Abstract
Background:
Turmeric (Curcuma longa) is reported to possess wide array of biological activities. Herbal Medicament (HM) is a standardized hexane-soluble fraction of C. longa and is well known for its neuroprotective effect.
Objective:
In this study, we attempted to synthesize a novel chemically modified bioactive fraction from HM (NCCL) along with isolation and characterization of a novel marker compound (I).
Materials and Methods:
NCCL was prepared from HM. The chemical structure of the marker compound isolated from NCCL was determined from 1D/2D nuclear magnetic resonance, mass spectroscopy, and Fourier transform infrared. The compound so isolated was subjected to in silico and in vitro screenings to test its inhibitory effect on estrogen receptors.
Results:
Molecular docking studies revealed that the binding poses of the compound I was energetically favorable. Among NCCL and compound I taken for in vitro studies, NCCL had exhibited good anti-cancer activity over compound I against MCF-7, MDA-MB-231, DU-145, and PC-3 cells.
Conclusion:
This is the first study about the synthesis of a chemically modified bioactive fraction which used a standardized extract since the preparation of the HM. It may be concluded that NCCL fraction having residual components induce more cell death than compound I alone. Thus, NCCL may be used as a potent therapeutic drug.
SUMMARY
In the present paper, a standardized hexane soluble fraction of Curcuma longa (HM) was chemically modified to give a novel bioactive fraction (NCCL). A novel marker compound was isolated from NCCL and was characerized using various spectral techniques. The compound so isolated was investigated for in-silico screenings. NCCL and isolated compound was subjected to in-vitro anti-cancer screenings against MCF 7, MDA MB 231 (breast adenocarcinoma) and DU 145 and PC 3 cell lines (androgen independent human prostate cancer cells). The virtual screenings reveals that isolated compound has shown favourable drug like properties. NCCL fraction having residual components induces more cell death in these four cancer cell lines than isolated compound alone.
Abbreviations used: HM: Herbal Medicament; NCCL: Chemically modified HM; FT-IR: Fourier transform-infrared spectroscopy; NMR: Nuclear magnetic resonance spectroscopy; MS: Mass spectroscopy; HPLC: High-performance liquid chromatography; ER: Estrogen receptor; MTT: 3-(4,5 dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide; MIC: Minimum inhibitory concentration; TAM: Tamoxifen KBr: Potassium bromide; DMSO: Dimethyl sulfoxide; ACN: Acetonitrile; PDB: Protein Data Bank; PDA: Photodiode array detector.
Keywords: Anti-cancer, Curcuma longa, isolation, molecular docking, synthesis
INTRODUCTION
Nature is one of the greatest chemist and plants are almost countless funds of charming chemical constituent. Natural products and their derivatives have been recognized for many years as a source of therapeutic agents. The use of plants for medicines worldwide still immensely exceeds the use of the current synthetic drug.[1] Turmeric is a spice derived from the rhizomes of Curcuma longa, which is a member of the ginger family (Zingiberaceae) which has been conventionally used in ayurvedic medicine and in the preparation of food.[2] C. longa contains various bioactive components such as tannins, phenols, saponins, alkaloids, flavonoids, glycosides, curcuminoids, and several volatiles oils including variety of sesquiterpenes possessing neuroprotective, antithrombotic, anti-inflammatory, and anti-cancer activities.[3,4,5,6] It has been reported to possess antifungal,[7] antibacterial,[8] insecticidal,[9] mosquitocidal,[10] antioxidant,[11] and anti-cancer activities.[3]
Our Institute had developed a standardized hexane-soluble extract of C. longa rhizomes known by the name of Herbal Medicament (HM), as an anti-stroke agent.[12] It has shown potential neuroprotective activity in neurovascular disorder, and the product was licensed to Themis Medicare Ltd., Mumbai for further development as an anti-stroke agent.
