Abstract
Background
Nutritional status has been related to clinical outcomes in patients with heart failure. We assessed the association between nutritional status, indexed by prognostic nutritional index (PNI), and survival in patients hospitalized for acute heart failure.
Methods and Results
A total of 1673 patients (age 76±13 years, 68% men) hospitalized for acute heart failure in a tertiary medical center were analyzed. PNI was calculated as 10×serum albumin (g/dL)+0.005×total lymphocyte count (per mm3). National Death Registry was linked to identify the clinical outcomes of all‐cause and cardiovascular death. With increasing tertiles of PNI, age and N‐terminal probrain natriuretic peptide decreased, and body mass index, estimated glomerular filtration rate, and hemoglobin increased. During a mean follow‐up duration of 31.5 months, a higher PNI tertile was related to better survival free from all‐cause and cardiovascular mortality in the total study population and in participants with either reduced or preserved left ventricular ejection fraction. After accounting for age, sex, estimated glomerular filtration rate, left ventricular ejection fraction, serum sodium level, and on‐admission systolic blood pressure, PNI was independently associated with cardiovascular death and total mortality (hazard ratio per 1 SD of the natural logarithm of the PNI: 0.76 [95% CI, 0.66–0.87] and 0.79 [95% CI, 0.73–0.87], respectively). In subgroup analyses stratified by age, sex, left ventricular ejection fraction, body mass index, or estimated glomerular filtration rate, PNI was consistently related to mortality.
Conclusions
PNI is independently associated with long‐term survival in patients hospitalized for acute heart failure with either reduced or preserved left ventricular ejection fraction.
Keywords: albumin, heart failure, lymphocyte, mortality, nutrition, prognosis, prognostic nutritional index
Subject Categories: Heart Failure
Clinical Perspective
What Is New?
Nutritional status, indexed by prognostic nutritional index, is a strong prognostic factor for long‐term outcomes in patients hospitalized for acute heart failure with either reduced or preserved left ventricular ejection fraction.
The prognostic nutritional index is correlated with cardiac hemodynamics, including right ventricular systolic pressure.
What Are the Clinical Implications?
Hospitalized heart failure patients with depressed prognostic nutritional index on admission, indicating an increased risk of total and cardiovascular mortality, may warrant closer follow‐up and more aggressive therapy.
Introduction
Malnutrition, which is associated with decreased immune system function,1 impaired respiratory function,2 and poor wound healing,3 is common in hospitalized older patients.4 Consequently, malnutrition correlates with increasing risks of postoperative morbidity and mortality,5 prolonged hospital stay,6 and poor quality of life.7 Protein‐energy malnutrition is prevalent and has been related to poor prognoses in patients with chronic illness, including end‐stage renal disease, malignancy,4 and advanced heart failure (HF).8
When Anker et al and Zapatero et al demonstrated that nutritional status was an independent prognostic factor in patients with either acute or chronic HF,8, 9 several nutritional screening tools such as the Mini Nutritional Assessment–Short Form (MNA‐SF) and Nutritional Risk Screening (NRS‐2002) were developed for assessing nutritional risks in patients with HF.10, 11 Given the complexity of computing these indexes and the subjectivity of the questionnaires, the validity and the generalizability of the nutritional indexes and questionnaires may vary according to the experience of the examiners and the presence of recall bias from the patients.
In contrast, some biochemical nutritional indicators, including body mass index, total cholesterol, serum albumin, and total lymphocyte count, were proposed to predict survival in patients with HF.12, 13, 14 The prognostic nutritional index (PNI), calculated from the serum albumin concentration and total lymphocyte count, is a simple and objective indicator of postoperative outcomes in patients undergoing cancer surgery5; however, the prognostic impact of PNI in patients with HF remained unexplored. The aim of this study was to investigate the clinical significance of PNI in patients hospitalized for acute HF (AHF).
Methods
The study population was retrieved from HARVEST, the Heart Failure Registry of Taipei Veterans General Hospital, in which patients hospitalized for AHF were enrolled.15 Patients with acute coronary syndrome, severe liver cirrhosis (Child‐Pugh score B or C), or sepsis16 were excluded. A total of 2663 patients who underwent echocardiography before discharge from October 2003 to December 2012 were analyzed. Medical history; findings from physical examination; and prescription, hematological, and biochemistry data were prospectively input in a web‐based electronic records system. The institutional review committee of Taipei Veterans General Hospital approved the use of the registry data for research purposes, and informed consent was waived, given the nature of an administrative registry.
Blood samples were obtained on admission and analyzed using the Beckman Coulter LH 780 analyzer for hematological data and a Roche Cobas 8000 for biochemical data. Renal function was expressed as the estimated glomerular filtration rate (eGFR) and calculated with the abbreviated MDRD (Modification of Diet in Renal Disease) formula.17 Chronic kidney disease was defined as eGFR <60 mL/min per 1.73 m2. Because the commercialized measure for NT‐proBNP (N‐terminal probrain natriuretic peptide) was available only after 2009, this analysis had missing values for NT‐proBNP. Left ventricular ejection fraction (LVEF) was obtained from 2‐dimensional guided M‐mode echocardiography in accordance with the recommendations of the American Society of Echocardiography.18 The ratio of transmitral flow velocity to mitral annulus motion velocity in early diastole (E/e′) and estimated right ventricular systolic pressure (RVSP) were also calculated. HF with reduced ejection fraction (HFrEF) or HF with preserved ejection fraction (HFpEF) was defined as LVEF <50% or ≥50%, respectively. PNI was calculated as 10×serum albumin (g/dL)+0.005×total lymphocyte count (per mm3).5, 19 Overall, 990 patients were excluded from this analysis for missing data on albumin or total lymphocyte count on the first morning of hospitalization.
