Abstract
Objective:
Nigella sativa is from botanical Ranunculaceae family and commonly known as black seed. Apoptotic effect of N. sativa and its apoptotic signaling pathways on U937 lymphoma cells are unknown.
Materials and Methods:
In this study, we investigated selective cytotoxic and apoptotic effects of N. sativa extract and its apoptotic mechanisms on U937 cells. In addition, we also studied selective cytotoxic activity of thymoquinone that is the most active essential oil of N. sativa.
Results:
Our results showed that N. sativa extract has selective cytotoxicity and apoptotic effects on U937 cells but not ECV304 control cells. However, thymoquinone had no significant cytotoxicity against on both cells. N. sativa extract increased significantly caspase-3, BAD, and p53 gene expressions in U937 cells.
Conclusions:
N. sativa may have anticancer drug potential and trigger p53-induced apoptosis in U937 lymphoma cells.
SUMMARY
This is the first study showing the apoptotic effect of Nigella sativa extract on U937 cells.
Abbreviations used: CI: Cytotoxicity index, DMEM: Dulbecco's Modified Eagle Medium, HL: Hodgkin's lymphoma, MTT: 3-(4,5-dimethy lthiazol-2yl)-2,5-diphenyl tetrazolium bromide, RPMI: Roswell Park Memorial Institute medium.
Keywords: Apoptosis, caspase-3, human lymphoma U937 cells, Nigella sativa, p53, thymoquinone
INTRODUCTION
Lymphoma is a type of cancer, and it is estimated to be responsible for 3% of all types of cancer worldwide.[1] Particular inherited disorders, immunosuppressive drug therapies, and certain viruses are counted as known risk factors for lymphoma because of the damage on patient's immune system.[1] The term lymphoma is firstly described by Thomas Hodgkin in 1832.[2] After Hodgkin's discovery, different kinds of lymphomas were characterized as well. The one that described by Thomas Hodgkin is called Hodgkin's lymphoma (HL) and the rest are all called as non-Hodgkin's lymphomas (NHLs). Both HL and NHL may occur in patients with cancer history. Chemotherapies and radiotherapies can be possible causes for lymphoma. As a matter of fact, these therapies may not suitable for the treatment of all types of cancer. Nigella sativa is one of the candidates for treatment or supportive agent that may be used for this purpose.
N. sativa is from botanical Ranunculaceae family and commonly known as black seed. It is regional to North Africa and Southwest Asia, but it is cultivated by Middle Eastern region, South Europe, India, Pakistan, Syria, Turkey, and Saudi Arabia.[3] It has countless biological effect such as anticancer, anti-inflammatory, analgesic, antidiabetic, and antimicrobial effect. Furthermore, it was used as a treatment of bronchitis, asthma, diarrhea, rheumatism, and skin disorders.[4] N. sativa contains various bioactive compounds including thymoquinone, p-cymene, cis-carveol, thymol, a-phellandrene, α-pinene, β-pinene, trans-anethole, a-longipinene, and longifolene.[5] Moreover, there are several studies reported anticancer effects of N. sativa extract against different cancer cell lines; however, anticancer effects of N. sativa against U937 cells have not been published, and this is the first study showing the apoptotic effect of N. sativa extract on U937 cells.
In this study, N. sativa extract and thymoquinone were assessed for their anticancer drug potential targeting human lymphoma cells. Cytotoxicity assay was applied to U937 lymphoma cells and examined in comparison with ECV304 as the control. The apoptotic effects of N. sativa and thymoquinone extract on intracellular signal nodes and apoptotic pathways in U937 cells include real-time polymerase chain reaction (RT-PCR) and DNA fragmentation assays.
MATERIALS AND METHODS
Extraction of Nigella sativa
Pulverized N. sativa was added into methanol with 10 mg/mL concentration. The extract was filtered using syringe tip filter (0.4 μM) after overnight incubation at room temperature.
Mammalian cell culture
Human lymphoma U937 cell line was used to examine cytotoxic potential of N. sativa extract. ECV304 human umbilical vein endothelial cell line was used as a control, noncancerous cellular model. Cell lines were obtained from the American Type Culture Collection.