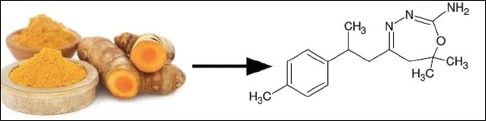
Depending on the wide therapeutic array of C. longa, its chemically modified fraction comprising compounds such as curcumene, zingiberene, β-bisabolene, β-sesquiphellandrene, curzerene, residual ar-turmerone, and some cyclic derivatives of the carbonyl compounds, for example, 7,7-dimethyl-5-(2-p-tolylpropyl)-6, 7-dihydro-1,3,4-oxadiazepin-2-amine (reaction product of ar-turmerone with semicarbazide; compound I), herein referred as NCCL was prepared. Since the anti-cancer effects of curcuminoids or turmerones are well explored[13,14,15,16] the effect of NCCL on these parameters, if any has been not evaluated so far.
Therefore, in this study, we describe the synthesis and anti-cancer screening of a novel chemically modified bioactive fraction NCCL from HM. During this study, we identified a novel marker compound (I) whose isolation, structural elucidation, in silico and in vitro screenings has been reported in this study.
Estrogens play crucial roles in breast and prostate cancer development. Therefore, much effort has been devoted to block estrogen formation.[17,18] Tamoxifen (TAM) is the most widely used therapy for antagonizing estrogen receptor (ER) function as it binds to the ER receptor and blocks downstream signaling.[19] Keeping this in mind, molecular docking study of the newly isolated compound was carried out. Oral bioavailability is a desirable property of compounds under investigation in the drug discovery process. Lipinski's rule of five is a simple model to forecast the absorption and intestinal permeability of a compound.[20,21] The isolated marker compound obeys the Lipinski rule of five along with obeying some additional parameters.[22] It was then evaluated for anti-cancer activity by 3-(4,5 dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay[23] against MCF-7, MDA-MB-231 (breast adenocarcinoma), DU-145 and PC-3 cells (androgen independent human prostate cancer cells). The results of this study are also documented herein.
MATERIALS AND METHODS
Hexane (95%), thiosemicarbazide (98%) was purchased from Sigma-Aldrich (St. Louis, MO, USA). HM was prepared in the Medicinal and Process Chemistry Division of Central Drug Research Institute, Lucknow, Uttar Pradesh, India. Milli-Q pure water was obtained from a Millipore Elix water purification system (Millipore India Pvt. Ltd., New Delhi, India). high-performance liquid chromatography (HPLC) grade methanol and acetonitrile (ACN) were purchased from Merck Ltd., (Mumbai, India). IR spectra was recorded using potassium bromide (KBr) discs, on a PerkinElmer Spectrum RX1 infrared (IR) spectrophotometer. 1H and 13C nuclear magnetic resonance (NMR) spectra were recorded in dimethyl sulfoxide (DMSO) on Bruker DRX-300 (400 MHz) and JEOL AL300 Fourier transform (FT)-NMR (400 MHz) systems; chemical shift (d) is reported in ppm using tetramethyl silane as an internal reference. Mass spectra were recorded on Agilent 6520 mass spectrometer. Elemental analysis was performed on Elementar's Vario EL III micro-analyzer.
Preparation of hexane soluble fraction (Herbal Medicament)
Dried and powdered rhizomes of the C. longa (25 Kg) was taken in a percolator and kept in freshly distilled hexane (40 L) for 48 h. After this, hexane was drained and filtered. The extraction was carried out two more times and combined filtrate was concentrated in vacuum at 40°C at 300-mile bar. Finally, when around 1 L of concentrate is left, it is dried over anhydrous sodium sulfate, filtered and further concentrated, first at 300 mb and then under high vacuum to completely remove solvent impurities. Yield of the oil: About 330 g (1.3%).[12]
Preparation of NCCL extract
NCCL was prepared from hexane soluble fraction of C. longa according to our patented method.[24] The hexane soluble fraction of C. longa (HM, 5 g) was mixed with semicarbazide hydrochloride (10 g) and ethyl alcohol (100 ml, 99% pure) with a few drops of glacial acetic acid, refluxed for 48 h. Further addition of semicarbazide hydrochloride (10 g) at an interval of 5–6 h was continued till the total amount of semicarbazide hydrochloride came up to 50 g. The completion of reaction was monitored by thin layer chromatography (TLC) and finally by HPLC for the confirmation of complete removal of carbonyl portion. The reaction mixture was cooled, filtered, residual portion was washed with ethyl alcohol and filtrate was concentrated under vacuum. Further HPLC grade hexane was added to the concentrate and refluxed for 5 h for the extraction of NCCL from ethanol. It was filtered again and concentrated under vacuum. No crystal formation was observed on retaining the NCCL.
