Abstract
Background:
In Ayurveda, five basic extraction procedures are mentioned in order of their decreasing potency. Swaras is considered as the most potent followed by, kalka, kwatha, fanta and hima.
Objective:
Present study was carried out to investigate the antioxidant and hepatoprotective potential of swaras and hima extracts of T.cordifolia and B. diffusa.
Materials and Methods:
Swaras and hima extracts of T. cordifolia and B. diffusa were prepared. Phytochemical screening and in vitro antioxidant activities was carried out using standard methods. Hepatoprotective efficacy of extracts were carried out in Swiss albino mice using paracetamol induced hepatotoxicity. Animals were administered with swaras and hima extracts of both plants at 200 mg/kg BW dose for 7 days and on 8th day hepatotoxicity was induced by intraperitoneal injection of paracetamol at 500 mg/kg BW. The degree of liver protection was determined by measuring the levels of liver enzymes followed by histopathology.
Results and Discussion:
The results of phytochemical, antioxidant and hepatoprotective activities showed that there were no significant difference between swaras and hima extracts. Both the extract of T. cordifolia were equally potent in reducing SGOT (P < 0.01) and ALP level (P < 0.001). Similar effects were observed with the Swaras and hima extracts of B. diffusa. Both the extracts reduced SGOT and ALP (P < 0.01). Histopathological findings among all the extracts were also more or less similar in lowering the paracetamol mitigated necrosis.
Conclusion:
The present study suggested that T. cordifolia and B. diffusa possess potential hepatoprotective activity irrespective of the extraction procedure.
SUMMARY
Aqueous extracts of Tinospora cordifolia and Boerhavia diffusa exhibited significant antioxidant and hepatoprotective activities
Aqueous extracts of both the plants were extracted using different extraction procedures mentioned in Ayurveda
Swaras and hima extracts of both the plants significantly reduced the deleterious effects of paracetamol, suggesting that both the plant extracts are equipotent
Acute toxicity of both the plant extracts did not produce any toxic effects.
Abbreviations used: TC swaras: T. cordifolia swaras; TC hima: T. cordifolia hima; BD swaras: B. diffusa swaras; BD hima: B. diffusa hima; BW: Body weight; LDL: Low-density lipoprotein; HDL: High-density lipoprotein; SGOT: Serum glutamate oxaloacetate transminase; SGPT: Serum glutamate pyruvate transminase; ALP: Alkaline phosphatase; I.P: Intraperitoneal; TAC: Total antioxidant capacity; DPPH: 2,2-diphenyl-1-picrylhydrazyl; TCA: Trichloro acetic acid; NO: Nitric oxide; TPC: Total phenolic content; NAPQI: N-acetyl-p-benzoquinone imine; PCM: Paracetamol.
Keywords: Antioxidant, Ayurveda, hepatoprotective, hima, histopathology, swaras
INTRODUCTION
Liver plays a vital role in various functions of the body such as metabolism, secretion, storage, and detoxification of endogenous and exogenous substances. Liver diseases are the major health problem worldwide due to unhealthy diet, pollution, environmental toxicants, infections, and sedentary lifestyle.[1] Paracetamol, a well known analgesic and antipyretic drug metabolizes in liver and produces harmful metabolites and free radicals that cause oxidative stress.
Medicinal plant-based drugs are considered as a potent therapeutic agent to reduce oxidative stress and help in the treatment of liver diseases. They contain various phytomolecules such as flavonoids and phenolic compounds, which are helpful in free radical scavenging, reduce oxidative stress, and help in regeneration of hepatocytes. Extractions of phytomolecules/bioactive agents from medicinal plants are well described in Ayurveda based on the potency known as “Panch vidhi kasaya kalpana.” “Swarasa,” the expressed juice of fresh plant; “Kalka,” fine paste of fresh plant; “Kwaatha,” the decoction;, “Faanta,” hot water infusion; and “Hima” or “Sheeta,” the cold water infusion. According to Ayurveda, swarasa is the most potent extract of any plant while hima is least potent.[2] Tinospora cordifolia (TC), commonly known as “Guduchi,” belongs to family Menispermaceae. Aqueous extract of T. cordifolia is widely used as household medicine in liver diseases. Boerhavia diffusa, belongs to family Nyctaginaceae, is commonly known as “Punarnava.” In Ayurveda and Unani systems, B. diffusa has been reported to be used in the treatment of jaundice and hepatitis.[3,4]
In the present study, swaras extract of both these plants was compared to the hima extracts. To the best of our knowledge, no previous study was conducted to validate the panch vidhi kasaya kalpna. The aim of the present study was to check the potency of both the plant extracts to substantiate the classical panch vidhi kasaya kalpana.
