Abstract
Background:
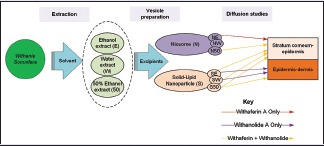
Withania somnifera is a medicinal plant native to India and is known to have anticancer properties. It has been investigated for its anti-melanoma properties, and since melanoma presents on the skin, it is prudent to probe the use of W. somnifera in topical formulations. To enhance topical drug delivery and to allow for controlled release, the use of niosomes and solid lipid nanoparticles (SLNs) as delivery vesicles were explored.
Objective:
The objective of this study is to determine the stability and topical delivery of W. somnifera crude extracts encapsulated in niosomes and SLNs.
Materials and Methods:
Water, ethanol, and 50% ethanol crude extracts of W. somnifera were prepared using 24 h soxhlet extraction which were each encapsulated in niosomes and SLNs. Franz cell diffusion studies were conducted with the encapsulated extracts to determine the release and skin penetration of the phytomolecules, withaferin A, and withanolide A.
Results:
The niosome and SLN formulations had average sizes ranging from 165.9 ± 9.4 to 304.6 ± 52.4 nm with the 50% ethanol extract formulations having the largest size. A small particle size seemed to have correlated with a low encapsulation efficiency (EE) of withaferin A, but a high EE of withanolide A. There was a significant difference (P < 0.05) between the amount of withaferin A and withanolide A that were released from each of the formulations, but only the SLN formulations managed to deliver withaferin A to the stratum corneum-epidermis and epidermis-dermis layers of the skin.
Conclusion:
SLNs and niosomes were able to encapsulate crude extracts of W. somnifera and release the marker compounds, withaferin A, and withanolide A, for delivery to certain layers in the skin.
SUMMARY
Withania somnifera crude extracts were prepared using ethanol, water, and 50% ethanol as solvents. These three extracts were then incorporated into niosomes and solid lipid nanoparticles (SLNs) for use in skin diffusion studies, thus resulting in six formulations (ethanol niosome, water niosome, 50% ethanol niosome, ethanol SLN, water SLN, and 50% ethanol SLN). The diffusion of two marker compounds (withaferin A and withanolide A) from the formulations into the skin was then determined.
Abbreviations used: API: Active pharmaceutical ingredient, ANOVA: Analysis of variance, ED: Epidermis-dermis, HPLC: High-performance liquid chromatography, HLB: Hydrophilic-lipophilic balance, NMR: Nuclear magnetic resonance spectroscopy, PDI: Polydispersity index, SLN: Solid lipid nanoparticle, SD: Standard deviation, SCE: Stratum corneum-epidermis, TEM: Transmission electron microscopy.
Keywords: Ashwagandha, niosomes, skin diffusion, solid lipid nanoparticles, stability, tape-stripping, Withania somnifera
INTRODUCTION
Withania somnifera (also known as Ashwagandha, Indian ginseng or winter cherry) is a plant well-known for its diverse medicinal properties in the Ayurveda system of natural medicine. Extracts from the plant leaves have a high anti-oxidant potential, and they contain a high concentration of bioactive compounds.[1,2] Therefore, the plant leaves were used in this study as the aim was to prepare formulations that contain different W. somnifera extracts for potential use in the treatment of skin conditions such as skin cancer (melanoma) and aging. The main bioactive compounds in W. somnifera are steroidal lactones collectively known as withanolides.[2,3] Throughout this study, the main focus was on withaferin A and withanolide A as bioactive marker molecules, which are known to be present in the leaves of W. somnifera.[4]
Some of the medicinal properties of W. somnifera that have been identified to date include antidiabetic, antihypertensive, antibacterial, antiaging, and anticancer properties.[5,6] The plant extract is currently available on the market as a powder, tonic, and as capsules.[2] In this study, it was decided to encapsulate different W. somnifera crude extracts in niosomes and solid lipid nanoparticles (SLNs) for topical delivery to the skin.
The skin is the body's first line of defense and is thus rather impermeable to any foreign substances.[7,8] Nanovesicles such as niosomes, SLNs, liposomes, ethosomes, and ufosomes are being investigated for use in the delivery of medicinal compounds to and through the skin.[9,10] Nanoparticles are advantageous in cancer therapy because they can aid in the transport of therapeutic agents through barriers such as the skin, improve the pharmacokinetic profile of medicinal agents, and they can be used for targeted drug delivery (e.g., dermal vs. transdermal delivery).[11,12] Niosomes are known to enhance the absorption of compounds through the skin, increase physicochemical stability of compounds and protect the skin from the potential irritating effects of medicinal compounds.[13,14] Various plant extracts have been successfully encapsulated in niosomes and delivered to the skin;[9,10] hence, the use of niosomes in the topical delivery of W. somnifera crude extract. SLNs have been reported to be suitable for topical drug delivery, resulting in reduced systemic delivery of medicinal compounds due to controlled and targeted drug delivery.[15,16]
The aim of this study was to prepare three different W. somnifera crude extracts and encapsulate these extracts in niosomes and SLNs for use in Franz cell diffusion studies. A stability assessment of the formulations was conducted to determine if certain marker molecules in the extracts remained stable in the niosomes and SLN.
MATERIALS AND METHODS
Materials
The withaferin A and withanolide A USP analytical standard compounds were purchased from ChromaDex (Irvine, California, USA). Ethanol and methanol for plant extractions and analytical standard preparation were purchased from Associated Chemical Enterprises (Johannesburg, South Africa). High-performance liquid chromatography (HPLC) grade acetonitrile and deuterated chloroform (CDCl3) were obtained from Merck Chemicals (Johannesburg, South Africa). The Compritol 888 ATO (glyceryl dibehenate) that was used for formulation of the SLN was a generous gift from Gattefossè (Lyon, France).
