Abstract
Background:
Increased levels of malondialdehyde (MDA) and 4-hydroxynonenal (HNE) are demonstrated in plasma of uremic patients. A study showed that the comparison of erythrocytes of healthy and diseased patients (obese, hypertensive, and Type 2 diabetics) with age is associated to a disturbed oxidant/antioxidant balance when obesity is associated with hypertension. 4-hydroxytyrosol is shown to significantly protect red blood cells (RBCs) from oxidative damage (4-HNE). In literature, there are partial discussions on the role of lipids and their oxidation products. The products of degradation of membrane proteins are observed as self-consisting products without interrelations with membrane lipids.
Objective:
The aim of this study is to evaluate the role of polyunsaturated fatty acid (PUFA) metabolites on oxidative damage (4-hydroxy-alkenals) in RBCs of hypertriglyceridemic patients after membrane treatment with 4-hydroxytyrosol.
Materials and Methods:
The authors optimize the isolation of RBC ghosts and spectrophotometric method to measure free 4-hydroxyalkenals in human RBC membranes and investigated the effect on oxidative damage in human erythrocyte membranes and in vitro 4-hydroxytyrosol treatment to evaluate the membrane lipids reducible by this phenol.
Results:
Plasma triglyceride levels in patients are clearly higher than in controls. Moreover, total membrane proteins data are similar to previous described. The normalized alkenals levels are significantly enhanced in hyperlipemic patients in comparison to normoglyceridemic controls. After the 4-hydroxytyrosol action, lipid metabolites substantially decrease. The ratio of oxidized lipids (MDA + HNE) and membrane proteins data are similar to previously described ones.
Conclusion:
According to experimental data, the accumulation of the alkenals in RBC membrane could be produced either by partial PUFA oxidation contained in glycerides and plasma glycerides and by glycerides into plasma membrane recycled RBC.
SUMMARY
Hypertriglyceridemia induces oxidative stress in human red blood cell (RBC) membranes
Oxidative stress causes increased plasma membrane total protein concentration and hydroxynonenal and malondialdehyde levels
The authors optimize the isolation of RBC ghosts and spectrophotometric method to measure free 4-hydroxyalkenals in human RBC membranes
After the reduction with 4-hydroxytyrosol, oxidized lipid concentration significantly decrease.
Abbreviations used: RBC: Red blood cell; MDA: Malondialdehyde; HNE\HAE: 4-hydroxyalkenals; LPO: Lipid peroxidation; ROS: Reactive oxygen species; ORAC: Oxygen Radical Absorbance Capacity.
Keywords: 4-hydroxyalkenals, 4-hydroxytyrosol, hypertriglyceridemia, lipid peroxidation, malondialdehyde, oxidative stress
INTRODUCTION
As described by Sommerburg et al., patients with end-stage renal failure undergoing hemodialysis are exposed to oxidative stress. Increased levels of malondialdehyde (MDA) and 4-hydroxynonenal (HNE) are demonstrated in plasma of uremic patients, indicating accelerated lipid peroxidation (LPO).[1] According to Breusing et al.,[2] human plasma samples exposed to UVA irradiation at different doses (0, 15 J, 20 J) where analyzed for MDA, 4-HNE, and isoprostanes contents, result in a variation of free HNE by HPLC analysis in an interlaboratory validation of methods of LPO measurement. A study of Constantin et al. investigated the effects of aging on the carbonyl stress and glutathione antioxidant defenses in red blood cells (RBCs) of obese Type 2 mellitus diabetic patients with/without hypertensive complications. The results showed that compared to RBCs of healthy controls, in obese Type 2 diabetics, aging is associated with: (i) an increase in the concentration and expression of carbonylated proteins, a marker of oxidative stress; (ii) a decrease of both nonenzymatic and enzymatic endogenous glutathione defences; (iii) a severely disturbed oxidant/antioxidant balance when obesity is associated with hypertension.[3] 4-hydroxytyrosol is shown to significantly protect RBCs from oxidative damage in a dose-dependent manner (20–3 μM).[4] 4-hydroxytyrosol inhibits 4-HNE-induced apoptosis, characterized by increased production of reactive oxygen species (ROS).[5]