MATERIALS AND METHODS
Plant materials
The fresh stems of T. cordifolia and whole plant of B. diffusa were collected and authenticated by the Department of Botany and Pharmacognosy, CSIR-Central Institute of Medicinal and Aromatic Plants, Lucknow.
Preparation of extracts
T. cordifolia and B. diffusa (italics) were freshly collected from farm of CSIR-CIMAP and Swaras was made after washing and grinding the plant materials. Hima extract was prepared by soaking the shade-dried plant material overnight in distilled water and filtered using muslin cloth.[5] Thereafter, extracts were concentrated on a rotary evaporator (BUTCHI; R-210, Switzerland) and lyophilized (Labconco, Fort Scott, KS, USA). The extract was then standardized as per the WHO guidelines.[6]
Preliminary phytochemical screening
The preliminary phytochemical study of the lyophilized extract was done by standard qualitative methods.[7]
Antioxidant activity
Total antioxidant capacity
One hundred microliter of different concentrations of samples (10–200 μg/ml) reacted with 1 ml TAC reagent (0.3 N sulfuric acid, 28 mM sodium phosphate, and 4 mM ammonium molybdate), incubated at 95°C for 90 min, and the absorbance was taken at 695 nm against blank.[8]
2,2-diphenyl-1-picrylhydrazyl free radical scavenging activity
One hundred microliter of 2,2-diphenyl-1-picrylhydrazyl (DPPH) solution (0.1 M in methanol) was added to 400 μl test samples. The mixture was shaken and incubated under dark for 30 min at room temperature. Absorbance was taken at 517 nm.[9]
Reducing power estimation
Samples of 200 μl were mixed with 200 μl phosphate buffer (300 mM, 6.6 pH) and 200 μl potassium ferricyanide (1% w/v). The mixture was incubated at 50°C for 20 min, cooled, and 200 μl of trichloro acetic acid (TCA, 10% w/v) was added. Then, the mixture was centrifuged at 3000 rpm for 5 min and 100 μl of the upper layer was collected. One hundred μl of double-distilled water and 20 μl of FeCl3 (0.1% w/v) were added, and absorbance was taken at 700 nm against blank.[10]
Nitric oxide radical scavenging activity
Two hundred microliter of 10 mM sodium nitroprusside was dissolved in 0.5 ml phosphate buffer saline (pH 7.4), mixed with 25 μl of sample, and incubated for 150 min. Fifty microliter of the incubated mixture was mixed in 100 μl sulfanilamide (1% in 5% phosphoric acid), and incubated for 5 min. Exactly 100 μl of 0.1% (a-napthyl)-ethylene diamine was added to the reaction mixture, incubated for 30 min, and the absorbance was measured at 546 nm.[11]
Hydroxyl radical scavenging activity
Fifty microliter sample was mixed with 50 μl of FeSO4.7H2O (10 mM), EDTA (10 mM), 2-deoxyribose (10 mM), and 250 μl phosphate buffer (0.1 M, 7.4 pH). Exactly 50 μl of H2O2 (10 mM) was added to the reaction mixture and incubated at 37°C for 4 h. Exactly 250 μl each of TCA (2.8%) and thiobarbituric acid (1%) was added and boiled for 10 min, cooled, and the absorbance was measured at 520 nm.[12]
Total phenolic content
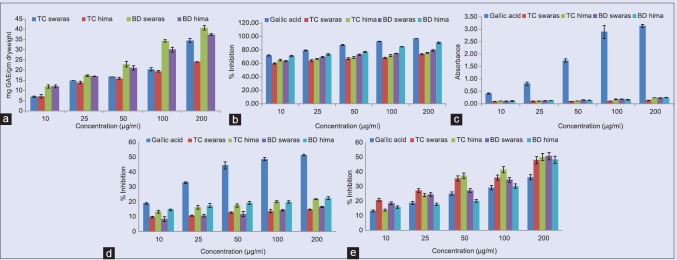
Ten microliter samples were mixed with 100 μl FCR (10% v/v) and 80 μl sodium carbonate (7.5%). The mixture was incubated at 40°C for 30 min.[13] The absorbance was measured at 765 nm using the equation obtained from a standard gallic acid calibration curve [Figure 1].