Preparation of plant extracts
W. somnifera leaves were purchased from Mountain Herb Estate Nursery (Kameeldrift-West, Pretoria, South Africa) and authenticated at the South African National Biodiversity Institute National Herbarium (Pretoria, South Africa). Plant leaves were cleaned, air-dried then crushed to a fine powder on receipt. A 24 h soxhlet extraction was used to prepare three separate crude extracts from the leaf powder using water, ethanol, and ethanol/water (50:50) as the solvents. After the soxhlet extraction, the ethanol was evaporated using a rotary evaporator, and the water was removed by using a freeze dryer (VirTis, Gardiner, NY, USA). The dry end-products were stored in glass containers, protected from light at −20°C.
Chemical characterization of Withania somnifera extracts with nuclear magnetic resonance spectroscopy
For each individual extract, approximately 50 mg of plant extract was weighed out and dissolved in 1.5 ml deuterated chloroform then filtered into a nuclear magnetic resonance (NMR) tube to remove any undissolved residue. Both 1H-NMR and C13-NMR spectra were obtained using an Avance III 600 Hz NMR Spectrometer (Bruker, Rheinstetten, Germany).
Chemical characterization of Withania somnifera extracts with high-performance liquid chromatography
The HPLC analytical method was developed in the Analytical Technology Laboratory of the North-West University, Potchefstroom, South Africa. This method was used for chemical fingerprinting of the plant extracts and for the detection of the marker compounds (withaferin A and withanolide A) throughout the study. The separation was carried out on an Agilent 1100 series HPLC equipped with a quarternary gradient pump, autosampler, diode array detector, and Chemstation A.10.01 data acquisition and analysis software (Agilent, Palo Alto, CA, USA) on a Venusil XBP C18 (2), 150 mm × 4.6 mm, 5 mm column (Agela Technologies, Newark, DE). A gradient elution method was used, in which mobile phase A was HPLC-grade water and mobile phase B was 100% acetonitrile. The run was started at 10% acetonitrile with a linear gradient to reach 100% acetonitrile after 10 min and holding to 20 min before reequilibrating at the start conditions. The flow rate, injection volume, detection wavelength, and stop time were set to 1 ml/min, 50 ml, 210 nm, and 22 min, respectively. The standard solutions and samples for chemical finger-printing were all prepared using analytical grade methanol and HPLC-grade water.
To obtain a chemical finger-print of W. somnifera crude extracts 10 mg of plant extract was dissolved in 1 ml of methanol with the aid of sonication and topped to 10 ml using Milli-Q water. The resulting solution was filtered then analyzed using HPLC.
Formulation of niosomes and solid lipid nanoparticles
The solvent injection method was utilized for the formulation of both the niosomes and SLN. Pando et al. reported that the solvent injection method for niosome formulation resulted in a higher resveratrol encapsulation efficiency (EE) and higher stability. Preformulation studies confirmed that this method of preparation of the nanoformulations was acceptable for encapsulation of the W. somnifera crude extracts.[17]
For the niosomes, a 2:1 mixture of surfactant (tween 80/span 60) and cholesterol (w/w) were dissolved in diethyl ether while the aqueous phase was heated to 60°C ± 2°C. The diethyl ether solution was slowly injected using a hypodermic needle into the preheated aqueous phase. When it came to the SLNs, a 2:1:1 mixture of surfactant, compritol 888ATO, and L-α-phosphatidylcholine (w/w) was weighed and dissolved in the organic solvent. This organic phase was then slowly injected into a preheated (60°C ± 2°C) aqueous phase. The organic and aqueous phases were continuously magnetically stirred, and the temperature maintained at 60°C ± 2°C until the organic solvent was driven off. The resulting formulation was cooled and sonicated on ice using a Hielscher UP 200ST sonicator (Hielscher Ultrasound Technology, Teltow, Germany). The ethanol and 50% ethanol extracts (2.0% w/w) were added to the organic phase while the water extract was added to the aqueous phase before the injection step. Zorzi et al. advise that a maximum of 2.0% crude extract should be incorporated into nanoformulations.[18] In total, six formulations were prepared as one niosome and one SLN formulation was prepared for each extract.
Physicochemical characterization of formulations
The physicochemical characteristics of the niosomes and SLN formulations that were assessed in this study include morphology, particle size, zeta-potential, polydispersity index (PDI), pH, and EE (withaferin A and withanolide A). Transmission electron microscopy (TEM) was used to visualize the morphology of the formulations. Zeta-potential, size, and PDI were measured using a Zetasizer Nano ZS (Malvern Instruments, Worcestershire, UK). Approximately 1 ml of each formulation was injected respectively into a disposable folded capillary cell for zeta-potential measurement, and the reading was taken using the Zetasizer Nano ZS. Freshly prepared formulations had their pH measured at 25°C using a Mettler Toledo pH meter (Mettler Toledo, Columbus, OH, USA). EE of the formulations was determined according to the method as described by Junyaprasert et al.[19] Briefly, the formulations were centrifuged in an Optima L-100XP ultracentrifuge (Beckman Coulter, Brea, California, USA) for 30 min at a speed of 30,000 rpm and temperature of 4°C. The supernatant was then diluted and analyzed using the HPLC analytical method for withaferin A and withanolide A. The percentage EE (%EE) was then calculated as follows:

Stability testing of formulations
A 3-month temperature stability assessment was conducted on lyophilized niosomes and SLNs. Niosomes and SLNs were formulated according to the described method, lyophilized using a VirTis freeze-dryer (Gardiner, NY, USA), and stored at room temperature for 3 months. The formulations were kept in temperature-controlled laboratories at a temperature of 22°C. The formulations were resuspended in Milli-Q water then particle size, zeta-potential, pH and %EE were measured after 0, 7, 14, 28, 56, and 84 days.
