Abstract
Purification and characterization of polyphenol oxidase (PPO) enzyme and determination of total phenolic contents during dormancy and sprouting stages in Crocus sativus corms were performed. PPO enzyme was purified by ammonium sulfate precipitation and ion-exchange chromatography using DEAE-Sephadex A25 and two isoenzymes were obtained on the SDS-PAGE, which corresponded to molecular weights of 70 and 54 kDa. The Km values of the enzyme were 4.87 and 2.12 mM for l-DOPA in dormancy and waking stages, respectively. Also, enzyme showed higher Vmax values of 0.026 (ΔOD.min-1) in dormancy compared with the value of 0.019 (ΔOD.min-1) in waking corms. Results showed an inverse correlation between phenolic contents and PPO activity. Accordingly, it can be concluded that as plant progressed through sprouting stage, in contrast to polyphenol oxidase activity, there was a significant increase in total amount of phenolic compounds, as determined by Folin-Ciocalteu method and water and aqueous ethanol extractions.
SUMMARY:
Purification of polyphenol oxidase enzyme using DEAE-Sephadex A25 in Crocus sativus corms.
Characterization of polyphenol oxidase enzyme.
Comparison of PPO enzyme characteristics in two different physiologic stages of dormancy and sprouting.
Determination of phenolic contents.
Correlation between phenolic contents and PPO activity during sprouting and dormancy.
Abbreviations used: PPO: Polyphenol Oxidase, DEAE-Sephadex: Diethylaminoethyl Sephadex, SDS-PAGE: Sodium Dodecyl Sulfate- Polyacrylamide Gel Electrophoresis, DOPA: Dihydroxyphenylalanine, PEG: Polyethylene Glycol
Keywords: Crocus sativus, DEAE-Sephadex A25, phenolics, polyphenol oxidase
INTRODUCTION
Crocus sativus L. (saffron) is a triploid plant cultivated in many countries such as Iran, Greece, and Spain.[1,2] C. sativus is a member of the Iridaceae family and is propagated by replacement of corms that are subterranean storage organs providing nutrients during sprouting. Thus, the survival of the corm is of utmost importance for plant.[3] In recent years, C. sativus was subjected to several studies from biomedical and biochemical to chemistry.[1,4,5]
Polyphenol oxidases [monophenol,3,4,-l-dihydroxyphenylalanine: oxygen oxidoreductase, EC 1.14.18.1, PPOs] are copper-containing oxidases[6,7] that catalyzes o-diphenols to o-diquinones using molecular oxygen.[8,9,10,11] This process includes two main reactions, monophenols undergo hydroxylation to form orthodiphenols, which, in turn, are oxidized to quinones.[12] It has been demonstrated that removal of copper as prosthetic groups from PPO enzyme results in an inactive form of enzyme that can reinstate its activity in presence of copper.[13] Although oxidation of phenolic compounds and melanin formation are main physiologic functions of enzyme, but the importance and significance of its activity in plant tissues are not completely understood. PPO is a widely distributed enzyme in higher plants tissues that is mainly localized in thylakoid membrane;[14,15,16,17] due to its plastidic localization, it is postulated to play a role in photosynthesis.[18] PPO enzyme is implicated to function in immunity reactions and defense mechanism against insects and plant pathogens,[19] plant components biosynthesis, enzymatic browning reactions, and free radical scavenging.[20,21,22] Also, it has been reported that an aurusidin synthase, a PPO homolog, is involved in developmental processes of flower petal.[23] PPO purification and characterization have been performed in different plants and results exhibited multiple forms of enzyme and high degree of variability, which likely depends on extraction condition, tissue, and physiologic stages.[24,25] Previous studies reported a wide range of molecular mass for isoforms of PPO enzyme from 32 to 200 kDa, but mostly they fall in the range of 35-70 kDa.[13]
Phenolic compounds are a diverse group of secondary metabolites widely distributed in plants derived from shikimate pathway and phenylpropanoid metabolism,[10,26,27] which are synthesized in response to ecologic and physiologic impacts.[28] Available experimental data on phenolic compounds demonstrated an important role of these compounds in plant defense and immunity, which is attributed to their antioxidant activity.[1,26]
PPO is clearly involved in phenolics oxidation in plants and the correlation between enzyme activity and phenolics level has been studied. The characterization of polyphenol oxidase and total phenolics in medlar fruit during two different ripening stages has been investigated. The results showed variations among species in pre- and postharvest stages, which can be due to season, maturity, and genotype of cultivars.[6] Also, a comparative study has been conducted between pear and peach in terms of PPO activity and phenolics levels and reported data present a good correlation between PPO activity and polyphenol contents. However, PPO activity and phenolics amount are highly affected by maturity stage and both internal and external factors.[24] In addition, an inverse trend of phenolics was reported in relation to PPO activity in watermelon and tomato plants subjected to heat and cold stresses. It has been demonstrated that PPO activity was inhibited under stress, which consequently accumulates the amount of soluble phenolics in tomato and watermelon.[11] The results from a study focused on olive fruit has recently reported an inverse trend between PPO levels and a particular phenolic compound, oleuroptin, which presents an important role of PPO in olive phenolics metabolism.[12]
However, there is no reported data on correlation between PPO enzyme and phenolics in C. sativus. The particular aim of this study is to purify and characterize PPO and determine the total amount of phenolics from saffron corms during dormancy and sprouting stages and elucidate whether there is a correlation between PPO activity and phenolic contents. The results of this work illustrate two isoforms of PPO enzyme in both dormancy and sprouting and demonstrate an inverse correlation between PPO activity and content of phenolics.
