Abstract
Background:
This study was designed to assess the in vitro anthelmintic activity of the fraction containing alkaloid from Prosopis juliflora pods on goat gastrointestinal nematodes using the egg hatch assay (EHA), larval migration inhibition assay (LMIA), and larval motility assay (LMA).
Materials and Methods:
The alkaloid-rich fraction (AF) – content juliprosopine as major alkaloid – was obtained from ethyl acetate extract after fractionation in Sephadex LH-20 chromatography column and its characterization were made by nuclear magnetic resonance analysis together with literature data comparison. The concentrations tested were 4.0, 2.67, 1.78, 1.19, and 0.79 mg/mL (EHA) and 4 mg/mL (LMIA and LMA). The in vitro cytotoxicity on Vero cell cultures was determined with the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide and trypan blue tests.
Results:
High ovicidal activity was observed with IC50 and IC90 values at 1.1 and 1.43 mg/mL for AF. On the other hand, this fraction showed low larvicidal activity and high toxic effect.
Conclusion:
Thus, P. juliflora pod alkaloid rich-fraction has ovicidal activity in vitro against goat gastrointestinal nematodes and cytotoxic in Vero cell cultures.
SUMMARY
Prosopis juliflora alkaloid-rich fraction (AF) showed in vitro anthelmintic effect against gastrointestinal nematodes of goats
The AF was more effective against eggs than third larval stage (L3) of gastrointestinal nematodes
The AF showed cytotoxicity activity on Vero cell line
The juliprosopine was the main alkaloid found in the AF from P. juliflora pods.
Abbreviations used: AF: Alkaloid-rich fraction; DMSO: Dimethyl sulfoxide; EE: Ethyl acetate extract; EHA: Egg hatch assay; IC50: Inhibitory concentration 50%; IC90: Inhibitory concentration 90%; L3: Infective larvae; LMA: Larval motility assay; LMIA: Larval migration inhibition assay; MTT: Bromide 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide; NMR: Nuclear magnetic resonance; PBS: Phosphate buffered saline; RPMI: Roswell Park Memorial Institute médium; TLC: Thin Layer Chromatography.
Keywords: Anthelmintic, goats, Prosopis juliflora, trichostrongyle nematodes
INTRODUCTION
Parasitic infections caused by gastrointestinal nematodes are among the common and economically important sanitary problems of small ruminant production in various parts of the world. This infection causes a reduction of weight and milk production, as well as diarrhea, anemia, and increased mortality in cases of high infection rate. The treatment is performed with the synthetic anthelmintic, yet the parasitic resistance to various drugs has increased, hindering the effective control of these parasites.[1] That leads to an increasing interest in alternative methods such as the natural products. Several studies have shown that plants act against nematodes.[2]
The Prosopis juliflora pod, popularly known as algaroba, is utilized as a supplementary food to goats in Northeastern Brazil. A previous study carried out by our research group reported the anthelmintic effect of methanol extracts of fruits from P. juliflora in goat fecal culture.[3] This plant is known to possess piperidine alkaloids which possess several biological activities.[4] The objectives of this study were to evaluate in vitro anthelmintic activity of the alkaloid rich fraction of P. juliflora pods on the egg and larval stages of trichostrongyle nematodes from goats, as well as the cytotoxicity effects on the Vero cells (African green monkey kidney epithelial cell line).
MATERIALS AND METHODS
Plant materials
Pods of P. juliflora were collected in the city of Senhor do Bonfim, state of Bahia, Brazil, in September 2011. A voucher specimen was deposited in the Botanic Laboratory of Dr. Antônio Nonato Marques, Company for the agriculture development of Bahia (EBDA), Salvador, Bahia (voucher number 5465).
