Abstract
Background:
Acacia catechu Willd (Fabaceae), commonly known as catechu, cachou, and black cutch, has been studied for its hepatoprotective, antipyretic, antidiarrheal, hypoglycemic, anti-inflammatory, immunomodulatory, antinociceptive, antimicrobial, free radical scavenging, and antioxidant activities.
Objective:
We evaluated the cytotoxic activity of ethanol extract of A. catechu seed (ACS) against SCC-25 human oral squamous carcinoma cell line.
Methods:
Cytotoxic effect of ACS extract was determined by 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay, using concentrations of 0.1–1000 μg/mL for 24 h. A. catechu ethanol seed extract was treated SCC-25 cells with 25 and 50 μg/mL. At the end of treatment period, apoptotic marker gene expressions such as caspase 8, 9, Bcl-2, Bax, and cytochrome c were evaluated by semiquantitative reverse transcription-polymerase chain reaction. Morphological changes of ACS treated SCC-25 cells was evaluated by acridine orange/ethidium bromide (AO/EB) dual staining. Nuclear morphology and DNA fragmentation was evaluated by propidium iodide (PI) staining.
Results:
A. catechu ethanol seed extract treatment caused cytotoxicity in SCC-25 cells with an IC50 value of 100 μg/mL. Apoptotic markers caspases 8 and 9, cytochrome c, Bax gene expressions were significantly increased upon ACS extract treatment indicate the apoptosis induction in SCC-25 cells. This treatment also caused significant downregulation of Bcl-2 gene expression. Staining with AO/EB and PI shows membrane blebbing, and nuclear membrane distortion further confirms the apoptosis induction by ACS treatment in SCC-25 cells.
Conclusion:
The ethanol seed extracts of A. catechu was found to be cytotoxic at lower concentrations and induced apoptosis in human oral squamous carcinoma SCC-25 cells.
SUMMARY
Acacia catechu ethanolic seed extract contains phytochemicals such as epicatechin, rutin, and quercetin
Acacia catechu seed (ACS) extract significantly (P < 0.001) inhibits the active proliferation of human oral squamous carcinoma (SCC-25) cells
ACS extract treatment to SCC-25 cells significantly modulated the gene expressions pertaining to apoptosis and propidium iodide and acridine orange/ethidium bromide staining also confirm the apoptosis induction
Antiproliferative and apoptosis inducing activities of ACS extract is correlated with phytochemical contents.
Abbreviations used: ACS: Acacia catechu seed extract; MTT: 3 (4,5 dimethylthiazol 2 yl) 2,5 diphenyltetrazolium bromide; DMSO: Dimethyl sulfoxide; AO/EO: Acridine orange/ethidium bromide; LC MS: Liquid chromatography mass spectrometry.
Keywords: Apoptosis, caspases, cytotoxicity, oral cancer
INTRODUCTION
Cancer prevalence is increasing every year and it is responsible for the third most cause of death in developing countries. In particular, oral cancer is the sixth most common cancer in the world and one of the significant public health issues worldwide. In India, oral cancer is the third most common type which accounts for over 30% of all cancers. In developing countries, it affects people from lower socioeconomic groups due to a higher exposure to risk factors such as the tobacco use.[1] However, oral cancer is a preventable disease, as aforesaid its causes are directly related to behavioral and lifestyle factors, including tobacco and alcohol.[2]
Effective anticancer treatment can prolong and improve the patient's quality of life. Surgery, radiotherapy, and chemotherapy are the major treatment modalities employed in oral cancer patients depending upon the stage of cancer. Radiation therapy has been used successfully as the primary modality for treating patients with early stage of oral cancer, and it is the standard of care for use as adjuvant therapy in postoperative cases of patients with advanced stage oral cancer as well.[3] Surgery or radiation is said to effective only when the tumor is localized and small in size, and chemotherapy could be effective for a small sized tumors.[4] More than 20 oral cytotoxic drugs are available for oral cancer including 6-mercaptopurine, methotrexate, and busulfan and are widely used in oral chemotherapy. The use of these oral chemotherapy drugs has been reported to have narrow clinical setting in which their use is indicated and besides, they might induce certain side effects.[5] Therefore, there is a real need for new anticancer drugs with reduced side effects that could treat chemotherapy-induced adverse effects mainly nausea, vomiting, and metallic taste. To overcome these side effects caused by cancer chemotherapy, several experimental studies have concretely reported the anticancer efficacy of medicinal plants against several human in vitro cancer cell lines and came out with promising results.[6,7] Plant phytochemicals are diverse group of compounds that are found naturally in fruits, vegetables, spices, and medicinal plants and they have implicated for their anticancer properties.[8]
Acacia catechu Willd (Fabaceae), commonly known as catechu, cachou, and black cutch, is a moderate size deciduous, thorny tree widely distributed in India. Various parts of this plant have been used since ancient times in Ayurvedic medicine.[7] Numerous natural bioactive compounds for instance 4-hydroxybenzoic acid, kaempferol, quercetin, 3,4,7-trihydroxyl-3,5-dimethoxyflavone, catechin, rutin, isorhamnetin, epicatechin, afzelechin, epiafzelechin, mesquitol, ophioglonin, aromadendrin, and phenol have been isolated from heartwood, bark, roots, leaves and stem of A. catechu and presence of the above active compounds have been implicated for its myriad biological effects.[9,10,11,12,13] The phytochemicals isolated from this plant have been widely studied for their cytotoxic potentials against variety of cancer cell lines and came out with promising results.[14,15,16] A. catechu has been studied for its hepatoprotective, antipyretic, antidiarrheal, hypoglycemic, anti-inflammatory, immunomodulatory, antinociceptive, antimicrobial, free radical scavenging, and antioxidant activities.[17,18,19,20,21,22] A. catechu is also studied for its anticancer and cytotoxic potentials against HeLa, COLO-205, and HT-1080 cell lines in vitro.[23] However, the anticancer efficacy of A. catechu seed (ACS) extract against human oral squamous carcinoma SCC-25 cells remains unknown. Hence, in the present study, we evaluated the anticancer effect of ACS extract in human oral squamous carcinoma SCC-25 cells.
