Abstract
IN BRIEF Cost-effective innovations to improve health and health care in patients with complex chronic diseases are urgently needed. Mobile health (mHealth) remote monitoring applications (apps) are a promising technology to meet this need. This article reports on a study evaluating patients’ use of a tablet device with an mHealth app and a cellular-enabled glucose meter that automatically uploaded blood glucose values to the app. Improvements were observed across all three components of the Patient Protection and Affordable Care Act’s “triple aim.” Self-rated wellness and numerous quality-of-care metrics improved, billed charges and paid claims decreased, but no changes in clinical endpoints were observed.
The United States spends more health care dollars per capita than any other nation (1), and 5% of its population uses 50% of these health care dollars (2). Health care reformers have identified patients with complex chronic diseases as a priority for innovative interventions. Chronic diseases and conditions are among the most common and costly of all health problems; the Centers for Disease Control and Prevention estimates that 7 of the top 10 causes of death in the United States are attributable to chronic diseases (3).
Interventions to improve both health care and wellness for these patients are urgently needed; it is likely that, if health improves, lower costs will follow. Proactive management of complex chronic conditions is necessary to achieve these goals. Mobile health (mHealth) interventions are a promising, relatively new solution to the proactive management issue, and data suggest that outcomes are improved using such interventions (4,5). The objective of this study was to use a pre-/post-intervention design to pilot test a new patient-friendly mHealth solution that incorporates multiple communication and connectivity features, including biometric monitoring, in a low-income population with complex chronic diseases.
Methods
This project used a newly developed mHealth application (app) to connect high-cost, high-utilizing Medicaid patients with diabetes to four nurse case managers at a large multispecialty clinic in the Pacific Northwest. Medicaid patients (or those dually eligible for Medicaid and Medicare) who were receiving primary care from the study clinic, had a diagnosis of either type 1 or type 2 diabetes, and were at least 18 years of age were eligible to participate. Patients with cognitive limitations requiring a caregiver were excluded.
Participants were given a tablet computer with the app installed and received initial training on its use. Technical support was available, both by phone and in person at the patients’ homes. The app includes text messaging; displays of real-time, interactive biometric data, including blood glucose, weight, and blood pressure; and provider alerts for out-of-range biometric readings or urgent messages.
Thirty-three patients were enrolled. Eight were lost to follow-up, and one was removed by her physician, leaving 24 who completed the project. All results reported here include these 24 patients only. Patients were enrolled in the project for 6–12 months during 2015–2016. This project was approved by the Oregon State University institutional review board as an exempt project (using medical records data) with a waiver of consent.
Data Sources
Patients completed a series of questionnaires at enrollment (pre-intervention) and again at the conclusion of the program (post-intervention). These included selected items from the Consumer Assessment of Healthcare Providers and Systems (CAHPS) (6), which addresses numerous domains related to patients’ experiences with the health care system, patient health behaviors, patient satisfaction, and self-rated wellness; the Patient Health Questionnaire-9 (PHQ-9) (7), which measures mental health symptoms; the abbreviated version of the World Health Organization Quality of Life instrument (WHOQOL-BREF) (8), which has five domains (overall quality of life and general health, physical health, psychological health, social relationships, and environment); and the Self-Efficacy for Managing Chronic Disease 6-item scale (9), which covers symptom control, role function, emotional functioning, and communicating with physicians. Details about each of these measures are presented in Table 1.
TABLE 1.
Summary of Measures Used for a Pre-/Post-Intervention Pilot Test of an mHealth App for Remote Case Management of Low-Income Patients With Diabetes
| Measure | Domains Assessed | Items (n) | Possible Range of Scores1 | Pre-Intervention Cronbach’s α | Post-Intervention Cronbach’s α |
|---|---|---|---|---|---|
| CAHPS | Accessibility of clinic staff | 3–62 | —3 | —3 | —3 |
| Provider knowledge | 2–32 | —3 | —3 | —3 | |
| Prescription drug behavior | 5–72 | —3 | —3 | —3 | |
| Diabetes-specific behaviors and quality of care | 9–122 | —3 | —3 | —3 | |
| Lifestyle behavior change plans | 4 | —3 | —3 | —3 | |
| Overall patient satisfaction | 4 | —3 | —3 | —3 | |
| Self-rated wellness | 6 | 6–27 | 0.852 | 0.807 | |
| PHQ-9 | Depressive symptoms | 9 | 0–27 | 0.784 | 0.931 |
| WHOQOL-BREF | Overall quality of life and general health | 2 | 2–10 | 0.820 | 0.742 |
| Physical health | 7 | 7–35 | 0.911 | 0.886 | |
| Psychological health | 6 | 6–30 | 0.729 | 0.890 | |
| Social relationships | 3 | 3–15 | 0.734 | 0.815 | |
| Environment | 8 | 8–40 | 0.770 | 0.785 | |
| Self-Efficacy for Managing Chronic Disease 6-item scale | Self-efficacy | 6 | 6–60 | 0.857 | 0.935 |
For all scales, higher scores indicate a more positive result (e.g., less depression or more social support).