Chromatographic method for fingerprinting of NCCL
Waters HPLC (Milford, MA, USA) system used was equipped with a binary gradient pump (Model 515, Waters), an autosampler injector (Model 2707, Waters) and a diode array detector (Model 2998, Waters). Waters HPLC interface and Empower software by Waters corporation, USA was utilized for data acquisition. The analytical column RP-18e B Lichrosphere® (250 mm × 4 mm, 5 μm, Merck, Germany) at 30°C ± 3°C was used for the study. The mobile phase included ACN water (70:30, v/v) and was degassed before analysis using a Millipore vacuum pump. Column effluent was monitored at 220 nm and 254 nm (photodiode array detection) with a total runtime of 30 min. The flow rate was maintained 1.0 ml/min. and injection volume remained 20 μL.
Isolation and identification of active marker compound (I) from NCCL
NCCL (200 mg) was dissolved in ACN (5 ml) and to it a few drops of water were added and injected onto a prep HPLC column C18 (250 mm, 25 mm, 15 μm, Phenominex). The flow rate was 12 mL/min with isocratic flow of ACN and water (50:50, v/v). The peaks were monitored at 220 and 254 nm and the total run time was 40 min. The fractions were collected according to their ascent in absorbance. The fractions containing compound I was separated and concentrated. This was repeated for 3 times with 200 mg of sample loading. These fractions were further analyzed through analytical HPLC. Its purity was established by TLC and HPLC. Compound I was found to be 99.58% pure. Compound I was characterized by employing different spectral techniques such as FTIR, 1H, and 13C NMR, mass.
Molecular docking studies (in silico)
Docking was used to predict the binding orientation of drug to their protein target to in-turn predict the affinity and activity of drug which includes docking of ligand to a set of grids describing the target protein. The compound I was docked into the nucleotide-binding pocket of the ERs structure using the program AutoDock 4.0. Interestingly, this method applies a Lamarckian model of genetics, in which environmental adaptations of an individual's phenotype are reverse transcribed into its genotype and become heritable traits (sic). We have considered Lamarckian genetic algorithm, search method which can handle ligands with more degrees of freedom than the Monte Carlo simulated annealing and traditional genetic algorithm method used in earlier versions of AutoDock, and that the Lamarckian genetic algorithm is the most efficient, reliable, and successful. AutoDock 4.0, combines energy evaluation through grids of affinity potential employing various search algorithms to find the suitable binding position for a ligand on a given protein. The structure of ligand molecule (compound I) was analyzed using ChemDraw Ultra 8.0 by CambridgeSoft Corporation (Cambridge Scientific Computing, Inc.), USA. 3D, coordinates were prepared using PRODRG server. The protein structure file (Protein Data Bank [PDB]: 2I0J and 2YJD) taken from PDB (www.rcsb.org/pdb) was edited by removing the heteroatoms, adding C-terminal oxygen. All torsions were allowed to rotate during docking. Steepest descent methods were applied for minimization, using default parameters. While docking, polar hydrogen's were added to ligand using the hydrogen's module in AutoDock tool and thereafter, Kollman united atom partial charges were assigned. Docking to ligand was carried out with standard docking protocol on the basis a population size of 150 randomly placed individuals; a maximum number of 2.5 × 107 energy evaluations, a mutation rate of 0.02, a crossover rate of 0.80 and an elitism value of 1. Fifteen independent docking runs were carried out for ligand and results were clustered according to the 1.0 Ǻ rmsd criteria. The grid maps representing the proteins were calculated using auto grid and grid size was set to 60 × 60 × 60 points with grid spacing of 0.375 Ǻ. The active site, i.e., Arg-394 and Glu-305 in the protein interacts with ligand of the substrate and gives increase to the catalytic activity to test ligand that helps in determining the binding pattern of the ligand to the active site of the ER. The docking results were interpreted according to the.pdb file. We have used the coordinates of the minimum energy run created in the.dlg which was determined using the rmsd table created in the.dlg file itself. The coordinate of the docked protein along with compound I was visualized using UCSF chimera within 6.5 Ǻ region.