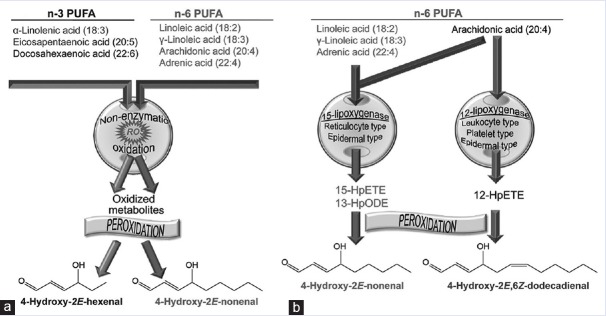
4-hydroxytyrosol shows a substantial degree of inhibition against ascorbate-Fe2+ induced LPO in rat liver microsomes.[6] In a previous study of Gallo and Martino[7] on RBC membranes damages by oxidant agents, authors demonstrated the conformational alterations and polyphenols repairing in the same structure. Among the known risk factors of atherosclerosis-associated oxidative stress are hyperlipidemia and/or hyperglycemia.[8,9,10] Currently, in the literature, there are only partial discussions on the role of lipids and their oxidation products as intermediates of their membrane structural damage and/or on the protective role in the same structures.[11,12] Furthermore, the products of degradation of membrane proteins are observed as self-consisting products without interrelations with membrane lipids. In this manuscript, we examine the role of triglycerides overload on degradation of membrane lipids in the presence of other reducing agents (protective effect of 4-hydroxytyrosol).[13,14,15] The studied membrane model is the RBC that is damaged from ROS.[16] The RBC is an experimental model of essential cellular structure. Hypertriglyceridemia denotes high blood levels of triglycerides, the most abundant fatty molecules in organisms. Elevated levels of triglycerides are associated with atherosclerosis and predispose to cardiovascular disease.[17] Oxidative stress, i.e., an altered balance between the production of free radicals and antioxidant defenses.[18] The peroxidation of n-3 and n-6 polyunsaturated fatty acids (PUFAs) and of their metabolites is a complex process. It is initiated by free oxygen radical-induced abstraction of a hydrogen atom from the lipid molecule followed by a series of nonenzymatic reactions that ultimately generate the reactive aldehyde species 4-hydroxyalkenals (HAE). 4-HNE has been implicated in various pathophysiological interactions due to their chemical reactivity and the formation of covalent adducts with macromolecules. The progressive accumulation of adducts alters normal cell functions that lead to cell death. At low and noncytotoxic concentrations, these molecules may function as signaling molecules in cells. This paper addresses the origin and cellular functions of 4-HAE.[19] The scavenging activity is studied after the “in vitro” treatment with 4-hydroxytyrosol that is classified as a phytochemical compound expressing strong antioxidant properties. The Oxygen Radical Absorbance Capacity index[20] for 4-hydroxytyrosol totaled (Trolox equivalent [TE] antioxidant capacity) 40,000 μmol TE/g that is about ten times greater than green tea at least twice compared to CoQ10. One study showed that a low dose of 4-hydroxytyrosol in rats reduces the consequences of the side effects of oxidative stress induced by smoking.[21] 4-hydroxytyrosol is shown to significantly protect RBCs from oxidative damage (LPO [4-HNE]). The authors[7] studied the protective effect of 4-hydroxytyrosol on human RBCs and oxidative damage so, the intent is to examine the role of triglycerides overload on degradation of membrane lipids in the presence of other reducing agents (protective effect of 4-hydroxytyrosol). For instance, in complete eukaryotic cells, the following damages can happen: 4-HAE are endogenous ligands for peroxisome proliferator-activated receptor-δ. 4-HNE affects p38 mitogen-activated protein kinases, c-Jun N-terminal kinase, protein kinase Cβ and -δ, cell cycle regulators, epithelial growth factor receptor, microRNAs, and the nuclear factor-kB [Figure 1].[22] Cytotoxic effects furthermore result from covalent interactions of 4-HNE with aminophospholipids and distort the bilayer structure of membranes and consequently alter their barrier and transport functions [Figure 2].[23,24]
Figure 1.
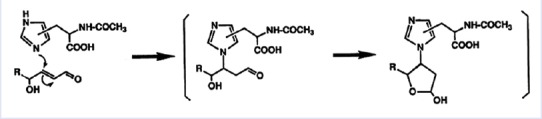
Proposed molecular mechanism of formation of hydroxynonenal-histidine adducts, according to Uchida and Stadtman, 1992
Figure 2.
