Abstract
Background:
Among the dietary polyphenolic, quercetin is the most common compound available in vegetables and fruits. The phytochemicals are used to treat diabetic wounds and diabetes, and specifically dietary polyphenols are being extensively studied for their anti-inflammatory and antioxidant abilities.
Objective:
The objective of the study was to assess the hypoglycemic, hypolipidemic, and wound healing potential of quercetin in streptozotocin (STZ)-induced diabetic Wistar rats.
Materials and Methods:
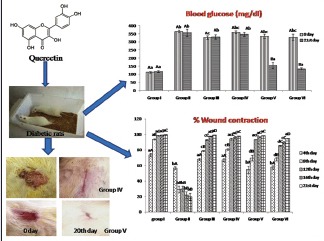
Induction of diabetes was done by intraperitoneally administration of STZ at the dose of 55 mg/kg in Wistar rats. An excision wound was created in diabetic rats that were treated with quercetin (100 mg/kg) orally and quercetin ointment topically to evaluate the antidiabetic and wound healing potential of quercetin.
Results:
Repeated oral administration of quercetin along with topical application of quercetin ointment in diabetic rats normalized the altered blood glucose, hydroxyproline, and glucosamine levels. Topical application of quercetin ointment alone on the excised wound was sufficient enough to heal the wound area in diabetic rats.
Conclusions:
The result of the present study indicates that quercetin produces hypoglycemic effect in STZ-induced diabetic rats and normalized plasma lipids and protein profiles. Besides, this quercetin also has an excellent wound healing property when applied topically on the wound area in diabetic rats.
SUMMARY
Quercetin has hypoglycaemic and hypolipidemic potential in streptozotocin induced diabetes in wistar rats
Dermal application along with oral administrations of quercetin has more effective in wound healing in diabetic animals
Histopathological studies of pancreas, skin and liver shows significant reduction in archaeological alterations on quercetin administrations in diabetic rats.
Abbreviation used: STZ: Streptozotocin; CMC: Carboxy methyl cellulose; HDL: High density lipoproteins; LDL: low density lipoproteins.
Keywords: Diabetes, hydroxyproline, quercetin, Wistar rats, wound healing
INTRODUCTION
The prevalence of noncommunicable diseases is expanding at an increasing rate.[1] Diabetes is the third killer of humankind after tumor, and cardiovascular ailments as a result of its high pervasiveness, morbidity, and mortality.[2] The prevalence of diabetes has risen steeply with an increase of mortality and morbidity rates.[3] Diabetes is a worldwide epidemic with an expected worldwide prevalence of 246 million people in 2007 and is predicted to rise to 300 million by 2025.[4] Diabetes is related with anomalous lipid/carbohydrate metabolism and is considered as the main risk factor for developing atherosclerosis and cardiovascular complications.[5]
Wounds have affected humans since ancient times, and the treatment and healing of wounds is a craftsmanship as old as humankind.[6] Increasing life expectancy coupled with change in lifestyle, wounds, particularly chronic wounds, increasingly affect elder patients.[7] The current assessments show that almost 6 million people are affected with interminable wounds causing great physiological and mental trauma.[8] The pathological conditions such as diabetes, immune disorders, ischemia, venous stasis, and injuries such as burns, frostbite, and gunshot wounds impair wound healing. Venous insufficiency related with diabetes build up a profound ulceration/injuries of the lower appendages.[9] Studies have demonstrated that there is a strong relationship between inflammatory processes and the development and advancement of diabetic complications.[10]
Numerous epidemiological reviews have recommended that intake of vegetable, fruits, red wine, and green tea on a regular basis, decreases the mortality rate.[11] Plants are a source of various bioactive compounds, and many of these compounds are customarily used to accelerate the process of healing.[12] Plants bioactive constituents, flavonoids, have received considerable attention in recent years. Flavonoids have been referred as “nature's biological response modifiers” as a result of their intrinsic capacity to alter the body response to allergens, viruses, and carcinogens.[13] The enthusiasm for dietary flavonoids has developed over the most recent 15 years after the publication of few logical reviews demonstrating an inverse correlation between the dietary utilization of flavonoids and abatement in frequency as well as mortality from cardiovascular diseases.[14] Among the tested bioflavonoid, quercetin is known to have the highest antioxidant activity.[15] Quercetin belongs to a class called flavonols that exists abundantly in onions, apples, berries, nuts, cauliflower, cabbage, red wine, and Chinese herbs.[16] Quercetin has been reported for an extensive variety of natural activities such as antioxidant, anti-inflammatory, antiatherosclerotic, and anticarcinogenic properties.[17,18] Keeping this in view the present study was conducted to evaluate the hypoglycemic, hypolipidemic, and wound healing potential of quercetin on diabetic wound in Wistar rats.
MATERIALS AND METHODS
Experimental animals
The study was conducted on healthy Wistar rats of either sex weighing 180–220 g obtained from Indian Institute of Integrative Medicine, Jammu, India. The rats were given standard pelleted ration and ad libitum drinking water. Before the beginning of the experiment, the rats were acclimatized to the research laboratory condition for a period of >3 weeks. The trial convention was affirmed and supervised by the Institutional Animal Ethics Committee.