METHODS
Reagents and chemicals
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT), dimethyl sulfoxide (DMSO) was purchased from Sigma Chemical Co. India. The other chemicals used in this study were purchased locally and were of Analar grade.
Plant collection and extract preparation
ACS was collected during the month of December 2015 from Hosur, Tamil Nadu, India, authenticated by Green Chem Lab, Bengaluru, Karnataka, India. Seeds were washed and shade dried for a week and was milled to fine powder. This seed powder was passed through 100 mesh sieve and stored in a sealed polythene bag 2.5 kg of powdered ACS were extracted with 10 L of ethanol, at 65°C, for 1 h, in a 20 L round bottom flask with Graham condenser attached. Condenser was cooled circulating with chilled water. After 1 h of extraction, round bottom flask was cooled to room temp and the extract were filtered and collected. The marc, an insoluble residue was extracted repeatedly with 10 L of ethanol, twice. The extracts were filtered and collected. The combined extracts was evaporated to dryness under reduced pressure in a Buchi rotary evaporator (Switzerland) at 65°C, to obtain 150 g of seed powder extract. The w/w yield of the prepared extract was 6%.
Phytochemical analysis by liquid chromatography-mass spectrometry
Presence of phytochemicals such as epicatechin, rutin, and quercetin were analyzed by liquid chromatography-mass spectrometry (LC-MS) method. High performance liquid chromatography system (Shimadzu LC-20AD Prominence gradient system) was equipped with column was a Phenomenex C-18, Luna, SS column, 150 mm × 4.6 mm. Five micron particle size. The mobile phases were (A) 0.1% formic acid in water (B): acetonitrile (gradient System) at a flow rate of 1000 μL/min. The LC conditions gradient system was 10% B during 0.01 min, a linear increase from 10% to 50% during 70 min. Detection was performed at 280 nm and injection rate was 10 μL. The LC instrument was equipped with MS instrument (Shimadzu LC MS 8030; triple quadrupole mass analyzer).
Cell culture
The SCC-25 human oral squamous carcinoma cell line was procured from ATCC. Cells were maintained in Dulbecco's minimum essential media and Ham's F-12 (1:1 ratio) supplemented with 10% fetal bovine serum with 100 units/mL penicillin and 100 μg/mL streptomycin. Cells were cultured in a humidified atmosphere with 5% CO2 at 37°C. Cells were grown in 75 cm2 culture flasks and after a few passages, cells were seeded for experiments. The experiments were done at 70%–80% confluence. Upon reaching confluence, cells were detached using 0.05% trypsin-ethylenediaminetetraacetic acid solution.
Cell treatment
A. catechu ethanol seed extract was dissolved in 0.1% DMSO (v/v). SCC-25 cells were plated at 10,000 cells/cm2. After 24 h, cells were fed with fresh expansion culture medium supplemented with different final concentrations of ACS extract (25 and 50 μg/mL) or the corresponding volumes of the vehicle. The ACS extract concentration used in this study was selected based on the evaluation of IC50 concentration. After 24 h of treatment, cells were collected by trypsin application. Total cell number was determined by counting each sample in triplicate under inverted microscope. Viability was also evaluated by the trypan blue dye exclusion assay.
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay
Cytotoxic effect of ACS extract on SCC-25 cells was assessed by MTT assay[24] using concentrations of 0.1–1000 μg/mL for 24 h. Cells were plated in 96-well plate at a concentration of 5 × 104 cells/well. After 24 h, cells were fed with fresh expansion culture medium supplemented with different final concentrations of ACS extract (0.1–1000 μg/well) and incubated for 24 h. Untreated cells served as control and received only 0.1% DMSO. At the end of treatment period, media from control, seed extract-treated cells was aspirated and 50 μL of MTT (5 mg/mL in phosphate-buffer saline [PBS]) was added to each well. Cells were then incubated inside the incubator. MTT was then discarded and the coloured crystals of produced formazan were dissolved in 150 μL of DMSO and mixed effectively. The purple blue formazan dye formed was measured using an ELISA reader (Bio-Rad, Hercules, CA, USA) at 570 nm.