Follow‐up
Clinical outcomes and mortality were acquired by linking the database to the National Death Registry, with a mean follow‐up duration of 31.5 months. The National Death Registry database registers valid information according to the International Classification of Diseases, Ninth Revision (ICD‐9). The ICD‐9 codes for cardiovascular death are 390 to 459. The accuracy of the coding in Taiwan's National Death Registry database has been validated.20
Statistical Analysis
Continuous variables are presented as mean and standard deviation (SD), and categorical variables are presented as number and percentage. Between‐group comparisons were achieved by 1‐way ANOVA for continuous variables and χ2 test for categorical variables. Because of the skewed distributions of PNI and NT‐proBNP levels, they were taken into natural logarithm transformations, respectively, before the linear regression analysis and Cox proportional hazards regression analysis. The missing NT‐proBNP values were analyzed by multiple imputations using the Markov Chain Monte Carlo method with a single chain and 20 imputations.
Linear regression analysis was used to evaluate the relationship between PNI and baseline characteristics. Multivariable linear regression analysis was conducted by stepwise forward selections, with age and sex being forced in the model. The Kaplan–Meier survival curve analysis was conducted to compare the prognoses of the tertiles of PNI. Cox proportional hazards models were used to evaluate the independence of PNI in predicting all‐cause or cardiovascular mortality after accounting for age, sex, renal function, LVEF, biochemical data, systolic blood pressure, and prescribed medications. To compare the prognostic impacts of PNI, albumin, and total lymphocyte count in addition to the clinical risks, the Akaike information criterion of the models and the net reclassification improvement were calculated. Participants with mortality risks of 7% at the 2 extremities in the study population were classified as low‐ and high‐risk groups. Participants were censored at the time of noncardiovascular death or the end of follow‐up in the analysis of cardiovascular death. Adequate nutritional status was defined as a PNI >44.8, which was the upper tertile of PNI. The 2‐sided differences were considered statistically significant at the 0.05 significance level. Statistical analyses were performed using IBM SPSS software version 21.0.
Results
A total of 1673 patients (mean age 76±13 years, 68% male, 48% HFrEF) were analyzed in this study. The baseline characteristics are shown in Table 1, stratified by the tertiles of PNI. In short, patients in the higher tertiles of PNI were younger; had higher BMI and prevalent coronary artery disease; and had higher lymphocyte counts, hemoglobin, and platelet counts. The sex distribution; prevalence of hypertension, diabetes mellitus, and prior stroke; and white blood cell counts were comparable among the PNI tertile groups. Serum levels of blood urea nitrogen, creatinine, and NT‐proBNP decreased, but eGFR, albumin, cholesterol, low‐density lipoprotein cholesterol, and triglyceride increased with increasing tertiles of PNI; however, serum levels of high‐density lipoprotein and glucose were not different between groups. In addition, patients in the higher tertiles of PNI also had lower E/e′ and RVSP but similar LVEF and the ratio of mitral peak velocity of early filling (E) to late filling (A) (E/A ratio).
Table 1.
Baseline Characteristics of the Cohort Study Population by Prognostic Nutritional Index Tertiles
| Variables | Total (n=1673) | Prognostic Nutrition Index | P Value | ||
|---|---|---|---|---|---|
| ≤39.3 (n=558) | 39.3 to 44.8 (n=557) | >44.8 (n=558) | |||
| Age, y | 75.8±13.2 | 77.5±13.0 | 76.5±12.2 | 73.4±14.0 | <0.001 |
| Men, n (%) | 1140 (68) | 382 (69) | 375 (67) | 383 (69) | 0.882 |
| BMI, kg/m2 | 24.6±5.0 | 23.8±4.6 | 24.3±5.0 | 26.2±5.2 | <0.001 |
| Systolic blood pressure, mm Hg | 143±33 | 146±35 | 142±32 | 141±31 | 0.071 |
| Heart rate, bpm | 91±24 | 91±24 | 92±24 | 91±23 | 0.794 |
| Comorbidities, n (%) | |||||
| Hypertension | 982 (59) | 323 (58) | 322 (58) | 337 (60) | 0.616 |