Figure 1.
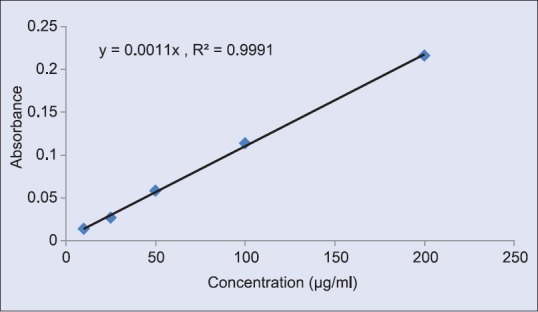
Standard curve of gallic acid
Acute toxicity study
Swiss albino mice (20–25g) were divided into three groups of six animals in each group. All the test extracts were administrated orally at single doses of 2000 μg/kg body weight (bw), following the guidelines of OECD-423.[14]
In vivo hepatoprotective activity
Test animals
Forty-two female S. albino mice weighing 25–30 g were obtained from CSIR-CIMAP, Lucknow. Animals were acclimatized at standard laboratory condition, fed with standard pellet diet (Dayal Industries, Lucknow, India) and water ad libitum. Institutional Animal Ethics Committee constituted under the CPCSEA, Government of India, New Delhi, approved the protocol of the experiment (AH-2012-10).
All the extracts were dissolved in distilled water and administered orally through intragastric tube daily for 7 days. Paracetamol overdose was used to induce liver toxicity in mice. Animals were randomly divided into seven groups of six animals each;
Group 1: Vehicle control, received distilled water orally
Group 2: Negative control, received distilled water
Group 3: Positive control, pretreated with Liv-52 for 7 days at 5.2 ml/kg.bw
Groups 4, 5, 6, and 7 served as test groups. Group 4 was pretreated with T. cordifolia swaras and Group 5 with T. cordifolia hima at 200 mg/kg.bw
Similarly, Group 6 was pretreated with B. diffusa swaras and Group 7 with B. diffusa hima at the dose of 200 mg/kg.bw.
On the 8th day, all the groups except Group 1 were given an intraperitoneal (IP) injection of paracetamol at 500 mg/kg.bw. After 2:30 h of paracetamol administration, 500 μl blood was collected from retro-orbital plexus. The serum was separated and stored at −20°C for experimental analysis.
Histopathological studies
Animals were sacrificed; liver was dissected out and washed with phosphate buffer (pH 7.4) for complete removal of blood stains and clot. Tissue was fixed in 10% formalin and embedded in molten paraffin wax followed by cutting of sections with microtome. Deparaffinized sections were then stained with hematoxylin and eosin and observed under a microscope for histopathological changes.[15]
Biochemical analysis
Serum was analyzed for different biochemical parameters using standard diagnostic kits as per manufacturer's protocol (Labkit from Merck Chemicals Private Ltd.).[16]
Statistical analysis
Statistical analyses were performed with the help of Instat GraphPad software (version 3.0, La Jolla, CA 92037 USA). Difference between means was calculated by one-way analysis of variance followed by Student–Newman–Keuls test as post hoc test (n = 6 mice/group). Results were considered statistically significant when P < 0.05.
