Abstract
Objective:
Novel nanovesicular gel of Berberis aristata extract was developed and evaluated for its anti-inflammatory and antipsoriatic activity.
Materials and Methods:
Transferosomes were prepared using soya phosphatidylcholine and edge activators (Tween 80, Span 80, and sodium deoxycholate) by a modified lipid film hydration technique using rotary evaporator and evaluated for various parameters. The quantification and standardization of extract have been carried out using its alkaloid content as berberine as biomarker. Topical application of imiquimod (IMQ) (immune modifier) on the shaved back of mice developed psoriasis-like inflammation followed by histopathological study of inflamed skin.
Results:
The size of transferosomes was in the range of 265–345 nm whereas polydispersity index ranges from 0.10 to 0.63, and for zeta potential, it was from −19.3 to −43.3 mV. Transferosomes were further added to Carbopol 934P for gel formation and subsequently evaluated for their physicochemical properties. Their efficacy against inflammation, IMQ-induced psoriasis, and skin sensitivity was compared with conventional formulation (commercial formulation-Angle Gloss, Phytolab Pvt. Ltd.). Percent inhibition of edema by transferosomal gel (55.76%) was more as compared to conventional gel of extract (33.5%) found out by Carrageenan-induced paw edema method. Primary irritation index was found to be <0.4 inferring its safe use for topical formulation.
Conclusion:
Histopathological report showed that, in psoriasis-induced animal treated with topical application of extract loaded transferosomal gel showed a marked reduction in thickness of epidermis, length of rete ridges as compared to conventional gel formulation. It can be inferred that B. aristata extract loaded transferosomal gel can function as potential anti-inflammatory and antipsoriatic formulation.
SUMMARY
The objective of the present research work was to prepare Berberis aristata extracts (roots, ethanolic 70%v/v) loaded transferosomal gel, to perform in vitro characterization and in vivo evaluation of their efficacy against inflammation as well as imiquimod (IMQ)-induced psoriasis in animals
The remarkable enhancement in the in vitro release efficiency of B. aristata extract loaded transferosomal gel resulted in improved anti-inflammatory activity. The prepared novel formulation of B. aristata has also shown its efficacy against IMQ-induced psoriasis.
Abbreviations used: SPC: Soyaphosphatidylcholine, PDI: Polydispersity index, IMQ: Imiquimod, EA: Edge activator, BE: Berberine, TEM: Transmission electron microscopy, PBS: Phosphate buffered saline, H and E: Hematoxylin and eosin, ZP: Zeta potential, EE: Entrapment efficiency.
Keywords: Anti-inflammatory, berberine, Berberis aristata, herbal vesicular gel, transferosomes, vesicles
INTRODUCTION
Psoriasis can be defined as chronic inflammatory, immune-mediated disorder characterized by marked changes in the skin like rashes, scales, red patches, and itchiness. The inflammatory processes included here may be linked with progression of morbidity and comorbid conditions which severely impair the patient's health.[1]
Histologically, psoriatic skin shows thickened epidermis, absence of granular layer, and epidermal cell nuclei in the superficial layers. A large number of inflammatory cells are present in all layers of the skin.
In psoriasis, the skin becomes rigid due to increase in the level of cholesterol and decrease in ceremides level which leads to a reduction of water content in the skin. This possesses a big challenge to make drug available to the target tissue using topical route.[2] A broad range of therapies are available for treatment of psoriasis; however, their side effects limit their application.[3] It has also been found that, for psoriasis-like inflammatory ailments, a large proportion of population is turning toward herbal remedies with a belief that they are free from any kind of side effects.[4]
Berberis aristata is one among many herbs that have been widely used in traditional medicines for skin diseases. B. aristata is an Indian medicinal plant officially noted in Ayurvedic and Siddha Pharmacopoeia of India, which belongs to the family Berberidaceae. It is an ayurvedic herb which is used since ancient times as anti-inflammatory agents for osteoporosis, joint pain, fever, eyes, and skin infection.[5] Topical instillation of aqueous extract of B. aristata showed potent anti-inflammatory activity against endotoxin-induced uveitis in rabbits as well as against turpentine liniment-induced ocular inflammation in rabbit's eyes.[6,7] Ethnobotanical studies indicate that Rasaut, decoction of B. aristatais, was commonly used to treat skin disorders.[8] Literature reports that Malani tribal communities from Himachal Pradesh, India, are also using B. aristata to cure skin disease.[9]
There are many reports existing in literature that emphasize various pharmacological potential of B. aristata extracts, but none of the existing literature reports the conversion of this extract into transferosomal gel and no studies have been done to confirm its antipsoriatic action.
Keeping in view the fact that reaching the psoriatic tissue through topical route is difficult and limitation of its existing treatment, novel B. aristata extracts (roots, ethanolic 70%v/v) loaded nanovesicular transferosomal gel have been prepared. The aim of the study was to observe the effect of these prepared nanovesicles on the skin disorders in particular psoriasis.
Imiquimod (IMQ) is a potent immune modifier upon daily spreading on shaved mice skin developed scaly inflamed skin which looks similar to plaque type psoriasis.[10] Distinctive feature of IMQ-induced psoriasis is epidermal hyperplasis, leukocyte infiltration, inflammation, and increased vascularity in dermis.[11] We attempted the mouse model to simulate the features of psoriasis using IMQ.
In the current study, for the quantification and standardization of B. aristata extract, its alkaloid content has been hypothesized as a biomarker.[5,12,13] They have been further evaluated for their efficacy against inflammation as well as IMQ-induced psoriasis in animals.