Anti-cancer activity (in vitro)
MCF-7, MDA-MB-231, PC-3, and DU-145 cells used in this study were obtained from the American Type Culture Collection, USA and were routinely maintained in Dulbecco's Modified Eagle Medium (Sigma-Aldrich) supplemented with 10% fetal bovine serum (Merck) and 1% antibiotic and antimycotic solution (Merck) at 37°C in a humidified incubator with 5% CO2. All stock solution of compounds (NCCL and compound I) was prepared in cell culture grade DMSO and stored in −20°C. Compounds were diluted in culture media before use in experiments. Cell viability was assessed using MTT assay, which is based on the reduction of MTT by mitochondrial dehydrogenases of viable cells to form a purple formazan product. In brief, MCF-7, MDA-MB-231, PC-3, and DU-145 cells (5 × 103/well) were plated in 96-well plates. After incubating for overnight, the cells were treated with different concentrations (200, 100, 50, 25, 12.5, and 6.25 μg/ml, in triplicates) of NCCL and compound I for 48 h. Subsequently, 10 μL of MTT (10 mg/mL) was added to each well and incubated for 3 h. The MTT formazan formed by viable cells was dissolved in 100 μL of DMSO and shaken for 10 min. The absorbance was measured at 540 nm on an ELISA reader. Each test was repeated at least 3 times. The concentration of the compound which gives the 50% growth inhibition value corresponds to IC50, calculated using GraphPad Prism 5 by GraphPad Software Inc.
RESULTS
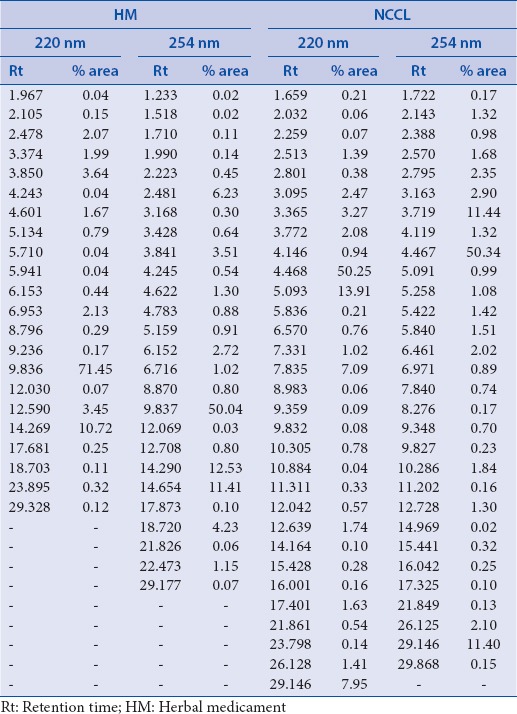
HPLC fingerprinting of blank ACN, HM, and NCCL was done and retention time and % peak area of HM and NCCL at 220 nm and 254 nm is given in Table 1.
Table 1.