| Diabetes mellitus | 619 (37) | 206 (37) | 207 (37) | 206 (37) | 0.996 |
| Coronary artery disease | 496 (30) | 130 (23) | 165 (30) | 201 (36) | <0.001 |
| Stroke | 141 (8) | 54 (10) | 44 (8) | 43 (8) | 0.426 |
| Systolic heart failure | 803 (48) | 256 (46) | 279 (50) | 268 (48) | 0.355 |
| Hematological and biochemical variables | |||||
| White blood cell count, 109/L | 7.6±3.2 | 7.5±3.3 | 7.6±3.4 | 7.7±2.7 | 0.797 |
| Lymphocyte count, 109/L | 1.3±0.7 | 0.9±0.4 | 1.2±0.5 | 1.8±0.9 | <0.001 |
| Hemoglobin, g/dL | 11.8±2.2 | 10.8±2.1 | 11.7±2.1 | 12.8±2.1 | <0.001 |
| Platelet count, 109/L | 204±94 | 195±92 | 199±90 | 217±98 | <0.001 |
| Blood urea nitrogen, mg/dL | 35±21 | 41±24 | 35±22 | 30±17 | <0.001 |
| Creatinine, mg/dL | 1.9±1.4 | 2.2±1.7 | 1.9±1.3 | 1.6±1.2 | <0.001 |
| eGFR, mL/min/1.73 m2 | 49±26 | 45±29 | 49±25 | 54±24 | <0.001 |
| Sodium, mmol/L | 139±5 | 138±5 | 139±5 | 139±4 | 0.005 |
| Potassium, mmol/L | 4.1±0.7 | 4.0±0.7 | 4.1±0.7 | 4.1±0.7 | 0.035 |
| Albumin, g/dL | 3.6±0.5 | 3.0±0.4 | 3.6±0.3 | 4.1±0.4 | <0.001 |
| Cholesterol, mg/dL | 155±43 | 145±43 | 152±41 | 166±42 | <0.001 |
| HDL cholesterol, mg/dL | 43±15 | 42±16 | 42±14 | 44±15 | 0.137 |
| LDL cholesterol, mg/dL | 94±34 | 85±33 | 92±33 | 102±34 | <0.001 |
| Triglyceride, mg/dL | 102±101 | 89±50 | 94±64 | 120±149 | <0.001 |
| Glucose, mg/dL | 170±96 | 172±108 | 165±85 | 170±83 | 0.811 |
| NT‐proBNP,a pg/mL (n=480) | 8.8±1.3 | 9.0±1.2 | 8.7±1.3 | 8.2±1.4 | <0.001 |
| Echocardiography | |||||
| LVEF, % | 50±20 | 50±21 | 50±18 | 50±20 | 0.745 |
| E/A ratio | 1.1±0.7 | 1.2±0.7 | 1.1±0.7 | 1.1±0.8 | 0.500 |
| E/e′ | 18±8 | 19±8 | 18±8 | 17±8 | 0.012 |
| RVSP, mm Hg | 44±17 | 48±17 | 45±16 | 41±16 | <0.001 |
| Prescribed medications, n (%) | |||||
| β‐Blockers | 1055 (63) | 336 (60) | 344 (62) | 375 (67) | 0.040 |
| RAS inhibitors | 1380 (83) | 452 (81) | 460 (83) | 468 (84) | 0.451 |
| Spironolactone | 942 (56) | 293 (53) | 319 (57) | 330 (59) | 0.071 |
| Loop diuretics | 1338 (80) | 445 (80) | 448 (80) | 445 (80) | 0.948 |
RAS inhibitors include angiotensin‐converting enzyme inhibitor or angiotensin receptor blockade. BMI indicates body mass index; E/e′, ratio of transmitral flow velocity to mitral annulus motion velocity in early diastole; eGFR, estimated glomerular filtration rate; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; LVEF, left ventricular ejection fraction; NT‐proBNP, N‐terminal probrain natriuretic peptide; RAS, renin–angiotensin system; RVSP, right ventricular systolic pressure.
NT‐proBNP was taken through natural logarithm transformation.
Correlates of PNI
In general, PNI negatively correlated with age, systolic blood pressure, blood urea nitrogen, E/e′, and RVSP and positively correlated with BMI, white blood cell count, hemoglobin, platelet count, eGFR, serum sodium, potassium, and cholesterol levels (Table 2). With fixed adjustments for age and sex, the forward stepwise multivariate linear regression analysis showed that BMI, systolic blood pressure, hemoglobin, cholesterol levels, and RVSP were independently related to PNI (Table 2).
Table 2.
Correlates of the Prognostic Nutritional Index by Univariate and Multivariate Linear Regression Analyses
| Univariate Analysis | Multivariate Analysisa | |||
|---|---|---|---|---|
| β Coefficient | P Value | β Coefficient | P Value | |
| Age, y | −0.149 | <0.001 | 0.039 | 0.517 |
| BMI, kg/m2 | 0.196 | <0.001 | 0.173 | 0.002 |
| Systolic blood pressure, mm Hg | −0.059 | 0.042 | −0.149 | 0.011 |
| White blood cell count, 109/L | 0.072 | 0.003 | ||
| Hemoglobin, g/dL | 0.397 | <0.001 | 0.372 | <0.001 |
| Platelet count, 109/L | 0.119 | <0.001 | ||
| Blood urea nitrogen, mg/dL | −0.202 | <0.001 | ||
| eGFR, mL/min per 1.73 m2 | 0.137 | <0.001 | ||
| Sodium, mmol/L | 0.097 | <0.001 | ||
| Potassium, mmol/L | 0.078 | 0.002 | ||
| Cholesterol, mg/dL | 0.208 | <0.001 | 0.195 | 0.001 |
| E/e′ | −0.115 | 0.003 | ||
| LVEF, % | −0.043 | 0.079 | ||
| RVSP, mm Hg | −0.164 | <0.001 | −0.142 | 0.012 |
BMI indicates body mass index; E/e′, ratio of transmitral flow velocity to mitral annulus motion velocity in early diastole; eGFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction; RVSP, right ventricular systolic pressure.