Induction of diabetes
Nearly 0.5% of streptozotocin (STZ) solution was prepared freshly in ice cold sodium citrate buffer (0.1M; pH 4.5) bearing strength of 10 mg/ml and was injected intraperitoneally to overnight fasted Wistar rats at the dose of 55 mg/kg body weight.[19,20,21] After STZ administration, there occurs a massive release of insulin from pancreas, so rats were kept on 0.5% glucose water to avoid hypoglycemic condition for the next 24 h.[22,23,24,25] After 72 h of STZ administrations, the blood glucose level was estimated using glucometer[24] and the rats indicating >225 mg/dl blood glucose level were considered as diabetic rats and included in the study.[25]
Creation of wound and its measurement
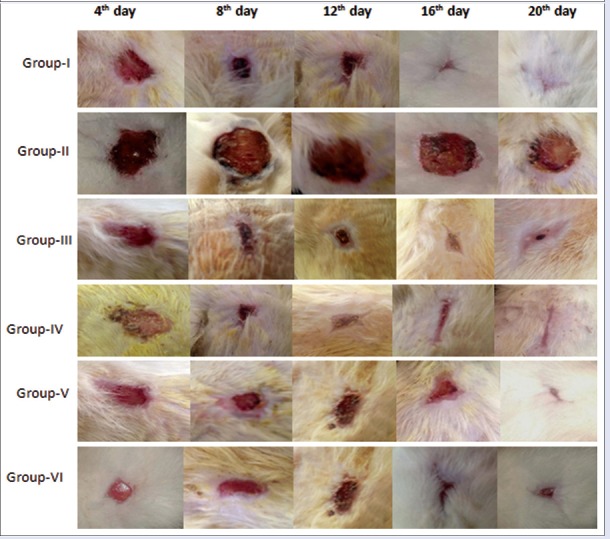
Group I to VI containing six Wistar rats in each were selected to create excision wound after anesthetizing with intramuscular administration of ketamine hydrochloride (75 mg/kg) and xylazine (10 mg/kg). The dorsal surface of rats was shaved and an area of 2 cm × 2 cm was marked using transparent tracing paper. A full thickness excision wound of area 2 cm × 2 cm (400 mm2) was made along the markings.[26] The wound was then measured on 4th, 8th, 12th, 16th, and 21st day of treatment. The percentage wound area contraction was calculated using formula:

Where “n” is the number of days.
Experimental design
Thirty diabetic rats and six healthy Wistar rats were chosen and divided into six groups containing six animals in each group. Induction of diabetes was done in all groups (Group II to Group VI) except in Group I which served as vehicle control group receiving 1 ml/day/rat carboxymethyl cellulose (CMC 0.01%) orally and petroleum jelly for 21 days. Group II diabetic rats were treated with CMC and petroleum jelly topically for 21 days. Group III was treated with 5% povidone ointment topically and CMC orally for 21 days. The diabetic rats of both Group IV and Group V received quercetin ointment topically, and in addition, Group V received quercetin orally after mixing it in CMC at the dose of 100 mg/kg body weight for 21 days. Group VI diabetic rats were treated with glibenclamide at the dose of 5 mg/kg body weight orally for 21 days. One gram of quercetin was mixed with 99 g of petroleum jelly to make quercetin ointment (1%).
Biochemical profile
The biochemical parameters, namely, blood glucose, total plasma proteins, albumin, total cholesterol, high-density lipoprotein (HDL), low-density lipoprotein (LDL) along with the levels of glucosamine,[27] and hydroxyproline[28] were estimated on 21st day of the experiment to analyze the effect of quercetin on wound induced in diabetic rats.
Histopathological studies
The histopathological studies of the skin, liver, and pancreatic tissues were performed in accordance with the standard method. Briefly, a small portion of tissue was quickly fixed in 10% formalin. After the formalin fixation, fixed tissues were embedded in paraffin, sectioned, stained with hematoxylin and eosin, and examined under a light microscope for further histopathological examination.
Statistical analysis
The statistical analysis was carried out in completely randomized design and the significance level was tested using Duncan multiple test[29] at 5% level (P < 0.05).
RESULTS
Effect of quercetin on blood glucose level
The blood glucose level was significantly (P < 0.05) increased in Group II, III, IV, V, and VI diabetic Wistar rats on day 0 compared to that of Group I Wistar rats [Figure 1]. After treating the diabetic rats of Group V with quercetin (100 mg/kg) and topical application of quercetin (1%), the blood glucose level was significantly reduced on 21st day of treatment and the levels were comparable to Group I control rats.
Figure 1.