MATERIALS AND METHODS
B. aristata extract was obtained as a gift sample from Aushadhi Herbals New Delhi, India. Berberine chloride dihydrate was obtained as a gift sample from Natural Remidies Pvt. Ltd., Bengaluru.
IMQ cream (Glenmark Pharmaceuticals) was purchased from local market. All other solvents and reagents used were of analytical grade.
Characterization of Berberis aristata extract
The high-performance thin layer chromatography characterization of B. aristata extract was performed according to our previously described method.[14]
Preparation of Berberis aristata extract loaded transferosomes
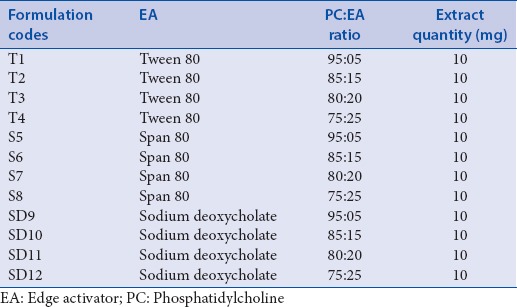
The transferosome formulations were prepared by modified lipid film hydration technique using rotary evaporator (IKA RV 10 digital).[15,16] Soyaphosphatidylcholine (SPC), edge activator (EA Tween 80/Span 80/sodium deoxycholate), and extract in different ratios were dissolved in 2–5 ml solvent mixture methanol and chloroform (2:1, v/v). The solvent was removed by rotary evaporation under reduced pressure above the lipid transition temperature to form a lipid film on the wall of flask. The thin film was stored overnight for complete evaporation of solvent. The film was then hydrated with phosphate-buffered saline (PBS) 7.4 pH with gentle shaking for 15 min and sonicated for 5 min. The transferosomal dispersion was further hydrated up to 1 h at 2°C–8°C and subjected to evaluation test.[17] Transferosomal dispersions were produced with different SPC:EA ratio (95:05, 85:15, 80:20, and 75:25) and three different EAs (Tween 80, Span 80, and Sodium deoxycholate) [Table 1].
Table 1.
Formulation table of Berberis aristata extract loaded transferosomes
Evaluation of transferosomes
All the prepared batches were evaluated for zeta potential (ZP), vesicular size, polydispersity index (PDI), percent entrapment efficiency (EE), and in vitro release study.
ZP, vesicle size, and PDI were measured by Zetasizer (Malvern Instruments, MAL 1021334, version 7.03).
Entrapment efficiency
Aliquots of transferosomal dispersions were subjected to centrifugation using cooling centrifuge (Remi C-24BL) at 12000 rpm. The clear supernatant was siphoned off carefully to separate the unentrapped extract. Sediment was treated with 1 ml of 0.1% Triton X-100 to lyse the vesicles and then diluted with PBS (pH 7.4). The EE was determined in terms of percentage berberine (BE) content in sediment. Since BE is one of the major and important alkaloids found in B. aristata which is responsible for its pharmacological property;[12] therefore, it was considered as a biomarker for estimations.[18,19]
The percent entrapment was calculated using the formula:
Percent Entrapment efficiency = (amount of BE in sediment)/(amount of BE in the extract added to transferosome) × 100.
Visualization of vesicles by phase contrast microscopy and transmission electron microscopy (TEM).
All the prepared batches were observed under phase contrast microscopy with the magnification power of ×100 (Olympus). Photographs of vesicles were taken using Olympus camera (Olympus MJU 9010).
Optimized batch was visualized using Jeol/JM 2100, Source LaB6 electron microscope (TEM) with an accelerating voltage of 200 kV for surface appearance and shape. The transferosomes were dispersed in distilled water and 10 μl of diluted dispersion was placed on carbon-coated grid.
In vitro diffusion studies
The dialysis membrane was placed in PBS (7.4 was selected because high release rate was obtained when compared to pH 5.4 and pH 6.8) for 6 h to attain saturation before starting permeation study. It was mounted between the donor and receptor compartment of the Franz diffusion cell (fabricated with glass) for in vitro diffusion studies. Transferosomal dispersion to be analyzed was placed into the donor cell compartment. The receptor chamber was filled with PBS (7.4) and was maintained at 37°C with continuous stirring. One milliliter aliquot of receptor phase solution was withdrawn at time intervals of 0.5, 1, 2, 4, 8, and 24 h, and the same volume of fresh medium was added back into the chamber. The quantification was done using ultraviolet spectrophotometer (Shimadzo Model No. 1800) at 203 nm.
Permeation data analysis
To study the release rate profile, the data obtained from in vitro drug release study were fitted in different kinetic equations: zero order as the cumulative percent of drug remaining versus time, first order as the log cumulative percentage of drug remaining versus time, Higuchi's model as the cumulative percent drug remaining versus square root of time, Hixson-Crowell cube root model and Korsmeyer–Peppas model as the log cumulative percentage of drug released versus log time.
Vesicle penetration study by fluorescence microscopy
The ability of fluorescence marker-loaded transferosome to penetrate through skin was observed by fluorescence microscopy. Franz diffusion cell was used to perform the study in a similar manner to the in vitro diffusion studies. The fluorescent probe rhodamine B was loaded in the vesicles along with extract during the hydration of the film with PBS 7.4 pH with gentle shaking for 15 min. Probe loaded vesicular dispersions were applied for 8 h to the dorsal skin of rats in Franz diffusion cell at 37°C. At the end of the experiment, skin was removed from cell and thoroughly washed with distilled water. Treated area was cut out from the skin and sectioned into 20 μ thickness tissues using cryotome (Thermo Scientific HM550). It is highly efficient instrument used for sectioning technique in routine and research. Skin specimens were observed under fluorescence microscope Leica DM2500, using Green Filter N2.1, position 3. The skin specimens (20 μm) were placed on glass slide irradiated with 540 nm and observed at magnification of ×100.