Dulbecco's Modified Eagle Medium (DMEM) (Sigma-Aldrich, D6429) and Roswell Park Memorial Institute medium (RPMI) (Biological Industries, 1640) were used for ECV304 and U937 cells, respectively. All mediums were supplemented with 10% fetal bovine serum (HyClone, SH3007003HI), 1% penicillin-streptomycin, 1% L-glutamine solution, and 0.1% minimum essential medium (MEM) nonessential amino acids solution (×100). All incubations were performed in an incubator (Thermo Fisher Scientific) with humidified atmosphere containing 5% CO2 at 37°C. 96-well plate, polystyrene cell culture flasks (TPP, 92096) were used for viability assays and cell cultures 2–4 days. Nearly 0.5% trypsin-ethylenediaminetetraacetic acid solution (Sigma-Aldrich, T3924) was used to remove adhesive ECV304 cells, washed once with serum-free medium and once with serous medium, and then, resuspended in DMEM at 5 × 105 cells/mL density. U937 cells were suspended in RPMI at 5 × 105 cells/ml density.
Cytotoxicity assay
The cytotoxic effects of N. sativa extract and thymoquinone against U937 lymphatic cells and ECV304 human endothelial cells were measured through 3-(4,5-dimethylthiazol-2yl)-2,5-diphenyl tetrazolium bromide (MTT) (Sigma-Aldrich, M-5655) assay.[6,7] 1 × 105 cells/mL were suspended in the medium supplemented with 10% of fetal bovine serum, 1% of penicillin-streptomycin, 1% of L-glutamine solution, and 0.1% of MEM nonessential amino acids solution (×100) at 37°C in a humidified atmosphere containing 5% CO2.
Stock solutions of the N. sativa extract were prepared as a final concentration of 10 mg/mL using methanol. Stock solution of the thymoquinone (2-isopropyl-5-methyl-1,4-benzoquinone) (Sigma-Aldrich) (10 mg/mL concentration) was prepared using dimethyl sulfoxide. Five different concentrations for both were prepared by serial dilutions of the stock solution: 5000, 500, 100, 50, and 10 μg/mL. Ten μL dilution of the N. sativa extracts and thymoquinone dispensed into 96-well plates, respectively, and 90 μL of the cells were added into each well. Final concentrations of 500, 50, 10, 5, and 1 μg/mL were used in the experiments (n = 6, for each). For the control wells, 10 μL medium was used instead of N. sativa extract. After 48 h of incubation, 10 μL of MTT (5 mg/mL) solutions in phosphate buffered saline were added into each well and incubated them for 3 h at 37°C. After the incubation, supernatants were from all wells and 100 μL of SDS (Sodium dodecyl sulfate) (pH 5.5) containing isopropyl alcohol was added to dissolve the formazan crystals formed by reduction of MTT in living cells. Then, microplates were left in the dark room for 10 min. Optical density of each well was measured with 570 nm on a microplate reader (Bio-Rad Benchmark, Philadelphia, USA). Cytotoxic index (CI) was calculated with the formula:
CI = 1– (OD (Optical density) [treated wells]/OD [control wells]) × 100%.
DNA fragmentation assay
After incubating with N. sativa extracts and thymoquinone for 24 h, U937 cells were collected by centrifugation at 13200 rpm for 20 s at room temperature; DNA fragmentation assay was applied according to a previous report.[8] Extracted genomic DNA was run in agarose gel electrophoresis (2%) for 40 min at 100 V, stained in an ethidium bromide solution (0.625 mg/mL), and analyzed on a gel imaging system (Fusion FX7-02).
