Abstract
Background:
Piperine was widely used in traditional medicine for inducing sterility and abortion.
Objective:
To evaluate the effect of the piperine on testis of male albino rats
Materials and Methods:
Adult male rats were divided into four groups (n = 12). Group I (control): Rats were given vehicle p.o. i.e. 0.5% carboxymethyl cellulose in normal saline daily for 60 days, Group II (ED): Rats received piperine at a dose of 10 mg/kg body weight (b.w.) daily, Group III (E4D): Rats received piperine at a dose of 10 mg/kg b.w. on every 4th day, Group IV (E7D): Rats received piperine at a dose of 10 mg/kg b.w. on every 7th day. Half of the animals from each group were sacrificed after the treatment period (60 days), and the remaining were kept for drug-free withdrawal period (60 days) and then sacrificed.
Results:
Piperine significantly decreased the reproductive organ weights in groups ED and E4D. Piperine induced hormonal imbalance by altering the serum levels of follicle-stimulating hormone, luteinizing hormone, sex hormone binding globulin, serum, and testicular testosterone in groups ED and E4D. Furthermore, piperine decreased the activity of germ cell markers and Leydig cellular steroidogenic enzymes in the groups ED and E4D after 60 days. All the above-altered values returned to normal levels after withdrawal period. Histopathological findings also supported the above findings.
Conclusion:
From the above data, it can be concluded that piperine could be a good lead molecule for the development of reversible oral male contraceptive.
SUMMARY
Piperine was employed for the contraceptive purposes in traditional medicine
Piperine significantly impaired the spermatogenesis by decreasing the testicular hormone synthesis in groups ED and E4D
Piperine disrupted the testicular antioxidant system by promoting the ROS production and hydroxyl radical generation in rat testis in groups ED and E4D
Histopathological evidence supported the disruption of spermatogenesis by piperine
All the effects of piperine after the treatment period (i.e. 60 days) were back to normal after the withdrawal period (i.e., after 120 days).
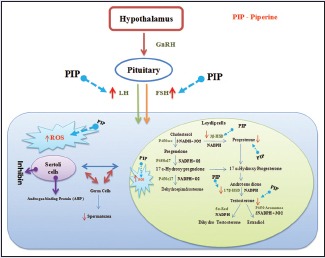
Keywords: Androgen binding protein, germ cell markers, male oral contraceptives, serum hormones, sex hormone binding globulin
INTRODUCTION
It is estimated that world's population will reach a staggering mark of 9.1 billion by 2050.[1] Even though sterilization is an effective and permanent means to control population explosion, social structure, health care infrastructure, and other factors prevailing in a particular region make it unsuitable in several circumstances. Alternatively, reversible or temporary contraception such as female oral contraceptives has gained popularity. Majority of men around the world are willing to take a contraceptive pill if such a method is available.[2] Despite this, very few contraceptive methods are available for men as compared to women. None of the hormonal contraceptives such as testosterone, testosterone ethanate, estrogen, anti-androgens, GnRH analogs, and dihydrotestosterone and chemical contraceptives such as depot medroxy progesterone acetate, cyproterone acetate, levonorgestrel, melatonin, a-chlorohydrin, metopirone, gossypol, and serotonin have the features of ideal male contraceptive.[2,3] Developing a reversible oral male contraceptive, which is rapidly effective, devoid of adverse events, does not influence the progeny, is acceptable to both the partners, and is still a dream for the researchers around the world. During the past century, natural products have served as a good source for modern drug discovery. Piperine is one such natural compound isolated from Piper nigrum Linn; Piper longum Linn. belongs to the family of Piperaceae. Piperine is responsible for the pungency of both the herbs.[4] A formulation containing these two herbs as active ingredients was employed for inducing menstruation and as abortifacient in Indian traditional medicine.[5] Preclinical studies revealed the effectiveness of piperine in treating ailments such as neuronal difficulties and depression.[6,7,8] Piperine is known to have antidiabetic,[9,10] anti-inflammatory,[11] antiplatelet,[12] antithyroid,[13] anti-leishmanial,[14] anti-asthmatic,[15] antidiarrheal,[16] antitumor,[17] anti-mutagenic,[18] and hepatoprotective activities.[19] Piperine is also known for its bioenhancing properties when simultaneously administered with gallic acid,[20] curcumin,[6] β-carotene,[21] tifferon,[22] and (−) epigallatocatechin-3-gallate.[23] It also enhances the bioavailability of clinically valuable drugs such as vasicine, sulfadiazine, isoniazid, ethambutol, phenobarbitone, phenytoin, dapsone, tetracyclines, rifampicin, pyrazinamide, carbamazepine, nimesulide, indomethacin, and ciprofloxacin.[24,25,26] Based on bioenhancing properties of piperine on antitubercular drugs such as isoniazid and rifampicin, synergistically acting formulations are available for clinical use in India since 2009.[4] A recent survey suggests that piperine is one among the 108 plant active constituents reported to have anti-fertility activity.[27] Furthermore, recent studies have proven the anti-fertility activity of piperine on male albino rats after 30 days of treatment.[28,29,30] However, the effect of piperine on complete spermatogenic cycle and its withdrawal effects were not investigated. The present study was undertaken to find out the effect of piperine on testes and prostate of male albino rats after the treatment (60 days) and drug-free withdrawal period (120 days) at a dose of 10 mg/kg body weight (b.w.).
MATERIALS AND METHODS
Chemicals
Piperine of 97% purity was obtained from Sigma-Aldrich (St. Louis, MO, USA). All other chemicals used for various assays were of analytical grade and were obtained from local commercial sources.
Animals
Healthy adult male albino rats of Wistar strain (age: 90 days) weighing 190–210 g procured from the Committee for the Purpose of Care and Supervision of Experimental Animals (CPCSEA) approved vendor (Biogen Laboratory Animal Facility, Bengaluru, Karnataka, India) were used for the present investigation. Animals were housed in polypropylene cages bedded with paddy husk and maintained under well-regulated light and dark (12 h: 12 h) schedule. Rats were fed with standard rat pellet diet (Om Sai Enterprises, Chennai, Tamil Nadu, India) and drinking water ad libitum.
Maintenance
All the protocols described in this research work were approved by Institutional Animal Ethical Committee (IAEC No: PU/IAEC/2014/25), Department of Biochemistry and Molecular Biology, Pondicherry University, Puducherry. All the animals were maintained according to the guidelines of CPCSEA, India.