RESULTS AND DISCUSSION
Preliminary phytochemical screening
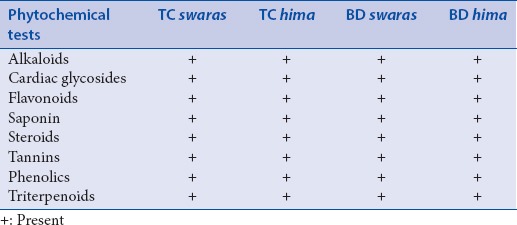
Swaras and hima extracts of T. cordifolia and B. diffusa showed the presence of major chemical compounds [Table 1].
Table 1.
Preliminary phytochemical screening
Antioxidant activity
The total antioxidant capacity of test extracts was increased with concentration [Figure 2a]. Free radical scavenging activity of all the test extracts was expressed in terms of percentage inhibition of DPPH radical. At all the concentrations of test solution, free radical was more or less inhibited [Figure 2b]. The IC50 values of swaras and hima extracts of T. cordifolia and B. diffusa are 57.77, 55.36, 53.43, and 48.38 μg/ml, respectively, as compared to gallic acid (44.96 μg/ml). Both the plant extracts did not exhibit any reducing power activity in comparison to gallic acid [Figure 2c]. Similarly, none of the test extracts scavenge nitric oxide radical (T. cordifolia: 309.77 and 214.84 μg/ml and B. diffusa: 310.27 and 204.54 μg/ml) in comparison to standard gallic acid (97.82 μg/ml) [Figure 2d]. However, all the test extracts showed scavenging activity against hydroxyl radical in a concentration-dependent manner. IC50 values of T. cordifolia extracts were 114.82 and 115.22 μg/ml and B. diffusa extracts were 123.53 and 145.13 μg/ml, as compared to gallic acid, 156.58 μg/ml [Figure 2e]. Total phenolic content was found to be 25.7, 29.70, 30.75, and 28.90 mg of gallic acid equivalent per gram dry weight for T. cordifolia and B. diffusa, respectively. These results showed that swaras and hima extracts of T. cordifolia and B. diffusa are equally potent in the inhibition of free radical generation.
Figure 2.
In vitro antioxidant activity: (a) total antioxidant capacity, (b) against 2,2-diphenyl-1-picrylhydrazyl radical, (c) reducing power estimation, (d) against nitric oxide radical, (e) against H2O2 radical
Acute toxicity study
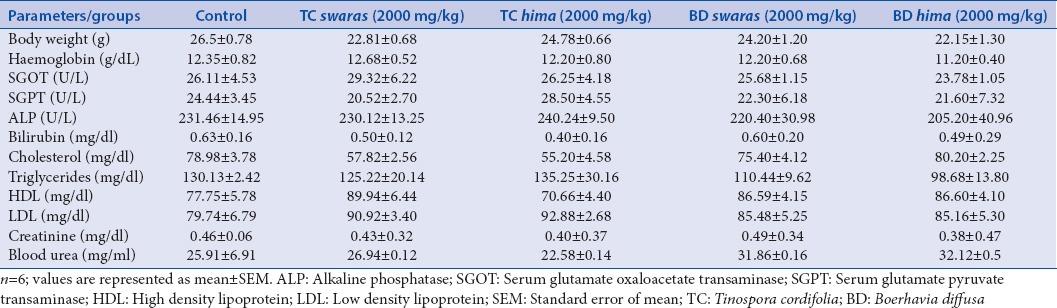
Oral administration of swaras and hima extracts of both the plants did not produce mortality in experimental mice at 2000 mg/kg.bw. Oral administration of all the extracts did not show noticeable change in body weight, food intake, and general behavior compared to the control group [Table 2].
Table 2.
Acute toxicity study on biochemical parameters
Hepatoprotective activity
The hepatoprotective efficacy of the swaras and hima extracts against paracetamol-induced hepatotoxicity is shown in Table 3. Paracetamol at 500 mg/kg IP significantly (P < 0.05) elevated the serum levels of serum glutamate oxaloacetate transminase (SGOT), serum glutamate pyruvate transminase (SGPT), alkaline phosphatase, bilirubin, total cholesterol, triglyceride, low-density lipoprotein (LDL), creatinine, urea, and uric acid, which confirmed that the paracetamol causes liver injury as well as disturbs the lipid profile and kidney functions at higher doses. On the other hand, the HDL level was decreased significantly. Pretreatment with Liv-52 syrup (5.2 ml/kg.bw) and swaras and hima extracts (200 mg/kg.bw) significantly (P < 0.001) decreases the elevated enzyme levels to normal level. The restoration effect of T. cordifolia and B. diffusa extracts is comparable with that of the standard drug Liv-52 syrup.