Acridine orange/ethidium bromide (dual staining)
Acridine orange/ethidium bromide (AO/EO) orange staining was carried out by the method of Gohel et al.[25] SCC-25 cells were plated at a density of 1 × 104 in 48-well plates. They were allowed to grow until they are 70%–80% confluent. After 24 h the cells were treated with 25 and 50 μg/mL of ACS extract for 24 h. The culture medium was aspirated from each well and cells were gently rinsed twice with PBS at room temperature. Then equal volumes of cells from control and ACS extract treated were mixed with 100 μL of dye mixture (1:1) of EO and AO and viewed immediately under inverted fluorescence microscope (Nikon [Ti series] at ×10 magnification). A minimum of 300 cells were counted in each sample at two different fields. The percentage of apoptotic cells was determined by (% of apoptotic cells = [total number of apoptotic cells/total number of cells counted] ×100).
Assessment of nuclear morphology after propidium iodide staining
Propidium iodide (PI) staining was carried out by the method of Chandramohan et al.[26] SCC-25 cells were plated at a density of 1 × 104 in 48 well plates. They were allowed to grow until they are 70%–80% confluent. Then, cells were treated with 25 and 50 μg/mL of ACS extract for 24 h. After 24 h, culture medium was aspirated and cells were gently rinsed twice with PBS at room temperature, before fixing in methanol: acetic acid (3:1 v/v) for 10 min, and stained with 10 μg/mL PI for 20 min. Nuclear morphology of apoptotic cells with condensed/fragmented nuclei was examined by fluorescence microscope and at least 1 × 103 cells were counted for assessing apoptotic cell death.
Gene expression analysis
Total RNA was extracted by trizol reagent according to the standard protocol. Concentration of the extracted RNA was determined and the integrity of RNA was visualized on a 1% agarose gel using a gel documentation system (Bio-Rad, Hercules, CA, USA). The first strand of cDNA was synthesized from 1 μg of total RNA by reverse transcriptase using M-MLV (Promega, Madison, WI, USA) and oligo (dT) primers (Promega, Madison, WI, USA) according to the manufacturer's protocol. Then, 2 μL of template cDNA was added to the final volume of 20 μL of reaction mixture. Reverse transcription-polymerase chain reaction (RT-PCR) cycle parameters included 10 min at 95°C followed by 40 cycles involving denaturation at 95°C for 15 s, annealing at 60°C for 20 s, and elongation at 72°C for 20 s. The sequences of the specific sets of primer for Bax, Bcl-2, cytochrome c, caspase 8, caspase 9 and GAPDH used in this study were taken from literatures. Expressions of selected genes were normalized to the GAPDH gene, which was used as an internal housekeeping control. All the RT-PCR experiments were performed in triplicate.
Statistical analysis
Data were expressed as mean ± standard error mean and analyzed by Tukey's test to determine the significance of differences between groups. P < 0.05, 0.01, and/or 0.001 was considered statistically significant.
RESULTS
Inhibitory effects of Acacia catechu seed extract against human SCC-25 oral carcinoma cells
The antiproliferative effect of ACS extract in SCC-25 cells was evaluated by MTT assay. ACS extract treatment for 24 h significantly (P < 0.001) inhibits the proliferation of oral squamous carcinoma cells. The maximum antiproliferative effect was found to be more than 80% at a maximum concentration used in this study i.e., 1000 μg/mL of ACS extract [Figure 1].
Figure 1.
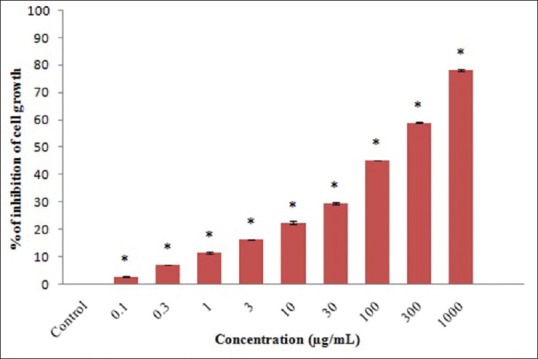
3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide assay analysis of cytotoxicity. Values are expressed as mean ± standard error mean (n = 3). Control compared with different Acacia catechu seed extract treated concentrations. *P < 0.001
Phytochemical analysis
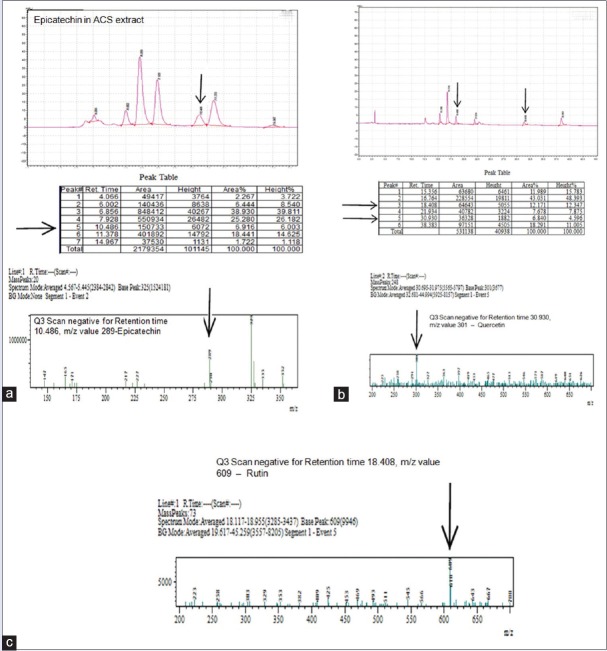
As shown in the chromatograms, we were able to separate the phytochemical compounds present in the ACS extract in 30 min. The presence of phytochemicals such as epicatechin, rutin, and quercetin were analyzed and confirmed by LC-MS with their corresponding standards. The retention times of epicatechin, rutin, and quercetin were separated on 10.486, 18.408, and 30.930 min, respectively. We thus confirmed that the ACS extract contain phytochemical such as epicatechin and small amounts of rutin and quercetin [Figure 2].