MATERIALS AND METHODS
Materials
Dormant and waking corms of C. sativus were obtained from the University of Tehran farm in August and March, respectively. After transferring corms to the laboratory, they were immediately depleted from their shooting leaves, rinsed, and stored at-70°C for later usage.
Reagents and chemicals
All chemicals and reagents used were analytical grade. Chemicals for enzymatic isolation and activity were obtained from Sigma-Aldrich (St. Louis, MO, USA). Folin-Ciocalteu was obtained from Merck (Darmstadt, Germany).
PPO extraction and purification
Thirty grams of saffron corm was ground in liquid nitrogen and homogenized in 20 mL of cold 0.1 M phosphate buffer (pH 6.5) containing 5% polyethylene glycol (PEG 1500) and 10 mM ascorbic acid. The crude extract was centrifuged at 10,000g for 45 min at 4°C and supernatant was collected.[29] Partial purification of PPO enzyme was carried out by 50 and 80% ammonium sulfate fractionation and centrifuged at 10,000g for 30 min. The pellet was later collected and dissolved in 5 mM phosphate buffer (pH 6.3) and dialyzed in the cellulose bag (MW cutoff > 12,000) at 4°C in the same buffer overnight.[30,31] The enzyme solution was transferred to DEAE-Sephadex A25 column, per-equilibrated with 5 mM phosphate buffer (pH 8.0). PPO enzyme was eluted with the same buffer containing 0.5 M NaCl and 3 mL fractions were collected. Then, protein levels of each fraction and PPO activity toward l-DOPA were determined.
Determination of protein
Protein level of each collected tube was determined by the method of Bradford[32] using bovine serum albumin as standard.
Enzymatic assay
PPO activity was monitored by measuring the increase in absorbance at 475 nm using 20 mM of l-DOPA in 0.1 M phosphate buffer (pH 6.8) with a spectrophotometer (Shimadzu UV-160).[5] The unit of enzyme activity was defined as an increase in absorbance of 0.001 min-1.[33]
Polyacrylamide gel electrophoresis
Polyacrylamide gel electrophoresis (SDS-PAGE) was conducted according to the method of Laemmli[34] to determine the molecular weight of purified enzyme. The purified enzyme was also subjected to native PAGE and the activity was localized by incubating the gel in 0.1 M phosphate buffer (pH 6.8) containing 10 mM l-DOPA for 30 min.[5]
Kinetic parameters
The Michaelis-Menten constant Km and maximum reaction velocity Vmax were obtained using l-DOPA as a substrate in the range of 1-35 mM under standard conditions by monitoring the absorbance increase at 475 nm. Data were plotted as 1/V and 1/[S] according to the method of Lineweaver and Burk.[35]
Total phenolics content
Total amount of phenolic compounds was determined by Folin-Ciocalteu method.[36] Ten grams of fresh corm was homogenized in 50 mL ethanol 50% v/v and distilled water separately and centrifuged at 10,000g for 45 min and supernatant was collected. Total phenolics content was determined quantitatively at 760 nm after incubating samples in Folin-Ciocalteu reagent for 45 min.[1]
RESULTS AND DISCUSSION
Purification of the enzyme
In this study, PPO was purified from saffron corm using Sephadex A25 ion-exchange column chromatography.[30,31] An analytical comparison was carried out between dormant and sprouting corms of C. sativus, and Figure 1 and 2 demonstrate the typical elution patterns of the PPO enzyme in waking and dormant corms, respectively. Fractions showing enzyme activity were pooled as purified polyphenol oxidase enzyme. As shown in Tables 1 and 2, PPO enzyme was purified 21.8- and 24.6-folds in waking and dormant corms, respectively.