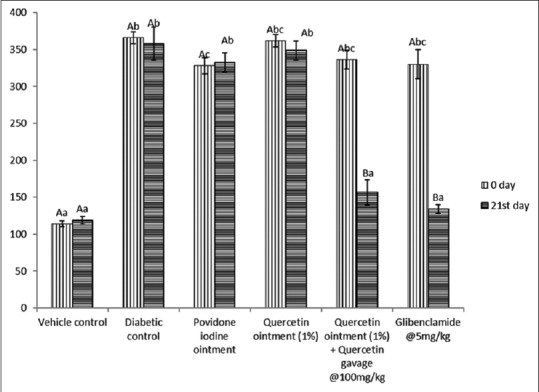
Effect of repeated oral administration of quercetin on the blood glucose level (mg/dl) of control and diabetic Wistar rats at 0 and 21st day of administration
Effect of quercetin on wound contraction
The present study revealed that the percentage wound contraction on 21st day was significantly (P < 0.05) decreased in Group II when compared to day 4 [Figures 2 and 3]. A significant increase in the percentage wound contraction was observed in Group III, IV, V, and VI after the treatment period of 21 days. The glucosamine levels of Group III and Group V were comparable with the level of Group I on 21st day of the experiment [Figure 4]. A significant decline was observed in Group II, Group IV, and Group VI glucosamine levels as compared to Group I. However, the glucosamine level of Group III, V, and VI was increased significantly as compared to Group II. The levels of hydroxyproline were significantly increased in Group II, Group IV, and Group VI as compared to the level of hydroxyproline in Group I. The hydroxyproline levels of Group III and Group V were comparable with the levels in Group I [Figure 4].
Figure 2.
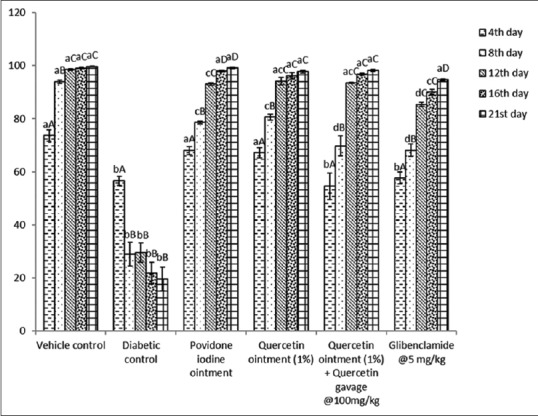
Effect of repeated oral administrations of quercetin on percent wound contraction in control and diabetic Wistar rats at different time intervals
Figure 3.
The effect of repeated oral administration of quercetin on wound contraction in Wistar rats at 4th, 8th, 12th, 16th, and 21th day of treatment period
Figure 4.
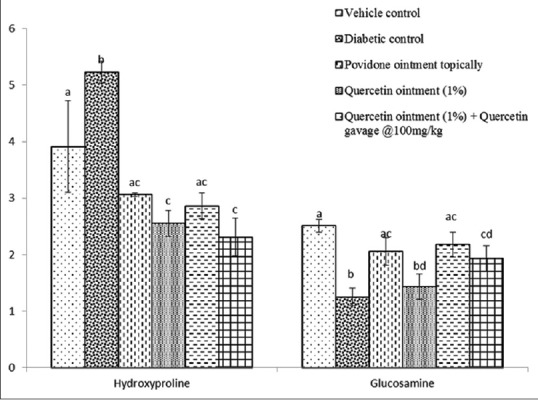
Effect of repeated oral administration of quercetin on hydroxyproline and glucosamine levels in control and diabetic Wistar rats
Effect of quercetin on lipid and total plasma protein profiles
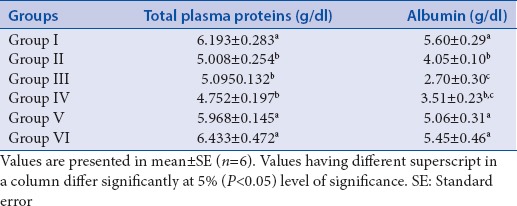
Group II, III, and IV showed a significant increase in the total cholesterol levels compared to Group I. However, in Group V and Group VI treated with quercetin and glibenclamide, respectively, for 21 days, a significant decline in the total cholesterol levels was observed as compared to Group II. The HDL cholesterol levels were significantly increased in Group V and VI when compared to Group II and the levels were comparable to Group I on 21st day of the experiment. However, a significant decline in HDL cholesterol levels was found in Group II, III, and IV as compared to Group I [Table 1]. The triglyceride levels and LDL cholesterol levels of Group V and VI showed a significant decrease from Group II, respectively, and the levels were comparable to Group I on 21st day of the experiment. A significant increase in the triglyceride and LDL cholesterol levels was found in Group II, III, and IV, respectively, compared to the level of triglyceride and LDL cholesterol level in Group I [Table 1]. The level of total plasma proteins in Group V and VI showed a significant increase compared to the level of total protein in Group II and the total protein levels of both Group V and VI were comparable with Group I. A significant decrease in the total protein level was found in Group II, III, and IV as compared to Group I. The albumin level of Group V and VI was significantly increased as compared to Group II. Furthermore, the level of albumin in Group V and VI was comparable with the level of albumin in Group I. There was a significant decline in the albumin levels of Group II, III, and IV as compared to Group I [Table 2].
Table 1.