The multivariate regression model was calculated by multiple linear regression stepwise analysis (included all variables with P<0.1), and with fixed adjusted for age and sex.
PNI and Clinical Outcomes
During a mean follow‐up of 31.5±27.6 months, 742 (44%) patients died. With increasing tertiles of PNI, total mortality and cardiovascular death decreased in the study population (Figure 1) and in patients with HFrEF and HFpEF (Figure 2). In addition, an adequate nutritional status of PNI >44.8 was associated with lower in‐hospital, 90‐day, and 4‐year mortality rates (Figure 3).
Figure 1.
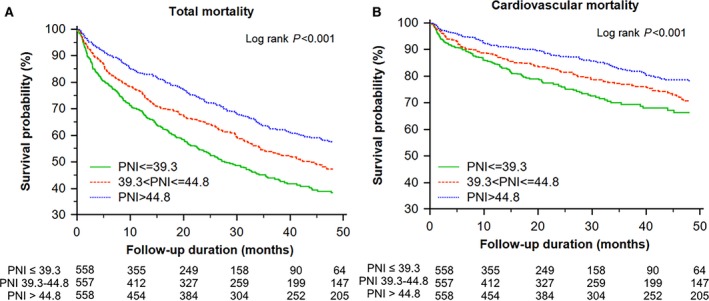
Kaplan–Meier survival curve analysis of total mortality (A) and cardiovascular mortality (B) across the tertiles of the prognostic nutritional index (PNI; >44.8, 44.8 to >39.3, and ≤39.3).
Figure 2.
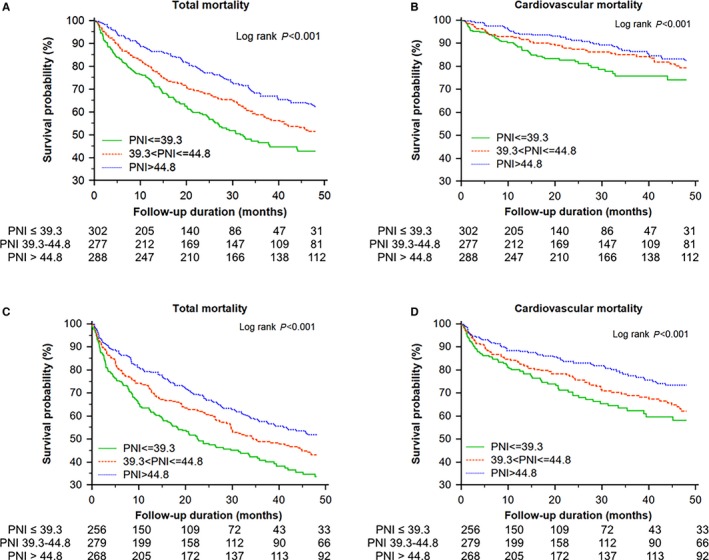
Kaplan–Meier survival curve analysis of total and cardiovascular mortality in patients with heart failure with preserved ejection fraction (HFpEF) or heart failure with reduced ejection fraction (HFrEF; left ventricular ejection fraction cutoff value: 50%; mean LVEF of HFrEF and HFpEF: 65% and 34%, respectively) across the tertiles of prognostic nutritional index (PNI). A, Total mortality in patients with HFpEF. B, Cardiovascular mortality in patients with HFpEF. C, Total mortality in patients with HFrEF. D, Cardiovascular mortality in patients with HFrEF.
Figure 3.
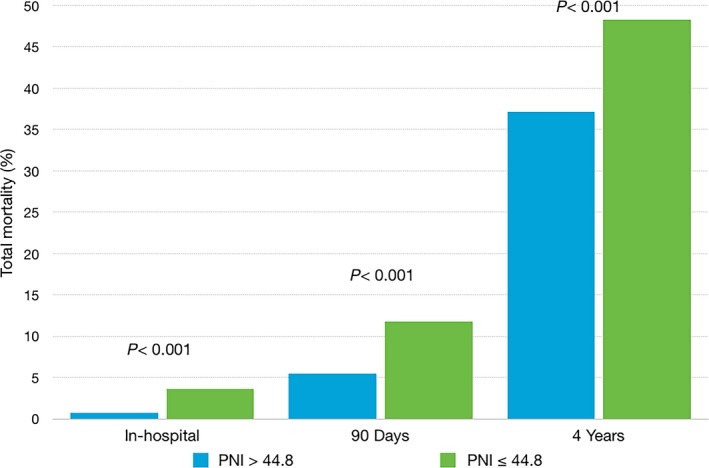
In‐hospital, 90‐day, and 4‐year cumulative mortality in patients with or without adequate nutritional status, defined by a prognostic nutritional index (PNI) of >44.8.