Figure 1.
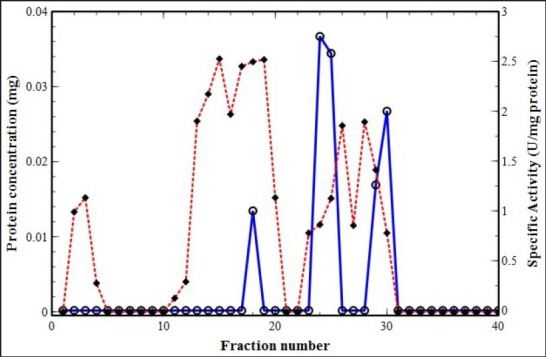
Ion-exchange chromatogram (Sephadex A25) of waking C. sativus corm. Protein concentration was monitored by UV-absorbance at λ = 280 nm (filled diamond connected by red dashed line) and PPO activity by absorbance at λ = 320 nm (hollow circle connected by blue line)
Figure 2.
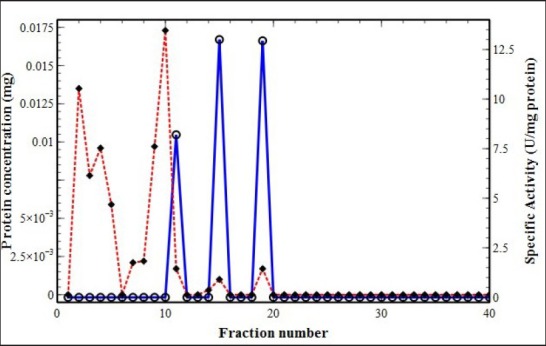
Ion-exchange chromatogram (Sephadex A25) of dormant C. sativus corm. Protein concentration was monitored by UV-absorbance at λ = 280 nm (filled diamond connected by red dashed line) and PPO activity by absorbance at λ = 320 nm (hollow circle connected by blue line)
Table 1.
Purification of polyphenol oxidase from waking C. sativus corm
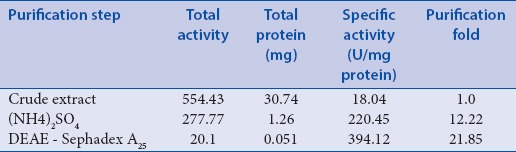
Table 2.
Purification of polyphenol oxidase from dormant C. sativus corm
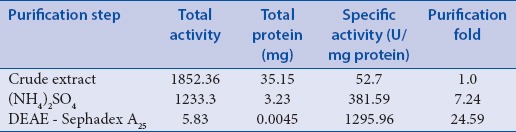
The results show a constant decrease in polyphenol oxidase activity in all steps of the purification as a result of multiple isoforms of PPO, which were lost in this procedure. However, the specific activity of enzyme increases in both samples, as it has been progressed through more purification. A comparison of specific activity of PPO between waking and dormant samples shows around 3.3-fold higher specific activity in dormant samples. This result is consistent with the previous study by Marri et al.,[37] which showed relatively higher activity of polyphenol oxidase in the early dormancy of potato tubers, which decreased significantly in mature tubers in early sprouting stage. These results seem to provide more evidence of the key role of PPO in dormancy, suggesting that PPO most likely protects plants against infection and disease.[13,37]
Several studies have also reported much higher activity of PPO enzyme in early stage of development compared with mature and developed plants.[13] It has been also reported that PPO activity is affected by experimental conditions and its sensitivity to extraction methods results in variations in different preparation procedures.[24]
Molecular mass determination
SDS-PAGE was applied to analyze the molecular weight of protein. The purified enzyme migrated on SDS-PAGE under denaturing condition showed two distinct bands in both samples corresponding to molecular masses of approximately 70 and 54 kDa, which reveals the presence of at least two isoforms of PPO enzyme [Figures 3 and 4].
Figure 3.