Treatment
The dose of the piperine was selected according to the investigations carried out by D’cruz and Mathur, 2005.[29] Adult male rats were divided into four groups with 12 animals (n = 12) in each group. Group I (control): Rats were given vehicle p.o., i.e., 0.5% carboxy methyl cellulose (CMC) in normal saline daily for 60 days. Group II: Rats were treated with piperine suspended in 0.5% CMC at a dose of 10 mg/kg b.w. p.o. daily for 60 days (group ED). Group III: Rats were treated with piperine suspended in 0.5% CMC at a dose of 10 mg/kg b.w. p.o. on every 4th day for 60 days (group E4D). Group IV: Rats were treated with piperine suspended in 0.5% CMC at a dose of 10 mg/kg b.w. p.o. on every 7th day for 60 days (group E7D). Half of the animals from each group were sacrificed after 60 days of treatment. Remaining animals were kept for the drug-free withdrawal period of another 60 days and then sacrificed (total of 120 days). In this study, treatment period was considered as Phase I and withdrawal period as Phase II.
Collection of serum
Blood samples were collected from control and treated groups after treatment and withdrawal periods from the retro-orbital plexus. Blood was centrifuged at 1500 ×g for 15 min. Serum was separated and stored at −20°C in microfuge tubes until use.
Organ weights
At the end of the treatment (60 days) and withdrawal periods (120 days), the testes and ventral prostate were dissected out and weighed.
Testis index, testicular coefficient, and gonadosomatic index
Testis index was calculated by dividing the left testis weight with the total b.w. and multiplying with 100.[31] Testicular coefficient was calculated by dividing total organ weight by b.w. and then multiplying it with 100.[32] Gonadosomatic index (GSI) was calculated by dividing gonad weight with total b.w. and then multiplying with 100, where gonad weight = (weight of the right testis + weight of the left testis)/2.[33]
Determination of serum and testicular hormones
Hormones such as follicular-stimulating hormone (FSH),[34] luteinizing hormone (LH),[34] testosterone (T),[35] progesterone (P),[36] and sex hormone-binding globulin (SHBG)[37] were assayed using standard methods.
Preparation of homogenates
Homogenates of testes were prepared separately in ice-cold normal saline by using glass-Teflon homogenizer. Supernatants obtained after centrifugation were used for the biochemical assays.
Determination of testicular parameters
Testicular testosterone, total cholesterol, total acid phosphatase (ACP), alkaline phosphatase (ALP), and prostatic ACP were assayed using standard methods.[35,38,39,40]
Isolation of Leydig cells
Isolation of Leydig cells was carried out by using methods reported by Anand et al., with little modifications.[41,42] Animals were sacrificed by cervical dislocation and testes were removed. Decapsulated testes were incubated in modified's Eagles medium containing 0.25 mg/ml collagenase and 10% penicillin and streptomycin solution for 15 min. The seminiferous tubules were allowed to settle under unit gravity for 5 min, and the supernatant containing the interstitial cells were removed. The tubules were resuspended again in the medium and allowed to resettle and supernatants were collected. Supernatants were pooled together and transferred the Leydig cells enriched media into falcon tubes. The tube was inverted for 10 min and then filtered using nylon mesh. RBCs were removed by washing with 0.84% NH4Cl for 15 min. Macrophages present in the pellet were removed by incubating the pellet in Falcon Petri dish containing 8 ml of complete media (Dulbecco's Modified Eagle's Medium, Hams F12 nutrient mixture in 1:1 ratio and 10% of fetal bovine serum [FBS]) for 45 min. Leydig cells were collected using the micropipette without disturbing the macrophages that settled at the bottom of Petri dish. Finally, Leydig cells were collected and counted using Neubauer chamber, cryopreserved using 95% of FBS and 5% of DMSO. The cells were counted using a hemocytometer and the purity was assessed by histochemical staining for the 3b-hydroxy steroid dehydrogenase (3b-HSD) using the procedure described by Sharpe and Cooper[43] It was observed that more than 95% of the cells were positively stained for Leydig cells [Figure 1].
Figure 1.
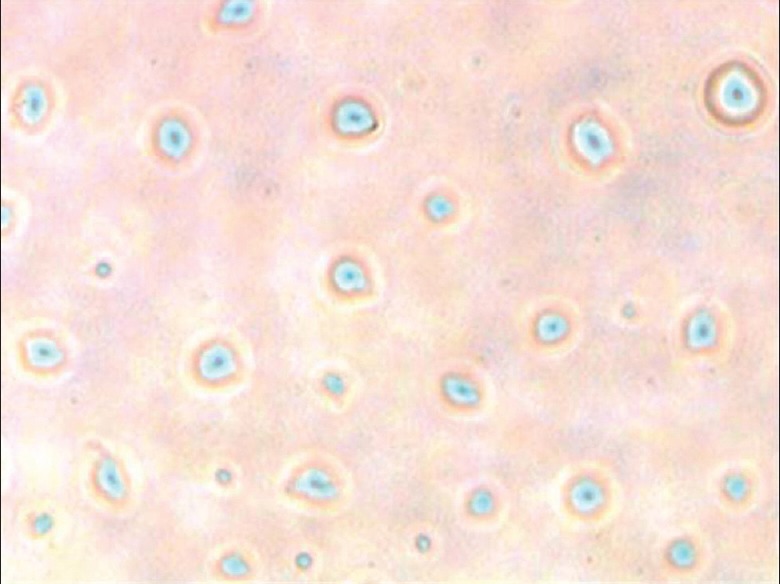
Immunohistochemical staining of Leydig cells (blue and dense blue color spots indicates the presence of Leydig cells)
Determination of steroidogenic enzymes, enzymatic and nonenzymatic antioxidants in testis and isolated Leydig cells
3b-HSD,[44] 17b-HSD,[44] glucose-6-phosphate dehydrogenase (G-6PD),[45] lactate dehydrogenase,[46] superoxide dismutase (SOD),[47] catalase (CAT),[48] lipid peroxidation (LPO),[49] glutathione-S-transferase,[50] reduced glutathione (GSH),[51] hydroxyl radical,[52] and g-glutamyl transpeptidase[53] were assayed according to the standard procedures.
Histopathological studies
At the end of the experimental schedule, one testis from each rat was taken and fixed in Bouin's fluid, and processed, and the paraffin sections of 4 mm were stained with hematoxylin and eosin stain and then examined under microscope.
Statistical analysis
The data were computed using Prism GraphPad software (GraphPad Company, USA) program version 6.0 and presented as mean ± standard deviation if applicable. Statistical analysis was performed using one-way analysis of variance method, followed by Student's t-test. The significance of differences was set at P < 0.05.
RESULTS AND DISCUSSION
Recent studies have reported the toxicity of piperine in testis and epididymis of male rats after treating them with different doses.[28,29,30] However, there is no information available about the effect of piperine for one complete spermatogenic cycle, i.e., 48 days. In addition, the effect of piperine after the withdrawal period, i.e., reversibility of piperine's activity, is not reported. Here, we report the effect of piperine on male albino rats after a treatment period of 60 days and drug-free withdrawal period of 60 days. Various parameters related to spermatogenesis and male fertility were investigated and is discussed below.