After accounting for age and sex, PNI was a significant predictor of cardiovascular mortality (hazard ratio per 1 SD: 0.81 [95% confidence interval, 0.73–0.90]) as well as total mortality (hazard ratio per 1 SD: 0.79 [95% confidence interval, 0.74–0.85]; Table 3, model 1). With adjustments for age, sex, eGFR, LVEF, serum sodium level, and systolic blood pressure, PNI was still an independent predictor of cardiovascular and total mortality (Table 3, model 2). With further adjustments for NT‐proBNP, PNI remained independently related to the outcomes (Table 3, model 4). Taking only the discharge survivors into account, PNI significantly correlated with both cardiovascular and total mortality, independent of age, sex, eGFR, LVEF, serum sodium level, systolic blood pressure, and on‐discharge medications (Table 3, model 3). Similarly, adequate nutritional status (PNI >44.8) was a strong independent predictor of cardiovascular and total mortality (Table 3).
Table 3.
Relative Risks of PNI for Cardiovascular Death and Total Mortality
| Cardiovascular Death | Total Mortality | |||
|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| PNI,a 1 SD=0.16 | ||||
| Model 1 | 0.81 (0.73–0.90) | <0.001 | 0.79 (0.74–0.85) | <0.001 |
| Model 2 | 0.76 (0.66–0.87) | <0.001 | 0.79 (0.73–0.87) | <0.001 |
| Model 3 | 0.76 (0.66–0.88) | <0.001 | 0.80 (0.73–0.88) | <0.001 |
| Model 4b | 0.86 (0.76–0.97) | 0.015 | 0.85 (0.78–0.92) | <0.001 |
| Adequate nutritional statusc | ||||
| Model 1 | 0.64 (0.50–0.81) | <0.001 | 0.65 (0.55–0.76) | <0.001 |
| Model 2 | 0.48 (0.34–0.67) | <0.001 | 0.65 (0.53–0.80) | <0.001 |
| Model 3 | 0.48 (0.34–0.68) | <0.001 | 0.66 (0.54–0.82) | <0.001 |
| Model 4b | 0.66 (0.51–0.85) | 0.001 | 0.69 (0.58–0.82) | <0.001 |
Model 1: adjusted for age and sex. Model 2: adjusted for age, sex, estimated glomerular filtration rate, left ventricular ejection fraction, sodium level, systolic blood pressure on admission. Model 3: model 2 plus use of β‐blocker, renin–angiotensin system blockade, and spironolactone (discharge survivor, n=1629). Model 4: model 2 plus NT‐proBNP. CI indicates confidence interval; HR, hazard ratio; NT‐proBNP, N‐terminal probrain natriuretic peptide; PNI, prognostic nutritional index.
PNI was taken through natural logarithm transformation.
The imputations for the missing NT‐proBNP values were conducted by using Markov chain Monte Carlo method with a single chain and 20 imputations.
Adequate nutritional status was defined as PNI >44.8
Although PNI and its components of albumin or total lymphocyte count were all independently related to mortality (Table 4), only PNI may significantly improve model prediction by showing the lowest Akaike information criterion and remarkable net reclassification improvement (2.4%, P=0.002).
Table 4.
Model Performance and the Predictive Values of the PNI, Albumin, or Total Lymphocyte Count for Total Mortality
| HR (95% CI) | P Value | AIC | NRI (95% CI) | |
|---|---|---|---|---|
| PNIa, 1 SD=0.16 | 0.79 (0.73–0.87) | <0.001 | 6738 | 2.4% (0.9–3.9%) |
| Albumin, 1 SD=0.53 mg/dL | 0.83 (0.76–0.90) | <0.001 | 8701 | 1.0% (−0.3% to 2.4%) |
| Total lymphocyte count, 1 SD=0.72×109/L | 0.81 (0.72–0.90) | <0.001 | 6920 | 0.9% (−0.2% to 2.0%) |
The Cox proportional hazard model has included age, sex, estimated glomerular filtration rate, left ventricular ejection fraction, sodium, and systolic blood pressure. AIC indicates Akaike information criterion; CI, confidence interval; HR, hazard ratio; NRI, net reclassification improvement; PNI, prognostic nutritional index.
PNI was taken through nature logarithm transformation.
Subgroup Analyses
With adjustments for age and sex, higher PNI was consistently associated with lower risks of total mortality in various subpopulations: age ≥75 or <75 years, male or female, HFrEF or HFpEF, BMI ≥24 or <24, and patients with or without chronic kidney disease (Figure 4).
Figure 4.
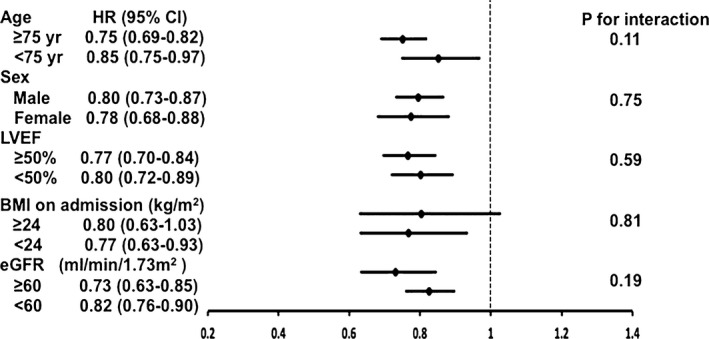
Hazard ratio (HR) and 95% confidence interval (CI) per 1‐standard deviation increase of prognostic nutritional index (PNI) for mortality in subgroup analyses after accounting for age and sex. PNI has taken natural logarithm transformation in the analyses. BMI indicates body mass index; eGFR, estimated glomerular filtration rate; LVEF, left ventricular ejection fraction.