Effect of repeated oral administration of quercetin on plasma lipid profile of control and diabetic rats
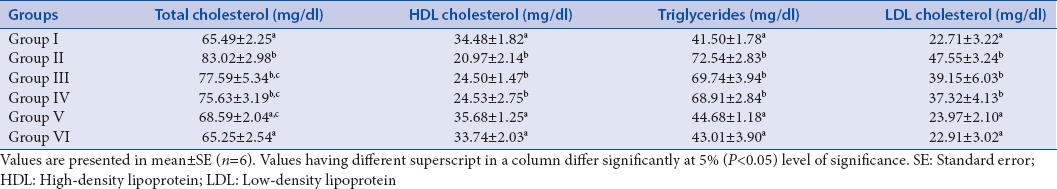
Table 2.
Effect of repeated oral administration of quercetin on plasma protein profile of control and diabetic Wistar rats
Effect of quercetin on histopathology of skin
A mild keratinization was seen in skin of Wistar rats of Group II. The keratinization along with the normal epidermis and hair follicles was seen in Group III. The keratinization along with the presence of inflammatory cells was seen in Group IV. In Group V, hyperkeratinization was seen and keratinization along with collagen fibers was observed in Group VI [Figure 5].
Figure 5.
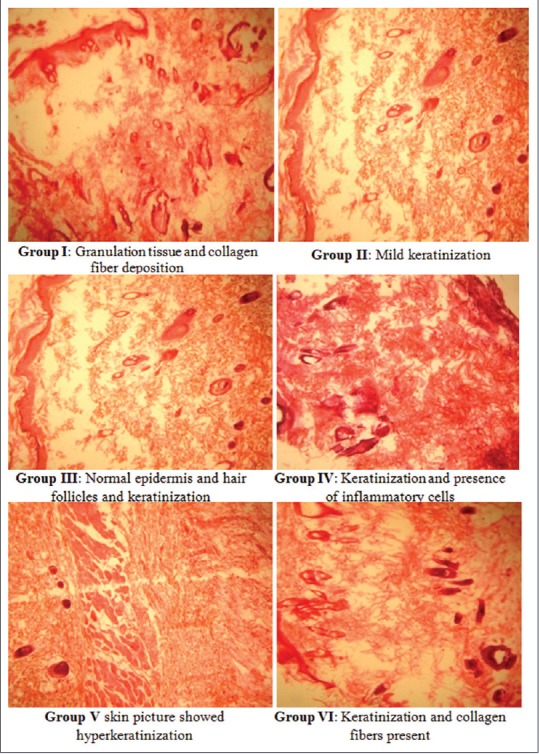
Photomicrograph of hematoxylin and eosin (H and E, ×100) stained sections of formalin-fixed skin of control and treated groups of diabetic Wistar rats
Effect of quercetin on histopathology of liver
In Group II, severe necrosis of hepatocytes was observed. Dilation of central vein and mild pericentral vacuolar degeneration of hepatocytes was observed in Group III. Binucleation of hepatocytes and fatty degeneration was seen in Group IV. In Group V, mild fibrotic changes were observed, and in Group VI, normal cord pattern of hepatocytes was seen [Figure 6].
Figure 6.
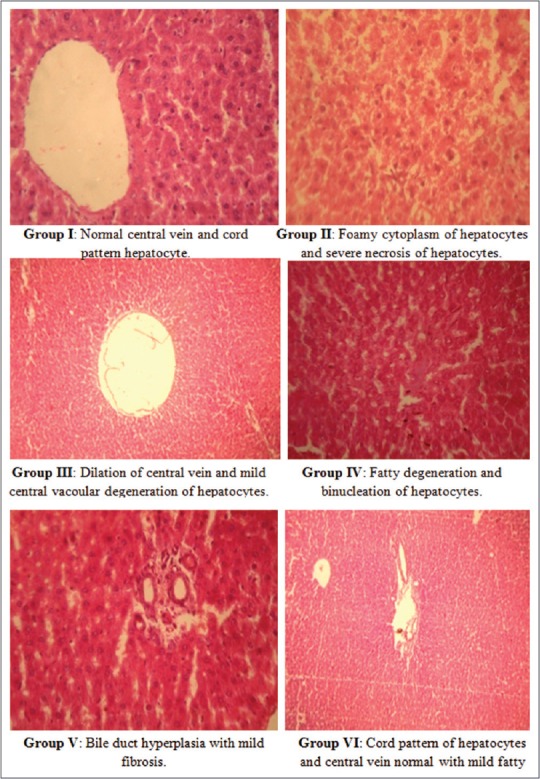
Photomicrograph of hematoxylin and eosin (H and E, ×100) stained sections of formalin-fixed liver of different groups in control and diabetic rats
Effect of quercetin on histopathology of pancreas
Severe degeneration and necrosis of acinar cells were seen in Group II. Degenerative changes such as cellular infiltration and fibrosis were seen in Group III and IV. Persistent necrosis of islets of Langerhans was seen in Group V even after treating the rats with quercetin (100 mg/kg). However, mild cellular infiltration was seen in Group VI after treating the rats with glibenclamide [Figure 7].
Figure 7.