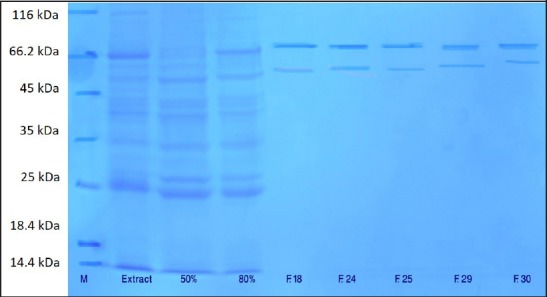
SDS-PAGE of polyphenol oxidase enzyme of waking saffron corm. Left to right: Marker (M), crude enzyme (extract), 50% ammonium sulfate fractionation (50%), 80% ammonium sulfate fractionation (80%), purified enzyme fractions (F18, F24, F25, F29, and F30)
Figure 4.
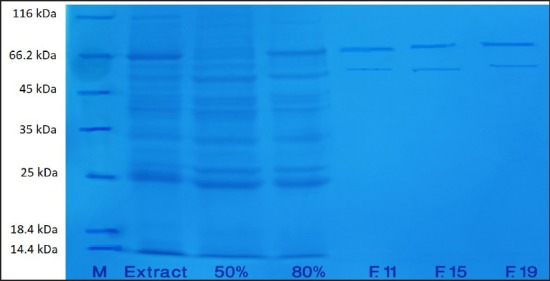
SDS-PAGE of polyphenol oxidase enzyme of dormant saffron corm. Left to right: Marker (M), crude enzyme (extract), 50% ammonium sulfate fractionation (50%), 80% ammonium sulfate fractionation (80%), purified enzyme fractions (F11, F15, and F19)
It has been shown that the molecular weight of PPO varies in different plant species and multiple forms of PPO have been illustrated in different plants such as banana, pineapple, potato, mushroom, lettuce, grape, spinach, and strawberry.[13] Although several studies agreed on the presence of multiple forms of PPO in plants, but the number of molecular forms of enzyme has been always under debate.[13] The molecular mass of PPO was reported to be around 65 kDa in mulberry plants and Chinese cabbage,[30,38] 69 kDa in potato tubers,[37] and 72 kDa in Indian tea leaf.[39] However, a higher molecular weight of 120 kDa has been reported in field been seeds.[40] Previous studies on peaches, apricots, and cherries demonstrated the synthesis of PPO as a 60-65 kDa protein, which can later form a 40-45 kDa protein through proteolysis procedure.[20] However, the results obtained from olive have shown two distinct bands with molecular weights of 55 and 36 kDa.[41]
Native PAGE also was performed in order to analyze the purified enzyme and two bands have been exhibited on the activity stained gel in the presence of l-DOPA as a substrate [Figure 5]. The result presented here confirms the presence of different isoforms of PPO enzyme in saffron corms in both sprouting and dormant corms, which is consistent with previous study performed by Saiedian et al.[5] on dormant corms of saffron. In general, a wide range of PPO isoforms have been reported in different plants. It has been shown that there are at least four isoforms of PPO in medlar plants[6,42] and kiwis;[13] however, three forms of PPO enzyme have been illustrated in peach[43] and artichoke.[31] Our result differs from these, but it is in good agreement with those studies demonstrated the presence of two PPO isoforms in cherry laurel,[44] eggplant,[45] and Bartlett pears.[46]
Figure 5.
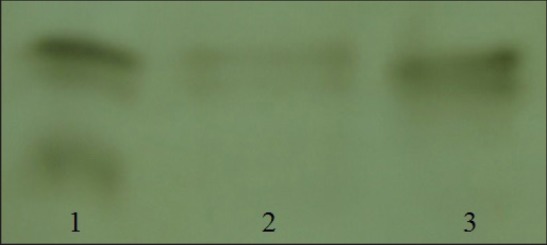
Native PAGE for PPO activity of crude extract and purified enzyme of saffron corms. Crude extract (lane 1); purified enzyme eluted from DEAE-Sephadex A25 of waking (lane 2); and dormant (lane 3) corms.
The variation in number and size of polyphenol oxidase enzyme forms could be due to differences in physiologic and developmental stages, subcellular localization, and formation of interfering compounds during purification process as a result of native enzyme modification.[13]
Enzyme kinetics
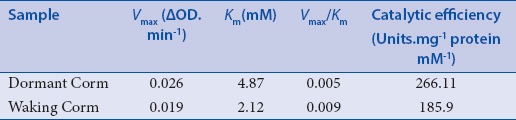
The maximum reaction velocity Vmax, Michaelis-Menten constant Km, and the value of Vmax/Km of PPO in both dormant and sprouting saffron corms were calculated using Lineweaver and Burk method.[35] In general, low values of Km between 1-10 have been shown for PPO suggesting the low affinity of enzyme for its substrate.[47] In this study, various concentrations of l-DOPA has been used as a substrate and its oxidation rate was measured by monitoring the absorbance increase at 475 nm and Table 3 shows the kinetic properties of PPO enzyme.