Retention time and percentage peak area of herbal medicament and NCCL at 220 and 254 nm
Compound I [Figure 1], was isolated from NCCL by preparative HPLC. The fractions containing marker compound were separated and concentrated under vacuum. The marker compound, I was characterized as 7,7-dimethyl-5-(2-p-tolylpropyl)-6,7-dihydro-1,3, 4-oxadiazepin-2-amine employing different spectral techniques such as FTIR, 1H and 13C NMR, Mass. The purity of compound I was checked by TLC and HPLC and was found >99% pure.
Figure 1.
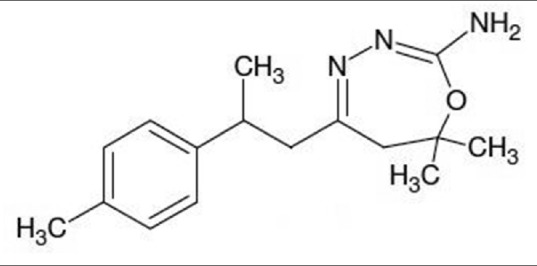
Structure of 7,7 dimethyl 5 (2 p tolylpropyl) 6, 7 dihydro 1,3,4 oxadiazepin 2 amine (compound I)
Infrared data
IR (KBr) (cm−1): 3411.7, 3015.2, 1667.2, 1564.5, 1514.7, 1448.0, 1216.1, 1054.7, 759.7, 669.1, 544.7.
Nuclear magnetic resonance data
1H NMR (400 MHz, DMSO-d6): d 7.13 (d, 2H, J = 7.68), d 7.08 (d, 2H, J = 7.56), d 5.93 (S, 2H), d 3.01 (dd, 1H, J = 6.90), d 2.5 (m, 4H), d 2.24 (s, 3H), d 1.31 (s, 3H), d 1.23(s, 3H), d 1.18 (d, 3H, J = 6.72).
13C NMR (400 MHz, DMSO-d6): d 155.41, 153.28, 142.96, 135.04, 128.86, 126.69, 61.66, 50.85, 37.98, 36.55, 26.00, 25.78, 22.29, 20.59.
Mass data
Mass: M/z 274.5 (M++1), 275.6 (M++2), HRMS m/z 274.1932 (M++1), 275.1956 (M++2).
Elemental analysis data
Anal. Calc. for C17H25N3O: C 71.04, H 8.77, N 14.62; Found: C 68.179, H 8.517, N 13.247.
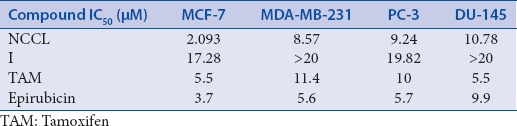
The binding poses in the docking studies are energetically favorable. The in vitro anticancer activity of NCCL and compound I was checked against four cancer cell lines namely MCF-7, MDA-MB-231, PC-3 and DU-145 at concentrations of 200, 100, 50, 25, 12.5, and 6.25 μg/ml, in triplicates. IC50 values in μM against various cell lines are given in Table 2.
Table 2.
IC50 values of NCCL and compound I in μM against various cell lines
DISCUSSION
NCCL was prepared from HM which is a hexane soluble fraction of C. longa. Compound I, 7, 7-dimethyl-5-(2-p-tolylpropyl)-6, 7-dihydro-1,3,4-oxadiazepin-2-amine (C17H25N3O), was isolated from NCCL by preparative HPLC. Compound I is characterized using 1D/2D NMR, mass spectroscopy (MS) and FT-IR techniques. The base peak in electrospray ionization-MS was found at M++1 (274.5). In IR spectra, a band at 3411.7 cm−1 indicated the N-H stretching of amine group, a strong band at 1514 cm−1 indicated the presence of conjugation in cyclic imine (C=N-). The presence of azine group (C=N-N=C) and amine group (−NH2) is indicated at by the presence of peaks at 1564 and 759 cm−1, respectively. Peaks at 1448 and 1054 cm-1 indicated the presence of methyl groups (R-CH3) and methyl benzene (C6H5-CH3), respectively. Cyclic ether (-C-O-C-) and cyclic alkene with internal double bonds are indicated by peak at 1216 and 669 cm−1, respectively. Peak at 544 cm−1 indicated the presence of monobranched alkane. 1H NMR spectra of compounds I gave a singlet between 2.24 and 1.18 ppm which indicated the presence of three protons of the methyl group (−CH3). Aiphatic-CH is indicated by the peak at 3.01 ppm. Broad singlet at 5.93 ppm was found indicating two protons attached to the nitrogen atom (−NH2). Peaks between 7.13 and 7.08 ppm indicated the presence of protons of the aromatic ring of compound. 13C spectra clearly supported the results obtained from 1H NMR spectra and also justified the number of carbon atoms in corresponding compounds.