Table 3.
Effect of swaras and hima extracts on serum biochemical parameters
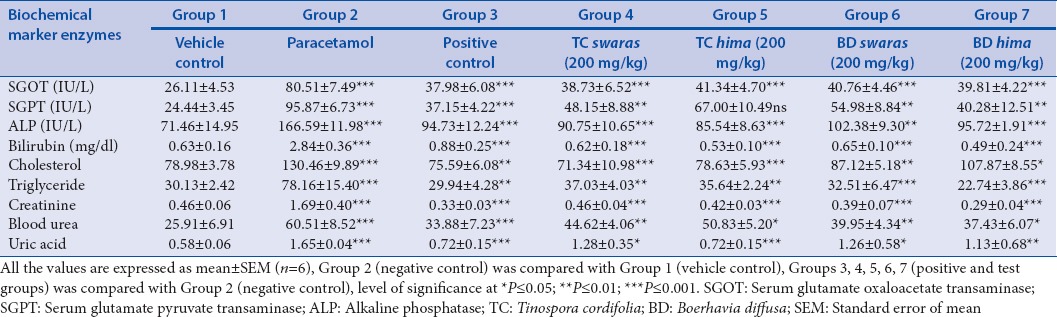
Biochemical marker levels were measured to evaluate liver injury.[17] Paracetamol-induced hepatotoxicity damages plasma membrane of hepatocytes which disturbs the transport mechanism of hepatocytes, leading to an increase in liver enzymes in blood circulation.[18] Treatment with Liv-52 syrup and swaras and hima extracts restored the increased levels of SGOT and SGPT likely by the repair of hepatic cell damage and stabilization of plasma membrane.[19] Increased ALP level is an indication of cellular cholestasis.[20] ALP level was significantly reduced in extract treated groups which indicate that plant extracts significantly protected the liver from parcetamol induced toxicity. Hyperbilirubinemia may occurs due to the over production of bilirubin which damage and reduce the ability to excrete normal amounts of bilirubin from liver.[21] The significant decrease in serum bilirubin level suggested the hepatoprotective potential of all the test extracts.
Paracetamol induced toxicity showed a significant (P < 0.001) increase in the levels of serum cholesterol, triglyceride, LDL, creatnine, urea, uric acid and decrease the level of HDL.[22] Oxidative stress involves in generation of oxygen derived species or N-acetyl-p-benzoquinone imine free radical, initiates lipid peroxidation.[23] It was reported earlier that the hypolipidemic drugs with antioxidant properties may prevent LDL peroxidation.[24] Increased serum LDL level was significantly reduced in swaras and hima extracts possibly due to their antioxidant property.[25] Paracetamol induced kidney damage was expressed by reduction in glomerular filtration rate which results increased levels of creatinine, urea and uric acid in serum. Oral administration of both swaras and hima extract significantly (P < 0.001) attenuated serum cholesterol, triglyceride, LDL, creatnine, urea and uric acid concentrations and increased the HDL concentrations compared to toxic control group. At 200 mg/kg, swaras and hima extract increased the HDL concentration, lowered the cholesterol and other parameters concentrations more than Liv-52. The rise in serum HDL concentrations and decrease in other lipid and kidney parameters suggests the stabilization and restoration of endoplasmic reticulum and kidney cells.
The hepatoprotective efficacy of the test extracts against paracetamol intoxication was further confirmed by histopathological examination of liver tissues [Figure 3].
Figure 3.