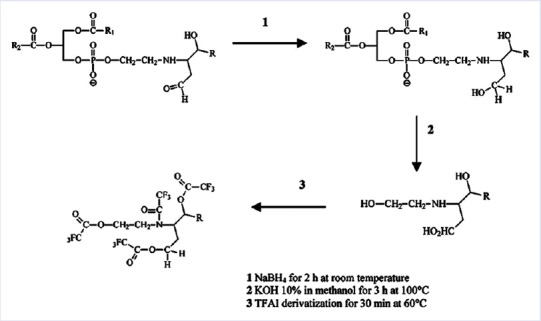
Scheme of the procedure used to evaluate ethanolamine phospholipid-alkenal Michael adducts by gas chromatography-mass spectrometry as their ethanolamine-alkenal residue, according to Bacot et al., 2007
MATERIALS AND METHODS
Patients
The studied population consisted of 10 patients with endogenous hypertriglyceridemia and ten normal controls who are recruited from the Health Center of the University of Calabria, Italy. All patients followed traditional local diet. The diagnosis of endogenous hypertriglyceridemia is based on the peroxidase-coupled method for the colorimetric determination of serum triglycerides.[25]
Blood samples
After fasting, blood is sampled on ethylenediaminetetraacetic acid (EDTA)-coated tubes, spun down at 1550 g for 10 min, plasma and leukocyte layers removed, and the packed RBCs layer used for the subsequent assays.
RBCs layer is lysed in 5 mM hypotonic phosphate buffer pH 8, containing in origin 1 mM EDTA (to complex all interfering cations, authors simplified the method increasing EDTA concentration at 100 mM) and 0.03 mM phenylmethylsulphonyl fluoride, followed by 20 min centrifugation at 16,000 g, the RBCs ghost sediment are resuspended by swirling while adding back sufficient of the fresh hypotonic buffer to reconstitute the original volume. The ratio of erythrocytes to washing solution is 1/40. The ghosts are washed 3 times subsequent to the hemolysis by sedimenting in a swinging-bucket rotor (16,000 g for 30 min), till free of hemoglobin. Aliquots of this should be taken for sample replicates, as well as for protein determination. 200 μL portions are required for use in this assay. Sample homogenates should be made as concentrated as possible. The concentration of protein in the homogenate should be determined. It is recommended that 0.2 μL of a homogenate containing 15–60 mg/mL protein be assayed for initial studies in a previously untested biological sample. The supernatant should be kept on ice before analyze or, if not analyzed immediately after preparation, frozen at − 70°C for longer storage.[3]
ASSAY OF TOTAL PROTEINS IN RED BLOOD CELLS GHOSTS
The determination of protein concentration is performed by the Biuret method.[26] The principle on which is based on the reaction of the peptide bond of proteins with Cu2+ ions. In alkaline solution, the formation of an indigo colored complex: The intensity of the color is directly proportional to the protein concentration. The standard used is bovine serum albumin from Sigma. Procedure: Samples prepared by plugging the tubes are shaken, and after 45 min it reads the absorbance of each sample at 540 nm on Shimadzu UV 2100 spectrophotometer.
Spectrophotometric analysis of free hydroxynonenal
Assay procedure for malondialdehyde ([concentrated hydrochloric acid] and malondialdehyde + hydroxyalkenals [concentrated methane sulfonic acid procedure])
All reagents are from Enzo Life Science. The standards (HNE standard: (E)–Hydroxynonenal IkB inhibitor) are prepared in clean glass test tubes or polypropylene microcentrifuge tubes. Preferably, standards should be run in triplicate. Add 200 μL of sample to a clean glass test tube or polypropylene microcentrifuge tube. Unknowns should be run in triplicate. Add 650 μL of diluted N-methyl-2-phenylindole reagent. Mix gently by vortexing the sample. Add 150 μL concentrated (12 N) HCl or methanesulfonic acid reagent. Mix well and stopper the tube. Incubate at 45°C for 60 min. Centrifuge turbid samples (e.g., 15,000 × g for 10 min) to obtain a clear supernatant. Transfer the clear supernatant to a cuvette. Measure the absorbance at 586 nm.[27]
In vitro 4-hydroxytyrosol treatment
The concentration of reducing agent is chosen according to literature data.[4] Reducing agent is provided from Sigma Chemical Co. (St. Louis, MO, USA) and used at the final concentration of 80 μM for 3 h at 37°C.