RNA isolation
After incubation for 24 h, 1 μL TRI Reagent (Sigma-Aldrich) was added onto U937 cells and incubated for 5 min at room temperature. Then, 200 μL of chloroform was added each of them and samples were mixed with vortex for 15 s. The samples were incubated for 3 min at room temperature. After the incubation, samples were centrifuged at 13200 rpm for 15 min at 4°C. Upper phase of samples was transferred a new microcentrifuge tube and 500 μL of isopropanol was added. Samples were incubated for 5 min at room temperature, and they were centrifuged at 13200 rpm for 10 min at 4°C. Supernatants were removed, and 750 μL of 75% ethanol was added. The samples were centrifuged at 13200 rpm for 5 min at 4°C. Then, samples were incubated for 10 min on ice to remove alcohol. RNA pellet was dissolved in 50 μL DEPC dH2O.
For cDNA synthesis, SensiFAST cDNA Synthesis Kit (Bioline) was used and protocol was performed according to the manufacturer's instructions.
Real-time polymerase chain reaction
RT-PCR was performed using Roche LightCycler® FastStart DNA Master SYBR Green I kit. Manufacturer's protocol was used for RT-PCR test. Reverse primers 5’- GCTGTCCACACACTCCATGCT-3’, 5’- CGTGTCACCGTCGTGGA-3’, 5’- CTGTAAGCTTCTGACATTTC-3’, 5’-GCATACTGTTTCAGCATGGCAC-3’, 5’- GAGCAACATTCAGCAGG-3’and forward primers 5’- AGCTCAGCCCACCCTTCAA-3’, 5’- AACGGTACTCCGCCACC-3’, 5’- GTGCCCGAGCGTTACCAGACC-3’, 5’- CAAACTTTTTCAGAGGGGATCG-3’, 5’- GTACGAACTGTGGCGACTCC-3’ were used to determine changes of AKT, p53, p38, caspase-3, and BAD gene expression levels, respectively.
Statistical analysis
Statistical analysis was performed using the Statistical Package for the Social Sciences software (IBM). Results were expressed as the mean ± standard deviation. Statistical differences were assessed by independent-samples t-test, with P < 0.05 as statistically significant.
RESULTS AND DISCUSSION
Cytotoxic activity of N. sativa extract against human lymphoma U937 and ECV304 human endothelial cells was shown in Figure 1. While the extract did not cause a cytotoxicity >20% to ECV304 cells, it showed significant cytotoxic effect around %50 against human lymphoma U937 cells at the concentration level of 500 μg/mL (P < 0.05). This result indicates that N. sativa extract has selective cytotoxic effects on U937 cells; one or a few chemical constituents of N. sativa extract has/have highly selective cytotoxic effects on U937 cells.
Figure 1.
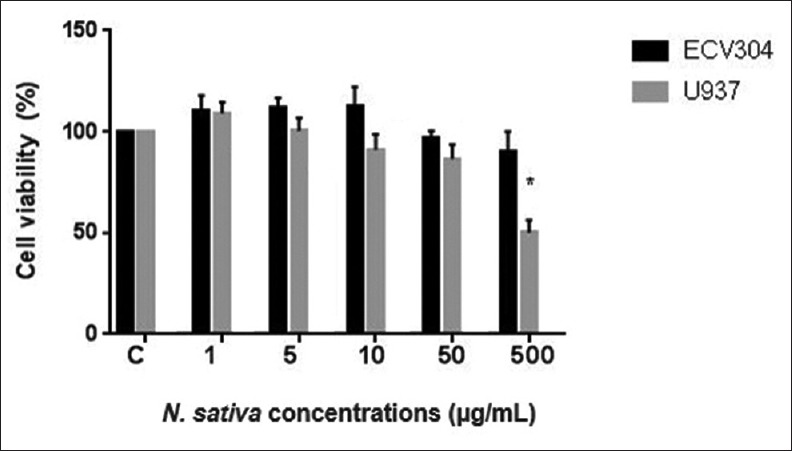
Cytotoxicity of Nigella sativa extract on U937 and ECV-304 cell lines (*P < 0.05)
On the other hand, one of the major chemical constituents of N. sativa extract, thymoquinone, has been shown as a cytotoxic agent for U937 and ECV304 cells at the same doses with N. sativa extract [Figure 2]. Interestingly, thymoquinone had no cytotoxic effect on U937 cells. However, it has been shown that it had a proliferative effect on both U937 and ECV304 cells.