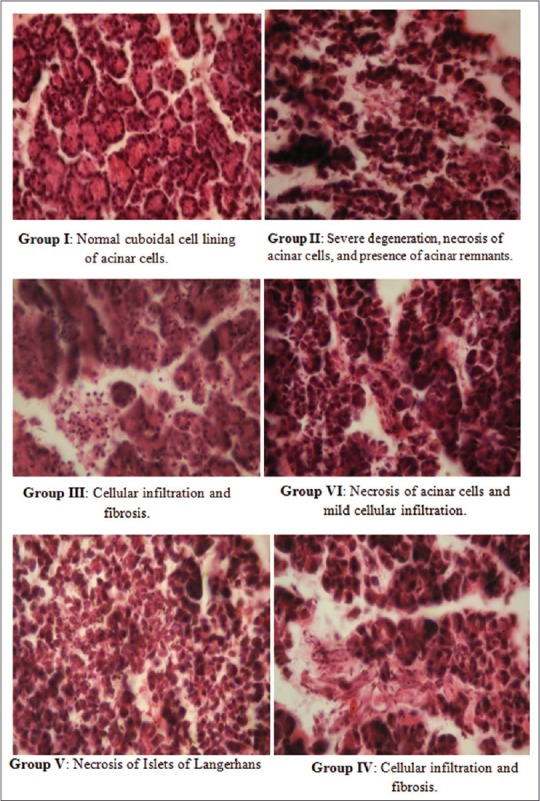
Photomicrograph of hematoxylin and eosin (H and E, ×100) stained sections of formalin-fixed pancreas of different groups in control and diabetic rats
DISCUSSION
Diabetes induced by STZ in Wistar rats serves as an efficient and effective model for studying antidiabetic potential of phytochemicals in animal model. In the present study, a single intraperitoneal administration of STZ significantly increased the glucose level of blood to >225 mg/dl. A similar increase in blood glucose level has been reported due to STZ-induced diabetes in Wistar rats.[30,31,32] A significant decline in blood glucose level was found when hyperglycemic rats were treated with quercetin indicating the hypoglycemic activity of quercetin. The hypoglycemic potential of polyphenols is mainly due to the decreased intestinal assimilation of dietary carbohydrates, regulation of enzymes involved in glucose metabolism, change of β-cell functioning, and insulin activity and stimulation of insulin secretion.[33] Kwon et al.[34] also reported a similar decrease in the blood glucose level and the possible mechanism due to which quercetin enhances glycemic control is because of decreased intestinal glucose assimilation at the level of glucose transporters (GLUTs). It is also thought that quercetin may block tyrosine kinase,[35] but as per Strobel et al.,[36] the inhibitory impact of quercetin on glucose uptake is because of direct activity on the transporter GLUT4 as opposed to its impact on cellular protein tyrosine kinase. Various studies have reported that oxidative stress results in the production of reactive nitrogen and oxygen species due to which the mitochondrial cell membrane potential changes resulting in release of cytochrome C and activating cell apoptosis.[37] The pancreatic cells of diabetic rats were regenerated after treating them with quercetin similar to that of Vessal et al.[38] who proposed that quercetin supplementation is beneficial in diminishing blood glucose concentration, advancing recovery of pancreatic islets, and increasing insulin release in diabetic rats. The possible mechanism may be because of the protective role of quercetin against oxidative damage, thus protecting the pancreatic β-cells.
The elevated blood glucose level in diabetes may cause endothelial damage with potential occlusion of capillary vessels as well as hyperglycemia-induced leukocyte dysfunction, decrease chemotaxis and phagocytosis resulting in impaired wound healing, and increased infection rate. Defective collagen metabolism in diabetes is thought to be a factor in delayed wound healing.[39] The impaired healing in diabetic rats is predominantly due to hypoxia, fibroblast and epidermal cell dysfunction, impairment in angiogenesis, neovascularization, and increased levels of metalloproteases.[40] Besides, this an increased production of reactive oxygen species (ROS), lipid peroxidation, and ineffective scavenging has a vital role in skin lesions and in modulation of fibroblast proliferation.[41] The histopathological study revealed well-organized collagen fibers, increase in fibroblast cells, and new blood vessel formation after the wound was treated with quercetin. The quercetin ointment (1%) alone and in combination with quercetin orally resulted in significant increase in wound contraction by enhanced epithelization and the possible mechanism of improved healing by quercetin is due to the ability of quercetin to improve the tissue antioxidant levels. Our findings were similar to the findings of Rajamanickam et al.[42] who evaluated the antibacterial and wound healing activity of quercetin. Maha et al.[43] also found that quercetin due to its antifibrotic and antihistaminic effect could be an effective treatment for hypertrophic scars. Ghorbani et al.[44] also reported that 2% quercetin cream was effective in treating phlebitis. Flavonoid helps in increasing collagen synthesis, promotes cross-linking of collagen, decreases the degradation of soluble collagen, promotes conversion of soluble collagen to insoluble collagen, and hinders the catabolism of soluble collagen. Disintegration of collagen releases free hydroxyproline and its peptides; subsequently, hydroxyproline estimation is used as an indicator for collagen turnover.[45]
Hyperlipidemia due to diabetes is mainly because of the increased mobilization of lipids from adipose tissue.[46] In diabetic condition, lipoprotein lipase is decreased due to which level of lipoproteins increases in blood.[47] In the present study, a significant decline in the levels of total cholesterol, triglyceride, and LDL cholesterol was seen along with a significant increase in the HDL cholesterol levels after treating STZ-induced rats with quercetin, and the results were in accordance with Kobori et al.[48] who found that quercetin resulted in diminished expression of peroxisome proliferator-activated receptor a and sterol regulatory element-binding-protein-1c, thus resulting in decreased synthesis of triglycerides in the liver of mice. Gnoni et al.[49] also reported that quercetin reduces the synthesis of triglycerides and fatty acid synthesis. Quercetin is also found to decrease the activity of acetyl-CoA carboxylase in rat hepatocytes and thus resulting in hypotriglyceridemic effect. Khaki[50] reported that quercetin has a defensive impact against putrefaction and apoptosis instigated by experimental ischemia and reperfusion in rat liver recommending that quercetin treatment may have a part at subcellular level in counteracting ischemia and reperfusion incited apoptosis. It likewise protects the hepatobiliary functions and the liver structure in hepatic damage.