Table 3.
Total phenolics of dormant and waking corms of C. sativus using Folin-Ciocalteu assay[1]

It has been reported that there is a wide variation in the PPO activity levels toward DOPA in plant species.[13] As it is shown in Table 3, the Vmax and Km values of saffron PPO during dormancy stage are 0.026 (ΔOD.min-1) and 4.87 mM, respectively. These results are in good agreement with those values reported in previous study on polyphenol oxidase enzyme extracted from dormant corms of saffron.[5] However, in this study, the kinetic properties of PPO extracted from waking corms were also determined, which are smaller than those in dormant corms. The exhibited values of Vmax and Km in waking stage are 0.017 (ΔOD.min-1) and 2.31 mM, respectively.
Phenolics content
In previous study on dormant and sprouting corms of saffron, we have extracted total phenolic compounds using distilled water and ethanol 80% based on method developed by Folin-Ciocalteu.[1,36]
As it has been depicted in Table 4, waking corms have higher amount of phenolics compared with dormant corms. These results are consistent with those obtained by Cevallos-Casals and Cineros-Zevallos,[48] which have shown a significantly higher content of phenolics in 7-day-old germinated seeds compare with dormancy stage. Also, the higher antiradical capacity and radical scavenging activity have been reported in sprouting stage in comparison with dormancy in saffron, alfalfa, and onion.[1,48] This phenomenon suggests the role of phenolics against oxidative reactions during the sprouting stage, which results in protection of hypocotyl growth against environmental stresses.[48] Moreover, the function of phenolics in plant resistance to biotic and abiotic stresses has been declared.[24]
Table 4.
Enzyme kinetics using l-DOPA as a substrate in both dormant and waking corms of C. sativus
In several studies, the correlation between PPO activity and the amount of phenolic compounds has been shown and the effect of pre- and postharvesting processes has been determined. The results from a study on loquat fruit (cv. Mogi) has shown a significant decrease in both PPO activity and phenolics content during fruit-ripening stage.[49] However, another study on different variety of loquat (cv. Algerie) has demonstrated an elevation in PPO activity along with and increased amount of phenolics in fully developed fruits.[6] In contrast, the concentration of phenolic compounds and PPO activity have illustrated a reduction during maturation of 15 different cultivars of peach.[22] The correlation study between PPO activity and phenolic contents in organically and conventionally grown peach and pear has shown a direct relationship.[24]
In this study, an inverse correlation has been observed between the concentration of phenolics and PPO activity in waking and dormancy stages of saffron corm. The obtained results are similar to those that have been reported in medlar,[6] olive fruits,[41] and red grape.[50] It has been also suggested that this inverse relationship between PPO activity and the amount of phenolics in medlar fruits during ripening and overripening stages is related to higher catalytic efficiency of enzyme in ripen fruits.[6] As it is shown in Tables 1 and 3, the specific activity and catalytic efficiency of purified enzyme are 394.12 (U/mg protein) and 185.9 (units/mg protein/mM) in waking corms, respectively, which are drastically smaller than those values of 1295.96 (U/mg protein) and 266.11 (Units/mg protein/mM) Tables 2 and 3 for dormant corms. On the other hand, the phenolics content was shown an inverse trend with PPO enzyme activity [Table 4]. It is well established that phenolic compounds can serve as substrates for PPO, so the reduction of total phenolic compounds in dormancy stage is most likely due to the higher rate of oxidation by polyphenol oxidase enzyme.[21]
Also, the seasonal effects on phenolics content have been studied widely in different plants including apple[51] and acerola,[52] and it has been shown that season and developmental stage can affect the formation, distribution, and activity of polyphenol oxidase enzyme as well as phenolics accumulation in plants.[6] Moreover, it has been shown that a group of phenolics, flavonoids, play an important role in development of plants due to regulation of auxin transport.[53]
CONCLUSION
The objective of this study was to evaluate the correlation between polyphenol oxidase activity and phenolics content in different physiologic stages of saffron corm. Despite the importance of polyphenol oxidase enzyme in plants, there have been limited studies on saffron corm. As described above, polyphenol oxidase has been purified using DEAE-Sephadex A25 column and kinetic characteristics of enzyme were determined. Also, two distinct isoforms of enzyme have been identified in both dormant and waking corms with molecular masses of 70 and 54 kDa. The findings presented here show an inverse correlation between polyphenol oxidase enzyme activity and phenolics content in dormancy and waking stages of C. sativus corms. The higher amount of phenols in waking stage suggests the importance of phenolics in germination. In addition, the lower content of phenolics in dormancy can be due to oxidation by polyphenol oxidase enzyme, which showed higher activity and catalytic efficiency during dormancy.