Statistical analysis
For statistical analysis, one-way ANOVA and Bonferroni post hoc test are used. Results are given as mean ± standard error of the mean on at least ten undependent determinations.
RESULTS
Authors studied the effect of oxidative stress of RBC ghosts by the method of Gèrard-Monnier et al.[27] The diagnostic criteria for endogenous hypertriglyceridemia are as follows: Total plasma triglyceride level > 200 mg/dL [Figure 3]. So that triglyceride plasma levels in patients are neatly higher than in controls. The mean values of triglyceride plasma levels in our casuistry are described in Figure 3. It is evident that data of the two groups are significantly different (P < 0.01).
Figure 3.
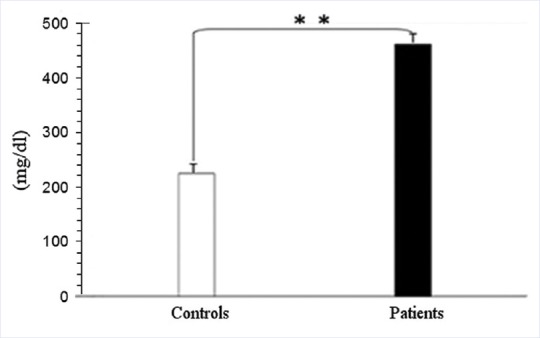
Plasma biochemical data of controls and hypertriglyceridemic patients (mean ± standard deviation). The mean values of triglyceride plasma levels. It is evident that data of the two groups are significantly different for P < 0.01 by one-way ANOVA and Bonferroni post hoc test. *P < 0.05; **P < 0.01 and ***P < 0.001
As described in Figure 4 total membrane proteins from samples of our casuistry are significantly different after extensive differential centrifugation of RBC ghosts. The mean values of total membrane protein levels in our casuistry are described in Figure 4. It is evident that the two grouped data are significantly different (P < 0.01). The proteins of pathological samples are about two times those of controls, showing that hypertriglyceridemic patients possess RBC ghosts that are richer in protein than reference ones. As described in Figure 5a and b the level of oxidation products of membrane lipids are referred as μM of MDA or HNE. MDA levels are always ten times those of HNE as referred in right-hand scale. In Figure 5a and b the scales are quite different (for instance: 0.00/0.85 in A and 0.000/0.033 in B for MDA). We can also observe that MDA levels of pathological samples are slightly higher than control ones but HNE levels of pathological samples are four times those of control ones. After the 4-hydroxytyrosol action both lipid metabolites substantially decrease. The mean values of total membrane protein levels in our casuistry are described in Figure 5. It is evident that for the two dosed substances each group of data is significantly different (P < 0.001) from the other ones. In Figure 6 are reported the total levels of MDA + HNE per milligram of total membrane proteins of RBC ghosts. As described the total oxidized lipid levels in RBC ghost membranes almost doubled in pathological samples in comparison to control ones. This shows that even if total membrane proteins dramatically increase in hypertriglyceridemic patients, also total oxidized lipids sufficiently increase in such samples so that ratio between patients RBC oxidized lipids to membrane proteins is always doubled. Such partial compensation of lipids in comparison to proteins from RBC membrane could be explained as an attempt of RBC metabolism to partially compensate ghost structure damages by oxidative stress. The ratio of total oxidized lipids to membrane proteins before and after 4-hydroxytyrosol treatment are referred in Figure 6. After 4-hydroxytyrosol treatment, the ratio of oxidized lipids to total membrane proteins decreases even more, but the values in our controls are always lower than in hypertriglyceridemic patients. The mean values of ratio of oxidized lipids (MDA + HNE) and membrane proteins in our casuistry are described in Figure 6. It is evident that the two groups of data are significantly different (P < 0.001). Neither Vitamin E nor Vitamin C are used because their ultraviolet–visible (UV-Vis) spectra superimpose to HNE determination method interfering with their evaluation.
Figure 4.