Figure 2.
Liquid chromatography-mass spectrometry analysis of epicatechin (a), quercetin (b) and rutin (c)
Apoptotic marker gene expressions
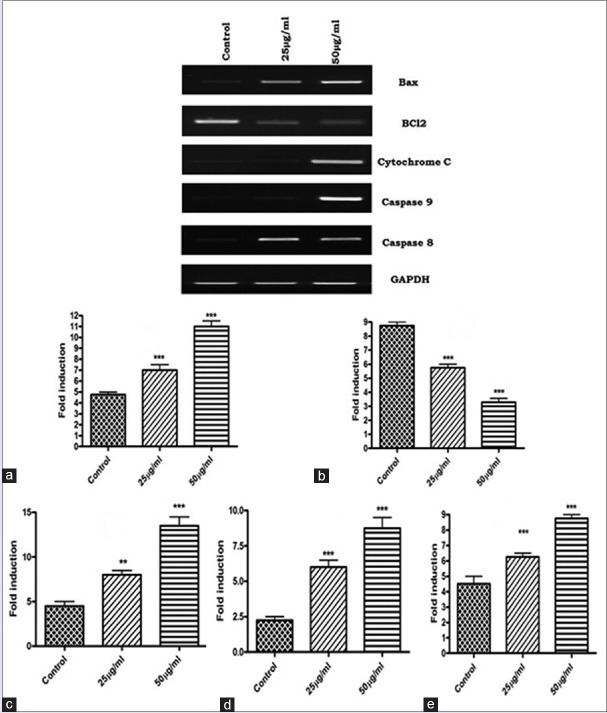
To examine the molecular mechanism underlying apoptosis process, we evaluated the gene expression analysis of cytochrome c, caspase 8 and caspase 9. The result shows that activation of caspase 8 and 9, and cytochrome c gene expressions in ACS extract treated groups were expressed highly than that of vehicle treated control. The data also shows that ACS extract caused significant down regulation of Bcl-2, an inhibitor of apoptosis and up regulation of Bax expression in SCC-25 cells [Figure 3a–e].
Figure 3.
Apoptotic marker gene analysis. (a) Bax; (b) B-bcl2; (c) cytochrome; (d) Caspase 9; (e) Caspase 8. Quantification of apoptotic marker gene expressions. Values are expressed as mean ± standard error mean (n = 3). **P < 0.01, ***P < 0.001
Acridine orange/ethidium bromide double-staining and fluorescent microscopy
To determine the effect of ACS treatment on the induction of apoptosis in SCC-25 cells, AO/EB immunofluorescence staining was performed to detect the number of apoptotic cells. Our result shows that no significant change and apoptosis was detected in the control group [Figure 4a]. Early-stage apoptotic cells, marked by yellow green AO nuclear staining, were detected in the experimental (25 μg/mL) ACS extract treatment [Figure 4b]. With increasing concentrations (50 μg/mL ACS), the number of early apoptotic cells increased along with late-stage apoptotic cells, orange nuclear EB staining, were also detected [Figure 4c]. The apoptotic cell number were significantly increased (P < 0.001) in dose dependent manner [Figure 4d].
Figure 4.
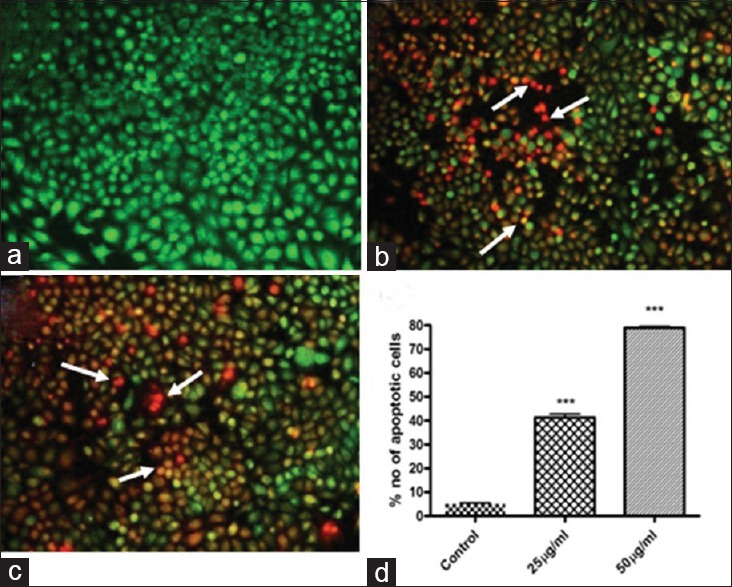
Apoptosis analysis by acridine orange/ethidium bromide (×10). (a) Negative control group (normal cells); (b) Nucleus showed yellow–green fluorescence by acridine orange staining (early apoptotic cells) and also showed orange fluorescence by ethidium bromide (late apoptotic cells) Acacia catechu seed extract 25 μg/ml treatment; (c) Nucleus showed yellow–green flurescence by acridine orange staining (early apoptotic cells) and showed orange fluorescence by ethidium bromide (late apoptotic cells) Acacia catechu seed extract 50 μg/ml treatment. (d) Quantification of apoptotic cells. Values are expressed as mean ± standard error mean (n = 3). ***P < 0.001
Nuclear fragmentation analysis by propidium iodide staining
Nuclear morphology was evaluated with membrane-permeable PI staining. The DMSO treated cells did not show any significant change in the morphology of SCC-25 cells [Figure 5a]. The ACS extract treated SCC-25 cells were reported to induce apoptosis [Figure 5b and c]. The ACS extract induced apoptotic cells displayed characteristic features of reduced size, intense fluorescence of condensed nuclear chromatin, and formation of membrane blebs. The number of apoptotic nuclei noticed was significantly (P < 0.001) increased after treatment with 25 and 50 μg/mL of ACS extract [Figure 5d].