After the achievement of isolation and characterization of the compound I, we performed in silico studies. To expose the specificity of the ERs (Er-α and Er-β) toward the target compounds, docking approach was carried out. Docking was used to predict the binding orientation of compound I to its protein target to in turn predict the affinity and activity of compound I which includes docking of ligand to a set of grids describing the target protein. The target compound I was docked into the nucleotide-binding pocket of the ERs alpha and beta (PDB: 2I0J and 2YJD). A Lamarckian genetic algorithm method, implemented in the program AutoDock 4.0, was employed. The docking of the ligand molecule (I) reveals that the inhibitor compound is exhibiting the bonding with one or the other amino acids such as Arg-394 and Glu-305 in the active pockets [Figure 2]. Compound no. I had shown the minimum binding energy of −8.72 and −9.31 kcal/mol with inhibition constant 405.35 and 149.38, respectively. The compound I had shown favorable drug-like properties and follow the Lipinski's rule of 5 with additional parameters predicted by Molsoft. The drug-likeness score of compound I is −0.82 with approximate molecular weight of 287, log P value of 4.11, log S value of −3.97, prostate-specific antigen value of 49.57, 3 hydrogen bond acceptors and 2 hydrogen bond donors.
Figure 2.
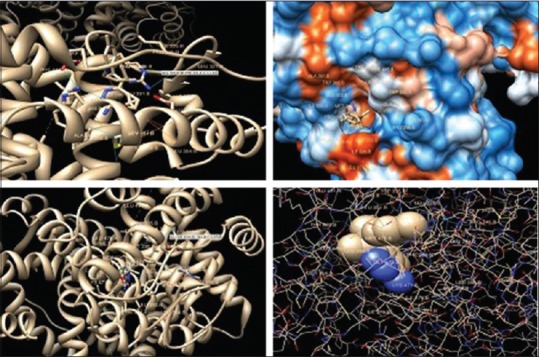
Docking of compound I into active site of estrogen receptors (estrogen receptor-α and estrogen receptor-β) showing bonding with the amino acids in the active pockets
With in silico results in hand, it was thought worthwhile to do in vitro studies to support the in silico studies. The in vitro anticancer activity of NCCL and compound I was checked against cancerous (breast and prostate) cells at different concentrations (200, 100, 50, 25, 12.5, and 6.25 μg/ml, in triplicates). The cell lines used in this investigation include MCF-7, MDA-MB-231, PC-3, and DU-145. NCCL significantly inhibited the proliferation of breast cancer cell MCF-7 (IC50~2.1 μM), and MDA-MB-231 (IC50~8.5 μM) as well as prostate cell lines PC-3 (IC50~9.2 μM) and DU-145 (IC50~10.7 μM) [Table 2].