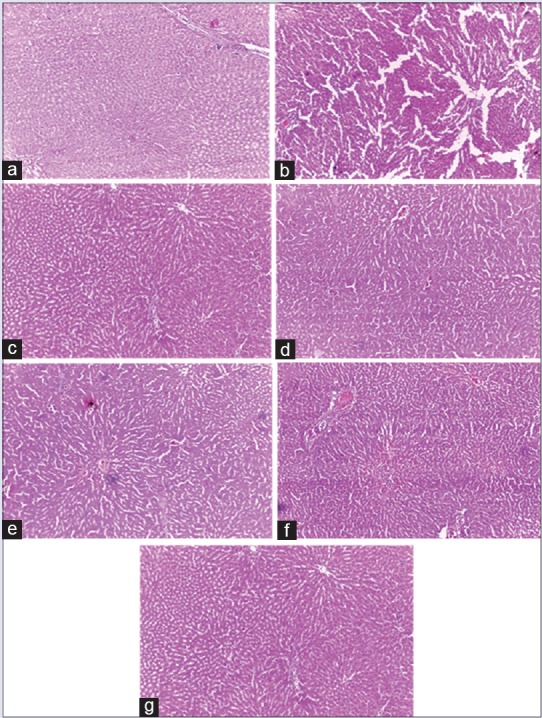
Histological observations (×40), (a) vehicle control (b) paracetamol (c) Liv. 52 (d) Tinospora cordifolia swaras (e) Tinospora cordifolia hima (f) Boerhavia diffusa swaras (g) Boerhavia diffusa hima
CONCLUSION
In the present study, swaras and hima extracts of T. cordifolia and B. diffusa express similar effects which are in contrast to the literature probably due to the presence of similar nature of compounds. Therefore the study suggests that both the hima and swaras extracts of any plant show similar effects.
Financial support and sponsorship
Financial assistance was provided by CSIR-CIMAP, Lucknow in house project OLP-08.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Rao BG, Rao YV, Rao TM. Hepatoprotective and antioxidant capacity of Melochia corchorifolia extracts. Asian Pac J Trop Med. 2013;6:537–43. doi: 10.1016/S1995-7645(13)60092-9. [DOI] [PubMed] [Google Scholar]
- 2.Savrikar SS, Ravishankar B. Bhaishajya Kalpanaa – The Ayurvedic pharmaceutics – An overview. Afr J Tradit Complement Altern Med. 2010;7:174–84. doi: 10.4314/ajtcam.v7i3.54773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Olaleye MT, Akinmoladun AC, Ogunboye AA, Akindahunsi AA. Antioxidant activity and hepatoprotective property of leaf extracts of Boerhavia diffusa Linn against acetaminophen-induced liver damage in rats. Food Chem Toxicol. 2010;48:2200–5. doi: 10.1016/j.fct.2010.05.047. [DOI] [PubMed] [Google Scholar]
- 4.Bhope SG, Gaikwad PS, Kuber VV, Patil MJ. RP-HPLC method for the simultaneous quantitation of boeravinone E and boeravinone B in Boerhavia diffusa extract and its formulation. Nat Prod Res. 2013;27:588–91. doi: 10.1080/14786419.2012.676550. [DOI] [PubMed] [Google Scholar]
- 5.Tripathi B. Saranghdhar Samhita. Varanasi, India: Chaukhambha Surbharti Prakashan; 2001. [Google Scholar]
- 6.Anonymous. Quality Control Methods of Plant Material. Geneva: World Health Organization; 2008. [Google Scholar]
- 7.Harborne J. Phytochemical Methods. London: Chapman and Hall, Ltd; 1973. p. 49. [Google Scholar]
- 8.Falleh H, Jalleli I, Ksouri R, Boulaaba M, Guyot S, Magné C, et al. Effect of salt treatment on phenolic compounds and antioxidant activity of two Mesembryanthemum edule provenances. Plant Physiol Biochem. 2012;52:1–8. doi: 10.1016/j.plaphy.2011.11.001. [DOI] [PubMed] [Google Scholar]
- 9.Yen GC, Chen HY. Antioxidant activity of various tea extracts in relation to their antimutagenicity. J Agric Food Chem. 1995;43:27–32. [Google Scholar]
- 10.Rainha N, Koci K, Coelho AV, Lima E, Baptista J, Fernandes-Ferreira M. HPLC-UV-ESI-MS analysis of phenolic compounds and antioxidant properties of Hypericum undulatum shoot cultures and wild-growing plants. Phytochemistry. 2013;86:83–91. doi: 10.1016/j.phytochem.2012.10.006. [DOI] [PubMed] [Google Scholar]
- 11.Rajesh KP, Manjunatha H, Krishna V, Swamy BE. Potential in vitro antioxidant and protective effects of Mesua ferrea Linn. bark extracts on induced oxidative damage. Ind Crops Prod. 2013;47:186–98. [Google Scholar]