Figure 5.
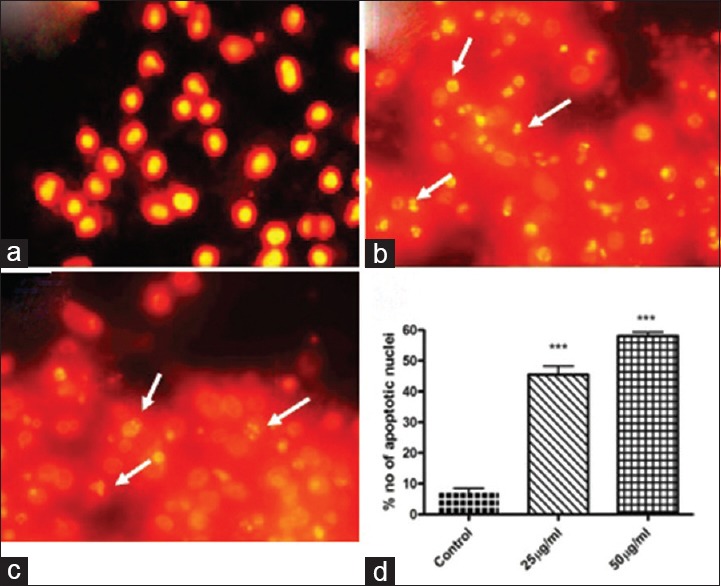
Nuclear morphology analysis by propidium iodide staining (×10). (a) Control; (b) Acacia catechu seed extract 25 μg/ml treatment; (c) Acacia catechu seed extract 50 μg/ml treatment. White arrow indicate the apoptotic cells with fragmented nuclei. (d) Quantification of apoptotic nuclei. Values are expressed as mean ± standard error mean (n = 3). ***P < 0.001
DISCUSSION
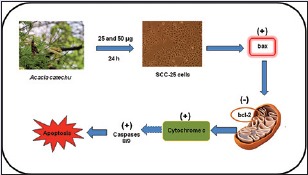
The plant-based drug discovery resulted primarily in the development of antioxidant, anticancer, and other anti-infectious agents and continues to contribute to the new leads in clinical trials.[27] Plant-based drug therapy is a new therapeutic modality which targets specific molecules in the cancer cell and modulates signaling pathways involved in carcinogenesis.[28] A. catechu has been extensively studied for its myriad beneficial effects against various etiologies.[7,21,23,29] Hence, in this study, we investigated the anticancer effect of ACS extract on human oral carcinoma cell lines. Probably, this could be the first study reporting the anticancer efficacy of ACS extract on human oral cancer cells.
In this study, ACS extract treatment significantly inhibits the proliferation of human oral squamous carcinoma cells in dose dependant manner. The profound antiproliferative efficacy of ACS extract found in this study could be due to presence of various phytochemical components in this plant as suggested by Alam et al. 2012.[29] Nevertheless, the data regarding phytochemicals present in ACS extract are not reported or scanty. In this study, we report that the presence of phytochemicals such as epicatechin, rutin, and quercetin in ACS extract. The cytotoxic effect of these phytochemicals isolated from different herbal plants was reported previously and was associated with their anticancer effects[30,31,32] and our current results are in agreement with these reports.
Apoptosis, programmed cell death plays a key role in the pathogenesis of various degenerative diseases and cancer. Induction of cancer cell apoptosis is one of the underlying principles of most current cancer therapies.[33] The apoptotic signals are complicated, and they are regulated at several levels. The tumor cells may use several molecular mechanisms to suppress apoptosis and acquire resistance to apoptotic agents, for instance, by the expression of antiapoptotic genes such as Bcl-2 or by the downregulation of proapoptotic gene expression such as Bax.[34] Bcl-2 is compartmentalized in an outer membrane of mitochondria, where it plays an important role in promoting cellular survival and inhibiting the actions of pro-apoptotic proteins (Bax). The pro-apoptotic proteins in the Bcl-2 family, including Bax, reported to interact with mitochondrial membrane to promote permeabilization, which leads to the loss in membrane potential which in turn release of cytochrome c that is one of the important signals in the apoptosis cascade.[35] These pro-apoptotic proteins (Bax) are in turn inhibited by the function of Bcl-2.[36] In light of the above reports, it is suggested that ACS treatment on SCC-25 cells could have increased the pro-apoptotic Bax gene expression and thus inturn decrease in Bcl-2 expression leading to apoptosis.