Effect of piperine on body and organ weights of rats
Rats treated with piperine at a dose of 10 mg/kg (group ED, E4D) for 60 days have significantly reduced testicular weights. It is already reported that the weight of the reproductive organs always give a good measure of the degree of spermatogenesis in rats;[54] moreover, it is suggested that significant decrease or increase in the absolute or relative weight of an organ after administration of a drug indicates the toxicity of the particular drug.[55,56] Hence, weights of the testis and prostate were also measured. The observed decrease in testicular weights in groups treated with piperine (ED, E4D) returned to normal levels after the drug-free withdrawal period. The decrease in the testicular weight after the daily treatment of piperine indicates the toxic effect of piperine on rat testis. No significant differences in the testis index were observed in all the treatment groups (ED, E4D, and E7D) compared to the control group [Table 1]. However, piperine caused a significant increase in the GSI in the group ED treated with piperine at a dose of 10 mg/kg b.w. for 60 days. GSI is inversely proportional to the reproductive efficiency of the rats[57] and testicular coefficient (TCT) value, which is directly proportional to reproductive toxicity in testes.[58] A marginal increase in the GSI in group E4D and E7D was observed after the treatment with piperine for 60 days. GSI and TCT in all the treated groups returned to normal levels after the drug-free withdrawal period of 60 days [Table 1].
Table 1.
Effect of piperine on rat body and organ weights
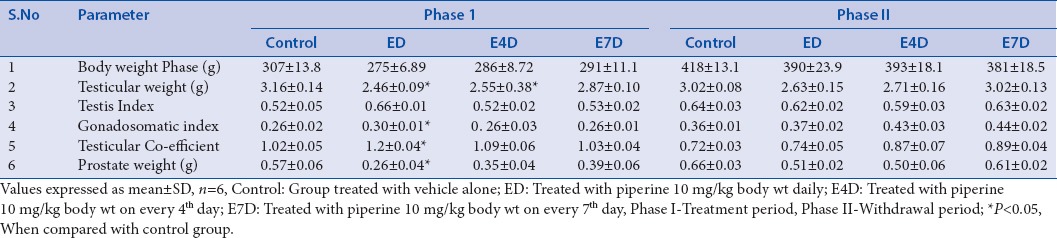
Decrease in the prostatic weight in the piperine-treated groups can be correlated with the deregulation of the steroid hormone status within the prostate gland[59] in comparison with the control group. Weights of the prostates were back to normal in all the groups after the withdrawal period; it may be due to the return of normal function in all the accessory organs [Table 1].
Effect of piperine on serum and testicular hormonal status of rats
No significant differences in the serum testosterone (T) levels were observed in all the groups (ED, E4D, and E7D) treated with piperine at a dose of 10 mg/kg, compared to the control group even after 60 days. However, significant reduction in the intra-testicular testosterone was observed in rats treated with piperine in group ED and E4D. This could be due to the nonspecific inhibition of NADPH-dependent cytochrome P450 enzyme, which plays an important role in the steroid hormone synthesis pathway through catalyzing the cholesterol side chain cleavage and other important hydroxylation reactions.[60,61,62] This decrease in testicular testosterone levels correlates with the earlier reports published by Malini et al. and D’cruz and Mathur.[28,29] The testicular testosterone levels returned to normal levels in all the groups treated with piperine after the drug-free withdrawal period of 60 days [Table 2].
Table 2.
Effect of piperine of serum hormones (Testosterone, Progesterone, FSH, LH and SHBG)
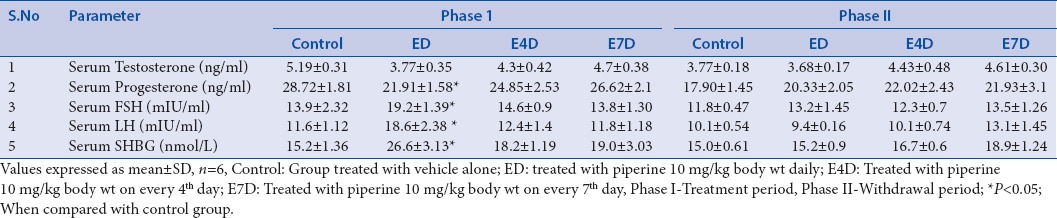
Piperine significantly increased the levels of the FSH and LH in group ED after 60 days of treatment. This could be due to the damage caused by the piperine to intra-testicular cells, i.e. Leydig cells and Sertoli cells, which in turn leads to a decrease in the testosterone biosynthesis or inadequate production of inhibin resulting in a decreased feedback signaling on pituitary gonadotropins.[28] This effect of piperine can be evidenced by a decreased serum testosterone concentration in the same group. However, increased levels of serum gonadotropins (FSH and LH) reinstated to normal values after the drug-free withdrawal period [Table 2].
Piperine caused only a minor hormonal imbalance in the group ED by increasing progesterone concentration. Progesterone plays a vital role in the estrogen balance and production of gonadotropins and also acts as a key regulator of male reproductive functions such as male sexual drive and ejaculatory potential.[63] However, progesterone levels were restored to normal level in all the piperine-treated groups after the withdrawal period [Table 2].
Serum SHBG was significantly increased in the group ED after the treatment period of 60 days, which further result in a decreased testosterone levels and reproductive functions.[64] Interactions of piperine with the active site amino acid residues of SHBG has already been reported in our previous in silico studies.[4]
Effect of piperine on functional status of reproductive organs of rats
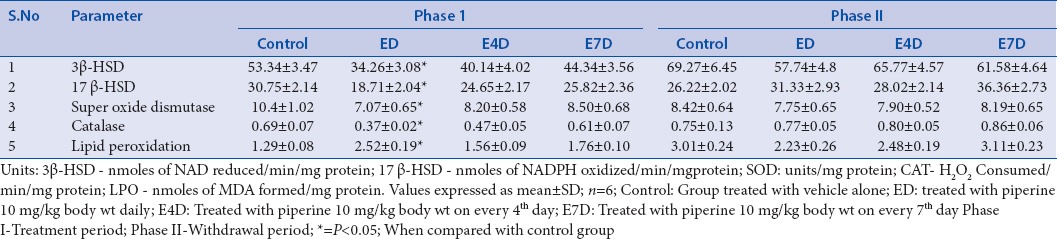
In our previous study, we have demonstrated the reversible spermatotoxic effect of piperine on male albino rats.[65] Piperine significantly impaired the testicular function of rats in the group ED and E4D through elevating the testicular cholesterol levels, resulting in the inhibition of the steroidogenesis after the treatment period.[66] A slight increase in the testicular cholesterol level was observed in the group E7D. Elevated cholesterol levels were returned to normal levels after the drug-free withdrawal period. Inhibition of the steroidogenesis was also evidenced by a decrease in the activity of two crucial enzymes, i.e. 3β-HSD and 17β-HSD, which are responsible for testosterone synthesis in group ED and E4D[67] [Table 3].
Table 3.