Figure 2.
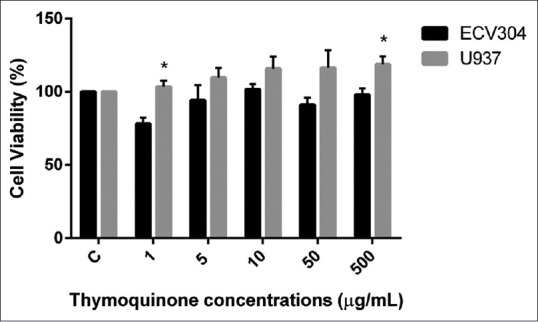
Cytotoxicity of thymoquinone on U937 and ECV-304 cell lines (*P < 0.05)
After the initial screen by MTT, to further study apoptotic effect of N. sativa extract on U937 cells, we checked the genomic DNA fragmentation in U937 cells incubated with N. sativa extract because fragmented genomic DNA is known to be a marker for apoptosis. When U937 cells were incubated 24 h with N. sativa extract, its genomic DNA were evidently fragmented, showing that the indicated extracts are apoptotic to these cells [Figure 3].
Figure 3.
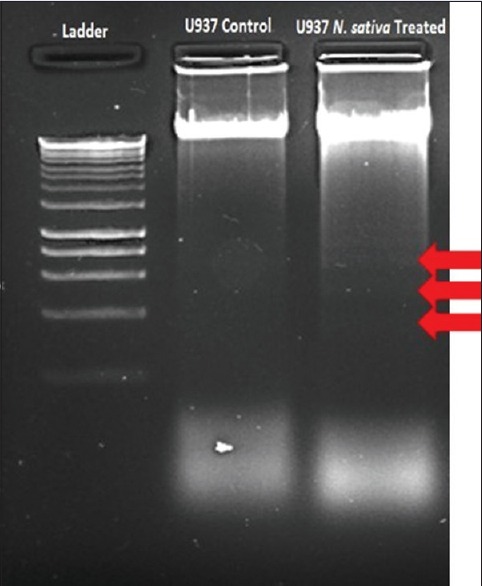
Screening apoptotic activity of Nigella sativa extract against U937 cells with DNA fragmentation method
The mechanisms behind the cytotoxic effects of N. sativa extract on U937 cells were further investigated by checking their specific roles on several major apoptotic signaling pathways. For this, N. sativa extract was incubated with U937 cells, and the changes in the gene expression levels of AKT, BAD, p38, p53, and caspase-3 were determined. Significant changes in the apoptotic signaling pathways were observed in U937 cells incubated with N. sativa extract [Figure 4]. N. sativa extract increased the expression levels of caspase-3, BAD, and p53 genes in U937 cells (fold changes; 3 ± 0.7, 5.9 ± 1.6, 16 ± 3, respectively) (P < 0.05). On the other hand, expression levels of AKT and p38 genes in U937 and ECV304 cells were similar in both cell lines. These results indicate that N. sativa extract induced apoptotic pathway in lymphoma cells without affecting healthy cells.
Figure 4.
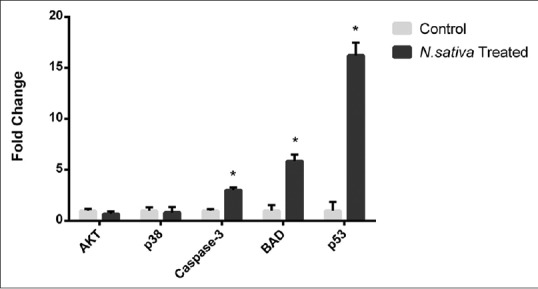
Real-time polymerase chain reaction analysis of AKT, BAD, p38, CDC42, p53, and caspase-3 gene expressions upon Nigella sativa treatment in U937 cells. This bar graph representing the fold changes of gene expression quantified by normalization to the GAPDH (*P < 0.05)
Various biological effects of N. sativa have been reported including anticancer and anti-inflammatory features in vitro and in vivo studies. Inhibitor effect of N. sativa on liver tumor development was reported, previously.[9,10] Anticancer effect of N. sativa extract was also reported using various cancer types.