Discussion
Main Findings
The present study demonstrated that nutrition status, based on PNI in patients with AHF, was independently associated with both short‐ and long‐term total and cardiovascular mortality. PNI, which was derived from serum albumin and total lymphocyte count, revealed more prognostic predictive power than its components alone. The associations remained true in patients with either HFrEF or HFpEF. In addition, PNI correlated with systolic blood pressure and RVSP, indicating an association between nutrition status and the preload and afterload of the left ventricle while RVSP was highly correlated with LV end‐diastolic pressure. The findings may support cardiointestinal interaction as a cause of cardiac cachexia.21 In addition, there was no interaction between PNI and age, BMI, or chronic kidney disease.
Determinants of Nutritional Status in HF
Cardiac cachexia is a serious presentation of the catabolic status in HF, when resting metabolic rate increases and gastrointestinal malabsorption prevails in patients with advanced disease.22 The pathophysiology may involve diminished perfusion of the gut and disturbed microcirculation of the intestine, resulting in local edema, abnormal mucosal permeability for endotoxin, and subsequent inflammation.21 PNI, which is calculated based on the serum albumin concentration and total lymphocyte count in the peripheral blood, may theoretically represent both malabsorption and chronic inflammation in HF. In the present study, PNI was independently associated with the known nutritional correlates, including BMI, hemoglobin, and cholesterol levels.13, 23 In addition, PNI was also related to systolic blood pressure and RVSP. Although systolic blood pressure is associated with cardiac performance,24 elevated RVSP correlates strongly with elevated end‐diastolic pressure of the right ventricle and right atrial pressure, which may be related to intestinal congestion and, thereafter, malnutrition.25 Intestinal congestion and redistribution of blood flow away from the splanchnic circulation due to increased sympathetic activity may contribute to intestinal mucosal barrier dysfunction, which causes lipopolysaccharide translocation into systemic circulation, and may worsen malnutrition in patients with AHF.22
Nutritional Status and the Prognosis of HF
Up to 50% of patients with HF are thought to be malnourished, and 15% are overtly cachectic. Cardiac cachexia, characterized by muscle wasting, inflammation, insulin resistance, weight loss, and anorexia, is an independent risk factor for mortality in patients with HF.8 In contrast, nutritional intervention may improve clinical outcomes with less hospitalization and medical cost, especially in older patients with HF.26 Consequently, a clinically significant nutritional index is indicated for risk stratification and clinical management of HF. In this study, we demonstrated that PNI, which is clinically and easily available, was an independent risk factor for cardiovascular death and total mortality in patients with AHF with either HFrEF or HFpEF. In subgroup analyses, we showed that PNI was independently related to mortality in younger and older patients, in men and women, in patients who were and were not overweight, and in patients with and without chronic kidney disease.
Nutritional Status in HF With Reduced or Preserved Ejection Fraction
Anker et al demonstrated that left ventricular systolic function was similar in HF patients who were either cachectic or noncachectic.27 Valentova et al further revealed that cardiac cachexia was associated with right ventricular dysfunction, resulting in consequent intestinal congestion, rather than left ventricular dysfunction.25, 28 In the present study, we also showed that PNI was associated with E/e′ and RVSP but not LVEF. Although Ikeda et al demonstrated the associations of intestinal wall thickness and E/e′ or left ventricular diastolic dysfunction,29 the present study may also contribute to the link of cardiac dysfunction, intestinal edema, and cardiac cachexia.25 Moreover, the previous study revealed that nutritional status was related to the prognoses of patients with HFrEF and HFpEF.30, 31 It is believed that the 2 phenotypes of HF may share a common mechanism of malnutrition–inflammation interaction related to congestive hemodynamics in the development of cardiac cachexia.
Study Limitation
This study had several limitations. Given the present study as a single‐center registry and an observational study, biases arose from internal and external validities. Because the mean age of 76 years and the in‐hospital mortality rate of 2.6% were similar to the published data,32 and we adjusted for all observed confounders, the study results might be generalizeable to other populations. Finally, the 990 patients with missing PNI data were characteristically different from the study population: They were older, more likely to have hypertension, had higher on‐admission systolic blood pressure, lower white blood cell counts, and better renal function. The missingness of PNI was not associated with mortality after accounting for characteristic differences. Further studies are needed to validate the clinical application of PNI in the management of HF.
Conclusions
On‐admission PNI is associated with total and cardiovascular mortality in patients hospitalized for AHF, independent of age, sex, eGFR, LVEF, sodium level, and systolic blood pressure. Advanced HF and congestive hemodynamics, characterized by elevated RVSP, would usually involve the intestinal wall edema and translocations of endotoxin; therefore, PNI, an index combining malnutrition and inflammation, is related to the mortality not only of HFrEF but also of HFpEF. PNI a simple index calculated from routine biochemistry and hemogram tests, has the potential to become a convenient tool for risk stratification and nutritional intervention in patients with HF.