Incorporation into hydrophilic gels
On the basis of EE, percentage drug release, PDI, and ZP, four transferosomal batches (T3, S6, S7, and SD11) were selected for incorporation into gel formulations. Carbopol 934P 0.5% w/v was soaked in a minimum amount of water for an hour, and then 10 ml of transferosomal dispersion containing B. aristata extract (10 mg) was added. It was then stirred continuously at 700 rpm in a closed vessel whose temperature was maintained at 30°C until homogeneous transferosomal gels was achieved. To maintain the neutral pH, triethanolamine was added slowly to the stirring mixture.
Evaluation of gel-viscosity and pH measurement
Viscosity of transferosomal gel formulations were measured using Brookfield viscometer (Model No. DV-III ULTRA) using spindle no. 6 at 100 rpm and pH measurements of the formulations were done using digital pH meter (Model RI-152-R).
Spreading diameter
The spreadability of gel formulation was determined by measuring the spreading diameter of 1 g of gel between two horizontal plates (20 cm × 20 cm) after 1 min. The standard weight applied on upper plate was 125 g.[20,21]
Drug content of the formed gels
Five hundred micrograms of gel was taken and dissolved in 50 ml of pH 7.4 PBS. The solution was then passed through the filter paper and 50 μl of the filtrate was withdrawn. The filtrate was diluted by adding 3.5 ml of distilled water and the drug content was measured spectrophotometrically at 203 nm (extract) against corresponding gel concentration.[22]
In vitro diffusion studies
The in vitro study for transferosomal gel was performed in the same way as it was carried out for transferosomes.
Experimental animals for psoriasis
Five-week-old male mice with no previous drug treatment were used for in vivo studies. The animals were fed on standard diet and water ad libitum.
Acclimatization to laboratory hygienic condition was done for 7 days before starting the experiment. Twenty-four mice were taken and divided into four groups (G1–G4) six animals in each group. Hairs on the dorsal portion of each mouse were removed using depilatory cream. The animal experimentation was performed according to Institutional Animal Ethical Committee of Era's Lucknow Medical College, Lucknow (Registration Number 1652/PO/a/12/CPCSEA).
Induction of psoriasis
This method was a slight modification from the study design by van der Fits.[10] The animals were shaved on the dorsal skin. Control group animals (G1) were treated with 50 mg of Vaseline on the dorsal skin surface for 11 days. IMQ cream (Glenmark Pharmaceuticals) 50 mg was applied topically on the dorsal skin surface of each mouse of Group 2–Group 4 to induce psoriasis once daily for 11 days.[23,24]
Treatment of psoriasis
B. aristata extract loaded transferosomal gel formulation (50 mg) was applied topically on Group 2 animals daily for 12 days. Its conventional gel formulation (50 mg) was applied to Group 3 animals. Group 4 animals were left untreated. In the control group, animal's application of Vaseline was continued. Body weight, food, and water intake of each animal were recorded daily.
Histological studies
The dorsal skin was excised from the mouse at the end of experiment. The skin samples were immersed in 10% formalin, embedded in paraffin wax, and sliced at a thickness of 3 μm using microtome (Leica RM2245). The samples were stained by hemoxylin and eosin (H and E) and observed under microscopy (Leica DM2500).
Photographs of ×40 magnification of H- and E-stained skin sections of normal, disease-induced, disease-induced animal treated with B. aristata extract loaded transferosomal gel formulation, and disease-induced animal treated with conventional gel formulation were taken.
Experimental animals for anti-inflammatory study
Wistar rats of either sex (150–200 g) with no previous drug treatment were used for in vivo studies. The animals were fed on a standard diet and water ad libitum.
Acclimatization to laboratory hygienic condition was done for 7 days before starting the experiment. The animal experimentation was performed according to Institutional Animal Ethical Committee of Era's Lucknow Medical College, Lucknow (Registration Number 1652/PO/a/12/CPCSEA).
Carrageenan-induced paw edema
Carrageenan-induced paw edema method was used for anti-inflammatory evaluation. Wistar albino rats were divided into three groups (n = 6). The first (control) and second (positive control) groups were treated with carbopol base gel and extract loaded transferosomal gel (optimized batch). Third group animals were treated with conventional gel.
All the treatments were given to the planter surface of the left hind paw of rats by gentle rubbing of 0.5 g of gel with the index finger after 60 min of induction of edema. Edema was induced by injecting 0.1 ml of Carragenan (1% w/v) in normal saline into the treated paws of all the rats. Paw edema was measured 0 h, 1, 2, 3, and 4 h after injection of Carragenan using plethysmograph.
The percent of inhibition of edema was calculated using the following formula:
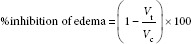
Where Vt is mean volume of paw edema in drug-treated group, Vc is mean volume of paw edema in control group.
Skin irritation study
Wistar rats of either sex (150–200 g) with no previous drug treatment were used for in vivo studies. The animals were fed on a standard diet and water ad libitum.