Oxidation of proteins due to ROS is one of the important and deleterious responses of membrane proteins implicated in the manifestation of critical illness. Some metabolic modifications prompt a negative nitrogen balance, improved proteolysis, and decrease the synthesis of proteins in experimental diabetes.[51] In the present study, an increase in total plasma proteins after the oral administration of quercetin may be due to reduced oxidation of plasma proteins. These findings were as per the study of Dene et al.,[52] who detailed that hypoglycemic impact of quercetin results in decreased glycation of cellular proteins.
CONCLUSION
Observations of the present study indicate that quercetin not only possesses hypoglycemic, hypolipidemic, potential but also an excellent wound healing property in diabetic rats.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
Authors thank the Dean, Faculty of Veterinary Science and Animal Husbandry, R S Pura Jammu, for giving vital facilities for conducting the research.
REFERENCES
- 1.Hossain P, Kawar B, El Nahas M. Obesity and diabetes in the developing world – A growing challenge. N Engl J Med. 2007;356:213–5. doi: 10.1056/NEJMp068177. [DOI] [PubMed] [Google Scholar]
- 2.Ahmad M, Prawez S, Sultana M, Raina R, Pankaj NK, Verma PK, et al. Anti-hypoglycemic, anti-hyperlipidemic and antioxidant potential of alcoholic extract of Sida cordifolia (areal part) in streptozotocin-induced-diabetes in Wistar rats. Proc Natl Acad Sci India Sect B Biol Sci. 2014;84:397–405. [Google Scholar]
- 3.Ergul A. Endothelin-1 and diabetic complications: Focus on the vasculature. Pharmacol Res. 2011;63:477–82. doi: 10.1016/j.phrs.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Whiting DR, Guariguata L, Weil C, Shaw J. IDF diabetes atlas: Global estimates of the prevalence of diabetes for 2011 and 2030. Diabetes Res Clin Pract. 2011;94:311–21. doi: 10.1016/j.diabres.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 5.Biddinger SB, Kahn CR. From mice to men: Insights into the insulin resistance syndromes. Annu Rev Physiol. 2006;68:123–58. doi: 10.1146/annurev.physiol.68.040104.124723. [DOI] [PubMed] [Google Scholar]
- 6.Robson MC, Steed DL, Franz MG. Wound healing: Biologic features and approaches to maximize healing trajectories. Curr Probl Surg. 2001;38:72–140. doi: 10.1067/msg.2001.111167. [DOI] [PubMed] [Google Scholar]
- 7.Tsala DE, Amadou D, Habtemariam S. Natural wound healing and bioactive natural products. Phytopharmacology. 2013;4:532–60. [Google Scholar]
- 8.Kumar B, Vijayakumar M, Govindarajan R, Pushpangadan P. Ethnopharmacological approaches to wound healing – Exploring medicinal plants of India. J Ethnopharmacol. 2007;114:103–13. doi: 10.1016/j.jep.2007.08.010. [DOI] [PubMed] [Google Scholar]
- 9.Pettet G, Chaplain MA, Mcelwain DL, Byrne HM. On the role of angiogenesis in wound healing. Proc Royal Soc London. 1996;263:1487–93. doi: 10.1098/rspb.1996.0217. [DOI] [PubMed] [Google Scholar]
- 10.Donath MY, Shoelson SE. Type 2 diabetes as an inflammatory disease. Nat Rev Immunol. 2011;11:98–107. doi: 10.1038/nri2925. [DOI] [PubMed] [Google Scholar]
- 11.Renaud S, de Lorgeril M. Wine, alcohol, platelets, and the French paradox for coronary heart disease. Lancet. 1992;339:1523–6. doi: 10.1016/0140-6736(92)91277-f. [DOI] [PubMed] [Google Scholar]
- 12.Jagetia GC, Rajanikant GK. Role of curcumin, a naturally occurring phenolic compound of turmeric in accelerating the repair of excision wound, in mice whole-body exposed to various doses of gamma-radiation. J Surg Res. 2004;120:127–38. doi: 10.1016/j.jss.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 13.Manivannana R, Sukumar D. The RBC membrane stabilisation in an in vitro method by the drug isolated from Leucas aspera. Int J Appl Sci Eng. 2007;5:133–8. [Google Scholar]
- 14.Karaca T, Cemek M, Kanter M. Lipid peroxidation and antioxidant levels, and alpha napthyl acetate esterase activity of peripheral blood lymphocytes in Mallard, Muscovy and Pekin ducks. Acta Vet Brno. 2006;75:33–8. [Google Scholar]