Skin preparation for skin diffusion studies
Caucasian, female, abdominal skin obtained from abdominoplasty patients was used for the skin diffusion studies. Informed consent was obtained from each patient, and the NWU Research Ethics Committee gave approval for obtaining, preparing, and using human excised skin for research purposes (Ethical approval number – NWU-00114-11-A5). The collected skin was inspected for imperfections such as holes and stretch marks so that such areas would be excluded from the experimental skin samples. The split-thickness skin at a thickness of 400 μm was prepared using a Zimmer® dermatome (Warsaw, IN, USA). The skin was placed on Whatmann® filter paper, wrapped in foil, placed in Ziploc® bags then frozen at −20°C for not more than 3 months.
Franz cell diffusion studies
Franz cell membrane diffusion studies were done to determine the withaferin A and withanolide A release characteristics of the niosomes and SLNs. Subsequent to the membrane diffusion studies, skin diffusion studies were performed to assess the diffusion of withaferin A and withanolide A into and through the skin. Static Franz diffusion cells with a diffusion area of 1.075 cm2 and receptor capacity of at least 2 ml were used for the membrane diffusion studies and the skin diffusion studies.
The formulations were prewarmed to 32°C (temperature at the surface of the skin),[20] and phosphate buffer solution (0.06 M NaOH and 0.08 M KH2PO4, pH 7.4) was prewarmed to 37°C (physiological temperature) in appropriately set water baths. This was done to mimic in vivo conditions.[20,21] The donor and receptor compartments were greased with Dow Corning® vacuum grease, and a magnetic stirring rod was placed inside the receptor compartment. A 0.45 μm polytetrafluoroethylene membrane filter (Whatman Plc, Maidstone, UK) or piece of skin (stratum corneum facing the donor compartment) was placed between the donor compartment and receptor phase. To avoid leaks, the two compartments were sealed and fastened together using vacuum grease and a horseshoe clamp. Two milliliters of buffer solution were added to the receptor compartment, and 1.0 ml of formulation was added to the donor compartment. Ten samples from the same skin donor (n = 10) were setup, and two Franz cells were setup with placebo formulations as the controls. The Franz cells were placed on a Franz cell stand in a 37°C water bath with a Variomag® magnetic stirrer to stir the receptor phase and keep it homogenous. The receptor phase was extracted at predetermined time intervals and replaced with fresh buffer. The extracted receptor phase was then analyzed using HPLC. Extractions for membrane diffusion studies were done every hour up to 6 h while a single extraction after 12 h was done for the skin diffusion studies.
Tape-stripping studies
The tape-stripping technique was used to determine the amounts of withaferin A and withanolide A that had permeated into the different skin layers. This technique works by selectively removing the upper skin layer and analyzing for the amount of compound within the stripped layer.[22,23] The method as described by Pellet et al.[24] was followed for the tape-stripping study. After the skin diffusion study was completed, the skin was cleaned using a paper towel to remove any unabsorbed drug. Thereafter, a piece of 3M Scotch® Magic tape was applied to the diffusion area, removed and discarded to strip off any unabsorbed compound on the skin surface. The stripping process was repeated with 15 pieces of tape, and these tape strips were all placed into a polytop containing 5 ml of phosphate buffer solution. The remaining piece of skin was cut into small pieces to increase surface area and placed into a polytop containing 5 ml of phosphate buffer solution. This process was repeated for each Franz cell, and the polytops were stored at 4°C overnight. On the following morning, the buffer solution was filtered into appropriately labeled HPLC vials, and the samples were analyzed using the HPLC analytical method.
Statistical analysis
Statistical analysis of the Franz cell diffusion data was done using Statistica data analysis software system (StatSoft Inc., version 12 [2015], Tulsa, Oklahoma, USA). The mean and median flux values were calculated for each experiment. A one-way and a two-way analysis of variance were done together with t-tests to determine any significant differences within and between the different experiments.
RESULTS AND DISCUSSION
Chemical characterization of Withania somnifera extracts
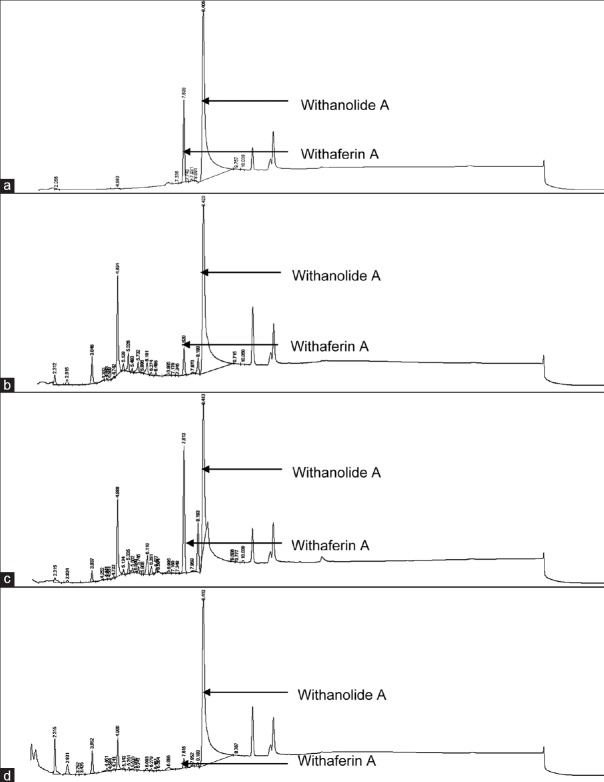
The HPLC analytical method was robust and suitable for use in the analysis of both withaferin A and withanolide A. Withaferin A eluted at approximately 7.5 min and withanolide A at 8.5 min. Figure 1 shows the chromatograms of the individual standard compounds and those of the crude extracts. The analysis of the crude plant extracts revealed that the withaferin A (w/w) content of the extracts was 0.98% w/w (water extract), 1.76% w/w (ethanol extract), and 4.55% w/w (50% ethanol extract), respectively. The withanolide A content of the three extracts was 5.04% w/w (water extract), 1.21% w/w (ethanol extract) and 3.04% w/w (50% ethanol extract), respectively.