Apoptosis is characterized by biochemical changes that include caspase activation, breakdown of DNA, and protein and membrane surface modifications that allow the apoptotic cell to be recognized and engulfed by phagocytic cells as well as morphologic changes, such as chromatin condensation, nuclear fragmentation and reduction of cell volume.[37] There are two major mechanisms exist that induce apoptosis by initiating the caspase cascade. The extrinsic pathway involves caspase 8 and the intrinsic pathway involves caspase 9 as the initiator caspase and plays a central role in the apoptosis.[38] In this study, apoptotic gene expression of caspases 8 and 9 increased significantly in ACS treated SCC-25 cells. In addition, cytochrome c expression also increased upon the above treatment. It has been reported that mitochondria, perhaps key regulators of apoptosis, have shown to be involved in integrating different pro-apoptotic pathways via release of cytochrome c into the cytosol.[39] The released cytochrome c is complexed with Apaf-1 and pro-caspase 9 in a deoxyadenosine triphosphate-dependent manner to form the “apoptosome” from which the release of activated caspase 9 further initiates the activation of caspase cascade leading to biochemical and morphological changes associated with apoptosis.[40] Therefore, release of cytochrome c from mitochondria is considered a key initial step in the apoptotic process. In light of these reports, it is suggested that the ACS treatment to SCC-25 cells could have caused the cytochrome c release in cytoplasm by altering of mitochondrial membrane potential that results in apoptosis via activation of caspase cascade.
The result of the above biochemical events may alter the morphologic appearance of dying cell through DNA damage. Hence, apoptosis was further confirmed by analyzing the nuclear morphology of ACS extract treated SCC-25 cells by PI staining. It has been reported that morphological hallmarks of apoptosis are consequence to DNA fragmentation and membrane blebbing.[41] In this study, the percentage of apoptotic nuclei after treatment with 25 and 50 μg/mL of ACS extract were increased significantly. Propidum iodide staining shows that ACS treatment could induce the nuclear morphological changes and DNA fragmentation as suggested by previous reports.[42] Further, staining of apoptotic cells with fluorescent dyes such as AO/EB is considered one of the methods for evaluating the morphology changes.[43,44] Not surprisingly, in this study, at both concentration of ACS treatments showed significant presence of early (yellow green fluorescence nucleus) and late apoptotic cells (orange colored nucleus) by AO/EB dual staining, which further confirm apoptosis induction. It has been reported previously that early apoptotic cells had fragmented DNA which exhibited intense green colored nuclei. While late apoptotic and necrotic cells DNA were fragmented and stained orange and red[25] and our present results are in agreement with this report.
CONCLUSION
In this study, we were able to show that ACS extracts triggers apoptosis in human oral squamous carcinoma SCC-25 cells through caspases activation involving dissipation of mitochondrial cytochrome c release into the cytosol. Expression of apoptosis initiator caspases and proapoptotic molecule Bax was significantly increased and decrease in the Bcl-2 gene expression was well correlated with apoptosis induction. Our phytochemical analysis and molecular investigations were well corroborated with morphological confirmation of apoptosis was provided by AO/EB dual staining and PI nuclear staining.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Coelho KR. Challenges of the oral cancer burden in India. J Cancer Epidemiol. 2012;2012:701932. doi: 10.1155/2012/701932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bavle RM, Venugopal R, Konda P, Muniswamappa S, Makarla S. Molecular classification of oral squamous cell carcinoma. J Clin Diagn Res. 2016;10:ZE18–21. doi: 10.7860/JCDR/2016/19967.8565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Day TA, Davis BK, Gillespie MB, Joe JK, Kibbey M, Martin-Harris B, et al. Oral cancer treatment. Curr Treat Options Oncol. 2003;4:27–41. doi: 10.1007/s11864-003-0029-4. [DOI] [PubMed] [Google Scholar]
- 4.Giacosa A, Morazzoni P, Bombardelli E, Riva A, Bianchi Porro G, Rondanelli M. Can nausea and vomiting be treated with ginger extract? Eur Rev Med Pharmacol Sci. 2015;19:1291–6. [PubMed] [Google Scholar]
