Cancer Cell. 2011;20(1):11–24. doi: 10.1016/j.ccr.2011.06.001. Epub 2011/07/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Kunimoto H, McKenney AS, Meydan C, Shank K, Nazir A, Rapaport F, et al. Aid is a key regulator of myeloid/erythroid differentiation and DNA methylation in hematopoietic stem/progenitor cells. Blood. 2017 doi: 10.1182/blood-2016-06-721977. Epub 2017/01/13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Xie H, Xu J, Hsu JH, Nguyen M, Fujiwara Y, Peng C, et al. Polycomb repressive complex 2 regulates normal hematopoietic stem cell function in a developmental-stage-specific manner. Cell Stem Cell. 2014;14(1):68–80. doi: 10.1016/j.stem.2013.10.001. Epub 2013/11/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Kamminga LM, Bystrykh LV, de Boer A, Houwer S, Douma J, Weersing E, et al. The Polycomb group gene Ezh2 prevents hematopoietic stem cell exhaustion. Blood. 2006;107(5):2170–9. doi: 10.1182/blood-2005-09-3585. Epub 2005/11/19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Sun D, Luo M, Jeong M, Rodriguez B, Xia Z, Hannah R, et al. Epigenomic profiling of young and aged HSCs reveals concerted changes during aging that reinforce self-renewal. Cell Stem Cell. 2014;14(5):673–88. doi: 10.1016/j.stem.2014.03.002. Epub 2014/05/06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Mortensen M, Soilleux EJ, Djordjevic G, Tripp R, Lutteropp M, Sadighi-Akha E, et al. The autophagy protein Atg7 is essential for hematopoietic stem cell maintenance. J Exp Med. 2011;208(3):455–67. doi: 10.1084/jem.20101145. Epub 2011/02/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Broxmeyer HE, O’Leary HA, Huang X, Mantel C. The importance of hypoxia and extra physiologic oxygen shock/stress for collection and processing of stem and progenitor cells to understand true physiology/pathology of these cells ex vivo. Curr Opin Hematol. 2015;22(4):273–8. doi: 10.1097/MOH.0000000000000144. Epub 2015/06/08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **78.Mohrin M, Shin J, Liu Y, Brown K, Luo H, Xi Y, et al. Stem cell aging. A mitochondrial UPR-mediated metabolic checkpoint regulates hematopoietic stem cell aging. Science. 2015;347(6228):1374–7. doi: 10.1126/science.aaa2361. Epub 2015/03/21. This study reveals that SIRT7, as a regulator of the mitochondria unfolded protein response, is critically required by aged HSCs, further implicating mitochondria (and organelle) homeostasis as critical for proper HSC function. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ivanova EA, Orekhov AN. The Role of Endoplasmic Reticulum Stress and Unfolded Protein Response in Atherosclerosis. Int J Mol Sci. 2016;17(2) doi: 10.3390/ijms17020193. Epub 2016/02/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Bertolotti A, Zhang Y, Hendershot LM, Harding HP, Ron D. Dynamic interaction of BiP and ER stress transducers in the unfolded-protein response. Nat Cell Biol. 2000;2(6):326–32. doi: 10.1038/35014014. Epub 2000/06/15. [DOI] [PubMed] [Google Scholar]
- 81.Wey S, Luo B, Lee AS. Acute inducible ablation of GRP78 reveals its role in hematopoietic stem cell survival, lymphogenesis and regulation of stress signaling. PLoS One. 2012;7(6):e39047. doi: 10.1371/journal.pone.0039047. Epub 2012/06/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **82.van Galen P, Kreso A, Mbong N, Kent DG, Fitzmaurice T, Chambers JE, et al. The unfolded protein response governs integrity of the haematopoietic stem-cell pool during stress. Nature. 2014;510(7504):268–72. doi: 10.1038/nature13228. Epub 2014/04/30. This study reveals a role for the endoplasmic reticulum unfolded protein response in HSC homeostasis. [DOI] [PubMed] [Google Scholar]
- **83.Taya Y, Ota Y, Wilkinson AC, Kanazawa A, Watarai H, et al. Depleting dietary valine permits nonmyeloablative mouse hematopoietic stem cell transplantation. Science. 2016;354(6316):1152–1155. doi: 10.1126/science.aag3145. Epub 2016/10/20. This study reveals that dietary restrictions, such as valine-deprivation, can be an effective means to open up space for transplanted HSCs in the BM niche of recipients, allowing for robust engraftment without irradiation or chemotherapy. [DOI] [PubMed] [Google Scholar]
- 84.Rowe RG, Daley GQ. Stem cells: Valine starvation leads to a hungry niche. Nature. 2017;541(7636):166–7. doi: 10.1038/nature21106. Epub 2016/12/16. [DOI] [PMC free article] [PubMed] [Google Scholar]