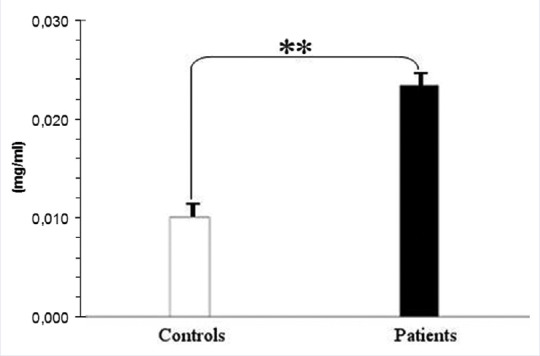
The quantitative assay of total proteins per blood ml from red blood cell ghosts. Mean values of proteins in red blood cell purified membranes. It is evident that data of the two groups are significantly different for P < 0.01 by one-way ANOVA and Bonferroni post hoc test. *P < 0.05; **P < 0.01 and ***P < 0.001
Figure 5.
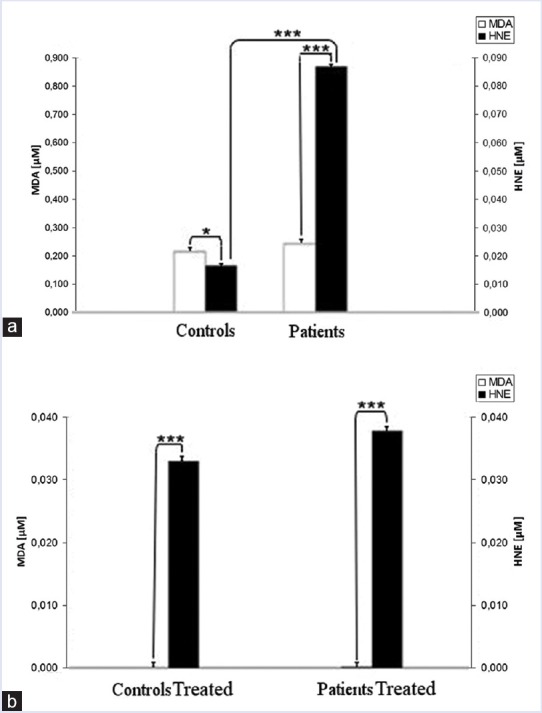
Human red blood cell membrane concentration of malondialdehyde and hydroxynonenal (μM absolute concentration in membrane samples) from controls and hypertriglyceridemic patients (a) and the same samples treated with 4-hydroxytyrosol (80 μM) (b). It is evident that for the two dosed substances each grouped data are significantly different for P < 0.001 by two ways ANOVA and Bonferroni post hoc test. *P < 0.05; **P < 0.01, and ***P < 0.001
Figure 6.
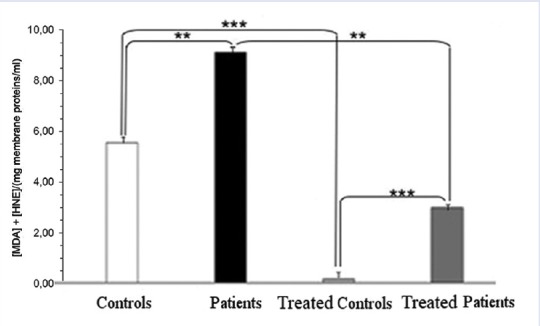
Ratio of oxidized lipids (malondialdehyde + hydroxynonenal) and membrane proteins. It is evident that all comparisons for the two dosed substances, each group of data is significantly different for P < 0.001 by two-ways ANOVA and Bonferroni post hoc test. *P < 0.05; **P < 0.01 and ***P < 0.001 from the others
DISCUSSION
According to our data in RBC, ghosts are present both HNE and MDA products [Figure 7].[19] As demonstrated by higher levels of HNE in comparison to MDA, according to Sommerburg et al.[1] The RBC membrane preparations are obtained increasing the efficiency of Fe2+ removal with an higher concentration of EDTA (100 mM). Spectrophotometric method (25) to measures free 4-HAE in human RBCs is optimized for the determination of alkenals [Figure 8] in membranes. Other kinds of reducing agents, such as Vitamin E, are not tested because their UV-Vis spectra superimpose to N-methyl-2-phenylindole product spectra.
Figure 7.
Mechanisms of 4-hydroxyalkenal production. Reactive oxygen species, reactive oxygen species. (a) Nonenzymatic generation of 4-hydroxy-2E-hexenal and 4-hydroxy-2E-nonenal (4-hydroxynonenal) from n-3 and n-6 polyunsaturated fatty acids, respectively. (b) lipoxygenase-initiated peroxidation of n-6 polyunsaturated fatty acids and generation of 4-hydroxynonenal and 4-hydroxy-2E,6Z-dodecadienal
Figure 8.