Financial support and sponsorship
Nil
Conflicts of interest
There are no conflicts of interest
REFERENCES
- 1.Esmaeili N, Ebrahimzadeh H, Abdi K, Safarian S. Determination of some phenolic compounds in Crocus sativus L. corms and its antioxidant activities study. Pharmacogn Mag. 2011;7:74–80. doi: 10.4103/0973-1296.75906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pitsikas N, Boultadakis A, Georgiadou G, Tarantilis P, Sakellaridis N. Effects of the active constituents of Crocus sativus L. crocins, in an animal model of anxiety. Phytomedicine. 2008;15:1135–9. doi: 10.1016/j.phymed.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 3.Vatankhah E, Niknam V, Ebrahimzadeh H. Activity of antioxidant enzyme during in vitro organogenesis in Crocus sativus. Biol Plant. 2010;54:509–14. [Google Scholar]
- 4.Amin B, Hosseinzadeh H. Evaluation of aqueous and ethanolic extracts of saffron Crocus sativuss L. and its constituents, safranal and crocin in allodynia and hyperalgesia induced by chronic constriction injury model of neuropathic pain in rats. Fitoterapia. 2012;83:888–95. doi: 10.1016/j.fitote.2012.03.022. [DOI] [PubMed] [Google Scholar]
- 5.Saiedian S, Keyhani E, Keyhani J. Polyphenol oxidase activity in dormant saffron (Crocus sativus L.). corm. Acta Physiol Plant. 2007;29:463–71. doi: 10.1021/jf063749n. [DOI] [PubMed] [Google Scholar]
- 6.Ayaz F, Demir O, Torun H, Kolcuoglu Y, Colak A. Characterization of polyphenoloxidase (PPO) and total phenolic contents in medlar (Mespilus germanica L.). fruit during ripening and over ripening. Food Chem. 2008;106:291–8. [Google Scholar]
- 7.Dicko M, Gruppen H, Traore AS, Voragen AGJ, van Berkel WJH. Phenolic compounds and related enzymes as determinants of sorghum for food use. Biotechnol Mol Biol Rev. 2006;1:21–38. [Google Scholar]
- 8.Sanchez-Ferrer A, Rodriguez-Lopez JN, Garcia-Canovas F, Garcia-Carmona F. Tyrosinase: a comprehensive review of its mechanism. Biochim Biophys Acta. 1995;1247:1–11. doi: 10.1016/0167-4838(94)00204-t. [DOI] [PubMed] [Google Scholar]
- 9.van Gelder CW, Flurkey WH, Wichers HJ. Sequence and structural features of plant and fungal tyrosinases. Photochemistry. 1997;45:1309–23. doi: 10.1016/s0031-9422(97)00186-6. [DOI] [PubMed] [Google Scholar]
- 10.Dicko MH, Hilhorst R, Gruppen H, Traore AS, Laane C, van Berkel WJH, et al. Comparison of content in phenolic compounds, polyphenol oxidase, and peroxidase in grains of fifty sorghum varieties from Burkina Faso. J Agric Food Chem. 2002;50:3780–8. doi: 10.1021/jf011642o. [DOI] [PubMed] [Google Scholar]
- 11.Rivero RM, Ruiz JM, Garcia PC, Lopez-Lefebre LR, Sanchez E, Romero L. Resistance to cold and heat stress: accumulation of phenolic compounds in tomato and watermelon plants. Plant Sci. 2001;160:315–21. doi: 10.1016/s0168-9452(00)00395-2. [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Rodriguez R, Romero-Segura C, Sanz C, Sanchez-Ortiz A, Perez AG. Role of polyphenol oxidase and peroxidase in shaping the phenolic profile of virgin olive oil. Food Res Int. 2011;44:629–35. [Google Scholar]
- 13.Yoruk R, Marshall MR. Physiochemical properties and function of plant polyphenol oxidase: a review. J Food Biochem. 2003;27:361–422. [Google Scholar]
- 14.Sherman TD, Vaughn KC, Duke SO. A limited survey of the phylogenetic distribution of polyphenol oxidase. Phytochemistry. 1991;30:2499–506. [Google Scholar]
- 15.Murata M, Kurokami C, Homma S. Purification and some properties of chlorogenic acid oxidase from apple (Malus pumila) Biosci Biotechnol Biochem. 1992;56:1705–10. doi: 10.1271/bbb.56.1705. [DOI] [PubMed] [Google Scholar]