Extraction and chemical characterization of the alkaloid-rich fraction
Air-dried and powdered pods (6 kg) of P. juliflora were macerated with hexane (10 L), ethyl acetate (6 L), and methanol (8 L) for 3 days at room temperature, successively. The yields of the extracts corresponded to 0.18% (hexane), 0.30% (ethyl acetate), and 3% (methanol). The extracts were concentrated under reduced pressure to yield the respective crude extracts. The presence of alkaloids was detected only in ethyl acetate extract (EE), through the orange spot visualization by Dragendorff's reagent in TLC.[5] The EE was subjected to open-column chromatography packed with Sephadex LH-20, eluted with ethanol (6 steps, 100 mL c.a.) to provide three fractions (F1, F2, and F3). The F2 fraction showed alkaloid-positive result (Dragendorff's reagent) and was called as the fraction containing alkaloids (AF). Further, AF was submitted to 13C nuclear magnetic resonance (NMR) analysis using a Bunker Avance III 500 MHz spectrometer (125 MHz) using CDCl3 deuterated chloroform as a solvent to identify the major alkaloid.
Anthelmintic assays
For parasitological tests, feces obtained from goats naturally infected with gastrointestinal nematodes were used, which contained predominantly eggs of Haemonchus spp. (fecal culture indicated: 92% of parasites from the genera Haemonchus and 8% of Trichostrongylus and Oesophagostomum). All procedures were conducted according to guidelines for animal ethics, and the study received the approval from the Ethics Committee for Animal Experimental of the Veterinary Medicine and Animal Science School, Federal University of Bahia (no. 36/2013).
An egg hatch assay (EHA) was conducted with eggs recovered from the feces of goats.[6] The concentrations of EE and AF, diluted in dimethyl sulfoxide (DMSO) (0.5%), were 4.0, 2.67, 1.78, 1.19, and 0.79 mg/mL. A negative control (DMSO – 0.5%) and a positive control (thiabendazole – 0.05 mg/mL) were added to each plate. Three experiments were performed with five replicates for each concentration and the control groups. The determination of concentrations of all tests was based on the results of previous pilot studies. The evaluation of larvicidal effect was performed with infective larvae (L3) using larval migration inhibition assay (LMIA)[7] and larval motility assay (LMA).[2] For the LMIA, the concentration of EE and AF was 4 mg/mL diluted in DMSO/PBS (phosphate buffered saline) (0.5%), and two controls were prepared: DMSO/PBS (0.5%) and levamisole (0.5 mg/mL). For the LMA, a suspension of infective larvae was distributed in 24-multiwell plates (50 larvae/100 μL/well) and 100 μL each of EE and AF (4 mg/mL) was added separately. A negative (DMSO 0.5%) and a positive (ivermectin 2 μg/mL) control was also prepared. Two experiments were conducted with six repetitions each.
Cytotoxicity assays
The Vero cell line was maintained at Roswell Park Memorial Institute (RPMI) medium supplemented with 10% fetal equine serum and antibiotics. The cells were cultured at 37°C in a humidified 5% CO2 incubator. The experiment was performed in 96-well plates and cells were incubated with EE extract (0.77–2.3 mg/mL) and AF (0.35–1 mg/mL) for 24 h. The control group was treated with DMSO diluted in the culture medium in the equivalent volume used for the treated group (0.1%). Three repetitions were performed with five replicates for each concentration and control. The viability of cells was estimated with the bromide 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT) assay.[8] Treated cells were incubated with MTT (1 mg/mL) for 2 h. Thereafter, cells were lysed with 20% sodium dodecyl sulfate/50% N, N-dimethylformamide (pH 4.7), and plates were kept overnight at 37°C to dissolve formazan crystals. The optical absorbance was measured using a wavelength (405–600 nm) plate reader. Results were shown as viability percentage of the control group. Cell viability was also determined by trypan blue assay. Treated cells were trypsinized, harvested, and pooled with floating cells and centrifuged at 5000 × g for 10 min. Cells were then suspended in 100 μL of RPMI and stained with trypan blue (0.1%). The number of viable and nonviable cells was determined in a Neubauer chamber. The viability percentage was calculated as: (Viable cells/the total cell count) × 100.