- 12.Ramalingam R, Nath AR, Madhavi BB, Nagulu M, Balasubramaniam A. Free radical scavenging and antiepileptic activity of Leucas lanata. J Pharm Res. 2013;6:368–72. [Google Scholar]
- 13.Dewan SM, Amin MN, Adnan T, Uddin SM, Shahid-Ud-Daula AF, Sarwar G, et al. Investigation of analgesic potential and in vitro antioxidant activity of two plants of Asteraceae family growing in Bangladesh. J Pharm Res. 2013;6:599–603. [Google Scholar]
- 14.OECD 423: Acute Oral Toxicity-Acute Toxic Class Method. OECD Guidelines for the Testing of Chemicals. 2001:1–14. [Google Scholar]
- 15.Bharti A, Manjusha C, Preeti A, Sunil K, Nitesh C, Surender S. Hepatoprotective potential of Saraca ashoka (Roxb.). De Wilde bark by carbon tetrachloride induced liver damage in rats. Bull Fac Pharm Cairo Univ. 2015;53:23–8. [Google Scholar]
- 16.Singh DP, Mani D. Protective effect of Triphala Rasayana against paracetamol-induced hepato-renal toxicity in mice. J Ayurveda Integr Med. 2015;6:181–6. doi: 10.4103/0975-9476.146553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Girish C, Koner BC, Jayanthi S, Rao KR, Rajesh B, Pradhan SC. Hepatoprotective activity of six polyherbal formulations in paracetamol induced liver toxicity in mice. Indian J Med Res. 2009;129:569–78. [PubMed] [Google Scholar]
- 18.Rajesh MG, Latha MS. Preliminary evaluation of the antihepatotoxic activity of Kamilari, a polyherbal formulation. J Ethnopharmacol. 2004;91:99–104. doi: 10.1016/j.jep.2003.12.011. [DOI] [PubMed] [Google Scholar]
- 19.Gini KC, Muraleedhara KG. Hepatoprotective effect of Spirulina lonar on paracetamol induced liver damage in rats. Asian J Exp Biol Sci. 2010;1:614–23. [Google Scholar]
- 20.Pagana KD, Pagana T. Mosby's Manual of Diagnostic and Laboratory Tests. St. Louis: C.V. Mosby Co. Inc; 1998. [Google Scholar]
- 21.Rang HP, Dale MM, Ritter JM, Moore PK. Handbook of Pharmacology. Vol. 5. Edinburgh, New York: Churchill Livingstone; 2003. pp. 71–8. [Google Scholar]
- 22.Gross AJ, Sizer IW. The oxidation of tyramine, tyrosine, and related compounds by peroxidase. J Biol Chem. 1959;234:1611–4. [PubMed] [Google Scholar]
- 23.Younes M, Sause C, Siegers CP, Lemoine R. Effect of deferrioxamine and diethyldithiocarbamate on paracetamol-induced hepato-and nephrotoxicity. The role of lipid peroxidation. J Appl Toxicol. 1988;8:261–5. doi: 10.1002/jat.2550080407. [DOI] [PubMed] [Google Scholar]
- 24.Daugherty A, Zweifel BS, Schonfeld G. The effects of probucol on the progression of atherosclerosis in mature Watanabe heritable hyperlipidaemic rabbits. Br J Pharmacol. 1991;103:1013–8. doi: 10.1111/j.1476-5381.1991.tb12293.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Anggadiredja J, Hasanudin Sidiq A, Pratomo S, Rudyansyah A. Screening of marine algae from Warambadi seashore of Sumba island of Indonesia for antibacterial activity. Phytomedicine. 1996;37(Suppl 3):1–37. [Google Scholar]