All animals were divided into three groups (n = 6). Group 1 (control) animals were treated with base gel. B. aristata loaded transferosomal gel (optimized batch) and conventional gel were applied on Group 2 and 3 animals, respectively. Skin irritation test was done according to the method of Driaze. Treated area was covered with gauze and wrapped with nonocclusive bandage. The bandage and test material were removed after 24 h and it was observed at 24 h and 48 h for erythema and edema.[25]
Statistical analysis
Differences in the in vitro release profile of prepared formulations were tested for significance using independent t-test using SPSS version 12.0. Difference was considered significant when P < 0.05. Graphs were prepared using GraphPad Prism 3 (GraphPad Software, Inc.).
RESULTS
High-performance thin layer chromatography
The presence of a specific peak for BE at Rf around 0.38 was recorded and considered as a positive result for B. aristata extract as already reported in our publication.[14]
Morphology
The phase contrast micrographs and TEM of optimized batch showed the surface morphology of transferosomes [Figure 1a and b].
Figure 1.
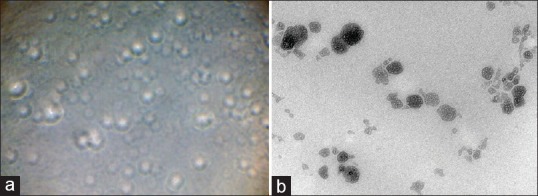
(a) Phase contrast microscopy image of transferosomal formulation (S7), (b) TEM image of transferosomal formulation (S7) (reproduction size at column width)
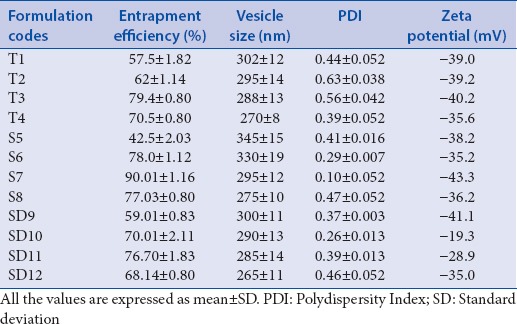
Vesicle size, polydispersity index, and zeta potential
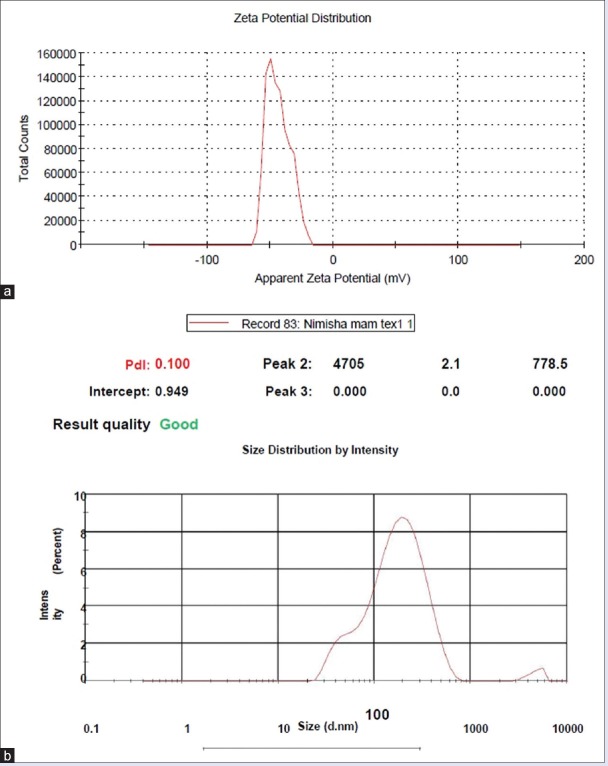
The size of transferosomes is in between 265 and 345 nm whereas PDI ranges from 0.10 to 0.63 and its ZP lies from −19.3 to −43.3 mV. The results are presented in Table 2.
Table 2.
Entrapment efficiency, vesicle size, polydispersity index, and zeta potential of the various transferosomal preparations
Entrapment efficiency
The range of EE of transferosomes was between 42.5% and 90.01% [Table 2].
Physicochemical properties of gel
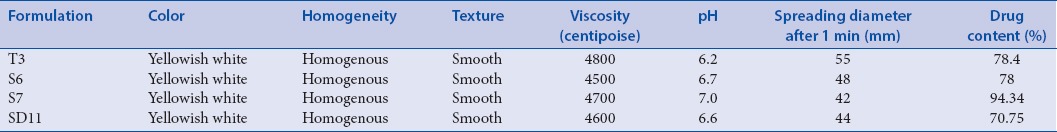
The results of all the physicochemical properties of the transferosomal gels are presented in Table 3.
Table 3.
Evaluation of physicochemical properties of Berberis aristata extract loaded transferosomal gel
Drug content
The drug content of the gels ranged between 78% and 94% was maximum in S7 formulation and minimum in SD11 [Table 4].
Table 4.
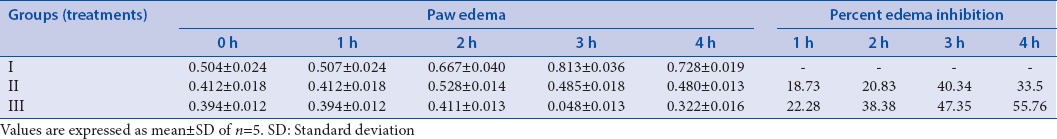
Evaluation of anti-inflammatory activity of Berberis aristata extract loaded transferosomal and conventional gel
Vesicle penetration study by fluorescence microscopy
Probe loaded transferosomal dispersions were able to permeate to deeper layers of the skin and this was confirmed by the presence of fluorescence in the skin specimens after analyzing under fluorescence microscope Leica DM2500, using Green Filter N2.1, position 3.