Effect of piperine on biochemical status of rat testis
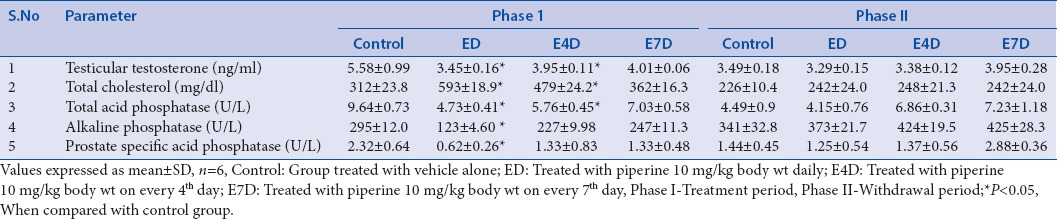
Administration of piperine caused a significant disruption in the membrane lipid bilayers of the plasma membrane, resulting in the leakage of ALP into the extracellular matrix, which results in the functional impairment of the testicular cells. A marked decrease in ALP activity in groups ED and E4D after the treatment period was observed as compared to the control group. This effect of the piperine may also be attributed due to its fungicidal properties.[68,69] Along with the decreased level of ALP, a decrease in the total ACPs was also observed in groups treated with piperine (ED and E4D), which confirms the piperine-mediated inhibition of steroidogenesis in rats. The degree of steroidogenesis is always dependent on the concentration of ACP.[70] Hence, a decrease in the level of ACP by piperine decreases the steroidogenesis. Piperine negatively affected the development of spermatocytes by decreasing the detachable ACP of lysosomal origin.[71] However, after the drug-free withdrawal period, both the ALP and ACP levels were restored to normal in all the piperine-treated groups.
A decrease in testicular γ-gluamyl tranpeptidase (γ-GT) activity was observed in piperine treated groups indicating the dysfunction of the Sertoli cell. This could be due to the inhibition of androgen receptor by piperine as the function of γ-GT depends upon the regulation of androgen receptor. Our in vitro study also supports the same.[4,72,73] Lactate dehydrogenase (LDH) plays a crucial role in the production of energy during spermatogenesis, and it is also necessary for the proper functioning of Sertoli cells.[74,75] LDH activity was found to be inhibited in groups ED and E4D, indicating suppression of testicular function. Further, piperine caused dysfunction of Leydig cells by inhibiting the activity of G-6PD in groups ED and E4D after the treatment period. Determination of G-6PD activity gives a good measure of the functional status of Leydig cells.[76] The activity of γ-GT, LDH, and G-6PD was restored to normal level after the drug-free withdrawal period [Table 4].
Table 4.
Effect of piperine on biochemical status of rat testis
Effect of piperine on enzymatic and nonenzymatic antioxidant status of rat testis
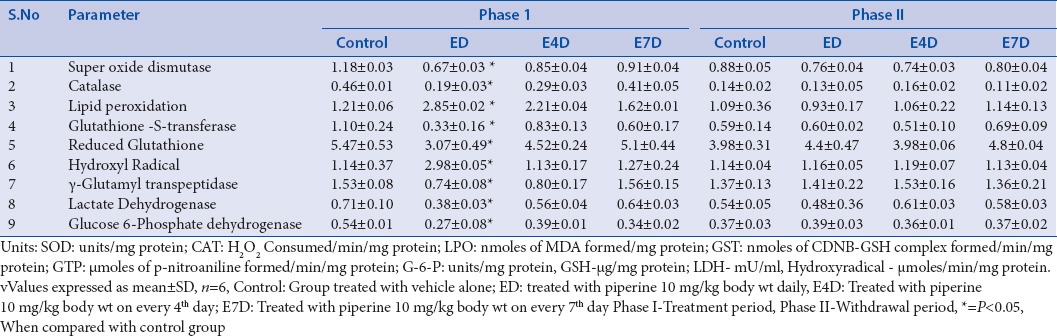
Oxidative damage caused to the rat testis can be shown by a decrease in the activities of two major antioxidant enzymes, namely, SOD and CAT, to a significant extent in the groups (ED, E4D and E7D) after the piperine treatment period of 60 days. SOD converts the superoxide radicals to hydrogen peroxide (H2O2) and CAT is crucial for the conversion of H2O2 into water. These two enzyme systems act together to prevent oxidative damage.[77] Piperine is known to accelerate the membrane LPO of lipids in rat testis.[28] In this study, significant increases in the malondialdehyde (MDA) levels were observed in the groups ED and E4D treated with piperine for 60 days. Increase in the MDA content of the tissue results in excessive generation of free radicals.[78] Piperine-mediated oxidative damage through the excessive generation of reactive oxygen species (ROS) was supported with an increase in the hydroxyl radical content in the testes of the rats treated with piperine for 60 days (groups ED and E4D).[79] Testicular toxicity of piperine can be correlated with a significant depletion in the reduced GSH levels in groups ED and E4D after the treatment period. Perturbation of intracellular GSH levels leads to a negative alteration in the cellular metabolism. This results in decreased detoxification potential and redox imbalance in the testicular tissue.[80] Moreover, declined levels of GSH may be associated with an enhanced LPO[81] [Table 4].
The extent of oxidative damage caused to the testis can be shown by a decrease in the activity of glutathione-S-transferase (GST) in the groups ED and E4D treated with piperine for 60 days. GST plays a vital role in the adaptive defensive mechanisms and works against the free radical induced oxidative damage and eliminates the toxic products by catalyzing the conjugation reactions.[80] On the contrary to its catalytic ability, GST is important for the functioning of the sperm.[82] All the above-mentioned altered activities of enzymes were restored to normal levels after the drug-free withdrawal period of 60 days [Table 4].
Effect of piperine on antioxidant status of the Leydig cells
Piperine significantly disrupted the antioxidant defensive mechanism of Leydig cells in groups ED and E4D by decreasing the activity of SOD and CAT after the 60 days treatment. SOD protects the Leydig cells from the free radical induced damage by converting superoxide anions into hydrogen peroxide (H2O2) and impairs the LPO. Whereas, CAT eliminates the lipid, protein, and DNA destructive H2O2 by its catalytic activity.[83] Increase in the ROS production can be shown with concomitant increase in MDA levels in groups ED and E4D.[84] The decreased activity of SOD and CAT and increased MDA content were reinstated to normal levels after the drug-free withdrawal period of 60 days [Table 5].
Table 5.
Effect of piperine on rat Leydig cells in phase I and phase II
Histopathological studies
Histology section of the group ED shows many desquamated spermatozoa with a decrease in the thickness of germ layer. The tubules showed both Type 1 and Type 2 spermatogonia and the rest composed of mostly mature spermatozoa. Primary spermatocytes are reduced in number compared to the control group. Section of the group E4D shows a decrease of 30% spermatozoa in the tubules, resulting in hypospermatogenesis. In contrary to this, section of group E7D shows a decrease of 10% spermatozoa in tubules, which represents mild hypospermatogenesis compared to the control group [Figure 2]. All the pathological changes appeared after treatment period returned to normal status after the drug-free withdrawal period of 60 days [Figure 3].