Branching logic was used, so not all patients were presented with all questions. For example, only those who answered “yes,” that they had contacted the clinic after business hours in the past 9 months saw the follow-up question about how long it then took to get an answer to their question.
Questions in this domain were treated as single items and not as a scale.
Medicaid claims data were obtained for all patients for the duration of their participation in the project, as well as for the corresponding timeframe before enrollment. Pre- and post-intervention monthly median charges and monthly median claims paid were compared. Patients were enrolled for different lengths of time; for each patient, we used a pre-intervention window of time that was the same length as the amount of time he or she was enrolled (the post-intervention window). To accommodate the lag inherent in claims data, the month of enrollment was counted in both pre- and post-intervention windows. For example, the program ended on 31 March 2016. For a patient who enrolled in September 2015, the post-intervention window was thus September 2015–March 2016 (8 months). The corresponding pre-intervention window was therefore February–September 2015 (8 months).
Data on billed amounts for prescription drugs were not available; thus, “billed charges” were for all other services only. Data were available on prescription benefits paid; thus, “paid claims” included payments for both prescriptions and all other services. For patients missing prescription data pre- but not post-intervention or vice-versa (n = 7), no prescription claims data were used; thus, for these patients, “paid claims” covered all other services only.
Patients were divided into two groups based on their pre-intervention window billed charges: those for whom charges during the costliest pre-intervention month were ≤$5,000 and those for whom these charges exceeded $5,000. These were used as proxies for identifying the sickest, as opposed to the most well-controlled, patients before the intervention.
For patients with diabetes, the app automatically records self-monitored blood glucose values via a cellular-enabled glucose meter, which was given to patients along with the tablet. Several months’ worth of glucose data were collected, allowing for observation of glycemic changes over time. No glucose data were available from the pre-intervention period.
Data Analysis
We calculated Cronbach’s α for all scales, separately for pre-intervention and post-intervention data (Table 1). We used matched analyses for all pre-/post-intervention comparisons and preferentially used nonparametric, bivariable statistics because of the small sample size. Ordinal and continuous variables were compared using the Wilcoxon signed ranks test, and dichotomous variables were compared using McNemar’s χ2 test. Because of the small sample size, we set α = 0.10. Analyses were conducted using SPSS version 23.0.1 (IBM Corp., Armonk, N.Y.) and S-Plus version 8.3 (Tibco Software, Inc., Palo Alto, Calif.) software.
Results
The mean age of patients in the study was 54.8 (SD 12.2) years. The vast majority (88%) were white and non-Hispanic, and all were low income by virtue of the study design. All had diabetes; three also had chronic obstructive pulmonary disease, one also had asthma, and three also had coronary artery disease.
The quality of care provided to participating patients improved over the course of the project, according to some metrics from the CAHPS scale. Among the 22 individuals who reported that they had “talked with clinic providers about health questions or concerns” both pre- and post-intervention, significantly more reported that they received an “easy-to-understand explanation about the next steps for these health questions or concerns” on the post-intervention survey. On the pre-intervention survey, 12 patients reported that they “always” received easy-to-understand explanations; this number increased to 19 on the post-intervention survey (P = 0.027). Among the 17 individuals who reported that clinic providers had discussed side effects of prescription medications both pre- and post-intervention, more patients reported that the explanations were “always easy to understand” on the post-intervention survey (5 vs. 9, P = 0.083). There was a significant increase in the number of patients who reported that clinic providers “always answered all of their questions about diabetes to their [the patient’s] satisfaction” (4 vs. 18, P = 0.002). Other CAHPS quality-of-care metrics did not change over the course of the project (data not shown).
Patients’ self-rated wellness im-proved. The median score on the CAHPS 6-item wellness scale pre-intervention was 16.5, compared to 18.7 post-intervention (P = 0.003). This improvement was driven largely by improvements on three of the six composite items: self-rated health (P = 0.046), the extent to which pain interferes with daily living (P = 0.001), and mobility (P = 0.009). Patients also improved when wellness was measured using the WHOQOL overall quality-of-life and general health domain (P = 0.071). No changes were observed as measured by the WHOQOL physical health, social relationships, or environment domains (data not shown).