In our study, the extract of N. sativa displayed significant cytotoxic activity around 50% against U937 lymphoma cells while it did not showed >20% cytotoxicity on ECV304 cells. Together with its selective cytotoxic properties, N. sativa can be an anticancer agent to treat lymphoma.
It was reported that alpha-hederin found in N. sativa extracts was a potential anticancer agent.[11,12] Salomi et al. showed that N. sativa extract has very cytotoxic effect on Ehrlich ascites carcinoma, Dalton's ascites lymphoma, and sarcoma 180.[13] N. sativa has a lot of other bioactive components such as thymoquinone, thymohydroquinone, dithymoquinone, p-cymene, carvacrol, 4-terpineol, t-anethol, sesquiterpene, longifolene, α-pinene, and thymol.[5] It also contains carotene, calcium, iron, and potassium.[14] Synergistic activity of this component can be responsible for cytotoxic activity of N. sativa extract against U937 cells. Duvoix et al. demonstrated the cytotoxic effect of trans-anethole against U937 with a high cell death percentage.[15] Fraternale et al. also tested p-cymene of Echinophora spinosa L and reported as a toxic compound for U937 cells.[16] In addition, thymoquinone, the most abundant component of N. sativa, has anticancer activity against several different cancer types and its phase studies have been started.[17-20] Salim et al. showed that thymoquinone has high cytotoxic effect on murine leukemia WEHI-3 cell line and induce apoptosis through Bcl-2 and Bax in vitro and in vivo.[21] Another study found that thymoquinone has anticancer effect on acute lymphocyte leukemic cell line and induces mitochondria-mediated apoptosis through Bcl-2 and Bax pathways.[22] Moreover, antiproliferative and proapoptotic effects of thymoquinone combination with topotecan on U937 cells were reported.[23] However, our results showed that thymoquinone has no cytotoxic effect on U937 cells, but it has proliferative effect on both U937 and ECV304 cells. Although we found that thymoquinone did not have anticancer effect against U937 cells. According to literature, various combinations of thymoquinone may lead to cytotoxic effect on U937 cells. In our study, commercial thymoquinone for the cytotoxicity experiments was used. There may be differences in between commercial thymoquinone and its isolated form from plant extract as chemical behavior and physical properties. Antagonistic or synergistic effects among essential oils can change cytotoxicity comparing with their single effects. Therefore, isolation of essential oils from the extract and their various combinations may contribute to find new anticancer agents.
To study the apoptotic effects of the extracts on U937 cells, DNA fragmentation assay was performed with treated and untreated cells. One of the hallmark features of apoptosis is fragmentation of nuclear DNA in a ladder electrophoretic pattern. Necrosis, on the other hand, is characterized by random digestion of DNA forming a “smear” on agarose gel.[24] As a result of DNA fragmentation assay, fragmented DNA was screened on the gel. The apoptotic effect of N. sativa was detected on U937 cells with DNA fragmentation assay.
To identify intracellular apoptotic signal pathways of N. sativa extract on U937 cells, changes in gene expressions of AKT, BAD, p38, p53, and caspase-3 were analyzed. We detected significant increased BAD, p53, and caspase-3 gene expression in U937 cells treated with N. sativa extract. According to our results, p53 may stimulate BAD and promote apoptosis through cytochrome c release from mitochondria in these cells. p53 tumor suppressor gene plays important roles in cell cycle control and apoptosis.[25] In addition, caspase-3 is a gene that has a central role in apoptosis and is activated by extrinsic or intrinsic mitochondrial pathways.[26] N. sativa extract may trigger apoptosis with the increase of p53 and caspase-3 gene expressions in U937 lymphoma cells. Alhazmi et al. (2014) showed that N. sativa extract increases of caspase-3, caspase-8, caspase-9, and p53 gene expressions in MCF-7 breast cancer cells.[27]
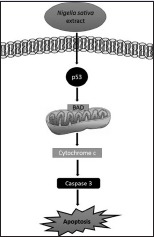
Furthermore, BAD gene is a member of Bcl-2 family and regulates programmed cell death.[28] With an apoptotic stimulus, BAD is dephosphorylated and induces cell death by targeting mitochondria. p53 releases BAD in mitochondria, and transient combination of p53 with BAD activates proapoptotic proteins.[29,30] Then, cytochrome c is released from mitochondria and caspase cascade is activated.[31] In this way, apoptosis is induced through p53, BAD, and caspase-3 activation. We analyzed and compared our results with the literature and predicted possible signaling pathways [Figure 5]. Based on our results, N. sativa extract can induce p53-induced mitochondria-mediated apoptosis in U937 cells.