- 16.Vaughn KC, Duke SO. Function of polyphenol oxidase in higher plants. Physiol Plant. 1984;60:106–12. [Google Scholar]
- 17.Mayer AM. Polyphenol oxidases in plants and fungi: going places? A review. Phytochemistry. 2006;67:2318–31. doi: 10.1016/j.phytochem.2006.08.006. [DOI] [PubMed] [Google Scholar]
- 18.Trebst A, Depka B. Polyphenol oxidase and photosynthesis research. Photosynth Res. 1995;46:41–4. doi: 10.1007/BF00020414. [DOI] [PubMed] [Google Scholar]
- 19.Constabel CP, Yip L, Patton JJ, Christopher ME. Polyphenol oxidase from hybrid poplar. Cloning and expression in response to wounding and herbivory. Plant Physiol. 2000;124:285–96. doi: 10.1104/pp.124.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gawlik-Dziki U, Zlotek U, Swieca M. Characterization of polyphenol oxidase from butter lettuce (Lactuca sativa var capitata L.) Food Chem. 2008;107:129–35. [Google Scholar]
- 21.Altunkaya A, Gokmen V. Effect of various inhibitors on enzymatic browning, antioxidant activity and total phenol content of fresh lettuce (Lactuca sativa) Food Chem. 2008;107:1173–9. [Google Scholar]
- 22.Lee CY, Kagan V, Jaworski AW, Brown SK. Enzymatic browning in relation to phenolic compounds and polyphenol oxidase activity among various peach cultivars. J Agric Food Chem. 1990;38:99–101. [Google Scholar]
- 23.Li L, Steffens J. Overexpression of polyphenol oxidase in transgenic tomato plants results in enhanced bacterial disease resistance. Planta. 2002;215:239–47. doi: 10.1007/s00425-002-0750-4. [DOI] [PubMed] [Google Scholar]
- 24.Carbonaro M, Mattera M. Polyphenol oxidase activity and polyphenol levels in organically and conventionally grown peach (Prunus persica L. cv. Regina bianca) and pear (Pyrus communis L., cv Williams) Food Chem. 2001;72:419–24. [Google Scholar]
- 25.Nicolas JJ, Richard-Forget FC, Goupy PM, Amiot M, Aubert SY. Enzymatic browning reactions in apple and apple products. Crit Rev Food Sci Nutr. 1994;34:109–57. doi: 10.1080/10408399409527653. [DOI] [PubMed] [Google Scholar]
- 26.Balasundram N, Sundram K, Samman S. Phenolic compounds in plants and agri-industrial by-products: antioxidant activity, occurrence, and potential uses. Food Chem. 2006;99:191–203. [Google Scholar]
- 27.Tomas-Barberan FA, Espin JC. Phenolic compounds and related enzymes as determinants of quality in fruits and vegetables. J Sci Food Agric. 2001;81:853–76. [Google Scholar]
- 28.Khoddami A, Wilkes MA, Roberts TH. Techniques for analysis of plant phenolic compounds. Molecules. 2013;18:2328–75. doi: 10.3390/molecules18022328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wesche-Ebeling P, Montgomery MW. Strawberry polyphenol oxidase: extraction and partial characterization. J Food Sci. 1990;55:1320–4. [Google Scholar]
- 30.Arslan O, Erzengin M, Sinan S, Ozensoy O. Purification of mulberry (Morus alba L.). polyphenol oxidase by affinity chromatography and investigation of its kinetic and electrophoretic properties. Food Chem. 2004;88:479–84. [Google Scholar]
- 31.Aydemir T. Partial purification and characterization of polyphenol oxidase from artichoke (Cynara scolymus L.). heads. Food Chem. 2004;87:59–67. [Google Scholar]
- 32.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- 33.Ho KK. Characterization of polyphenol oxidase from aerial roots of an orchid Aranda’Christine 130’. Plant Physiol Biochem. 1999;37:841–8. doi: 10.1016/s0981-9428(99)00114-x. [DOI] [PubMed] [Google Scholar]
- 34.Laemmli UK. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–5. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 35.Lineweaver H, Burk D. The determination of enzyme dissociation constants. JACS. 1934;56:658–66. [Google Scholar]