Figure 2.
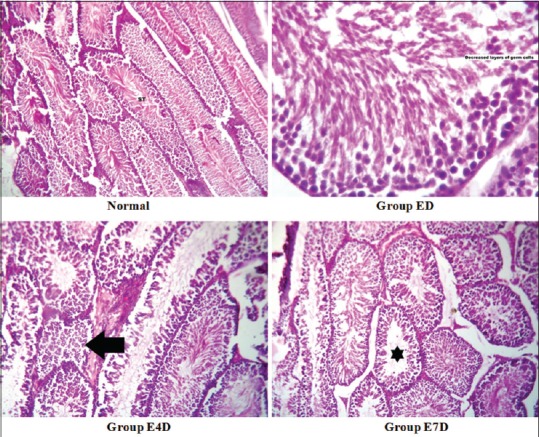
Histopathological sections of testis after the treatment period Legend: Control: Seminiferous tubules showing normal spermatogenesis (H and E, ×100), ED: Seminiferous tubules showing decreased thickness of germ cells due to fewer spermatocytes (H and E, ×400), E4D: Many seminiferous tubules show normal spermatogenesis while some others show no mature spermatozoa (hypospermatogenesis – left arrow) (H and E, ×100), E7D: Most seminiferous tubules show normal spermatogenesis while very few show no mature spermatozoa (mild hypospermatogenesis - star) (H and E, ×100)
Figure 3.
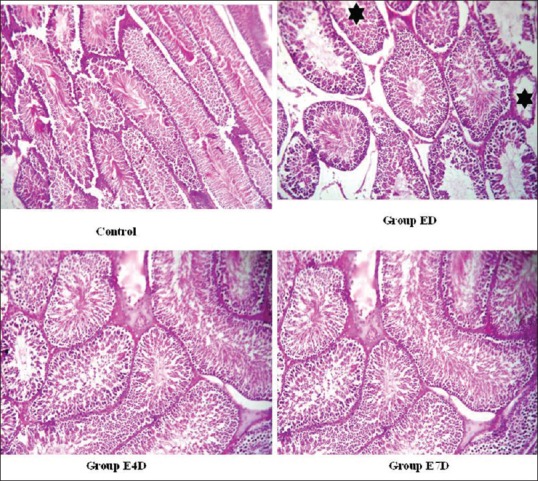
Histopathological sections of testis after the withdrawal period Legend: Control: - seminiferous tubules showing normal spermatogenesis (H and E, ×100), ED: Most seminiferous tubules show normal spermatogenesis while very few show no mature spermatozoa (mild hypospermatogenesis - star) (H and E, ×100), E4D: Seminiferous tubules showing normal spermatogenesis (H and E, ×100), E7D: Seminiferous tubules showing normal spermatogenesis (H and E, ×100)
CONCLUSION
Compared to the synthetic molecules, natural and herbal products have been more successful in delivering safer drugs for the human kind. The present study was aimed to evaluate the effect and reversibility of piperine mediated testicular toxicity in male albino rats. Various parameters related to male fertility such as weights of the testis and prostate, serum, and intra-testicular testosterone levels, and serum gonadotropin levels were measured. Furthermore, functional status of testis was ascertained by measuring the steroidogenesis-related parameters such as activity levels of enzymes 3b-hydroxy and 17b-HSD, ALP, ACPs, γ-GT, lactate dehydrogenase, and G-6PD. The extent of oxidative damage to testis caused by piperine treatment was monitored by measuring the activity levels of SOD, CAT, and GST enzymes; further, levels of MDA, ROS, and reduced glutathione levels were measured.
Our study result suggests that piperine disrupted the functional integrity of the testis by altering the germ cell markers, antioxidant status, and testicular hormones. These biochemical study results were further reinforced by histopathological observation of hypospermatogenesis on piperine treatment. However, the toxic effects of piperine were reversed after the drug-free withdrawal period of 60 days, suggesting reversible nature of piperine effect on male fertility. From the above results, it can be concluded that piperine can be used as a lead molecule for the development of reversible oral male contraceptive agent.
Financial support and sponsorship
Authors thank the Department of Biotechnology, Govt. of India, for the financial support for the DBT-IPLS program (Grant No : BT/PR14554/INF/22/125/2010), Pondicherry University, Puducherry, India. Authors also thank Professor T.G. Shrivatsav, NIHFW, Munirka, New Delhi for Providing the Testosterone and Progesterone Elisa kits.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Mruk DD. New perspectives in non-hormonal male contraception. Trends Endocrinol Metab. 2008;19:57–64. doi: 10.1016/j.tem.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 2.Pasqualotto FF, Lucon AM, Pasqualotto EB, Arap S. Trends in male contraception. Rev Hosp Clin Fac Med Sao Paulo. 2003;58:275–83. doi: 10.1590/s0041-87812003000500007. [DOI] [PubMed] [Google Scholar]
- 3.Vijayakumar RS, Nalini N. Piperine, an active principle from Piper nigrum modulates hormonal and apo lipoprotein profiles in hyperlipidemic rats. J Basic Clin Physiol Pharmacol. 2006;17:71–86. doi: 10.1515/jbcpp.2006.17.2.71. [DOI] [PubMed] [Google Scholar]
- 4.Chinta G, Ramya Chandar Charles M, Klopcic I, Sollner Dolenc M, Periyasamy L, Selvaraj Coumar M. In silico and in vitro investigation of the Piperine's male contraceptive effect: Docking and molecular dynamics simulation studies in androgen-binding protein and androgen receptor. Planta Med. 2015;81:804–12. doi: 10.1055/s-0035-1546082. [DOI] [PubMed] [Google Scholar]
- 5.Johri RK, Thusu N, Khajuria A, Zutshi U. Piperine-mediated changes in the permeability of rat intestinal epithelial cells. The status of gamma-glutamyl transpeptidase activity, uptake of amino acids and lipid peroxidation. Biochem Pharmacol. 1992;43:1401–7. doi: 10.1016/0006-2952(92)90195-o. [DOI] [PubMed] [Google Scholar]
- 6.Kulkarni SK, Bhutani MK, Bishnoi M. Antidepressant activity of curcumin: Involvement of serotonin and dopamine system. Psychopharmacology (Berl) 2008;201:435–42. doi: 10.1007/s00213-008-1300-y. [DOI] [PubMed] [Google Scholar]
- 7.Li S, Wang C, Li W, Koike K, Nikaido T, Wang MW. Antidepressant-like effects of piperine and its derivative, antiepilepsirine. J Asian Nat Prod Res. 2007;9:421–30. doi: 10.1080/10286020500384302. [DOI] [PubMed] [Google Scholar]
- 8.Li S, Wang C, Wang M, Li W, Matsumoto K, Tang Y. Antidepressant like effects of piperine in chronic mild stress treated mice and its possible mechanisms. Life Sci. 2007;80:1373–81. doi: 10.1016/j.lfs.2006.12.027. [DOI] [PubMed] [Google Scholar]
- 9.Atal S, Agrawal RP, Vyas S, Phadnis P, Rai N. Evaluation of the effect of piperine per se on blood glucose level in alloxan-induced diabetic mice. Acta Pol Pharm. 2012;69:965–9. [PubMed] [Google Scholar]