Sources of Funding
The study was supported by Taipei Veterans General Hospital (V100C‐145, V101C‐092, V102C‐119, V103B‐017, V104C‐172), Ministry of Science and Technology (MOST 103‐2314‐B‐010‐050‐MY2), and Ministry of Health and Welfare, Taiwan with grant (MOHW‐104‐TDU‐B‐211‐113003 and MOHW‐105‐TDU‐B‐211‐133017, MOHW106‐TDU‐B‐211‐113001) and the death registry.
Disclosures
None.
(J Am Heart Assoc. 2017;6:e004876 DOI: 10.1161/JAHA.116.004876.)28649089
References
- 1. Calder PC. Feeding the immune system. Proc Nutr Soc. 2013;72:299–309. [DOI] [PubMed] [Google Scholar]
- 2. Grant JP. Nutrition care of patients with acute and chronic respiratory failure. Nutr Clin Pract. 1994;9:11–17. [DOI] [PubMed] [Google Scholar]
- 3. Chow O, Barbul A. Immunonutrition: role in wound healing and tissue regeneration. Adv Wound Care. 2014;3:46–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cederholm T, Hellstrom K. Nutritional status in recently hospitalized and free‐living elderly subjects. Gerontology. 1992;38:105–110. [DOI] [PubMed] [Google Scholar]
- 5. Jeon HG, Choi DK, Sung HH, Jeong BC, Seo SI, Jeon SS, Choi HY, Lee HM. Preoperative prognostic nutritional index is a significant predictor of survival in renal cell carcinoma patients undergoing nephrectomy. Ann Surg Oncol. 2016;23:321–327. [DOI] [PubMed] [Google Scholar]
- 6. Allard JP, Keller H, Jeejeebhoy KN, Laporte M, Duerksen DR, Gramlich L, Payette H, Bernier P, Davidson B, Teterina A, Lou W. Decline in nutritional status is associated with prolonged length of stay in hospitalized patients admitted for 7 days or more: a prospective cohort study. Clin Nutr. 2016;35:144–152. [DOI] [PubMed] [Google Scholar]
- 7. Larsson J, Akerlind I, Permerth J, Hornqvist JO. The relation between nutritional state and quality of life in surgical patients. Eur J Surg. 1994;160:329–334. [PubMed] [Google Scholar]
- 8. Anker SD, Ponikowski P, Varney S, Chua TP, Clark AL, Webb‐Peploe KM, Harrington D, Kox WJ, Poole‐Wilson PA, Coats AJ. Wasting as independent risk factor for mortality in chronic heart failure. Lancet. 1997;349:1050–1053. [DOI] [PubMed] [Google Scholar]
- 9. Zapatero A, Barba R, Gonzalez N, Losa JE, Plaza S, Canora J, Marco J. Influence of obesity and malnutrition on acute heart failure. Rev Esp Cardiol. 2012;65:421–426. [DOI] [PubMed] [Google Scholar]
- 10. Yost G, Gregory M, Bhat G. Short‐form nutrition assessment in patients with advanced heart failure evaluated for ventricular assist device placement or cardiac transplantation. Nutr Clin Pract. 2014;29:686–691. [DOI] [PubMed] [Google Scholar]
- 11. Tevik K, Thurmer H, Husby MI, de Soysa AK, Helvik AS. Nutritional risk screening in hospitalized patients with heart failure. Clin Nutr. 2015;34:257–264. [DOI] [PubMed] [Google Scholar]
- 12. Horwich TB, Kalantar‐Zadeh K, MacLellan RW, Fonarow GC. Albumin levels predict survival in patients with systolic heart failure. Am Heart J. 2008;155:883–889. [DOI] [PubMed] [Google Scholar]
- 13. Rauchhaus M, Clark AL, Doehner W, Davos C, Bolger A, Sharma R, Coats AJ, Anker SD. The relationship between cholesterol and survival in patients with chronic heart failure. J Am Coll Cardiol. 2003;42:1933–1940. [DOI] [PubMed] [Google Scholar]
- 14. Ommen SR, Hodge DO, Rodeheffer RJ, McGregor CG, Thomson SP, Gibbons RJ. Predictive power of the relative lymphocyte concentration in patients with advanced heart failure. Circulation. 1998;97:19–22. [DOI] [PubMed] [Google Scholar]
- 15. Huang WM, Hsu PF, Cheng HM, Lu DY, Cheng YL, Guo CY, Sung SH, Yu WC, Chen CH. Determinants and prognostic impact of hyperuricemia in hospitalization for acute heart failure. Circ J. 2016;80:404–410. [DOI] [PubMed] [Google Scholar]
- 16. Levy MM, Fink MP, Marshall JC, Abraham E, Angus D, Cook D, Cohen J, Opal SM, Vincent JL, Ramsay G; Sccm/Esicm/Accp/Ats/Sis . 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Crit Care Med. 2003;31:1250–1256. [DOI] [PubMed] [Google Scholar]
- 17. Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. [DOI] [PubMed] [Google Scholar]