Statistical analysis
The results obtained from the parasitological and cytotoxic studies were analyzed through ANOVA followed by Tukey's test, with a 5% significance level. The IC50 and IC90 were calculated from the nonlinear regression analysis using the GraphPrism program (version 5.0, San Diego - California, USA).
RESULTS AND DISCUSSION
The alkaloid-rich fraction (AF) was obtained from EE from P. juliflora pods after fractionation on Sephadex LH-20 column. The juliprosopine chemical structure [Figure 1] – in the alkaloid-rich fraction – was characterized through NMR analysis in comparison with literature data.[9,10] Juliprosopine is a common alkaloid found in Prosopis species.[4]
Figure 1.
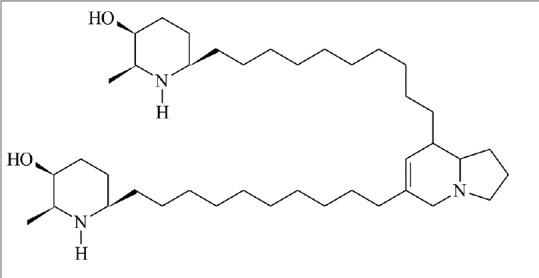
Chemical structure of juliprosopine
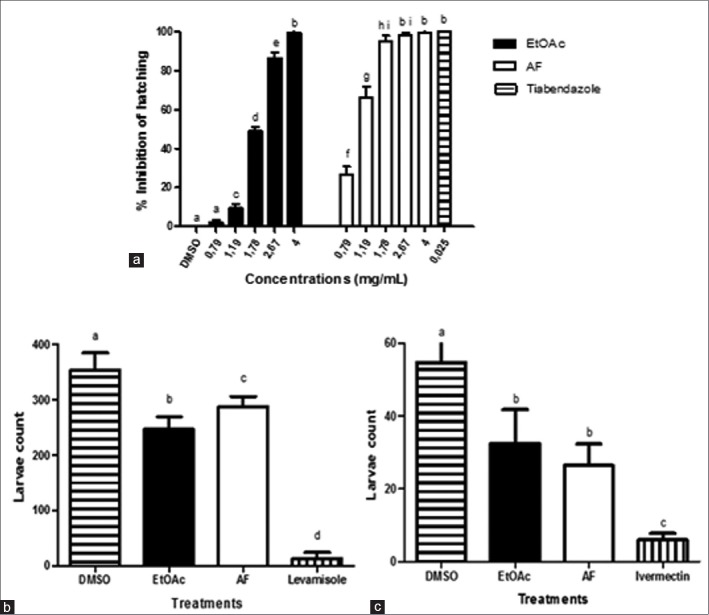
Figure 2 shows the anthelmintic activity of the EE and AF. In the EHA, the IC50 and IC90 values equaled 1.9 and 2.9 mg/mL (EE) and 1.1 and 1.43 mg/mL (AF), respectively. High anthelmintic activity was also reported for the methanol extract obtained from P. juliflora pods (253.7 mg/mL) in an in vitro assay of coprocultures with goat gastrointestinal nematodes.[3] This difference in the concentration used may be attributed to the distinct methodologies applied and the different extraction procedures. The values of IC50 and IC90 for the AF were two times lower when compared to the EE that suggests that the chemical compounds responsible for the ovicidal activity of P. juliflora can be alkaloids. Studies conducted with piperidine alkaloids isolated from this plant demonstrated a variety of biological activities including antibacterial, antifungal,[11] and cytotoxic.[12] The mechanisms of action of active compounds from plants upon the eggs of gastrointestinal nematodes are not yet completely understood. However, the environmental stimuli on the host lead to the release of enzymes by L1 larvae, which degrade the egg membrane.[13] The action of alkaloids of P. juliflora may be due to the inhibition of these enzymes activities.