Patients’ mental health improved. Among the 24 patients with complete pre-/post-intervention data on the PHQ-9, the median score dropped from 10.5 to 5.5, although this was not statistically significant (P = 0.12). The degree of depressive symptoms reported by patients was also improved from pre- to post-intervention; eight patients’ scores were consistent with moderate or severe depression on the pre-intervention survey, whereas only four patients were in this category on the post-intervention survey. There were no changes in scores on the WHOQOL psychological health domain (data not shown).
Patients’ self-efficacy to manage their chronic disease(s) did not change over the course of this project, although a bimodal distribution was observed, with some patients reporting extremely high levels of self-efficacy and others reporting very low levels (data not shown).
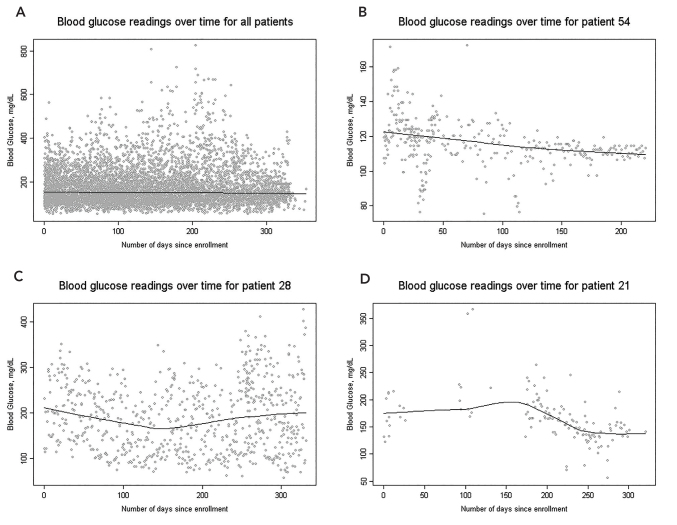
Self-monitored blood glucose values overall did not change by a clinically significant amount (+0.17 mg/dL per week, 95% CI 0.011–0.32) (Figure 1A). However, this overall statistic masks some large changes that occurred at the individual level. Some patients’ blood glucose control improved substantially, both in terms of overall average and decreased variability (Figure 1B), whereas other patients’ control worsened (Figure 1C). Adherence to self-monitoring regimens appears to be associated with control (Figure 1D).
FIGURE 1.
Blood glucose readings over time, with superimposed LOESS trend lines. Data came from a pilot project of a telehealth intervention connecting nurse case managers to Medicaid patients with diabetes served by a multispecialty clinic in the Pacific Northwest. A) All patients enrolled in the pilot project. B) Overall average blood glucose for this patient improved over time, and variability in blood glucose decreased over time. Both of these are indicators of better disease control. C) There was no change in average blood glucose variability over time for this patient. D) This patient initially was not deeply engaged in the program; once complete patient engagement occurred, blood glucose control improved.
Finally, there was a cost savings associated with this program, and the savings was more apparent among those patients who had poor diabetes control at baseline (Table 2). None of the changes in median bills charged or claims paid per patient per month were statistically significant, although, with a highly variable measure such as health care costs, the statistical power associated with a small sample size is quite low. We estimate that it costs $225 per patient per month to implement the program—$175 for nurse case-manager salaries and $50 for software, hardware, and technical support.
TABLE 2.
Summary of Cost Data for a Pre-/Post-Intervention Pilot Test of an mHealth App for Remote Case Management of Low-Income Patients With Diabetes
| Billed Charges Per Patient Per Month | Claims Paid Per Patient Per Month | |||||||
|---|---|---|---|---|---|---|---|---|
| Pre-Intervention ($)1 | Post-Intervention ($)1 | Change ($) | Change (%) | Pre-Intervention ($)1 | Post-Intervention ($)1 | Change ($) | Change (%) | |
| All patients | 1,894 (942–3,615) | 1,374 (515–2,785) | –520 | –27.5 | 577 (246–1,438) | 397 (155–1,398) | –180 | –31.2 |
| Patients with well- controlled diabetes at baseline2 | 947 (288–1,338) | 626 (412–1,430) | –321 | –33.4 | 459 (165–1,244) | 377 (123–1,302) | –82 | –17.9 |
| Patients with uncontrolled diabetes at baseline3 | 3,615 (2,395–8,132) | 1,779 (1,375–8,663) | –1,836 | –50.8 | 939 (246–2,440) | 429 (155–3,516) | –510 | –54.3 |
Data are shown as median (interquartile range).