In vitro diffusion studies
Percent cumulative amount of drug release in 24 h ranges from 76% to 99% for transferosomal gel whereas only 40% for pure extract. In vitro drug release studies transferosomal gel formulations (T3, S6, S7, and SD11) and conventional gel were showed that cumulative percent drug release for B. aristata extract loaded transferosomal gel is significantly higher than (P < 0.5) B. aristata extract loaded conventional gel.
Anti-inflammatory activity and skin irritation test
Intraplantar administration of Carragenan in the right hind paw of rats produced edema. B. aristata extract was able to produce a significant reduction in paw volume when compared with control.
Percent inhibition of edema by conventional gel of extract (33.5%) was significantly less than transferosomal gel (optimized batch-55.76%). Primary irritation index for both the gel formulation comes out to be <0.4 which shows negligible irritation and the preparations are safe for topical use [Table 4].
Histopathological observations for psoriatic skin
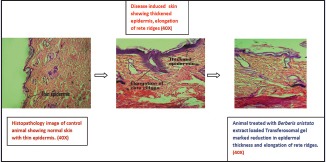
Compared with the control, dorsal skin sections of diseased-induced animal showed increased epidermal thickness, elongation of rete ridges, and capillary loop dilation. This confirms the successful development of disease-induced animal model.
Topical application of B. aristata extract loaded transferosomal gel formulation showed a marked reduction in thickness of epidermis, less elongation of rete ridges with capillary loop dilation. However, conventional gel formulation-treated animal showed nominal decrease in epidermal thickness [Figure 2a–d].
Figure 2.
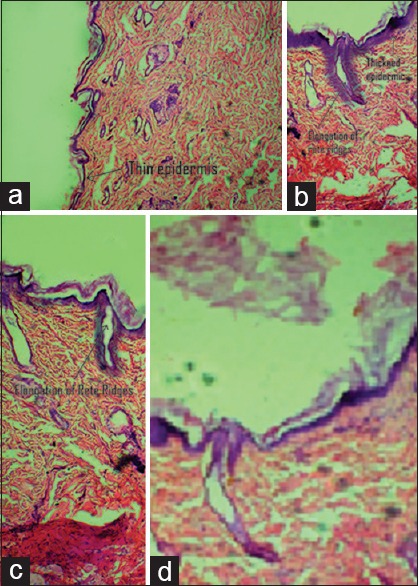
(a) Histopathology image of control animal's skin section with thin epidermis, (b) histopathology image of disease-induced (imiquimod treated) skin showing thickened epidermis, elongation of rete ridges, (c) histopathology image of animal treated with conventional gel showing nominal reduction in epidermis and rete ridges, (d) animal treated with Berberis aristata extract loaded transferosomal gel marked reduction in epidermal thickness and elongation of rete ridges (reproduction size: all at column width)
DISCUSSION
Transdermal delivery is most preferred and commonly used for psoriasis as it bypasses first-pass metabolism. The challenge to conventional topical delivery is its transport across the skin barrier. In psoriasis, the condition is more complicated because the rigid and dehydrated skin poses more resistance. To overcome this problem, Mental et al., 2009, proposed the delivery of drugs through lipoidal carrier systems.
Transferosomes are one such nanovesicular lipoidal carrier which is being widely used for transdermal delivery. They easily pass through the skin and increase the accumulation of drug in the skin. Ghanbarzadeh and Arami, 2013, had reported that the steady state transdermal rate of diclofenac sodium transfersomes is increased 2–3 times as compared to normal liposome. It has also been reported that, compared to vesicular dispersions, incorporation of vesicular formulation into gel preparation could significantly increase flux and Kp up to 3 times (P < 0.05).[26] In the present study, B. aristata extract loaded transferosomal formulations were prepared for the treatment of psoriasis and inflammation.
Size and shape of the vesicle is a crucial factor in the therapeutic performance of transdermal drug delivery systems. Phase contrast microscopy showed the surface morphology of transferosomes [Figure 1a]. TEM of optimized batch (S7) showed spherical vesicles [Figure 1b]. The structural image showed a light center space and enclosed by a dark heavy boundary which is completely intact.
In general, particle size increased along with surfactants possessing lower hydrophile–lipophile balance (HLB). Surfactants interact with lipid head groups in the membrane and increase packing density of layer, leading to high surface free energy.[27] The HLB value of these surfactants such as Span 80, Tween 80, and sodium deoxycholate are 4.3, 15, and 16, respectively. Their affinity with lipids is in the order of S >T>SD.
Since Span 80 has highest affinity with lipids, it resulted in expanded size of Span 80 transferosomes. However, no significant difference in size between transferosomal formulations containing Tween 80 and sodium deoxycholate was observed. The size of the vesicles was also affected by EA and phospholipids concentration.
It was also found that vesicle size of B. aristata extract loaded transferosomes decreased with increase in EA concentration and increased with increase in phospholipid concentration.
PDI was considered for evaluation of homogeneity of prepared transferosomes on the basis of their particle size distribution. The transferosomal preparations showing PDI between 0 and 1 were selected [Table 2].
Stability of transferosomal formulation can be assessed by its ZP. The negative ZP is responsible for enhanced percutaneous permeation of drug. All the transferosomal formulations were found to have negative ZP (−19.3 to − 43.3 mV) due to the net charge of the lipid composition in the formulation.