- 36.Folin O, Ciocalteu V. On tyrosine and tryptophane determinations in proteins. J Biol Chem. 1927;73:627–50. [Google Scholar]
- 37.Marri C, Frazzoli A, Hochkoeppler A, Poggi V. Purification of a polyphenol oxidase isoform from potato (Solanum tuberosum) tubers. Phytochemistry. 2003;63:745–52. doi: 10.1016/s0031-9422(03)00353-4. [DOI] [PubMed] [Google Scholar]
- 38.Nagai T, Suzuki N. Partial purification of polyphenol oxidase from Chinese cabbage Brassica rapa L. J Agric Food Chem. 2001;49:3922–6. doi: 10.1021/jf000694v. [DOI] [PubMed] [Google Scholar]
- 39.Halder J, Tamuli P, Bhaduri A. Isolation and characterization of polyphenol oxidase from Indian tea leaf (Camellia sinensis) JNB. 1998;9:75–80. [Google Scholar]
- 40.Paul B, Gowda LR. Purification and characterization of a polyphenol oxidase from the seeds of field bean (Dolichos lablab) J Agric Food Chem. 2000;48:3839–46. doi: 10.1021/jf000296s. [DOI] [PubMed] [Google Scholar]
- 41.Ortega-Garcia F, Blanco S, Peinado MA, Peragon J. Polyphenol oxidase and its relationship with oleuropein concentration in fruits and leaves of olive (Olea europaea) cv. Picual trees during fruit ripening. Tree Physiol. 2008;28:45–54. doi: 10.1093/treephys/28.1.45. [DOI] [PubMed] [Google Scholar]
- 42.Dincer B, Colak A, Aydin N, Kadioglu A, Güner S. Characterization of polyphenoloxidase from medlar fruits (Mespilus germanica L. Rosaceae) Food Chem. 2002;77:1–7. [Google Scholar]
- 43.Flurkey WH. Polypeptide composition and amino-terminal sequence of broad bean polyphenoloxidase. Plant Physiol. 1989;91:481–3. doi: 10.1104/pp.91.2.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Colak A, Ozen A, Dincer B, Guner S, Ayaz FA. Diphenolases from two cultivars of cherry laurel (Laurocerasus officinalis Roem.). fruits at an early stage of maturation. Food Chem. 2005;90:801–7. [Google Scholar]
- 45.Mishra BB, Gautam S, Sharma A. Purification and characterization of polyphenol oxidase (PPO) from eggplant (Solanum melongena) Food Chem. 2012;134:1855–61. doi: 10.1016/j.foodchem.2012.03.098. [DOI] [PubMed] [Google Scholar]
- 46.De Jesus Rivas N, Whitaker JR. Purification and some properties of two polyphenol oxidases from Bartlett pears. Plant Physiol. 1973;52:501–7. doi: 10.1104/pp.52.5.501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mazzafera P, Robinson SP. Characterization of polyphenol oxidase in coffee. Phytochemistry. 2000;55:285–96. doi: 10.1016/s0031-9422(00)00332-0. [DOI] [PubMed] [Google Scholar]
- 48.Cevallos-Casals BA, Cisneros-Zevallos L. Impact of germination on phenolic content and antioxidant activity of 13 edible seed species. Food Chem. 2010;119:1485–90. [Google Scholar]
- 49.Ding CK, Chachin K, Ueda Y, Imahori Y. Purification and properties of polyphenol oxidase from loquat fruit. J Agric Food Chem. 1998;46:4144–9. [Google Scholar]
- 50.Orak HH. Total antioxidant activities, phenolics, anthocyanins, polyphenoloxidase activities of selected red grape cultivars and their correlations. Sci Horticult. 2007;111:235–41. [Google Scholar]
- 51.Burda S, Oleszek W, Lee CY. Phenolic compounds and their changes in apples during maturation and cold storage. J Agric Food Chem. 1990;38:945–8. [Google Scholar]
- 52.Lima VL, Melo EA, Maciel MIS, Prazeres FG, Musser RS, Lima DE. Total phenolic and carotenoid contents in acerola genotypes harvested at three ripening stages. Food Chem. 2005;90:565–8. [Google Scholar]
- 53.Brown DE, Rashotte AM, Murphy AS, Normanly J, Tague BW, Peer WA, et al. Flavonoids act as negative regulators of auxin transport in vivo in Arabidopsis. Plant Physiol. 2001;126:524–35. doi: 10.1104/pp.126.2.524. [DOI] [PMC free article] [PubMed] [Google Scholar]