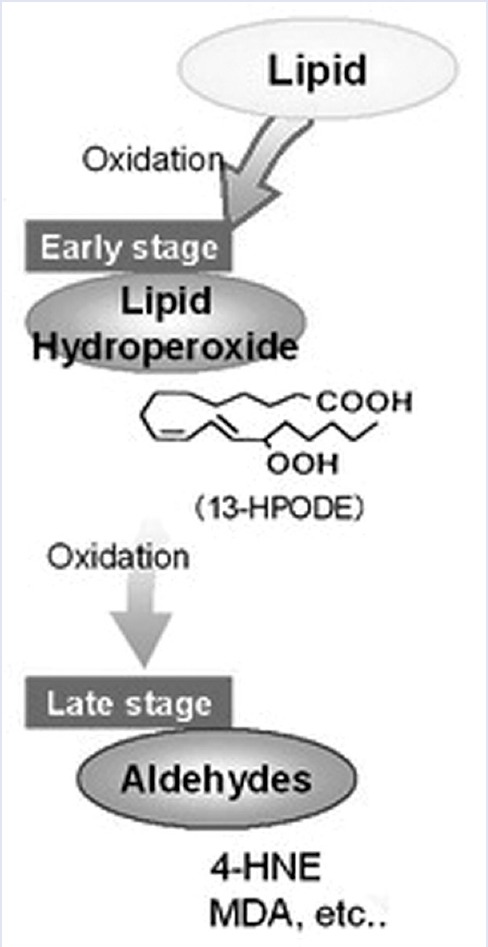
Stages of lipid peroxidation (modified by authors scheme of Abd El-Aal HAHM. Lipid Peroxidation End-products as a Key of Oxidative Stress: Effect of Antioxidant on Their Production and Transfer of Free Radicals. In: Catala A, editor. Biochemistry, Genetics and Molecular Biology “Lipid Peroxidation”. Winchester (GB): InTech; 2012. p. 63-88)
In membranes of hypertriglyceridemic patients, the levels (triglycerides, total membrane proteins, total aldehydes and their ratio) are higher than in control samples. All the data decrease relevantly (P < 0.001) after the 4-hydroxytyrosol treatment, especially those of alkenals [Figure 9].
Figure 9.
4-hydroxytyrosol ultraviolet–visible absorbance spectrum peak at 280 nm (Klen TJ. Olive fruit phenols in olive oil processing: the fate and antioxidant potential. Thesis, University of Nova Gorica, Nova Gorica, Slovenia; 2014)
CONCLUSION
According to experimental data, the accumulation of the alkenals in RBC membrane could be produced either by partial PUFA oxidation contained in glycerides and plasma glycerides and by glycerides into recycled plasma membrane in RBC neogenesis [Figure 10]. According to these hypotheses, the increased charge of triglycerides in plasma forces its metabolism toward either incorporation in cell membrane of degradative oxidation. This last pathway induces increase of oxidative product such as alkenals and MDA. Furthermore, free alkenals can be dissolved in lipid membrane bilayer degrading their structures. This last process favors increased level of macromolecular assemblies in circulation that enhances microcirculation damage. Such last data could be studied in following works.
Figure 10.
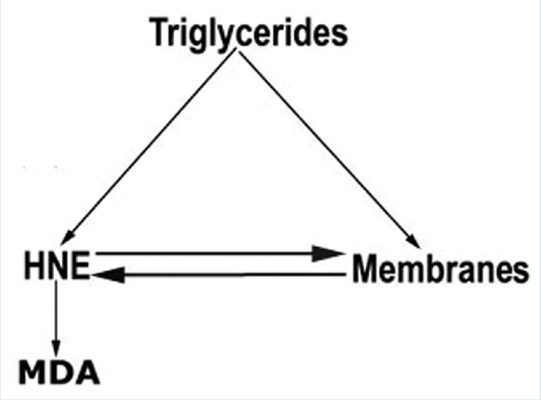
Graphical resume of main metabolic steps of fatty acids in cell membrane
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
The authors would like to acknowledge Prof. Domenico Sturino for his mother language support and technical revision of manuscript.