No monthly charges pre-intervention were >$5,000.
At least one monthly charge pre-intervention was >$5,000.
Discussion
In this small pilot study of an mHealth management platform for Medicaid patients with diabetes, we observed improvements across all three areas of the Patient Protection and Affordable Care Act’s “triple aim”: improvements in quality of care, improvements in self-rated health and mental health, and decreased costs. Although we cannot attribute causality to the intervention because of the small, nonrandomized sample, our data nonetheless suggest that 1) mHealth apps connecting patients to nurse case managers can be used successfully in a low-income, chronically ill population, and 2) such apps may improve both care and outcomes without a large increase in costs in this traditionally hard-to-reach population. This may be especially true in those patients whose disease is not well controlled.
Our project used nurse case managers to connect with patients via the app; additional cost savings could be realized by using a triage system and other, less highly skilled personnel (e.g., medical assistants) as the first point of contact for patients. It is also possible that, over time, improved knowledge and adherence would decrease the number of provider contacts necessary to maintain patient gains in health, allowing the provider team to serve more patients.
Limitations of this project include the small sample size, multiple comparisons, and the pre-/post-intervention design. Given the promising results of this pilot study, next steps would include evaluating the program in a larger sample using a randomized trial design.
Chronic diseases progress, making it difficult to assess improvements on an individual level becaquse most patients’ disease states worsen over time. That we were able to observe significant improvements in all areas of the triple aim from the pre-project period to the post-project period with a small sample is promising.
Acknowledgments
The authors gratefully acknowledge the work of the four nurse case managers who provided remote care for patients in this project: Erin Bartek, Laurie Kerp, Kathy Nepper, and Lindsay Rickli.
Funding
Funding for this project was provided by the Intercommunity Health Network, the Coordinated Care Organization/Medicaid Payer for Linn, Benton, and Lincoln Counties in Oregon.
Duality of Interest
M.L.B. is a paid consultant (statistics and research methods) for, and M.A.M. is the chief medical officer of, Kannact, Inc., the company that produces the software used in this study. R.W. and B.B. were employed by Kannact, Inc., at the time of the study. No other potential conflicts of interest relevant to this article were reported.
Author Contributions
M.L.B. was responsible for data analysis and wrote the first draft of the manuscript. J.L. assisted with data collection and analysis and edited the manuscript. R.W. assisted with data collection and deploying the app and hardware and edited the manuscript. B.B. provided technical support to patients using the app, was primarily responsible for deploying the app and hardware, and edited the manuscript. M.A.M. was responsible for obtaining funding and coordinating with the clinical site, collaborated with M.L.B. on interpretation of results, and edited the manuscript. M.L.B. is the guarantor of this work and, as such, had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
References
- 1.World Health Organization Spending on health: a global overview [Internet]. Available from http://www.who.int/mediacentre/factsheets/fs319/en. Accessed 18 January 2017
- 2.Wier LM, Andrews RM. The national hospital bill: the most expensive conditions by payer, 2008 (Health Care and Utilization Project statistical brief #107). Rockville, Md, Agency for Healthcare Research and Quality, 2011 [PubMed] [Google Scholar]
- 3.Centers for Disease Control and Prevention Chronic disease overview [Internet]. Available from http://www.cdc.gov/chronicdisease/overview. Accessed 23 February 2016
- 4.Wild SH, Hanley J, Lewis SC, et al. . Supported telemonitoring and glycemic control in people with type 2 diabetes: the Telescot Diabetes pragmatic multicenter randomized controlled trial. PLoS Med 2016;13:e1002098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jackson CL, Bolen S, Brancati FL, Batts-Turner ML, Gary TL. A systematic review of interactive computer-assisted technology in diabetes care. Interactive information technology in diabetes care. J Gen Intern Med 2006;21:105–110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Agency for Healthcare Research & Quality CAHPS home page [Internet]. Available from https://www.ahrq.gov/cahps/index.html. Accessed 18 January 2017
- 7.Richardson LP, McCauley E, Grossman DC, et al. . Evaluation of the Patient Health Questionnaire-9 item for detecting major depression among adolescents. Pediatrics 2010;126:1117–1123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.World Health Organization WHO Quality of Life-BREF (WHOQOL-BREF) [Internet]. Available from http://www.who.int/substance_abuse/research_tools/whoqolbref/en. Accessed 18 January 2017
- 9.Stanford University School of Medicine Self-Efficacy for Managing Chronic Disease 6-Item Scale [Internet]. Available from http://patienteducation.stanford.edu/research/secd6.html. Accessed 18 January 2017