- 18. Sahn DJ, DeMaria A, Kisslo J, Weyman A. Recommendations regarding quantitation in M‐mode echocardiography: results of a survey of echocardiographic measurements. Circulation. 1978;58:1072–1083. [DOI] [PubMed] [Google Scholar]
- 19. Mohri Y, Inoue Y, Tanaka K, Hiro J, Uchida K, Kusunoki M. Prognostic nutritional index predicts postoperative outcome in colorectal cancer. World J Surg. 2013;37:2688–2692. [DOI] [PubMed] [Google Scholar]
- 20. Lu TH, Lee MC, Chou MC. Accuracy of cause‐of‐death coding in Taiwan: types of miscoding and effects on mortality statistics. Int J Epidemiol. 2000;29:336–343. [DOI] [PubMed] [Google Scholar]
- 21. Sandek A, Swidsinski A, Schroedl W, Watson A, Valentova M, Herrmann R, Scherbakov N, Cramer L, Rauchhaus M, Grosse‐Herrenthey A, Krueger M, von Haehling S, Doehner W, Anker SD, Bauditz J. Intestinal blood flow in patients with chronic heart failure: a link with bacterial growth, gastrointestinal symptoms, and cachexia. J Am Coll Cardiol. 2014;64:1092–1102. [DOI] [PubMed] [Google Scholar]
- 22. Sundaram V, Fang JC. Gastrointestinal and liver issues in heart failure. Circulation. 2016;133:1696–1703. [DOI] [PubMed] [Google Scholar]
- 23. Mitrache C, Passweg JR, Libura J, Petrikkos L, Seiler WO, Gratwohl A, Stahelin HB, Tichelli A. Anemia: an indicator for malnutrition in the elderly. Ann Hematol. 2001;80:295–298. [DOI] [PubMed] [Google Scholar]
- 24. Gheorghiade M, Abraham WT, Albert NM, Greenberg BH, O'Connor CM, She L, Stough WG, Yancy CW, Young JB, Fonarow GC; Investigators O‐H and Coordinators . Systolic blood pressure at admission, clinical characteristics, and outcomes in patients hospitalized with acute heart failure. JAMA. 2006;296:2217–2226. [DOI] [PubMed] [Google Scholar]
- 25. Valentova M, von Haehling S, Bauditz J, Doehner W, Ebner N, Bekfani T, Elsner S, Sliziuk V, Scherbakov N, Murin J, Anker SD, Sandek A. Intestinal congestion and right ventricular dysfunction: a link with appetite loss, inflammation, and cachexia in chronic heart failure. Eur Heart J. 2016;37:1684–1691. [DOI] [PubMed] [Google Scholar]
- 26. Rich MW, Beckham V, Wittenberg C, Leven CL, Freedland KE, Carney RM. A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure. N Engl J Med. 1995;333:1190–1195. [DOI] [PubMed] [Google Scholar]
- 27. Anker SD, Coats AJ. Cardiac cachexia: a syndrome with impaired survival and immune and neuroendocrine activation. Chest. 1999;115:836–847. [DOI] [PubMed] [Google Scholar]
- 28. Valentova M, von Haehling S, Krause C, Ebner N, Steinbeck L, Cramer L, Doehner W, Murin J, Anker SD, Sandek A. Cardiac cachexia is associated with right ventricular failure and liver dysfunction. Int J Cardiol. 2013;169:219–224. [DOI] [PubMed] [Google Scholar]
- 29. Ikeda Y, Ishii S, Fujita T, Iida Y, Kaida T, Nabeta T, Maekawa E, Yanagisawa T, Koitabashi T, Takeuchi I, Inomata T, Ako J. Prognostic impact of intestinal wall thickening in hospitalized patients with heart failure. Int J Cardiol. 2017;230:120–126. [DOI] [PubMed] [Google Scholar]
- 30. Kinugasa Y, Kato M, Sugihara S, Hirai M, Yamada K, Yanagihara K, Yamamoto K. Geriatric nutritional risk index predicts functional dependency and mortality in patients with heart failure with preserved ejection fraction. Circ J. 2013;77:705–711. [DOI] [PubMed] [Google Scholar]
- 31. Liu M, Chan CP, Yan BP, Zhang Q, Lam YY, Li RJ, Sanderson JE, Coats AJ, Sun JP, Yip GW, Yu CM. Albumin levels predict survival in patients with heart failure and preserved ejection fraction. Eur J Heart Fail. 2012;14:39–44. [DOI] [PubMed] [Google Scholar]
- 32. Shiraishi Y, Kohsaka S, Abe T, Mizuno A, Goda A, Izumi Y, Yagawa M, Akita K, Sawano M, Inohara T, Takei M, Kohno T, Higuchi S, Yamazoe M, Mahara K, Fukuda K, Yoshikawa T; West Tokyo Heart Failure Registry I . Validation of the Get With The Guideline‐Heart Failure risk score in Japanese patients and the potential improvement of its discrimination ability by the inclusion of B‐type natriuretic peptide level. Am Heart J. 2016;171:33–39. [DOI] [PubMed] [Google Scholar]