- 10.Atal S, Atal S, Vyas S, Phadnis P. Bio-enhancing effect of piperine with metformin on lowering blood glucose level in alloxan induced diabetic mice. Pharmacognosy Res. 2016;8:56–60. doi: 10.4103/0974-8490.171096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mujumdar AM, Dhuley JN, Deshmukh VK, Raman PH, Naik SR. Anti-inflammatory activity of piperine. Jpn J Med Sci Biol. 1990;43:95–100. doi: 10.7883/yoken1952.43.95. [DOI] [PubMed] [Google Scholar]
- 12.Park BS, Son DJ, Park YH, Kim TW, Lee SE. Antiplatelet effects of acidamides isolated from the fruits of Piper longum L. Phytomedicine. 2007;14:853–5. doi: 10.1016/j.phymed.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 13.Panda S, Kar A. Piperine lowers the serum concentrations of thyroid hormones, glucose and hepatic 5’D activity in adult male mice. Horm Metab Res. 2003;35:523–6. doi: 10.1055/s-2003-42652. [DOI] [PubMed] [Google Scholar]
- 14.Veerareddy PR, Vobalaboina V, Nahid A. Formulation and evaluation of oil-in-water emulsions of piperine in visceral leishmaniasis. Pharmazie. 2004;59:194–7. [PubMed] [Google Scholar]
- 15.Kim SH, Lee YC. Piperine inhibits eosinophil infiltration and airway hyperresponsiveness by suppressing T cell activity and Th2 cytokine production in the ovalbumin-induced asthma model. J Pharm Pharmacol. 2009;61:353–9. doi: 10.1211/jpp/61.03.0010. [DOI] [PubMed] [Google Scholar]
- 16.Bajad S, Bedi KL, Singla AK, Johri RK. Antidiarrhoeal activity of piperine in mice. Planta Med. 2001;67:284–7. doi: 10.1055/s-2001-11999. [DOI] [PubMed] [Google Scholar]
- 17.Samykutty A, Shetty AV, Dakshinamoorthy G, Bartik MM, Johnson GL, Webb B, et al. Piperine, a bioactive component of pepper spice exerts therapeutic effects on androgen dependent and androgen independent prostate cancer cells. PLoS One. 2013;8:e65889. doi: 10.1371/journal.pone.0065889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.El Hamss R, Idaomar M, Alonso-Moraga A, Muñoz Serrano A. Antimutagenic properties of bell and black peppers. Food Chem Toxicol. 2003;41:41–7. doi: 10.1016/s0278-6915(02)00216-8. [DOI] [PubMed] [Google Scholar]
- 19.Koul IB, Kapil A. Evaluation of the liver protective potential of piperine, an active principle of black and long peppers. Planta Med. 1993;59:413–7. doi: 10.1055/s-2006-959721. [DOI] [PubMed] [Google Scholar]
- 20.Zhao JQ, Du GZ, Xiong YC, Wen YF, Bhadauria M, Nirala SK. Attenuation of beryllium induced hepatorenal dysfunction and oxidative stress in rodents by combined effect of gallic acid and piperine. Arch Pharm Res. 2007;30:1575–83. doi: 10.1007/BF02977327. [DOI] [PubMed] [Google Scholar]
- 21.Badmadev V, Majid M, Norkus PE. Piperine, an alkaloid derived from black pepper increases serum response of beta carotene during 14 day oral beta carotene supplementation. Nutr Res. 1999;19:381–8. [Google Scholar]
- 22.Nirala SK, Bhadauria M, Mathur R, Mathur A. Influence of alpha-tocopherol, propolis and piperine on therapeutic potential of tiferron against beryllium induced toxic manifestations. J Appl Toxicol. 2008;28:44–54. doi: 10.1002/jat.1250. [DOI] [PubMed] [Google Scholar]
- 23.Lambert JD, Hong J, Kim DH, Mishin VM, Yang CS. Piperine enhances the bioavailability of the tea polyphenol (-)-epigallocatechin-3-gallate in mice. J Nutr. 2004;134:1948–52. doi: 10.1093/jn/134.8.1948. [DOI] [PubMed] [Google Scholar]
- 24.Chinta G, Safiulla BS, Mohane SC, Latha P. Piperine: A comprehensive review of its preclinical and clinical investigations. Curr Bioact Compd. 2015;11:156–69. [Google Scholar]
- 25.Han HK. The effects of black pepper on the intestinal absorption and hepatic metabolism of drugs. Expert Opin Drug Metab Toxicol. 2011;7:721–9. doi: 10.1517/17425255.2011.570332. [DOI] [PubMed] [Google Scholar]
- 26.Patil UK, Singh A, Chakraborthy AK. Role of piperine as a bioavailability enhancer. Int J Recent Adv Pharm Res. 2011;4:16–23. [Google Scholar]
- 27.Gupta RS, Kachhawa JB, Chaudhary R. Antispermatogenic, antiandrogenic activities of Albizia lebbeck (L.). Benth bark extract in male albino rats. Phytomedicine. 2006;13:277–83. doi: 10.1016/j.phymed.2004.11.008. [DOI] [PubMed] [Google Scholar]
- 28.Malini T, Manimaran RR, Arunakaran J, Aruldhas MM, Govindarajulu P. Effects of piperine on testis of albino rats. J Ethnopharmacol. 1999;64:219–25. doi: 10.1016/s0378-8741(98)00128-7. [DOI] [PubMed] [Google Scholar]
- 29.D’cruz SC, Mathur PP. Effect of piperine on the epididymis of adult male rats. Asian J Androl. 2005;7:363–8. doi: 10.1111/j.1745-7262.2005.00059.x. [DOI] [PubMed] [Google Scholar]
- 30.D’Cruz SC, Vaithinathan S, Saradha B, Mathur PP. Piperine activates testicular apoptosis in adult rats. J Biochem Mol Toxicol. 2008;22:382–8. doi: 10.1002/jbt.20251. [DOI] [PubMed] [Google Scholar]
- 31.Dikhil MA, Saleh AQ, Moneim AE. Effect of pomegranate (Punica granatum L.). juice and methanolic peel extract on testis of male rats. Pak J Zool. 2013;45:1343–9. [Google Scholar]
- 32.Yu ZZ, Chen J, Shou PQ, Feng L. Effects of micronutrients on the reproduction of infertility rat model induced by adenine. Int J Clin Exp Med. 2014;7:2754–62. [PMC free article] [PubMed] [Google Scholar]
- 33.Latif R, Lodhi GM, Aslam M. Effects of amlodipine on serum testosterone, testicular weight and gonado-somatic index in adult rats. J Ayub Med Coll Abbottabad. 2008;20:8–10. [PubMed] [Google Scholar]
- 34.Engvall E. Enzyme immunoassay ELISA and EMIT. Methods Enzymol. 1980;70:419–39. doi: 10.1016/s0076-6879(80)70067-8. [DOI] [PubMed] [Google Scholar]