CONCLUSION
We have reported for the first time, the synthesis of a novel chemically modified bioactive fraction from HM (NCCL) and isolation of a novel marker compound I, 7,7-dimethyl-5-(2-p-tolylpropyl)-6, 7-dihydro-1,3,4-oxadiazepin-2-amine (reaction product of ar-turmerone with semicarbazide). Their inhibitory effects on ERs were evaluated using in silico followed by in vitro studies. A molecular docking study has revealed that compound I have minimum binding energy with favorable drug-likeness scores and may be considered a good estrogen blocker. The order of NCCL sensitivity was MCF-7 cells > MDA-MB-231 cells and PC-3 > DU-145 cell. NCCL fraction having residual components induces more cell death than compound I alone. NCCL turned out to be a potent anticancer agent over compound I against these four cancer cell lines and has emerged as a promising alternative to the marketed TAM.
Further work is progress in our laboratory to evaluate their in vivo anticancer potential and in-depth mechanistic aspects.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Stratton CF, Newman DJ, Tan DS. Cheminformatic comparison of approved drugs from natural product versus synthetic origins. Bioorg Med Chem Lett. 2015;25:4802–7. doi: 10.1016/j.bmcl.2015.07.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Prasad S, Aggarwal BB. Turmeric, the Golden Spice: From Traditional Medicine to Modern Medicine. 2nd ed. Boca Raton, FL: CRC Press, Taylor and Francis; 2011. [PubMed] [Google Scholar]
- 3.Sandur SK, Pandey MK, Sung B, Ahn KS, Murakami A, Sethi G, et al. Curcumin, demethoxycurcumin, bisdemethoxycurcumin, tetrahydrocurcumin and turmerones differentially regulate anti-inflammatory and anti-proliferative responses through a ROS-independent mechanism. Carcinogenesis. 2007;28:1765–73. doi: 10.1093/carcin/bgm123. [DOI] [PubMed] [Google Scholar]
- 4.Dohare P, Garg P, Sharma U, Jagannathan NR, Ray M. Neuroprotective efficacy and therapeutic window of curcuma oil: In rat embolic stroke model. BMC Complement Altern Med. 2008;8:55. doi: 10.1186/1472-6882-8-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Prakash P, Misra A, Surin WR, Jain M, Bhatta RS, Pal R, et al. Anti-platelet effects of Curcuma oil in experimental models of myocardial ischemia-reperfusion and thrombosis. Thromb Res. 2011;127:111–8. doi: 10.1016/j.thromres.2010.11.007. [DOI] [PubMed] [Google Scholar]
- 6.Rana M, Reddy SS, Maurya P, Singh V, Chaturvedi S, Kaur K, et al. Turmerone enriched standardized Curcuma longa extract alleviates LPS induced inflammation and cytokine production by regulating TLR4–IRAK1–ROS–MAPK–NFkB axis. J Funct Foods. 2015;16:152–63. [Google Scholar]
- 7.Apisariyakul A, Vanittanakom N, Buddhasukh D. Antifungal activity of turmeric oil extracted from Curcuma longa (Zingiberaceae) J Ethnopharmacol. 1995;49:163–9. doi: 10.1016/0378-8741(95)01320-2. [DOI] [PubMed] [Google Scholar]
- 8.Negi PS, Jayaprakasha GK, Jagan Mohan Rao L, Sakariah KK. Antibacterial activity of turmeric oil: A byproduct from curcumin manufacture. J Agric Food Chem. 1999;47:4297–300. doi: 10.1021/jf990308d. [DOI] [PubMed] [Google Scholar]
- 9.Tripathi AK, Prajapati V, Verma N, Bahl JR, Bansal RP, Khanuja SP, et al. Bioactivities of the leaf essential oil of Curcuma longa (var. ch-66) on three species of stored-product beetles (Coleoptera) J Econ Entomol. 2002;95:183–9. doi: 10.1603/0022-0493-95.1.183. [DOI] [PubMed] [Google Scholar]