The EE in transferosomes depends on the EA concentration in the lipid bilayer. Transferosomal formulations containing varying percent of Span 80, Tween 80, and sodium deoxycholate (5%–25%) were evaluated for their EE. The EE of T1 to T4 was found to be 57.5%–70.5% whereas it was 42.5%–77.03% for S5 to S8 and, for SD9 to SD 12, it ranges between 49.01% and 68.14%.
Initially, with increasing EA (5%–20%), there was an increase in EE, but it was observed that above 20% concentration of EAs EE decreased. This may be due to micelles formation in the vesicle membranes at higher EA concentration. This corroborates with the report which states that micelles have a lower drug carrying capacity and poor skin permeation due to their internal structural feature.[28]
On the basis of EE, percentage drug release, PDI, and ZP four transferosomal batches (T3, S6, S7, and SD11) were selected for in vitro release profile as well as incorporation into gel formulations.
Four batches (S6, S7, SD11, and T3) of transferosomal dispersion and pure extract were subjected to in vitro diffusion studies.
The cumulative amount of drug release was calculated for each formulation, and it was found that percent cumulative amount of drug release in 24 h ranges from 76% to 99% for the respective batches taken which was significantly higher than for pure extract (P < 0.05) [Figure 3].
Figure 3.
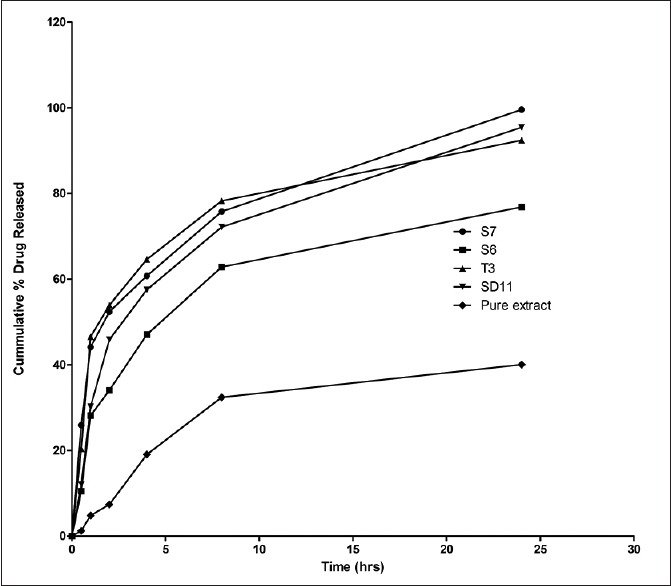
In vitro release profile of Berberis aristata loaded of transferosomal dispersion (S6, S7, SD11, and T3) and pure extract (reproduction size at column width)
Among the four selected transferosomal batches, batch S7 (Span 80, 20%) showed maximum drug release up to 24 h, may be due to highest EE. This is also in accordance with the results reported by Agarwal et al., which states that, at optimum EA concentration, (20%) the EA molecules get linked with SPC bilayer, thus enabling partitioning of drug across the vesicle easily and higher percentage drug release. The batch also depicted EE, vesicle size, ZP, and PDI as 90.01%, 295 nm, −43.3, and 0.10, respectively, so it was selected as an optimized batch [Figure 4a and b].
Figure 4.
(a) The zeta potential of Batch S7. (b) The size and size distribution of Batch S7 (reproduction size at column width)
Transferosomes with composition (PC:EA ratio 80:20, extract quantity 10 mg) and fluorescent probe rhodamine B 0.03% were prepared and were subjected to vesicle penetration study by fluorescence microscopy. Probe loaded transferosomal dispersions were able to permeate to deeper layers of the skin and this was confirmed by the presence of fluorescence in the skin specimens (20 μm) after analyzing under fluorescence microscope Leica DM2500, using Green Filter N2.1, position 3 [Figure 5].
Figure 5.
Fluorescence micrograph image (reproduction size at column width)
The prepared gels were evaluated for physical appearance, pH, spreadability, viscosity, and drug content. Gels were found to be smooth, homogenous, yellowish white in color, pH lying in the normal skin pH range, easily spreadable, and viscosity ranging between 4500 and 4800 cps [Table 3].
Transferosomal gel formulations (T3, S6, S7, and SD11) and conventional gel were also evaluated for their in vitro drug release studies. Results showed that cumulative percent drug release for B. aristata extract loaded transferosomal gel is significantly higher than (P < 0.5) B. aristata extract loaded conventional gel [Figure 6].
Figure 6.
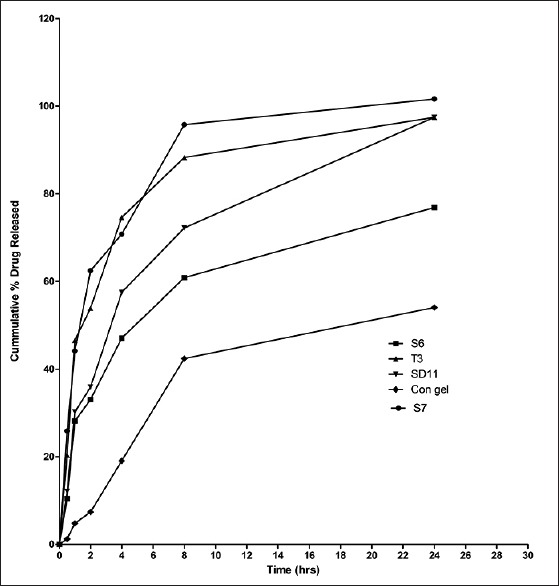
Comparative in vitro release profile of transferosomal gel (T3, S6, S7, and SD11) preparations and conventional gel (reproduction size at column width)
These results suggested that transferosomal gel can enhance the skin permeation of drug.