Figure 2.
In vitro effect of the ethyl acetate extract and AF from Prosopis juliflora on eggs and larvae of gastrointestinal nematodes of goats. (a) Percentage of egg hatching inhibition. (b) Number of third-stage larvae of gastrointestinal nematodes recovered in the migration assay. (c) Larvae mobile recovered in the motility assay
The treatment of larvae (L3) with EE and AF led to a significant reduction in the number of mobile larvae compared to the negative control (P < 0.05). However, this effect was lower than that observed in the positive controls [Figure 2]. Variations in the activities of the eggs and larvae may be due to differences in enzyme components and structure of the membranes of the parasite stages. Another study conducted with plant has also found the same variations.[14]
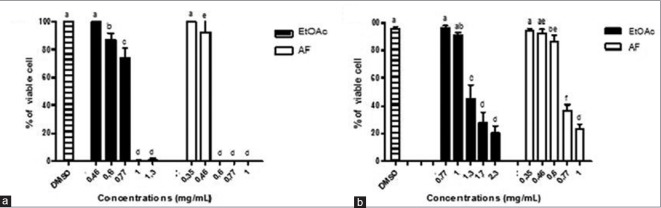
In cytotoxicity assays, the EE and AF promoted a reduction in cell viability in a concentration-dependent manner [Figure 3]. The IC50 values for the extract and the fraction (trypan blue test) were 1.3 and 0.73 mg/mL, respectively. The microscopic evaluation of the culture cells treated with EE (1.3 to 2.3 mg/mL) and AF (0.77 and 1.0 mg/mL) showed a high proportion of cytoplasmatic vacuoles. Substantial cell degradation was observed only in the cultures treated with high concentrations of the EE and AF. The previous investigation also found the presence of vacuoles and mitochondrial damage in the neuronal cells treated with the AF of P. juliflora and that the toxicity of the AF was more pronounced in neuronal cells than in glial cells.[12]
Figure 3.
Viability percentage of Vero cell posttreatment with ethyl acetate extract and AF from Prosopis juliflora, in the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (a) and trypan blue (b) tests
The difference between the concentrations needs to consider that an antiparasitic activity and the cytotoxic effect of the EE and the AF of the plant can be based on the classical principles of toxicity evaluation of xenobiotics. Every drug, being either natural or synthetic, can be responsible for adverse effects, depending on factors such as the target organ, the dose, concentration, and the exposure conditions such as frequency and duration.[15] The cytotoxicity assays conducted in the present work are initial studies to evaluate the toxicity of the extract and the AF of P. juliflora.
The EE and AF from the pods of P. juliflora showed in vitro ovicidal activity on the gastrointestinal nematodes of goats and cytotoxic effects on Vero cell cultures. The anthelmintic and cytotoxic effects are possibly associated with the presence of alkaloids in this plant. In vivo and in vitro additional studies about the toxicity of the plant as well as juliprosopine are necessary to assure its therapeutic utilization against gastrointestinal parasites.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
The authors are grateful to Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES), Fundação de Amparo à Pesquisa do Estado da Bahia (FAPESB), and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq) for the grants and fellowship.