- 15.Morel I, Lescoat G, Cogrel P, Sergent O, Pasdeloup N, Brissot P, et al. Antioxidant and iron-chelating activities of the flavonoids catechin, quercetin and diosmetin on iron-loaded rat hepatocyte cultures. Biochem Pharmacol. 1993;45:13–9. doi: 10.1016/0006-2952(93)90371-3. [DOI] [PubMed] [Google Scholar]
- 16.Naderi GA, Asgary S, Sarraf-Zadegan N, Shirvany H. Anti-oxidant effect of flavonoids on the susceptibility of LDL oxidation. Mol Cell Biochem. 2003;246:193–6. [PubMed] [Google Scholar]
- 17.Lotito SB, Frei B. Dietary flavonoids attenuate tumor necrosis factor alpha-induced adhesion molecule expression in human aortic endothelial cells. Structure-function relationships and activity after first pass metabolism. J Biol Chem. 2006;281:37102–10. doi: 10.1074/jbc.M606804200. [DOI] [PubMed] [Google Scholar]
- 18.Mamani-Matsuda M, Kauss T, Al-Kharrat A, Rambert J, Fawaz F, Thiolat D, et al. Therapeutic and preventive properties of quercetin in experimental arthritis correlate with decreased macrophage inflammatory mediators. Biochem Pharmacol. 2006;72:1304–10. doi: 10.1016/j.bcp.2006.08.001. [DOI] [PubMed] [Google Scholar]
- 19.Chang KJ. Effect of taurine and beta-alanine on morphological changes of pancreas in streptozotocin-induced rats. Adv Exp Med Biol. 2000;483:571–7. doi: 10.1007/0-306-46838-7_61. [DOI] [PubMed] [Google Scholar]
- 20.Ramesh B, Pugalendi KV. Impact of umbelliferone (7-hydrxycoumarin) on hepatic marker enzymes in STZ diabetic rats. Indian J Pharmacol. 2006;38:209–10. [Google Scholar]
- 21.Gayathri M, Kannabiran K. Hypoglycemic activity of Hemidesmus indicus R. Br. On streptozotocin-induced diabetic rats. Int J Diabetes Dec Ctries. 2008;28:6–10. doi: 10.4103/0973-3930.41979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Akbarzadeh A, Norouzian D, Mehrabi MR, Jamshidi SH, Farhangi A, Allahverdi A, et al. Induction of diabetes by Streptozotocin in rats. Indian J Clin Biochem. 2007;22:60–4. doi: 10.1007/BF02913315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abeeleh MA, Ismail ZB, Alzaben KR, Abu-Halaweh SA, Al-Essa MK, Abuabeeleh J, et al. Induction of diabetes mellitus in rats using intraperitoneal streptozotocin: A comparison between 2 strains of rats. Eur J Sci Res. 2009;32:466–75. [Google Scholar]
- 24.Karunanayake EH, Hearse DJ, Mellows G. The metabolic fate and elimination of streptozotocin. Biochem Soc Trans. 1975;3:410–4. doi: 10.1042/bst0030410. [DOI] [PubMed] [Google Scholar]
- 25.Sokeng RD, Lontsi D, Moundipa PF, Jatsa HB, Watcho P, Kamtchouing P. Hypoglycemic effect of Annacardium occidentale L. methanol extract and fractions on streptozotocin-induced diabetic rats. Global J Pharmacol. 2007;1:1–5. [Google Scholar]
- 26.Ahanger AA, Prawez S, Leo MD, Kathirvel K, Kumar D, Tandan SK, et al. Pro-healing potential of hemin: An inducer of heme oxygenase-1. Eur J Pharmacol. 2010;645:165–70. doi: 10.1016/j.ejphar.2010.06.048. [DOI] [PubMed] [Google Scholar]
- 27.Woessner JF., Jr The determination of hydroxyproline in tissue and protein samples containing small proportions of this amino acid. Arch Biochem Biophys. 1961;93:440–7. doi: 10.1016/0003-9861(61)90291-0. [DOI] [PubMed] [Google Scholar]
- 28.Rondle CJ, Morgan WT. The determination of glucosamine and galactosamine. Biochem J. 1955;61:586–9. doi: 10.1042/bj0610586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Duncan DB. Multiple range and multiple F tests. Biometrics. 1955;11:1–42. [Google Scholar]
- 30.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–20. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 31.Bandawane D, Juvekar A, Juveka M. Antidiabetic and antihyperlipidemic effect of Alstonia scholaris Linn bark in streptozotocin induced diabetic rats. Indian Journal of Pharmaceutical Education and Research. 2011;45:114–20. [Google Scholar]
- 32.Khan TY, Raina R, Verma PK, Jyoti MK. Phytochemical constituents and antidiabetic potential of Ipomea carnea Jacq leaves extracts in Wistar rats. J exp Integr Med. 2014;4:137–42. [Google Scholar]