Figure 1.
High-performance liquid chromatography chromatograms of withaferin A and withanolide A standards (a), ethanol extract (b), 50% ethanol extract (c), and water extract (d) for high-performance liquid chromatography finger-printing
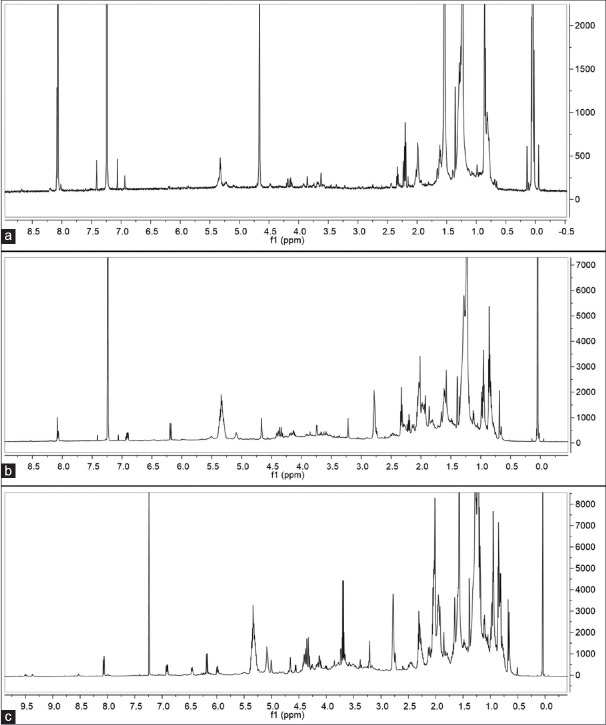
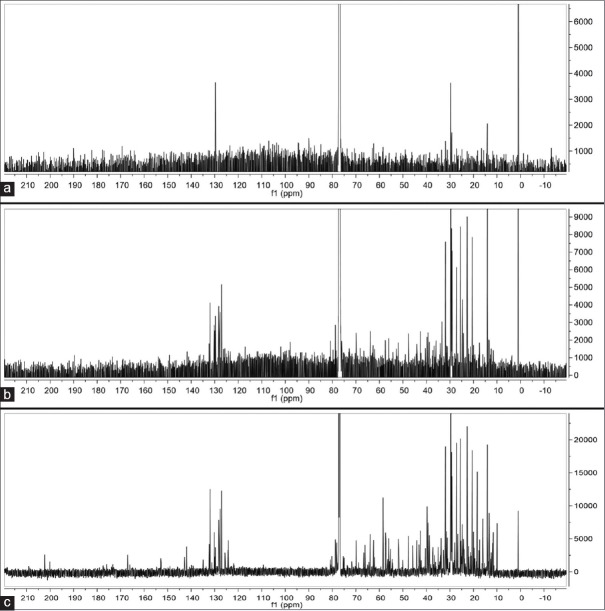
The 1H-NMR and C13-NMR spectra of the different W. somnifera crude extracts are shown in Figures 2 and 3, respectively.
Figure 2.
1H-nuclear magnetic resonance spectra of water (a), 50% ethanol (b), and ethanol (c) crude extracts for nuclear magnetic resonance fingerprinting
Figure 3.
C13-nuclear magnetic resonance spectra of water (a), 50% ethanol (b), and ethanol (c) crude extracts for nuclear magnetic resonance fingerprinting
Physicochemical characterization of formulations
The physicochemical properties of all the formulations are summarized in Table 1. The mean of three independent experiments is shown ± standard deviation. Figure 4 shows the TEM micrographs of the formulated placebo niosomes and SLNs.
Table 1.
Average values for the physicochemical properties of freshly prepared formulations±standard deviation (n=3)
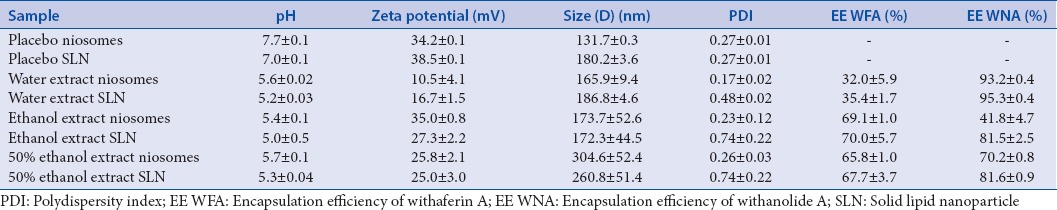
Figure 4.