REFERENCES
- 1.Sommerburg O, Grune T, Hampl H, Riedel E, van Kuijk FJ, Ehrich JH, et al. Does long-term treatment of renal anaemia with recombinant erythropoietin influence oxidative stress in haemodialysed patients? Nephrol Dial Transplant. 1998;13:2583–7. doi: 10.1093/ndt/13.10.2583. [DOI] [PubMed] [Google Scholar]
- 2.Breusing N, Grune T, Andrisic L, Atalay M, Bartosz G, Biasi F, et al. An inter-laboratory validation of methods of lipid peroxidation measurement in UVA-treated human plasma samples. Free Radic Res. 2010;44:1203–15. doi: 10.3109/10715762.2010.499907. [DOI] [PubMed] [Google Scholar]
- 3.Constantin A, Constantinescu E, Dumitrescu M, Calin A, Popov D. Effects of ageing on carbonyl stress and antioxidant defense in RBCs of obese Type 2 diabetic patients. J Cell Mol Med. 2005;9:683–91. doi: 10.1111/j.1582-4934.2005.tb00498.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Paiva-Martins F, Fernandes J, Rocha S, Nascimento H, Vitorino R, Amado F, et al. Effects of olive oil polyphenols on erythrocyte oxidative damage. Mol Nutr Food Res. 2009;53:609–16. doi: 10.1002/mnfr.200800276. [DOI] [PubMed] [Google Scholar]
- 5.Bali EB, Ergin V, Rackova L, Bayraktar O, Küçükboyaci N, Karasu Ç. Olive leaf extracts protect cardiomyocytes against 4-hydroxynonenal-induced toxicity in vitro: Comparison with oleuropein, hydroxytyrosol, and quercetin. Planta Med. 2014;80:984–92. doi: 10.1055/s-0034-1382881. [DOI] [PubMed] [Google Scholar]
- 6.Gutierrez VR, de la Puerta R, Catalá A. The effect of tyrosol, hydroxytyrosol and oleuropein on the non-enzymatic lipid peroxidation of rat liver microsomes. Mol Cell Biochem. 2001;217:35–41. doi: 10.1023/a:1007219931090. [DOI] [PubMed] [Google Scholar]
- 7.Gallo G, Martino G. In vitro action of 2.2’-azobis-2 amidinopropan dihydrochloride, red wine polyphenols, resveratrol and catechin on anion permeability for chloride in human red blood cell. Free Radic Antioxid. 2014;4:13–7. [Google Scholar]
- 8.Couillard C, Pomerleau S, Ruel G, Archer WR, Bergeron J, Couture P, et al. Associations between hypertriglyceridemia, dietary fat intake, oxidative stress, and endothelial activation in men. Nutrition. 2006;22:600–8. doi: 10.1016/j.nut.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 9.de Man FH, Jonkers IJ, Schwedhelm E, Smelt AH, Onkenhout W, van Duyvenvoorde W, et al. Normal oxidative stress and enhanced lipoprotein resistance to in vitro oxidation in hypertriglyceridemia: Effects of bezafibrate therapy. Arterioscler Thromb Vasc Biol. 2000;20:2434–40. doi: 10.1161/01.atv.20.11.2434. [DOI] [PubMed] [Google Scholar]
- 10.Ceriello A, Quagliaro L, Piconi L, Assaloni R, Da Ros R, Maier A, et al. Effect of postprandial hypertriglyceridemia and hyperglycemia on circulating adhesion molecules and oxidative stress generation and the possible role of simvastatin treatment. Diabetes. 2004;53:701–10. doi: 10.2337/diabetes.53.3.701. [DOI] [PubMed] [Google Scholar]
- 11.Richard D, Kefi K, Barbe U, Bausero P, Visioli F. Polyunsaturated fatty acids as antioxidants. Pharmacol Res. 2008;57:451–5. doi: 10.1016/j.phrs.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 12.Ambrozova G, Pekarova M, Lojek A. Effect of polyunsaturated fatty acids on the reactive oxygen and nitrogen species production by raw 264.7 macrophages. Eur J Nutr. 2010;49:133–9. doi: 10.1007/s00394-009-0057-3. [DOI] [PubMed] [Google Scholar]