- 35.Shrivastav TG, Basu A, Kariya KP. One step enzyme linked immunosorbent assay for direct estimation of serum testosterone. J Immunoassay Immunochem. 2003;24:205–17. doi: 10.1081/IAS-120020085. [DOI] [PubMed] [Google Scholar]
- 36.Shrivastav TG, Chaube SK, Kariya KP, Prasad PK, Kumar D. Influence of different length spacers containing enzyme conjugate on functional parameters of progesterone ELISA. J Immunoassay Immunochem. 2013;34:94–108. doi: 10.1080/15321819.2012.686470. [DOI] [PubMed] [Google Scholar]
- 37.Moore JW, Bulbrook RD. The epidemiology and function of sex hormone-binding globulin. Oxf Rev Reprod Biol. 1988;10:180–236. [PubMed] [Google Scholar]
- 38.Hillmann G. Continuous photometric measurement of prostate acid phosphatase activity. Z Klin Chem Klin Biochem. 1971;9:273–4. [PubMed] [Google Scholar]
- 39.Allain CC, Poon LS, Chan CS, Richmond W, Fu PC. Enzymatic determination of total serum cholesterol. Clin Chem. 1974;20:470–5. [PubMed] [Google Scholar]
- 40.Bessey OA, Lowry OH, Brock MJ. A method for the rapid determination of alkaline phosphates with five cubic millimeters of serum. J Biol Chem. 1946;164:321–9. [PubMed] [Google Scholar]
- 41.Anand H, Misro MM, Sharma SB, Prakash S. siRNA as a tool to delineate pathway channelization in H(2)O(2) induced apoptosis of primary Leydig cells in vitro. Apoptosis. 2012;17:1131–43. doi: 10.1007/s10495-012-0749-7. [DOI] [PubMed] [Google Scholar]
- 42.Khan S, Teerds K, Dorrington J. Growth factor requirements for DNA synthesis by Leydig cells from the immature rat. Biol Reprod. 1992;46:335–41. doi: 10.1095/biolreprod46.3.335. [DOI] [PubMed] [Google Scholar]
- 43.Sharpe RM, Cooper I. Variation in the steroidogenic responsiveness of isolated rat Leydig cells. J Reprod Fertil. 1982;65:475–81. doi: 10.1530/jrf.0.0650475. [DOI] [PubMed] [Google Scholar]
- 44.Bergmeyer HU, editor. Methods of Enzymatic Analysis. New York: Academic Press; 1974. β-hydroxy steroid dehydrogenase; pp. 447–89. [Google Scholar]
- 45.Beutler E. Selectivity of proteases as a basis for tissue distribution of enzymes in hereditary deficiencies. Proc Natl Acad Sci U S A. 1983;80:3767–8. doi: 10.1073/pnas.80.12.3767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.King J, editor. Practical Clinical Enzymology. London: Van Nostrand Company Ltd; 1965. The dehydrogenases or oxidoreductases – Lactate dehydrogenase; pp. 83–93. [Google Scholar]
- 47.Marklund S, Marklund G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and a convenient assay for superoxide dismutase. Eur J Biochem. 1974;47:469–74. doi: 10.1111/j.1432-1033.1974.tb03714.x. [DOI] [PubMed] [Google Scholar]
- 48.Sinha AK. Colorimetric assay of catalase. Anal Biochem. 1972;47:389–94. doi: 10.1016/0003-2697(72)90132-7. [DOI] [PubMed] [Google Scholar]
- 49.Devasagayam TP, Tarachand U. Decreased lipid peroxidation in the rat kidney during gestation. Biochem Biophys Res Commun. 1987;145:134–8. doi: 10.1016/0006-291x(87)91297-6. [DOI] [PubMed] [Google Scholar]
- 50.Habig WH, Pabst MJ, Jakoby WB. Glutathione S-transferases. The first enzymatic step in mercapturic acid formation. J Biol Chem. 1974;249:7130–9. [PubMed] [Google Scholar]
- 51.Moron MS, Depierre JW, Mannervik B. Levels of glutathione, glutathione reductase and glutathione S-transferase activities in rat lung and liver. Biochim Biophys Acta. 1979;582:67–78. doi: 10.1016/0304-4165(79)90289-7. [DOI] [PubMed] [Google Scholar]
- 52.Puntarulo S, Cederbaum AI. Production of reactive oxygen species by microsomes enriched in specific human cytochrome P450 enzymes. Free Radic Biol Med. 1998;24:1324–30. doi: 10.1016/s0891-5849(97)00463-2. [DOI] [PubMed] [Google Scholar]
- 53.Orlowski M, Meister A. Isolation of gamma-glutamyl transpeptidase from hog kidney. J Biol Chem. 1965;240:338–47. [PubMed] [Google Scholar]
- 54.Raji Y, Ifabunmi OS, Akinsomisoye OS, Morakinyo AO, Oloyo AK. Gonadal responses to antipsychotic drugs: Chlorpromazine and thioridazine reversibly suppress testicular functions in albino rats. Int J Pharmacol. 2005;1:287–92. [Google Scholar]
- 55.Maina MB, Garba SH, Jacks TW. Histological evaluation of the rats testis following administration of a herbal tea mixture. J Pharmacol Toxicol. 2008;3:464–70. [Google Scholar]
- 56.Simmons JE, Yang RS, Berman E. Evaluation of the nephrotoxicity of complex mixtures containing organics and metals: Advantages and disadvantages of the use of real-world complex mixtures. Environ Health Perspect. 1995;103(Suppl 1):67–71. doi: 10.1289/ehp.95103s167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Silva AA, Essica SD, de Oliveira RR, Sílvia RA, Valdemiro AS, Elizabeth NM. Evaluation of quantitative parameters of Leydig cell in diabetic adults rats. Acta Sci Biol Sci. 2014;36:483–9. [Google Scholar]
- 58.Yuan L, Fan M, Zhan P. Dose-dependent effects of pentabrominated diphenyl ethers on sexual hormone and histology of male reproductive system in rats. J Biomed Sci Eng. 2012;5:302–6. [Google Scholar]
- 59.Weber KS, Setchell KD, Stocco DM, Lephart ED. Dietary soy-phytoestrogens decrease testosterone levels and prostate weight without altering LH, prostate 5alpha-reductase or testicular steroidogenic acute regulatory peptide levels in adult male Sprague-Dawley rats. J Endocrinol. 2001;170:591–9. doi: 10.1677/joe.0.1700591. [DOI] [PubMed] [Google Scholar]
- 60.Miller WL. Molecular biology of steroid hormone synthesis. Endocr Rev. 1988;9:295–318. doi: 10.1210/edrv-9-3-295. [DOI] [PubMed] [Google Scholar]