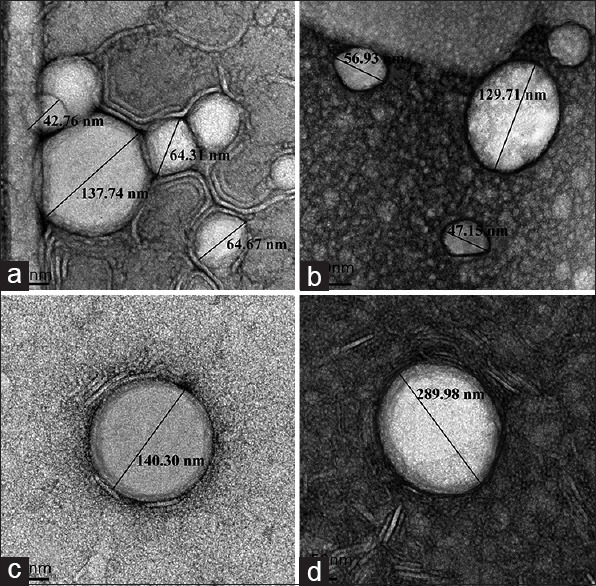
Transmission electron micrographs of placebo niosomes (a and b) and placebo solid lipid nanoparticles (c and d)
The 50% ethanol formulations displayed with relatively larger average particle sizes as compared to the other formulations. The different chemical compositions of the crude extracts possibly played a role as the 50% ethanol extract was expected to contain both polar and nonpolar compounds due to the presence of both an aqueous solvent and an organic solvent during the extraction process. All the freshly prepared formulations had pH values that were between 5.017 and 5.709 which is considered safe for topical application as the skin's pH lies between 4 and 6.[21,25] SLNs were generally the least homogenous and this was probably due to the high lipid content of the SLNs. It is possible that the lipids were affected by the energy released during the sonication process resulting in aggregation of some particles, thus resulting in relatively higher PDI values. Becker Peres et al. found that a long sonication time (90 s) resulted in an increase in particle size. This was possibly due to slight destabilization which resulted in very small droplets that could not be completely covered by the surfactant in the formulation.[26] The presence of water-soluble compounds in the water extracts possibly contributed to the low absolute zeta-potential values of the water extract formulations by reducing the cohesive properties of the formulations. Use of a higher concentration of a high hydrophilic-lipophilic balance surfactant may be able to improve the issues to do with the stability of the water extract formulations. These water extract formulations had a very low percentage encapsulation of withaferin A but a high withanolide A percentage encapsulation. It is apparent that a change in formulation (SLN vs. niosome) did not cause any major changes in particle size and EE of withaferin A. The SLNs exhibited a slightly higher encapsulation of both withaferin A and withanolide A than the respective niosome formulations. This effect of the SLNs was more apparent for the withanolide A as compared to the withaferin A and it may have been due to the different physicochemical properties of the two compounds. Both vesicle types managed to encapsulate withaferin A and withanolide A from all the extracts. The highest percentage encapsulation (95.3%) that was obtained was for withanolide A from the water extract SLNs. It is, however, possible that the nonencapsulated extract compounds could have been solubilized in the external aqueous phase or adsorbed on the surface of the carrier vesicles instead of being encapsulated in the vesicles.[18]
Stability testing of formulations
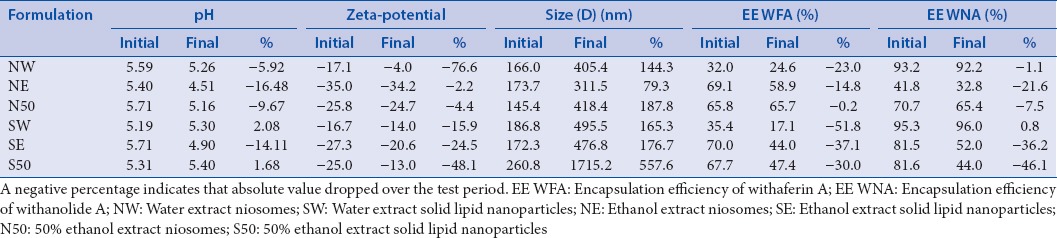
The changes that transpired over the 84 days test period have been summarized in Table 2. The changes in the pH values of the formulations ranged from 0.089 (50% ethanol extract SLNs) to 0.890 (ethanol extract niosomes) over the 3 months period. The final values were still within an acceptable range for topical application. Zeta-potential measurements of some of the formulations fluctuated over the 3 months period with the changes per formulation ranging from 0.78 mV (ethanol extract niosomes) to 13.12 mV (water extract niosomes). The formulations which had the most electronegative initial zeta-potential values (ethanol extract niosomes and SLNs) exhibited the smallest fluctuations with respect to zeta-potential measurements, thus implying that these formulations were relatively stable colloidal systems. At the end of the 3-month testing period, all the average particle sizes were above 300 nm with the ethanol extract niosomes having the smallest change (137.73 nm) and the 50% ethanol extract SLN having the largest size increase (1454.33 nm). The changes in the PDI values ranged from 0.001 (water extract SLN) to 0.185 (50% ethanol extract SLN). Changes in percentage encapsulation of withaferin A ranged from 2.03% (50% ethanol extract niosomes) to 26.00% (ethanol extract SLN) while the changes for withanolide A ranged from 0.72% (water extract SLN) to 37.61% (50% ethanol extract SLN). Percentage encapsulation efficiencies of both withaferin A and withanolide A generally varied the most with the SLN formulations as compared to the niosome formulations. SLNs stored at 4°C are said to have better stability as compared to SLNs stored at room temperature; therefore, the higher storage temperature may have been responsible for the instability that was observed.[27] Stability problems of nanovesicles are usually due to postformulation expulsion of the active pharmaceutical ingredient (API) and particle aggregation. To increase the stability of nanoformulations, it may be necessary to increase surfactant content as this increases the physical stability of the nanoparticles and also results in a high concentration of smaller nanoparticles.[28] The relative instability of the formulations that was observed may have been due to the presence of sodium cholate which is capable of accelerating degradation of formulations in the long-term.[29]
Table 2.
Mean initial (day 0) and final (day 84) physicochemical values recorded for the stability study with an indication of percentage change over the period
Initial physicochemical characterization was done on freshly prepared samples while the stability experiments were done after freeze-drying. The formulations in this study were freeze-dried in the absence of a lyoprotectant such as sucrose, mannitol, or trehalose and this may have been responsible for some of the instability issues encountered.[30] Reports have been made that lyophilization of nanoformulations can cause instability with respect to particle aggregation, physical properties, osmolarity, pH, and drug loading.[31] The absence of lyoprotectants during freeze-drying can also affect the EE of compounds in liposomes, and a similar phenomenon may also occur with encapsulation in niosomes and SLNs.[30] The presence of many unidentified compounds in the crude extract may have also contributed to the physicochemical changes that were detected.