Figure 5.
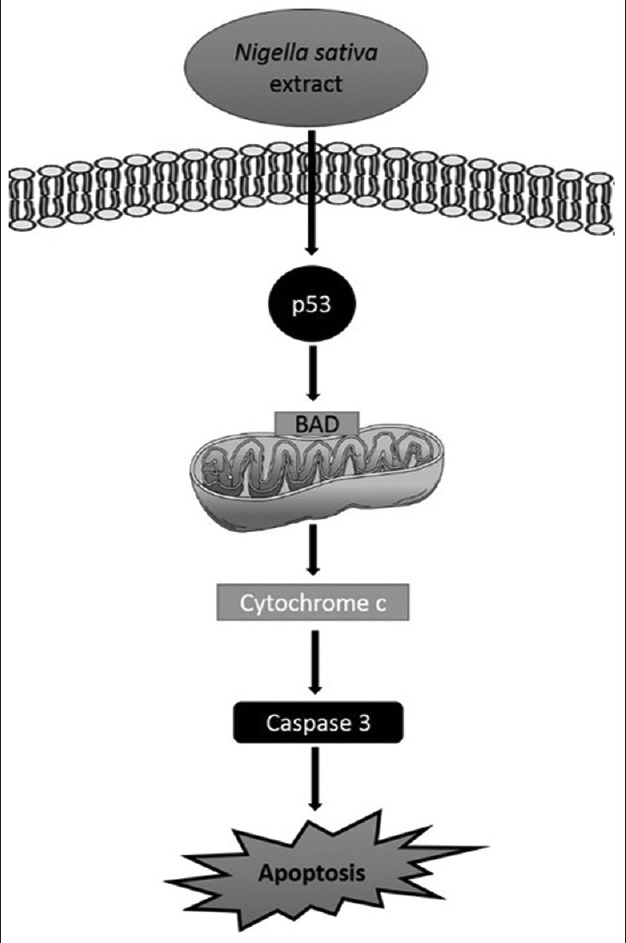
Our predicted possible apoptotic pathway of Nigella sativa extract on U937 cells
CONCLUSIONS
N. sativa may have anticancer drug potential for U937 lymphoma cells and trigger p53-induced mitochondria-mediated apoptosis in these cells. Chemical composition of N. sativa extract can be analyzed in next studies, and common molecules found in especially essential oils may be individually tested on lymphoma cells to find the most suitable compound and/or synergistic combination of the molecules in advance.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Marcus R, Sweetenham JW, Williams ME. Lymphoma: Pathology, Diagnosis and Treatment. Cambridge: Cambridge University Press; 2007. [Google Scholar]
- 2.Stone MJ. Thomas Hodgkin: Medical İmmortal and Uncompromising İdealist. Vol. 18. Baylor University Medical Center Proceedings; 2005. p. 368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khare CP. Encyclopedia of Indian Medicinal Plants. New York: Springe-Verlag Berlin Heidelberg; 2004. [Google Scholar]
- 4.Assayed ME. Radioprotective effects of black seed (Nigella sativa) oil against hemopoietic damage and immunosuppression in gamma-irradiated rats. Immunopharmacol Immunotoxicol. 2010;32:284–96. doi: 10.3109/08923970903307552. [DOI] [PubMed] [Google Scholar]
- 5.Sarwar A, Latif Z. GC-MS characterisation and antibacterial activity evaluation of Nigella sativa oil against diverse strains of Salmonella. Nat Prod Res. 2015;29:447–51. doi: 10.1080/14786419.2014.947493. [DOI] [PubMed] [Google Scholar]
- 6.Atasever-Arslan B, Yilancioglu K, Bekaroglu MG, Taskin E, Altinoz E, Cetiner S. Cytotoxic effect of extract from Dunaliella salina against SH-SY5Y neuroblastoma cells. Gen Physiol Biophys. 2015;34:201–7. doi: 10.4149/gpb_2014034. [DOI] [PubMed] [Google Scholar]
- 7.Pirildar S, Sütlüpinar N, Atasever B, Erdem-Kuruca S, Papouskova B, Simánek V. Chemical constituents of the different parts of Colchicum baytopiorum and their cytotoxic activities on K562 and HL60 cell lines. Pharm Biol. 2010;48:32–9. doi: 10.3109/13880200903029373. [DOI] [PubMed] [Google Scholar]
- 8.Atasever-Arslan B, Yilancioglu K, Kalkan Z, Timucin AC, Gür H, Isik FB, et al. Screening of new antileukemic agents from essential oils of algae extracts and computational modeling of their interactions with intracellular signaling nodes. Eur J Pharm Sci. 2016;83:120–31. doi: 10.1016/j.ejps.2015.12.001. [DOI] [PubMed] [Google Scholar]