- 33.Bahadoran Z, Mirmiran P, Azizi F. Dietary polyphenols as potential nutraceuticals in management of diabetes: A review. J Diabetes Metab Disord. 2013;12:43. doi: 10.1186/2251-6581-12-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kwon O, Eck P, Chen S, Corpe CP, Lee JH, Kruhlak M, et al. Inhibition of the intestinal glucose transporter GLUT2 by flavonoids. FASEB J. 2007;21:366–77. doi: 10.1096/fj.06-6620com. [DOI] [PubMed] [Google Scholar]
- 35.Elberg G, Li J, Leibovitch A, Shechter Y. Non-receptor cytosolic protein tyrosine kinases from various rat tissues. Biochim Biophys Acta. 1995;1269:299–306. doi: 10.1016/0167-4889(95)00124-8. [DOI] [PubMed] [Google Scholar]
- 36.Strobel P, Allard C, Perez-Acle T, Calderon R, Aldunate R, Leighton F, et al. Myricetin, quercetin and catechin-gallate inhibit glucose uptake in isolated rat adipocytes. Biochem J. 2005;386:471–8. doi: 10.1042/BJ20040703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yamg R, Zhang ZF, Pei X, Han X, Wang J, Wang L, et al. Immunomodulatory effects of marine oligopeptide preparation from chum salmon (Oncorhynchus keta) in mice. Food Chem. 2009;113:464–70. [Google Scholar]
- 38.Vessal M, Hemmati M, Vasei M. Antidiabetic effects of quercetin in streptozocin-induced diabetic rats. Comp Biochem Physiol C Toxicol Pharmacol. 2003;135C:357–64. doi: 10.1016/s1532-0456(03)00140-6. [DOI] [PubMed] [Google Scholar]
- 39.Decker RT, Sirois DA, Mobley CC. Nutrition and oral medicine. In: Winkler MF, Makowski S, editors. Wound Healing. Totowa: Humana Press; 2005. pp. 273–83. [Google Scholar]
- 40.Lodhi S, Singhai AK. Wound healing effect of flavonoid rich fraction and luteolin isolated from Martynia annua linn. on streptozotocin induced diabetic rats. Asian Pac J Trop Med. 2013;6:253–9. doi: 10.1016/S1995-7645(13)60053-X. [DOI] [PubMed] [Google Scholar]
- 41.Winyars PG, Blake DR, Evans CH. Free Radicals and Inflammation. Switzerland: Birkhauser; 2009. pp. 195–203. [Google Scholar]
- 42.Rajamanickam M, Kalaivanan P, Sivagnanam I. Antibacterial and wound healing activities of quercetin-3-O-A-L-Rhamnopyranosyl-(1-6)-b-D-glucopyranoside isolated from Salvia leucantha. Int J Pharm Sci Rev Res. 2013;22:264–8. [Google Scholar]
- 43.Maha F, El-Goweini MD, Nagwa M, Nour-el-din MD. Effect of quercetin on excessive dermal scaring. Egypt Dermatol Online J. 2005;1:1–10. [Google Scholar]
- 44.Ghorbani S, Foadoddini M, Frad MH, Mahdiabadi MA, Vejdan SA. The effects of quercetin topical cream on phlebitis caused by peripheral intravenous catheters: A randomized controlled trial. Mod Care J. 2016;13:e8857. [Google Scholar]
- 45.Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991;40:405–12. doi: 10.2337/diab.40.4.405. [DOI] [PubMed] [Google Scholar]
- 46.Arvind K, Pradeepa R, Deepa R, Mohan V. Diabetes and coronary artery disease. Indian J Med Res. 2002;116:163–76. [PubMed] [Google Scholar]
- 47.Johnston CS, Gaas CA. Vinegar: Medicinal uses and antiglycemic effect. MedGenMed. 2006;8:61. [PMC free article] [PubMed] [Google Scholar]
- 48.Kobori M, Masumoto S, Akimoto Y, Takahashi Y. Dietary quercetin alleviates diabetic symptoms and reduces streptozotocin-induced disturbance of hepatic gene expression in mice. Mol Nutr Food Res. 2009;53:859–68. doi: 10.1002/mnfr.200800310. [DOI] [PubMed] [Google Scholar]
- 49.Gnoni GV, Paglialonga G, Siculella L. Quercetin inhibits fatty acid and triacylglycerol synthesis in rat-liver cells. Eur J Clin Invest. 2009;39:761–8. doi: 10.1111/j.1365-2362.2009.02167.x. [DOI] [PubMed] [Google Scholar]
- 50.Khaki A. Protective effect of quercetin against necrosis and apoptosis induced by experimental ischemia and reperfusion in rat liver. Afr J Pharm Pharmacol. 2009;4:22–6. [Google Scholar]
- 51.Pathak A, Dhawan D. Effect of lithium on levels of blood urea and creatinine in diabetic rats. Med Sci Rev. 1998;26:855–6. [Google Scholar]
- 52.Dene BA, Maritim AC, Sanders RA, Watkins JB., 3rd Effects of antioxidant treatment on normal and diabetic rat retinal enzyme activities. J Ocul Pharmacol Ther. 2005;21:28–35. doi: 10.1089/jop.2005.21.28. [DOI] [PubMed] [Google Scholar]