Franz cell diffusion studies
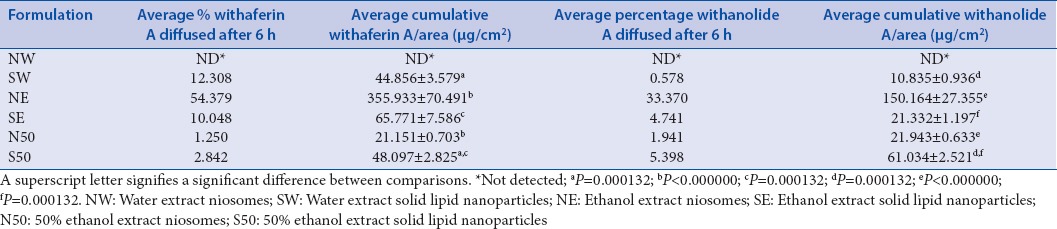
The average percentage release of withaferin A and that of withanolide A after the 6 h membrane diffusion was calculated after the membrane release experiment and is represented in Table 3. Average cumulative amount of withaferin A released per unit area is also shown as is that of withanolide A.
Table 3.
Total amount of marker compound released as a percentage of initial amount in donor formulation and average cumulative amount of marker compound released after the 6 h membrane diffusion studies±standard deviation (n=10)
After the 12 h, skin diffusion study neither withaferin A nor withanolide A was detected in the receptor phase. This led to the assumption that the compounds had only been retained within the skin and had not permeated through the skin to reach receptor phase, so the tape-stripping study was conducted to determine the quantities of these marker molecules in the distinctive layers of the skin.
Tape-stripping studies
The average concentration of each compound that was detected in the stratum corneum epidermis and in the epidermis-dermis was calculated and tabulated in Table 4. A comparison was made between the amount of marker compound that reached the two skin layers, and a statistically significant difference was detected. This implied that the difference was due to effect of the physical, biological, and chemical differences between the stratum corneum epidermis and epidermis-dermis. The extent of the skin penetration of the marker compounds was different for each formulation. Only the 50% ethanol extract SLNs managed to deliver both withaferin A and withanolide A to both the epidermis and dermis. Permeation to the epidermis-dermis level was only achieved by the SLN formulations. It is thus conceivable that the SLNs had a greater ability to deliver withaferin A and withanolide A to the deeper skin layers as compared to the niosomes. In any study, it is imperative to select the most appropriate nanocarrier as it will result in the required amounts of API reaching the desired skin layers.[21]
Table 4.
Average concentrations of withaferin A and withanolide A that remained in the epidermis and dermis after the 12 h skin diffusion studies (n=10)
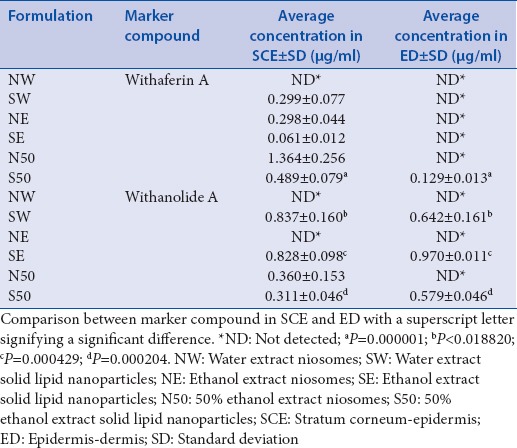
The 50% ethanol SLNs were the only formulation which was able to deliver both withaferin A and withanolide A to the stratum corneum–epidermis and the epidermis–dermis. These results suggested that the 50% ethanol SLN formulation was the optimum formulation as it was capable of delivering the two marker compounds to the target skin layers for topical cancer chemotherapy. Melanoma penetrates vertically into the dermis before metastasizing; therefore, delivery of an API into the dermis is ideal for potential skin cancer treatment. The 50% ethanol niosomes, however, depicted the highest average concentration of withaferin A in the stratum corneum-epidermis (1.364 μg/ml), followed by 50% ethanol SLNs (0.489 μg/ml), water SLNs (0.299 μg/ml), ethanol niosomes (0.298 μg/ml), and finally, the ethanol SLNs (0.061 μg/ml). The withaferin A content of the extracts influenced the permeation of withaferin A into the skin as is reflected by the 50% ethanol SLNs and niosomes resulting in the highest concentrations of withaferin A in the stratum corneum-epidermis. The 50% ethanol extract contained 4.55% withaferin A which was considerably higher than the withaferin A content of the water (0.98%) and ethanol (1.76%) extracts.