- 9.Abdel-Hamid NM, Abdel-Ghany MI, Nazmy MH, Amgad SW. Can methanolic extract of Nigella sativa seed affect glyco-regulatory enzymes in experimental hepatocellular carcinoma? Environ Health Prev Med. 2013;18:49–56. doi: 10.1007/s12199-012-0292-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fathy M, Nikaido T. In vivo modulation of iNOS pathway in hepatocellular carcinoma by Nigella sativa. Environ Health Prev Med. 2013;18:377–85. doi: 10.1007/s12199-013-0336-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Al-Jassir MS. Chemical composition and microflora of black cumin (Nigella sativa L.). seeds growing in Saudi Arabia. Food Chem. 1992;45:239–42. [Google Scholar]
- 12.Malik S, Hasan SS, Choudhary MI, Ni CZ, Clardy J. Nigellidine – A new indazole alkaloid from the seeds of Nigella sativa. Tetrahedron Lett. 1995;36:1993–6. [Google Scholar]
- 13.Salomi NJ, Nair SC, Jayawardhanan KK, Varghese CD, Panikkar KR. Antitumour principles from Nigella sativa seeds. Cancer Lett. 1992;63:41–6. doi: 10.1016/0304-3835(92)90087-c. [DOI] [PubMed] [Google Scholar]
- 14.Salem HB, Nefzaoui A, Salem LB. Sheep and Goat Nutrition: İntake, Digestion, Quality of Products and Rangelands. Zarazoga: CIHEAM-IAMZ; 2000. Sheep and goat preferences for Mediterranean fodder shrubs. Relationship with the nutritive characteristics; pp. 155–9. [Google Scholar]
- 15.Duvoix A, Delhalle S, Blasius R, Schnekenburger M, Morceau F, Fougère M, et al. Effect of chemopreventive agents on glutathione S-transferase P1-1 gene expression mechanisms via activating protein 1 and nuclear factor kappaB inhibition. Biochem Pharmacol. 2004;68:1101–11. doi: 10.1016/j.bcp.2004.05.032. [DOI] [PubMed] [Google Scholar]
- 16.Fraternale D, Ricci D, Calcabrini C, Guescini M, Martinelli C, Sestili P. Cytotoxic activity of essential oils of aerial parts and ripe fruits of Echinophora spinosa (Apiaceae) Nat Prod Commun. 2013;8:1645–9. [PubMed] [Google Scholar]
- 17.Banerjee S, Padhye S, Azmi A, Wang Z, Philip PA, Kucuk O, et al. Review on molecular and therapeutic potential of thymoquinone in cancer. Nutr Cancer. 2010;62:938–46. doi: 10.1080/01635581.2010.509832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al Amri AM, Bamosa AO. Phase I safety and clinical activity study of thymoquinone in patients with advanced refractory malignant disease. Shiraz E Med J. 2009;10(3):107–11. [Google Scholar]
- 19.Abukhader MM. Thymoquinone in the clinical treatment of cancer: Fact or fiction? Pharmacogn Rev. 2013;7:117–20. doi: 10.4103/0973-7847.120509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Singh S, Das SS, Singh G, Schuff C, de Lampasona MP, Catalán CA. Composition in vitro antioxidant and antimicrobial activities of essential oil and oleoresins obtained from black cumin seeds (Nigella sativa L.) Biomed Res Int. 2014;2014:918209. doi: 