- 10.Roth GN, Chandra A, Nair MG. Novel bioactivities of Curcuma longa constituents. J Nat Prod. 1998;61:542–5. doi: 10.1021/np970459f. [DOI] [PubMed] [Google Scholar]
- 11.Jayaprakasha GK, Jena BS, Negi PS, Sakariah KK. Evaluation of antioxidant activities and antimutagenicity of turmeric oil: A byproduct from curcumin production. Z Naturforsch C. 2002;57:828–35. doi: 10.1515/znc-2002-9-1013. [DOI] [PubMed] [Google Scholar]
- 12.Ray M, Pal R, Singh S, Khanna NM. Herbal Medicaments for the Treatment of Neurocerebrovascular Disorders. US Patent 1814. 2006 Jan;No. 699(31) [Google Scholar]
- 13.Simon A, Allais DP, Duroux JL, Basly JP, Durand-Fontanier S, Delage C. Inhibitory effect of curcuminoids on MCF-7 cell proliferation and structure-activity relationships. Cancer Lett. 1998;129:111–6. doi: 10.1016/s0304-3835(98)00092-5. [DOI] [PubMed] [Google Scholar]
- 14.Shao ZM, Shen ZZ, Liu CH, Sartippour MR, Go VL, Heber D, et al. Curcumin exerts multiple suppressive effects on human breast carcinoma cells. Int J Cancer. 2002;98:234–40. doi: 10.1002/ijc.10183. [DOI] [PubMed] [Google Scholar]
- 15.Aggarwal BB, Kumar A, Bharti AC. Anticancer potential of curcumin: Preclinical and clinical studies. Anticancer Res. 2003;23:363–98. [PubMed] [Google Scholar]
- 16.Naksuriya O, Okonogi S, Schiffelers RM, Hennink WE. Curcumin nanoformulations: A review of pharmaceutical properties and preclinical studies and clinical data related to cancer treatment. Biomaterials. 2014;35:3365–83. doi: 10.1016/j.biomaterials.2013.12.090. [DOI] [PubMed] [Google Scholar]
- 17.Reddy BS, Banerjee R. 17Beta-estradiol-associated stealth-liposomal delivery of anticancer gene to breast cancer cells. Angew Chem Int Ed Engl. 2005;44:6723–7. doi: 10.1002/anie.200501793. [DOI] [PubMed] [Google Scholar]
- 18.Carruba G. Estrogen and prostate cancer: An eclipsed truth in an androgen-dominated scenario. J Cell Biochem. 2007;102:899–911. doi: 10.1002/jcb.21529. [DOI] [PubMed] [Google Scholar]
- 19.Baumann CK, Castiglione-Gertsch M. Estrogen receptor modulators and down regulators: Optimal use in postmenopausal women with breast cancer. Drugs. 2007;67:2335–53. doi: 10.2165/00003495-200767160-00004. [DOI] [PubMed] [Google Scholar]
- 20.Lipinski CA. Drug-like properties and the causes of poor solubility and poor permeability. J Pharmacol Toxicol Methods. 2000;44:235–49. doi: 10.1016/s1056-8719(00)00107-6. [DOI] [PubMed] [Google Scholar]
- 21.Lipinski CA, Lombardo F, Dominy BW, Feeney PJ. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv Drug Deliv Rev. 2001;46:3–26. doi: 10.1016/s0169-409x(00)00129-0. [DOI] [PubMed] [Google Scholar]
- 22.Vistoli G, Pedretti A, Testa B. Assessing drug-likeness – What are we missing? Drug Discov Today. 2008;13:285–94. doi: 10.1016/j.drudis.2007.11.007. [DOI] [PubMed] [Google Scholar]
- 23.Narender T, Sukanya P, Sharma K, Bathula SR. Apoptosis and DNA intercalating activities of novel emodin derivatives. RSC Adv. 2013;3:6123–31. doi: 10.1016/j.phymed.2013.03.015. [DOI] [PubMed] [Google Scholar]
- 24.Dwivedi AK, Naqvi A, Malasoni R, Rana M, Pandey RR, Srivastava A, et al. Indian Patent Application No. 1940/DEL/2014, Published 31 Aug. 2016 [Google Scholar]