- 61.Reen RK, Singh J. In vitro and in vivo inhibition of pulmonary cytochrome P450 activities by piperine, a major ingredient of piper species. Indian J Exp Biol. 1991;29:568–73. [PubMed] [Google Scholar]
- 62.Reen RK, Wiebel FJ, Singh J. Piperine inhibits aflatoxin B1-induced cytotoxicity and genotoxicity in V79 Chinese hamster cells genetically engineered to express rat cytochrome P4502B1. J Ethnopharmacol. 1997;58:165–73. doi: 10.1016/s0378-8741(97)00104-9. [DOI] [PubMed] [Google Scholar]
- 63.Alvarenga TA, Andersen ML, Tufik S. Influence of progesterone on sexual performance in male rats. J Sex Med. 2010;7:2435–44. doi: 10.1111/j.1743-6109.2010.01851.x. [DOI] [PubMed] [Google Scholar]
- 64.Harris ID, Fronczak C, Roth L, Meacham RB. Fertility and the aging male. Rev Urol. 2011;13:e184–90. [PMC free article] [PubMed] [Google Scholar]
- 65.Chinta G, Periyasamy L. Reversible anti-spermatogenic effect of piperine on epididymis and seminal vesicles of albino rats. Drug Res (Stuttg) 2016;66:420–6. doi: 10.1055/s-0042-108186. [DOI] [PubMed] [Google Scholar]
- 66.Kaur R, Dhanju CK, Kaur K. Effects of dietary selenium on biochemical composition of rat testis. Indian J Exp Biol. 1999;37:509–11. [PubMed] [Google Scholar]
- 67.Ghosh S, Dasgupta S. Gentamicin induced inhibition of steroidogenic enzymes in rat testis. Indian J Physiol Pharmacol. 1999;43:247–50. [PubMed] [Google Scholar]
- 68.Ananthan G, Kumaran B. Effect of mancozeb on the specific activities of testicular phosphates and protective role of Vitamin c in albino rats. BEPLS. 2013;2:56–61. [Google Scholar]
- 69.Castillo F, Hernández D, Gallegos R, Rodrıguez Aguilar CN. Antifungal properties of bioactive compounds from plants, fungicides for plant and animal disease. In: Dhanasekaran D, editor. Fungicides for Plant and Animal Diseases. China: In-Tech; 2012. pp. 1–27. [Google Scholar]
- 70.Mathur PP, Chattopadhyay S. Involvement of lysosomal enzymes in flutamide-induced stimulation of rat testis. Andrologia. 1982;14:171–6. doi: 10.1111/j.1439-0272.1982.tb03120.x. [DOI] [PubMed] [Google Scholar]
- 71.Chitra KC, Latchoumycandane C, Mathur PP. Chronic effect of endosulfan on the testicular functions of rat. Asian J Androl. 1999;1:203–6. [PubMed] [Google Scholar]
- 72.Olayinka E, Ore A. Hepatotoxicity, nephrotoxicity and oxidative stress in rat testis following exposure to haloxyfop-p-methyl ester, an aryloxyphenoxypropionate herbicide. Toxics. 2015;3:373–89. doi: 10.3390/toxics3040373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sherins RJ, Hodgen GD. Testicular gamma glutamyl-transpeptidase: An index of Sertoli cell function in man. J Reprod Fertil. 1976;48:191–3. doi: 10.1530/jrf.0.0480191. [DOI] [PubMed] [Google Scholar]
- 74.El-Kashoury AA. Influence of subchronic exposure of profenofos on biochemical markers and microelements in testicular tissue of rats. Nat Sci. 2009;7:16–29. [Google Scholar]
- 75.Zhang B, Lin S. Effects of 3,4-dichloroaniline on testicle enzymes as biological markers in rats. Biomed Environ Sci. 2009;22:40–3. doi: 10.1016/S0895-3988(09)60020-9. [DOI] [PubMed] [Google Scholar]
- 76.Heywood L, Blackshaw A. Lactate-dehydrogenase activity in the rat testis: A comparison between fluorometric assay of freeze-dried sections and histochemical localization with phenazine methosulphate. J Histochem Cytochem. 1978;26:967–72. doi: 10.1177/26.11.722053. [DOI] [PubMed] [Google Scholar]
- 77.Kalender S, Uzun FG, Demir F, Uzunhisarcikli M, Aslanturk A. Mercuric chloride-induced testicular toxicity in rats and the protective role of sodium selenite and vitamin E. Food Chem Toxicol. 2013;55:456–62. doi: 10.1016/j.fct.2013.01.024. [DOI] [PubMed] [Google Scholar]
- 78.Rahal A, Kumar A, Singh V, Yadav B, Tiwari R, Chakraborty S, et al. Oxidative stress, prooxidants, and antioxidants: The interplay. Biomed Res Int. 2014;2014:761264. doi: 10.1155/2014/761264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Vigueras-Villaseñor RM, Ojeda I, Gutierrez-Pérez O, Chavez-Saldaña M, Cuevas O, Maria DS, et al. Protective effect of α-tocopherol on damage to rat testes by experimental cryptorchidism. Int J Exp Pathol. 2011;92:131–9. doi: 10.1111/j.1365-2613.2010.00757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Maneesh M, Jayalekshmi H, Dutta S, Chakrabarti A, Vasudevan DM. Role of oxidative stress in ethanol induced germ cell apoptosis – An experimental study in rats. Indian J Clin Biochem. 2005;20:62–7. doi: 10.1007/BF02867402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Khan RA. Protective effects of Launaea procumbens on rat testis damage by CCl4. Lipids Health Dis. 2012;11:103. doi: 10.1186/1476-511X-11-103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Gopalakrishnan B, Aravinda S, Pawshe CH, Totey SM, Nagpal S, Salunke DM, et al. Studies on glutathione S-transferases important for sperm function: Evidence of catalytic activity-independent functions. Biochem J. 1998;329(Pt 2):231–41. doi: 10.1042/bj3290231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Aitken RJ, Roman SD. Antioxidant systems and oxidative stress in the testes. Oxid Med Cell Longev. 2008;1:15–24. doi: 10.4161/oxim.1.1.6843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Gautam DK, Misro MM, Chaki SP, Sehgal N. H2O2 at physiological concentrations modulates Leydig cell function inducing oxidative stress and apoptosis. Apoptosis. 2006;11:39–46. doi: 10.1007/s10495-005-3087-1. [DOI] [PubMed] [Google Scholar]