10.1155/2014/918209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Salim LZ, Othman R, Abdulla MA, Al-Jashamy K, Ali HM, Hassandarvish P, et al. Thymoquinone inhibits murine leukemia WEHI-3 cells in vivo and in vitro. PLoS One. 2014;9:e115340. doi: 10.1371/journal.pone.0115340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Salim LZ, Mohan S, Othman R, Abdelwahab SI, Kamalidehghan B, Sheikh BY, et al. Thymoquinone induces mitochondria-mediated apoptosis in acute lymphoblastic leukaemia in vitro. Molecules. 2013;18:11219–40. doi: 10.3390/molecules180911219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khalife R, El-Hayek S, Tarras O, Hodroj MH, Rizk S. Antiproliferative and proapoptotic effects of topotecan in combination with thymoquinone on acute myelogenous leukemia. Clin Lymphoma Myeloma Leuk. 2014;14(Suppl):S46–55. doi: 10.1016/j.clml.2014.04.014. [DOI] [PubMed] [Google Scholar]
- 24.Saraste A, Pulkki K. Morphologic and biochemical hallmarks of apoptosis. Cardiovasc Res. 2000;45:528–37. doi: 10.1016/s0008-6363(99)00384-3. [DOI] [PubMed] [Google Scholar]
- 25.Levine AJ. Introduction: The changing directions of p53 research. Genes Cancer. 2011;2:382–4. doi: 10.1177/1947601911413463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Porter AG, Jänicke RU. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999;6:99–104. doi: 10.1038/sj.cdd.4400476. [DOI] [PubMed] [Google Scholar]
- 27.Alhazmi MI, Hasan TN, Shafi G, Al-Assaf AH, Alfawaz MA, Alshatwi AA. Roles of p53 and caspases in induction of apoptosis in MCF- 7 breast cancer cells treated with a methanolic extract of Nigella sativa seeds. Asian Pac J Cancer Prev. 2014;15:9655–60. doi: 10.7314/apjcp.2014.15.22.9655. [DOI] [PubMed] [Google Scholar]
- 28.Yang E, Zha J, Jockel J, Boise LH, Thompson CB, Korsmeyer SJ. Bad, a heterodimeric partner for Bcl-XL and Bcl-2, displaces Bax and promotes cell death. Cell. 1995;80:285–91. doi: 10.1016/0092-8674(95)90411-5. [DOI] [PubMed] [Google Scholar]
- 29.Jiang P, Du W, Heese K, Wu M. The Bad guy cooperates with good cop p53: Bad is transcriptionally up-regulated by p53 and forms a Bad/p53 complex at the mitochondria to induce apoptosis. Mol Cell Biol. 2006;26:9071–82. doi: 10.1128/MCB.01025-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Xu-Monette ZY, Medeiros LJ, Li Y, Orlowski RZ, Andreeff M, Bueso-Ramos CE, et al. Dysfunction of the TP53 tumor suppressor gene in lymphoid malignancies. Blood. 2012;119:3668–83. doi: 10.1182/blood-2011-11-366062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jiang P, Du W, Wu M. p53 and Bad: Remote strangers become close friends. Cell Res. 2007;17:283–5. doi: 10.1038/cr.2007.19. [DOI] [PubMed] [Google Scholar]