REFERENCES
- 1.Hoste H, Torres-Acosta JF. Non chemical control of helminths in ruminants: Adapting solutions for changing worms in a changing world. Vet Parasitol. 2011;180:144–54. doi: 10.1016/j.vetpar.2011.05.035. [DOI] [PubMed] [Google Scholar]
- 2.Ferreira LE, Castro PM, Chagas AC, França SC, Beleboni RO. In vitro anthelmintic activity of aqueous leaf extract of Annona muricata L. (Annonaceae) against Haemonchus contortus from sheep. Exp Parasitol. 2013;134:327–32. doi: 10.1016/j.exppara.2013.03.032. [DOI] [PubMed] [Google Scholar]
- 3.Batatinha MJ, Almeida GN, Domingues LF, Simas MM, Botura MB, Cruz AC, et al. Effects of aqueous and methanol extracts of algarroba on cultures of larvae of gastrointestinal nematodes in goats. Ciênc Anim Bras. 2011;12:514–9. [Google Scholar]
- 4.Sathiya M, Muthuchelian K. Anti-tumor potential of total alkaloid extract of Prosopis juliflora DC. leaves against Molt-4 cells in vitro. Afr J Biotechnol. 2011;10:8881–8. [Google Scholar]
- 5.Wamburu RW, Kareru PG, Mbaria JM, Njonge FK, Nyaga G, Rechab SO. Acute and sub-acute toxicological evaluation of ethanolic leaves extract of Prosopis juliflora (Fabaceae) J Nat Sci Res. 2013;3:8–15. [Google Scholar]
- 6.Coles GC, Bauer C, Borgsteede FH, Geerts S, Klei TR, Taylor MA, et al. World Association for the Advancement of Veterinary Parasitology (W.A.A.V.P.) methods for the detection of anthelmintic resistance in nematodes of veterinary importance. Vet Parasitol. 1992;44:35–44. doi: 10.1016/0304-4017(92)90141-u. [DOI] [PubMed] [Google Scholar]
- 7.Molento MB, Prichard RK. Effect of multidrug resistance modulators on the activity of ivermectin and moxidectin against selected strains of Haemonchus contortus infective larvae. Pesqui Vet Bras. 2001;21:117–21. [Google Scholar]
- 8.Hansen MB, Nielsen SE, Berg K. Re-examination and further development of a precise and rapid dye method for measuring cell growth/cell kill. J Immunol Methods. 1989;119:203–10. doi: 10.1016/0022-1759(89)90397-9. [DOI] [PubMed] [Google Scholar]
- 9.Dos Santos ET, Pereira ML, da Silva CF, Souza-Neta LC, Geris R, Martins D, et al. Antibacterial activity of the alkaloid-enriched extract from Prosopis juliflora pods and its influence on in vitro ruminal digestion. Int J Mol Sci. 2013;14:8496–516. doi: 10.3390/ijms14048496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ahmad VU, Sultana A, Qazi S. Alkaloids from the leaves of Prosopis juliflora. J Nat Prod. 1989;52:497–501. [Google Scholar]
- 11.Aqeel A, Khursheed AK, Viqaruddin A, Sabiha Q. Antimicrobial activity of julifloricine isolated from Prosopis juliflora. Arzneimittelforschung. 1989;39:652–5. [PubMed] [Google Scholar]
- 12.Silva VD, Pitanga BP, Nascimento RP, Souza CS, Coelho PL, Menezes-Filho N, et al. Juliprosopine and juliprosine from Prosopis juliflora leaves induce mitochondrial damage and cytoplasmic vacuolation on cocultured glial cells and neurons. Chem Res Toxicol. 2013;26:1810–20. doi: 10.1021/tx4001573. [DOI] [PubMed] [Google Scholar]
- 13.Mansfield LS, Gamble HR, Fetterer RH. Characterization of the eggshell of Haemonchus contortus– I. Structural components. Comp Biochem Physiol B. 1992;103:681–6. doi: 10.1016/0305-0491(92)90390-d. [DOI] [PubMed] [Google Scholar]
- 14.Botura MB, dos Santos JD, da Silva GD, de Lima HG, de Oliveira JV, de Almeida MA, et al. In vitro ovicidal and larvicidal activity of Agave sisalana Perr. (sisal) on gastrointestinal nematodes of goats. Vet Parasitol. 2013;192:211–7. doi: 10.1016/j.vetpar.2012.10.012. [DOI] [PubMed] [Google Scholar]
- 15.Klaassen CD, Watkins JB. Casarett & Doull's Essentials of Toxicology. New York: McGraw-Hill; 2012. [Google Scholar]