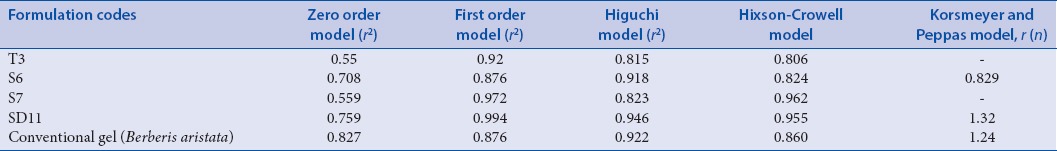
The data obtained from in vitro release study of transferosomal gel and conventional gel was fitted in different kinetic equations to access the release rate profile. Both the batches S6 and SD11 were best fitted for the Higuchi kinetic model as the r2 value was higher than other values. This implies slow and steady release by the process of diffusion as proposed by Higuchi. Batch S7 showed first-order release kinetics inferring that the rate of diffusion is directly proportional to concentration of drug [Table 5].
Table 5.
Kinetic assessment of release profile of Berberis aristata extract loaded transferosomal gel
Anti-inflammatory activity and skin irritation study was done for optimized batch transferosomal gel (Batch S7) and conventional gel. Percent inhibition of edema for Batch S7 transferosomal gel was 55.76% as compared to conventional gel which showed only 33.5% inhibition. This study implies that transferosomal gel has greater potential to decrease inflammation. Primary irritation was <0.4 indicating negligible irritation in all the three groups. Hence, the developed formulations are free from skin irritation. Histopathological study revealed that topical application of B. aristata extract loaded transferosomal gel formulation causes a marked reduction in thickness of epidermis and elongation of rete ridges than compared to conventional gel formulation in psoriasis-induced animal. This may be due to enhanced permeation ability of B. aristata extract loaded transferosomal gel through psoriatic skin [Figure 2a–d, and Table 4].
CONCLUSION
Plants have always been a component of mankind's health-care system. There is great potential in herbal drug candidates although very few examples exist in literature that emphasize on novel delivery system bearing herbal extracts and phytoconstituents. Novel herbal delivery systems not only increase the therapeutic efficacy by reducing toxicity but also increase bioavailability. The aim of the present study was to prepare transferosomes of herbal extract of B. aristata for treating inflammation and psoriasis. The remarkable enhancement in the in vitro release efficiency of B. aristata extract loaded transferosomal gel resulted in improved anti-inflammatory activity. The prepared novel formulation of B. aristata has also shown its efficacy against IMQ-induced psoriasis. We conclude that this system may be a better therapeutic agent as compared to the conventional system for psoriasis as well as inflammation. However, further biological and clinical studies have to be carried out to establish the mechanism of action.
Financial support and sponsorship
This study was financially supported by Amity Institute of Pharmacy, Amity University, Lucknow Campus, Uttar Pradesh, India.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Reich K. The concept of psoriasis as a systemic inflammation: Implications for disease management. J Eur Acad Dermatol Venereol. 2012;26(Suppl 2):3–11. doi: 10.1111/j.1468-3083.2011.04410.x. [DOI] [PubMed] [Google Scholar]
- 2.Katare OP, Raza K, Singh B, Dogra S. Novel drug delivery systems in topical treatment of psoriasis: Rigors and vigors. Indian J Dermatol Venereol Leprol. 2010;76:612–21. doi: 10.4103/0378-6323.72451. [DOI] [PubMed] [Google Scholar]
- 3.Syed TA, Ahmad SA, Holt AH, Ahmad SA, Ahmad SH, Afzal M. Management of psoriasis with Aloe vera extract in a hydrophilic cream: A placebo-controlled, double-blind study. Trop Med Int Health. 1996;1:505–9. doi: 10.1046/j.1365-3156.1996.d01-91.x. [DOI] [PubMed] [Google Scholar]
- 4.Sidhu K, Pannu K. Indigenous use of medicinal plants for health care. Ethno Med. 2010;4:145–8. [Google Scholar]
- 5.Potdar D, Hirwani RR, Dhulap S. Phyto-chemical and pharmacological applications of Berberis aristata. Fitoterapia. 2012;83:817–30. doi: 10.1016/j.fitote.2012.04.012. [DOI] [PubMed] [Google Scholar]
- 6.Gupta SK, Agarwal R, Srivastava S, Agarwal P, Agrawal SS, Saxena R, et al. The anti-inflammatory effects of Curcuma longa and Berberis aristata in endotoxin-induced uveitis in rabbits. Invest Ophthalmol Vis Sci. 2008;49:4036–40. doi: 10.1167/iovs.07-1186. [DOI] [PubMed] [Google Scholar]
- 7.Abdul L, Abdul R, Sukul RR, Nazish S. Anti-inflammatory and antihistaminic study of a unani eye drop formulation. Ophthalmol Eye Dis. 2010;2:17–22. doi: 10.4137/oed.s3612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Meena AK, Bansal P, Kumar S. Plants-herbal wealth as a potential source of ayurvedic drugs. Asian J Tradit Med. 2009;4:152–70. [Google Scholar]