With respect to withanolide A, all the SLN formulations resulted in withanolide A reaching the stratum corneum-epidermis and the epidermis-dermis while the 50% ethanol extract niosomes only managed to result in withanolide A reaching the stratum corneum-epidermis. The SLNs were clearly superior to niosomes in terms of the ability to deliver withaferin A and withanolide A to the deeper skin layer (epidermis-dermis). This is similar to what was observed by Dwivedi et al., in the topical delivery of artemisone whereby SLNs delivered artemisone to the stratum corneum-epidermis and epidermis-dermis while the niosomes only delivered artemisone to the stratum corneum-epidermis.[32] The lack of ability to penetrate right through the skin barrier may be the reason why niosomes have been conventionally used for topical delivery of APIs to the stratum corneum versus transdermal delivery of APIs.[10] The occlusive effect of SLNs which inhibits transepidermal water loss may have influenced this observed result of the SLNs.[31]
None of the marker compounds were detected in stratum corneum-epidermis or the epidermis-dermis after the water extract niosome diffusion study, which was consistent with the membrane release and skin diffusion results. The lack of information with respect to all the phytocompounds in all the crude extracts makes it difficult to account for all the differences that were observed. This, however, reflects that relative high variation can be expected when it comes to the medicinal use of plant extracts as there is no set standard for composition and expected effects or outcomes. There is a need for standardized plant extracts or methods for extract preparation so as to ensure that the expected treatment outcomes are achieved.[33] The use of pure compounds to avoid issues due to complex mixtures may be tempting, but it is has been found that isolation of pure compounds at times resulted in loss of activity, chemical instability, and eliminates possible synergism.[18]
CONCLUSION
The results of the membrane release studies showed that withaferin A and withanolide A were released from the niosome and SLN formulations to varying extents. Therefore, these compounds would be available for diffusion into and through the skin. The different release characteristics of the formulations and the differences in the skin samples were partly responsible for the differences that were observed for the skin diffusion studies.[34]
During the 12 h skin diffusion study, relatively low concentrations of withaferin A and withanolide A diffused into the skin. Possibly a longer time frame would have resulted in higher concentrations of compound being detected since SLNs are said to allow for sustained release of encapsulated APIs into the skin as the API must firstly diffuse through the solid lipid matrix.[35] It has been suggested that nanovesicles above 20 nm in diameter do not permeate the skin but rather accumulate in hair follicles where they act as API reservoirs. It is possible that the marker compounds in this study were slowly being released from the nanovesicle reservoirs and penetrating the skin barrier to reach the stratum corneum epidermis and epidermis-dermis.[8] Withaferin A and withanolide A being fairly lipophilic compounds could easily overcome the stratum corneum barrier, but the aqueous layer beneath the horny layer was possibly the biggest deterrent when it came to reaching the deeper layers (dermis).[34] In this study, it was also revealed that a high EE does not necessarily correlate with a high drug release and skin permeation as other researchers have also reported.[17]
Financial support and sponsorship
The authors would like to sincerely thank the Centre of Excellence for Pharmaceutical Sciences, Faculty of Health Sciences, North-West University, South Africa and the National Research Foundation of South-Africa for their financial contribution to this project (Grant # - CPRR13091742482). Any opinion, findings and conclusions or recommendations expressed in this material are those of the authors and therefore the NRF does not accept any liability in regard thereto.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Fernando ID, Abeysinghe DC, Dharmadasa RM. Determination of phenolic contents and antioxidant capacity of different parts of Withania somnifera (L.). Dunal from three different growth stages. Ind Crops Prod. 2013;50:537–9. [Google Scholar]
- 2.Kulkarni SK, Dhir A. Withania somnifera: An Indian ginseng. Prog Neuropsychopharmacol Biol Psychiatry. 2008;32:1093–105. doi: 10.1016/j.pnpbp.2007.09.011. [DOI] [PubMed] [Google Scholar]
- 3.Ganzera M, Choudhary MI, Khan IA. Quantitative HPLC analysis of withanolides in Withania somnifera. Fitoterapia. 2003;74:68–76. doi: 10.1016/s0367-326x(02)00325-8. [DOI] [PubMed] [Google Scholar]
- 4.Malik F, Kumar A, Bhushan S, Mondhe DM, Pal HC, Sharma R, et al. Immune modulation and apoptosis induction: Two sides of antitumoural activity of a standardised herbal formulation of Withania somnifera. Eur J Cancer. 2009;45:1494–509. doi: 10.1016/j.ejca.2009.01.034. [DOI] [PubMed] [Google Scholar]
- 5.Nagella P, Murthy HN. Establishment of cell suspension cultures of Withania somnifera for the production of withanolide A. Bioresour Technol. 2010;101:6735–9. doi: 10.1016/j.biortech.2010.03.078. [DOI] [PubMed] [Google Scholar]
- 6.Winters M. Ancient medicine, modern use Withania somnifera and its potential role in integrative oncology. Altern Med Rev. 2006;11:269–77. [PubMed] [Google Scholar]
- 7.Lam PL, Gambari R. Advanced progress of microencapsulation technologies In vivo and in vitro models for studying oral and transdermal drug deliveries. J Control Release. 2014;178:25–45. doi: 10.1016/j.jconrel.2013.12.028. [DOI] [PubMed] [Google Scholar]
- 8.Prow TW, Grice JE, Lin LL, Faye R, Butler M, Becker W, et al. Nanoparticles and microparticles for skin drug delivery. Adv Drug Deliv Rev. 2011;63:470–91. doi: 10.1016/j.addr.2011.01.012. [DOI] [PubMed] [Google Scholar]