- 5.O’Neill VJ, Twelves CJ. Oral cancer treatment: Developments in chemotherapy and beyond. Br J Cancer. 2002;87:933–7. doi: 10.1038/sj.bjc.6600591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Elattar TM, Virji AS. The inhibitory effect of curcumin, genistein, quercetin and cisplatin on the growth of oral cancer cells in vitro. Anticancer Res. 2000;20:1733–8. [PubMed] [Google Scholar]
- 7.Ghate NB, Hazra B, Sarkar R, Mandal N. Heartwood extract of Acacia catechu induces apoptosis in human breast carcinoma by altering bax/bcl-2 ratio. Pharmacogn Mag. 2014;10:27–33. doi: 10.4103/0973-1296.126654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Khalid EB, Ayman EE, Rahman H, Abdelkarim G, Najda A. Natural products against cancer angiogenesis. Tumour Biol. 2016;37:14513–36. doi: 10.1007/s13277-016-5364-8. [DOI] [PubMed] [Google Scholar]
- 9.Duval A, Avérous L. Characterization and physicochemical properties of condensed tannins from Acacia catechu. J Agric Food Chem. 2016;64:1751–60. doi: 10.1021/acs.jafc.5b05671. [DOI] [PubMed] [Google Scholar]
- 10.Li X, Wang H, Liu C, Chen R. Chemical constituents of Acacia catechu. Zhongguo Zhong Yao Za Zhi. 2010;35:1425–7. [PubMed] [Google Scholar]
- 11.Hye MA, Taher MA, Ali MY, Ali MU, Zaman S. Isolation of (+)-catechin from Acacia catechu (cutch tree) by a convenient method. J Sci Res. 2009;1:300–5. [Google Scholar]
- 12.Shen D, Wu Q, Wang M, Yang Y, Lavoie EJ, Simon JE. Determination of the predominant catechins in Acacia catechu by liquid chromatography/electrospray ionization-mass spectrometry. J Agric Food Chem. 2006;54:3219–24. doi: 10.1021/jf0531499. [DOI] [PubMed] [Google Scholar]
- 13.Sharma P, Dayal R, Ayyar KS. Chemical constituents of Acacia catechu leaves. J Indian Chem Soc. 1997;74:60. [Google Scholar]
- 14.Shang W, Lu W, Han M, Qiao J. The interactions of anticancer agents with tea catechins: Current evidence from preclinical studies. Anticancer Agents Med Chem. 2014;14:1343–50. doi: 10.2174/1871520614666140829123651. [DOI] [PubMed] [Google Scholar]
- 15.Kim SH, Choi KC. Anti-cancer effect and underlying mechanism(s) of kaempferol, a phytoestrogen, on the regulation of apoptosis in diverse cancer cell models. Toxicol Res. 2013;29:229–34. doi: 10.5487/TR.2013.29.4.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zheng SY, Li Y, Jiang D, Zhao J, Ge JF. Anticancer effect and apoptosis induction by quercetin in the human lung cancer cell line A-549. Mol Med Rep. 2012;5:822–6. doi: 10.3892/mmr.2011.726. [DOI] [PubMed] [Google Scholar]
- 17.Jayasekhar P, Mohanan PV, Rathinam K. Hepatoprotective activity of ethyl acetate extract of Acacia catechu. Indian J Pharmacol. 1997;29:426–8. [Google Scholar]
- 18.Ray D, Sharatchandra KH, Thokchom IS. Antipyretic, antidiarrhoeal, hypoglycaemic and hepatoprotective activities of ethyl acetate extract of Acacia catechu Willd. in albino rats. Indian J Pharmacol. 2006;38:408–13. [Google Scholar]
- 19.Burnett BP, Jia Q, Zhao Y, Levy RM. A medicinal extract of Scutellaria baicalensis and Acacia catechu acts as a dual inhibitor of cyclooxygenase and 5-lipoxygenase to reduce inflammation. J Med Food. 2007;10:442–51. doi: 10.1089/jmf.2006.255. [DOI] [PubMed] [Google Scholar]
- 20.Ismail S, Asad M. Immunomodulatory activity of Acacia catechu. Indian J Physiol Pharmacol. 2009;53:25–33. [PubMed] [Google Scholar]
- 21.Lakshmi T, Aravind Kumar S. Preliminary phytochemical analysis & in vitro antibacterial activity of Acacia catechu Willd bark against Streptococcus mitis, Streptococcus sanguis and Lactobacillus acidophilus. Int J Phytomed. 2011;3:579–84. [Google Scholar]
- 22.Rahmatullah M, Hossain M, Mahmud A, Sultana N, Rahman SM, Islam MR, et al. Antihyperglycemic and antinociceptive activity evaluation of ‘khoyer’prepared from boiling the wood of Acacia catechu in water. Afr J Tradit Complement Altern Med. 2013;10:1–5. doi: 10.4314/ajtcam.v10i4.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Nadumane VK, Nair S. Evaluation of anticancer and cytotoxic potentials of Acacia catechu extracts in vitro. J Nat Pharm. 2011;2:190–5. [Google Scholar]
- 24.Safadi FF, Xu J, Smock SL, Kanaan RA, Selim AH, Odgren PR, et al. Expression of connective tissue growth factor in bone: Its role in osteoblast proliferation and differentiation in vitro and bone formation in vivo. J Cell Physiol. 2003;196:51–62. doi: 10.1002/jcp.10319. [DOI] [PubMed] [Google Scholar]
- 25.Gohel A, McCarthy MB, Gronowicz G. Estrogen prevents glucocorticoid-induced apoptosis in osteoblasts in vivo and in vitro. Endocrinology. 1999;140:5339–47. doi: 10.1210/endo.140.11.7135. [DOI] [PubMed] [Google Scholar]