- 13.Gavino VC, Miller JS, Ikharebha SO, Milo GE, Cornwell DG. Effect of polyunsaturated fatty acids and antioxidants on lipid peroxidation in tissue cultures. J Lipid Res. 1981;22:763–9. [PubMed] [Google Scholar]
- 14.McAnulty SR, Nieman DC, Fox-Rabinovich M, Duran V, McAnulty LS, Henson DA, et al. Effect of n-3 fatty acids and antioxidants on oxidative stress after exercise. Med Sci Sports Exerc. 2010;42:1704–11. doi: 10.1249/MSS.0b013e3181d85bd1. [DOI] [PubMed] [Google Scholar]
- 15.Eritsland J. Safety considerations of polyunsaturated fatty acids. Am J Clin Nutr. 2000;71(1 Suppl):197S–201S. doi: 10.1093/ajcn/71.1.197S. [DOI] [PubMed] [Google Scholar]
- 16.Balogh N, Gaál T, Ribiczeyné PS, Petri A. Biochemical and antioxidant changes in plasma and erythrocytes of pentathlon horses before and after exercise. Vet Clin Pathol. 2001;30:214–218. doi: 10.1111/j.1939-165x.2001.tb00434.x. [DOI] [PubMed] [Google Scholar]
- 17.Pejic RN, Lee DT. Hypertriglyceridemia. J Am Board Fam Med. 2006;19:310–6. doi: 10.3122/jabfm.19.3.310. [DOI] [PubMed] [Google Scholar]
- 18.Chang T, Wu L. Methylglyoxal, oxidative stress, and hypertension. Can J Physiol Pharmacol. 2006;84:1229–38. doi: 10.1139/y06-077. [DOI] [PubMed] [Google Scholar]
- 19.Riahi Y, Cohen G, Shamni O, Sasson S. Signaling and cytotoxic functions of 4-hydroxyalkenals. Am J Physiol Endocrinol Metab. 2010;299:E879–86. doi: 10.1152/ajpendo.00508.2010. [DOI] [PubMed] [Google Scholar]
- 20.Cao G, Alessio HM, Cutler RG. Oxygen-radical absorbance capacity assay for antioxidants. Free Radic Biol Med. 1993;14:303–11. doi: 10.1016/0891-5849(93)90027-r. [DOI] [PubMed] [Google Scholar]
- 21.Awasthi YC, Yang Y, Tiwari NK, Patrick B, Sharma A, Li J, et al. Regulation of 4-hydroxynonenal-mediated signaling by glutathione S-transferases. Free Radic Biol Med. 2004;37:607–19. doi: 10.1016/j.freeradbiomed.2004.05.033. [DOI] [PubMed] [Google Scholar]
- 22.Uchida K, Stadtman ER. Modification of histidine residues in proteins by reaction with 4-hydroxynonenal. Proc Natl Acad Sci U S A. 1992;89:4544–8. doi: 10.1073/pnas.89.10.4544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bacot S, Bernoud-Hubac N, Chantegrel B, Deshayes C, Doutheau A, Ponsin G, et al. Evidence for in situ ethanolamine phospholipid adducts with hydroxy-alkenals. J Lipid Res. 2007;48:816–25. doi: 10.1194/jlr.M600340-JLR200. [DOI] [PubMed] [Google Scholar]
- 24.Cohen G, Riahi Y, Sunda V, Deplano S, Chatgilialoglu C, Ferreri C, et al. Signaling properties of 4-hydroxyalkenals formed by lipid peroxidation in diabetes. Free Radic Biol Med. 2013;65:978–87. doi: 10.1016/j.freeradbiomed.2013.08.163. [DOI] [PubMed] [Google Scholar]
- 25.McGowan MW, Artiss JD, Strandbergh DR, Zak B. A peroxidase-coupled method for the colorimetric determination of serum triglycerides. Clin Chem. 1983;29:538–42. [PubMed] [Google Scholar]
- 26.Datta SP, Leberman R, Rabin BR. The chelation of metal ions by dipeptides and related substances. Part 5 – cupric complexes of sarcosyl and leucyl ligands. Trans Faraday Soc. 1959;55:2141–51. [Google Scholar]
- 27.Gérard-Monnier D, Erdelmeier I, Régnard K, Moze-Henry N, Yadan JC, Chaudière J. Reactions of 1-methyl-2-phenylindole with malondialdehyde and 4-hydroxyalkenals. Analytical applications to a colorimetric assay of lipid peroxidation. Chem Res Toxicol. 1998;11:1176–83. doi: 10.1021/tx9701790. [DOI] [PubMed] [Google Scholar]