- 9.Yeh MI, Huang HC, Liaw JH, Huang MC, Huang KF, Hsu FL. Dermal delivery by niosomes of black tea extract as a sunscreen agent. Int J Dermatol. 2013;52:239–45. doi: 10.1111/j.1365-4632.2012.05587.x. [DOI] [PubMed] [Google Scholar]
- 10.Manosroi A, Chankhampan C, Manosroi W, Manosroi J. Transdermal absorption enhancement of papain loaded in elastic niosomes incorporated in gel for scar treatment. Eur J Pharm Sci. 2013;48:474–83. doi: 10.1016/j.ejps.2012.12.010. [DOI] [PubMed] [Google Scholar]
- 11.Sanna V, Siddiqui IA, Sechi M, Mukhtar H. Nanoformulation of natural products for prevention and therapy of prostate cancer. Cancer Lett. 2013;334:142–51. doi: 10.1016/j.canlet.2012.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Estanqueiro M, Amaral MH, Conceição J, Sousa Lobo JM. Nanotechnological carriers for cancer chemotherapy: The state of the art. Colloids Surf B Biointerfaces. 2015;126:631–48. doi: 10.1016/j.colsurfb.2014.12.041. [DOI] [PubMed] [Google Scholar]
- 13.Manosroi A, Chankhampan C, Manosroi J, Manosroi W. Enhancement of chemical stability and transdermal absorption of salmon calcitonin loaded in elastic niosomes. Adv Sci Lett. 2012;5:314–9. [Google Scholar]
- 14.Paolino D, Cosco D, Muzzalupo R, Trapasso E, Picci N, Fresta M. Innovative bola-surfactant niosomes as topical delivery systems of 5-fluorouracil for the treatment of skin cancer. Int J Pharm. 2008;353:233–42. doi: 10.1016/j.ijpharm.2007.11.037. [DOI] [PubMed] [Google Scholar]
- 15.Jain S, Jain S, Khare P, Gulbake A, Bansal D, Jain SK. Design and development of solid lipid nanoparticles for topical delivery of an anti-fungal agent. Drug Deliv. 2010;17:443–51. doi: 10.3109/10717544.2010.483252. [DOI] [PubMed] [Google Scholar]
- 16.Musicanti C, Gasco P. Solid lipid nanoparticles. In: Bhushan B, editor. Encyclopedia of Nanotechnology. Dordrecht: Springer; 2012. pp. 2471–87. [Google Scholar]
- 17.Pando D, Matos M, Gutiérrez G, Pazos C. Formulation of resveratrol entrapped niosomes for topical use. Colloids Surf B Biointerfaces. 2015;128:398–404. doi: 10.1016/j.colsurfb.2015.02.037. [DOI] [PubMed] [Google Scholar]
- 18.Zorzi GK, Carvalho EL, von Poser GL, Teixeira HF. Review: On the use of nanotechnology-based strategies for association of complex matrices from plant extracts. The Brazilian Journal of Pharmacognosy. 2015;25:426–36. [Google Scholar]
- 19.Junyaprasert VB, Teeranachaideekul V, Supaperm T. Effect of charged and non-ionic membrane additives on physicochemical properties and stability of niosomes. AAPS PharmSciTech. 2008;9:851–9. doi: 10.1208/s12249-008-9121-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Williams AC. Transdermal and Topical Drug Delivery. London: Pharmaceutical Press; 2003. [Google Scholar]
- 21.Clares B, Calpena AC, Parra A, Abrego G, Alvarado H, Fangueiro JF, et al. Nanoemulsions (NEs), liposomes (LPs) and solid lipid nanoparticles (SLNs) for retinyl palmitate: Effect on skin permeation. Int J Pharm. 2014;473:591–8. doi: 10.1016/j.ijpharm.2014.08.001. [DOI] [PubMed] [Google Scholar]
- 22.Surber C, Schwarb FP, Smith EW. Tape-stripping technique. In: Bronaugh RL, Maibach HI, editors. Percutaneous Absorption: Drugs-Cosmetics-Mechanisms-Methodology. 3rd ed. New York: Marcel Dekker; 1999. pp. 395–409. [Google Scholar]
- 23.Brain KR, Walters KA, Watkinson AC. Methods for studying percutaneous absorption. In: Walters KA, editor. Dermatological and Transdermal Formulations. Vol. 119. New York: Marcel Dekker; 2002. pp. 197–269. [Google Scholar]
- 24.Pellett MA, Roberts MS, Hadgraft J. Supersaturated solutions evaluated with an in vitro stratum corneum tape stripping technique. Int J Pharm. 1997;151:91–8. [Google Scholar]
- 25.Ali SM, Yosipovitch G. Skin pH: From basic science to basic skin care. Acta Derm Venereol. 2013;93:261–7. doi: 10.2340/00015555-1531. [DOI] [PubMed] [Google Scholar]
- 26.Becker Peres L, Becker Peres L, de Araújo PH, Sayer C. Solid lipid nanoparticles for encapsulation of hydrophilic drugs by an organic solvent free double emulsion technique. Colloids Surf B Biointerfaces. 2016;140:317–23. doi: 10.1016/j.colsurfb.2015.12.033. [DOI] [PubMed] [Google Scholar]
- 27.Geszke-Moritz M, Moritz M. Solid lipid nanoparticles as attractive drug vehicles: Composition, properties and therapeutic strategies. Mater Sci Eng C Mater Biol Appl. 2016;68:982–94. doi: 10.1016/j.msec.2016.05.119. [DOI] [PubMed] [Google Scholar]
- 28.Shah RB, Khan MA. Nanopharmaceuticals: Challenges and regulatory perspective. In: de Villiers MM, Aramwit P, Kwon GS, editors. Nanotechnology in Drug Delivery. New York: Springer; 2009. pp. 621–46. [Google Scholar]
- 29.Mehnert W, Mäder K. Solid lipid nanoparticles. Adv Drug Deliv Rev. 2012;64:83–101. doi: 10.1016/s0169-409x(01)00105-3. [DOI] [PubMed] [Google Scholar]
- 30.Hua TC, Liu BL, Zhang H. Freeze-Drying of Pharmaceutical and Food Products. Boca Raton: CRC Press; 2010. [Google Scholar]
- 31.Majuru S, Oyewumi MO. Nanotechnology in drug development and life cycle management. In: de Villiers MM, Aramwit P, Kwon GS, editors. Nanotechnology in Drug Delivery. New York: Springer; 2009. pp. 597–620. [Google Scholar]
- 32.Dwivedi A, Mazumder A, Fox LT, Brümmer A, Gerber M, du Preez JL, et al. In vitro skin permeation of artemisone and its nano-vesicular formulations. Int J Pharm. 2016;503:1–7. doi: 10.1016/j.ijpharm.2016.02.041. [DOI] [PubMed] [Google Scholar]
- 33.Aqil F, Munagala R, Jeyabalan J, Vadhanam MV. Bioavailability of phytochemicals and its enhancement by drug delivery systems. Cancer Lett. 2013;334:133–41. doi: 10.1016/j.canlet.2013.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Flaten GE, Palac Z, Engesland A, Filipovic-Grcic J, Vanic Ž, Škalko-Basnet N. In vitro skin models as a tool in optimization of drug formulation. Eur J Pharm Sci. 2015;75:10–24. doi: 10.1016/j.ejps.2015.02.018. [DOI] [PubMed] [Google Scholar]
- 35.Godin B, Touitou E. Dermal and transdermal delivery. In: Bhushan B, editor. Encyclopedia of Nanotechnology. Netherlands: Springer; 2012. pp. 517–26. [Google Scholar]