- 26.Chandramohan KV, Gunasekaran P, Varalakshmi E, Hara Y, Nagini S. In vitro evaluation of the anticancer effect of lactoferrin and tea polyphenol combination on oral carcinoma cells. Cell Biol Int. 2007;31:599–608. doi: 10.1016/j.cellbi.2006.11.034. [DOI] [PubMed] [Google Scholar]
- 27.Ezhilarasan D, Sokal E, Karthikeyan S, Najimi M. Plant derived antioxidants and antifibrotic drugs: Past, present and future. J Coast Life Med. 2014;2:738–45. [Google Scholar]
- 28.Sutandyo N. New paradigm in treating cancer: Right on target. Acta Med Indones. 2016;48:139–44. [PubMed] [Google Scholar]
- 29.Alam S, Khatri M, Tiwari M. In vitro cytotoxic activity of methanolic extract of the bark of Acacia catechu against human oral and lung cancer cell lines. J Pharm Res. 2012;5:4487–91. [Google Scholar]
- 30.Sugantha Priya E, Selvakumar K, Bavithra S, Elumalai P, Arunkumar R, Raja Singh P, et al. Anti-cancer activity of quercetin in neuroblastoma: An in vitro approach. Neurol Sci. 2014;35:163–70. doi: 10.1007/s10072-013-1462-1. [DOI] [PubMed] [Google Scholar]
- 31.Babich H, Krupka ME, Nissim HA, Zuckerbraun HL. Differential in vitro cytotoxicity of (-)-epicatechin gallate (ECG) to cancer and normal cells from the human oral cavity. Toxicol In Vitro. 2005;19:231–42. doi: 10.1016/j.tiv.2004.09.001. [DOI] [PubMed] [Google Scholar]
- 32.Cristina Marcarini J, Ferreira Tsuboy MS, Cabral Luiz R, Regina Ribeiro L, Beatriz Hoffmann-Campo C, Ségio Mantovani M. Investigation of cytotoxic, apoptosis-inducing, genotoxic and protective effects of the flavonoid rutin in HTC hepatic cells. Exp Toxicol Pathol. 2011;63:459–65. doi: 10.1016/j.etp.2010.03.005. [DOI] [PubMed] [Google Scholar]
- 33.Westhoff MA, Marschall N, Debatin KM. Novel approaches to apoptosis-inducing therapies. Adv Exp Med Biol. 2016;930:173–204. doi: 10.1007/978-3-319-39406-0_8. [DOI] [PubMed] [Google Scholar]
- 34.Hassan M, Watari H, AbuAlmaaty A, Ohba Y, Sakuragi N. Apoptosis and molecular targeting therapy in cancer. Biomed Res Int. 2014;2014:150845. doi: 10.1155/2014/150845. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 35.Gogvadze V, Orrenius S, Zhivotovsky B. Multiple pathways of cytochrome c release from mitochondria in apoptosis. Biochim Biophys Acta. 2006;1757:639–47. doi: 10.1016/j.bbabio.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 36.Hardwick JM, Soane L. Multiple functions of BCL-2 family proteins. Cold Spring Harb Perspect Biol. 2013;5:pii: A008722. doi: 10.1101/cshperspect.a008722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Galluzzi L, Vitale I, Abrams JM, Alnemri ES, Baehrecke EH, Blagosklonny MV, et al. Molecular definitions of cell death subroutines: Recommendations of the Nomenclature Committee on Cell Death 2012. Cell Death Differ. 2012;19:107–20. doi: 10.1038/cdd.2011.96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Pastorino JG, Chen ST, Tafani M, Snyder JW, Farber JL. The overexpression of Bax produces cell death upon induction of the mitochondrial permeability transition. J Biol Chem. 1998;273:7770–5. doi: 10.1074/jbc.273.13.7770. [DOI] [PubMed] [Google Scholar]
- 39.Ott M, Robertson JD, Gogvadze V, Zhivotovsky B, Orrenius S. Cytochrome c release from mitochondria proceeds by a two-step process. Proc Natl Acad Sci U S A. 2002;99:1259–63. doi: 10.1073/pnas.241655498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhou M, Li Y, Hu Q, Bai XC, Huang W, Yan C, et al. Atomic structure of the apoptosome: Mechanism of cytochrome c- and dATP-mediated activation of Apaf-1. Genes Dev. 2015;29:2349–61. doi: 10.1101/gad.272278.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.McIlwain DR, Berger T, Mak TW. Caspase functions in cell death and disease. Cold Spring Harb Perspect Biol. 2015;7:pii: A026716. doi: 10.1101/cshperspect.a026716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Riccardi C, Nicoletti I. Analysis of apoptosis by propidium iodide staining and flow cytometry. Nat Protoc. 2006;1:1458–61. doi: 10.1038/nprot.2006.238. [DOI] [PubMed] [Google Scholar]
- 43.Ho K, Yazan LS, Ismail N, Ismail M. Apoptosis and cell cycle arrest of human colorectal cancer cell line HT-29 induced by vanillin. Cancer Epidemiol. 2009;33:155–60. doi: 10.1016/j.canep.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 44.Savitskiy VP, Shman TV, Potapnev MP. Comparative measurement of spontaneous apoptosis in pediatric acute leukemia by different techniques. Cytometry B Clin Cytom. 2003;56:16–22. doi: 10.1002/cyto.b.10056. [DOI] [PubMed] [Google Scholar]