- 9.Sharma A, Dubey A, Gupta P, Yadav R, Saraogi R. Transferosome: Novel drug delivery system. Int J Biol Pharm Res. 2012;3:722–8. [Google Scholar]
- 10.van der Fits L, Mourits S, Voerman JS, Kant M, Boon L, Laman JD, et al. Imiquimod-induced psoriasis-like skin inflammation in mice is mediated via the IL-23/IL-17 axis. J Immunol. 2009;182:5836–45. doi: 10.4049/jimmunol.0802999. [DOI] [PubMed] [Google Scholar]
- 11.Lin YK, Yang SH, Chen CC, Kao HC, Fang JY. Using imiquimod-induced psoriasis-like skin as a model to measure the skin penetration of anti-psoriatic drugs. PLoS One. 2015;10:e0137890. doi: 10.1371/journal.pone.0137890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sharma PK, Chauhan NS, Lal B. Studies on plant associated indigenous knowledge among the Malanis of Kullu district, Himachal Pradesh. Indian J Tradit Knowl. 2005;4:403–8. [Google Scholar]
- 13.Saraf S, Jeswani G, Kaur CD, Saraf S. Development of novel herbal cosmetic cream with Curcuma longa extract loaded transfersomes for antiwrinkle effect. Afr J Pharm Pharmacol. 2011;5:1054–62. [Google Scholar]
- 14.Nimisha Fatima Z, Kaur C. Formulation and Performance evaluation of Berberis aristata extract loaded ethosomal gel. Asian J Pharm. 2017;11:1–9. [Google Scholar]
- 15.Elsayed MM, Abdallah OY, Naggar VF, Khalafallah NM. Deformable liposomes and ethosomes: Mechanism of enhanced skin delivery. Int J Pharm. 2006;322:60–6. doi: 10.1016/j.ijpharm.2006.05.027. [DOI] [PubMed] [Google Scholar]
- 16.Jain S, Jain P, Umamaheshwari R, Jain N. Transfersomes – A novel vesicular carrier for enhanced transdermal delivery: Development, characterization, and performance evaluation. Drug Dev Ind Pharm. 2003;29:1013–26. doi: 10.1081/ddc-120025458. [DOI] [PubMed] [Google Scholar]
- 17.Kaur CD, Saraf S. Topical vesicular formulations of Curcuma longa extract on recuperating the ultraviolet radiation-damaged skin. J Cosmet Dermatol. 2011;10:260–5. doi: 10.1111/j.1473-2165.2011.00586.x. [DOI] [PubMed] [Google Scholar]
- 18.Kaur CD, Saraf S. Photoprotective herbal extract loaded nanovesicular creams inhibiting ultraviolet radiations induced photoaging. Int J Drug Deliv. 2011;3:699. [Google Scholar]
- 19.Duangjit S, Opanasopit P, Rojanarata T, Ngawhirunpat T. Evaluation of meloxicam-loaded cationic transfersomes as transdermal drug delivery carriers. AAPS PharmSciTech. 2013;14:133–40. doi: 10.1208/s12249-012-9904-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Khan NR, Wong TW. Microwave-aided skin drug penetration and retention of 5-fluorouracil-loaded ethosomes. Expert Opin Drug Deliv. 2016;13:1209–19. doi: 10.1080/17425247.2016.1193152. [DOI] [PubMed] [Google Scholar]
- 21.Srivastava N, Singh K, Amrit K. Formulation and evaluation of Seabuckthorn leaf extract loaded ethosomal gel. Asian J Pharm Clin Res. 2015;8:309–12. [Google Scholar]
- 22.Misal J, Dixit G, Gulkari V. Formulation and evaluation of herbal gel. Indian J Nat Prod Res. 2012;3:501–5. [Google Scholar]
- 23.Ellis J, Parlapally S, Cherukupalli N, Bhumireddy SR, Sripadi P, Anisetti R, et al. Chemical profiling and anti-psoriatic activity of methanolic extract of Andrographis nallamalayana. Natural Product Res. 2015;30:1256–61. doi: 10.1080/14786419.2015.1054825. [DOI] [PubMed] [Google Scholar]
- 24.Parlapally S, Cherukupalli N, Bhumireddy SR, Sripadi P, Anisetti R, Giri CC, et al. Chemical profiling and anti-psoriatic activity of methanolic extract of Andrographis nallamalayana JL Ellis. Nat Prod Res. 2016;30:1256–61. doi: 10.1080/14786419.2015.1054825. [DOI] [PubMed] [Google Scholar]
- 25.More B, Sakharwade S, Tembhurne S, Sakarkar D. Evaluation for skin irritancy testing of developed formulations containing extract of Butea monosperma for its topical application. Int J Toxicol Appl Pharmacol. 2013;3:10–3. [Google Scholar]
- 26.Ghanbarzadeh S, Arami S. Enhanced transdermal delivery of diclofenac sodium via conventional liposomes, ethosomes, and transfersomes. Biomed Res Int. 2013;2013:616810. doi: 10.1155/2013/616810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lichtenberg D, Opatowski E, Kozlov MM. Phase boundaries in mixtures of membrane-forming amphiphiles and micelle-forming amphiphiles. Biochim Biophys Acta. 2000;1508:1–19. doi: 10.1016/s0304-4157(00)00004-6. [DOI] [PubMed] [Google Scholar]
- 28.Yoshioka T, Sternberg B, Florence AT. Preparation and properties of vesicles (niosomes) of sorbitan monoesters (Span 20, 40, 60 and 80) and a sorbitan triester (Span 85) Int J Pharm. 1994;105:1–6. [Google Scholar]