Abstract
Background
Brugada syndrome (BrS) is an inherited primary arrhythmia disorder leading to sudden cardiac arrest. SCN5A, encoding the α‐subunit of the cardiac sodium channel (Nav1.5), is the most common pathogenic gene of BrS. An implantable cardioverter defibrillator (ICD) is the standard treatment for secondary prevention. This study aimed to evaluate association of the SCN5A variant with this cardiac conduction disturbance and appropriate ICD shock therapy in Thai symptomatic BrS patients with ICD implants.
Methods and Results
Symptomatic BrS patients diagnosed at university hospital were enrolled from 2008 to 2011. The primary outcome of the study was an appropriate ICD shock defined as having non‐pacing‐associated ICD shock after the occurrence of ventricular tachycardia or ventricular fibrillation. Associations between SCN5A polymorphisms, cardiac conduction disturbance, and potential confounding factors associated with appropriate ICD shock therapy were analyzed. All 40 symptomatic BrS patients (median age, 43 years) with ICD implantations were followed for 24 months. There were 16 patients (40%) who had the appropriate ICD shock therapy after ICD treatment. An independent factor associated with appropriate ICD shock therapy was SCN5A‐R1193Q with an adjusted hazard ratio of 10.550 (95% CI, 1.631–68.232).
Conclusions
SCN5A‐R1193Q is associated with cardiac conduction disturbances. It may be a genetic marker associated with ventricular arrhythmia leading to appropriate ICD shock therapy in symptomatic BrS patients with ICD treatment. Because of the small sample size of study population and the appropriate ICD shock outcome, further large studies are needed to confirm the results of this study.
Keywords: appropriate implantable cardioverter defibrillator shock therapy, Brugada syndrome, genetics, implantable cardioverter defibrillator, SCN5A R1193Q
Subject Categories: Sudden Cardiac Death; Arrhythmias; Genetic, Association Studies
Clinical Perspective
What is New?
SCN5A‐R1193Q is a genetic marker associated with cardiac conduction defects and ventricular fibrillation in symptomatic BrS patients.
What are the Clinical Implications?
Cardiologists should be aware that BrS patients with the SCN5A‐R1193Q polymorphism may require more careful monitoring of medications or electrolyte abnormalities to prevent ventricular arrhythmias.
Introduction
Brugada syndrome (BrS) is diagnosed in patients with an ST‐segment elevation with type 1 morphology of more than 2 mm in more than 1 lead among the right precordial leads V1 and/or V2 positioned in the second, third, or fourth intercostal spaces occurring either spontaneously or after provocative drug testing with intravenous administration of sodium‐channel blockers.1, 2 In Thailand, there were survivors from sudden cardiac arrest (SCA) who were found to have an ECG profile and symptoms characteristic of BrS, and some of whom had a documented family history of SCA.3, 4 Most patients were young, born and raised in the northeastern part of Thailand without any previous medical history. It is believed that BrS is an inherited disease attributed to genetic penetrance in family members. Currently, implantable cardioverter defibrillator (ICD) is the standard treatment for secondary prevention post‐SCA or syncope of unknown origin.5, 6 The ICD is an effective treatment to prevent cardiac arrest from recurrent ventricular tachycardia or ventricular fibrillation (VF) in BrS patients.
The SCN5A gene mutations were found in 20% to 25% of BrS patients and 0% to 0.08% in general population.1 There are 4 known common polymorphisms of the SCN5A gene related to BrS, including R34C, H558R, S1103Y, and R1193Q.7, 8 These SCN5A polymorphisms could decrease expression of sodium‐channel proteins and alter gating properties resulting in prolongation of the QRS duration and slow conduction in the heart.7, 9 The SCN5A mutations may be associated with early and frequent VF recurrence or SCA in BrS patients, which may be related to fibrosis in the right ventricular outflow tract epicardial surface.10, 11 Most studies on SCN5A mutations included both asymptomatic and symptomatic BrS patients.8, 9, 10, 11 This study was aimed to investigate the association of SCN5A mutations and appropriate ICD shock therapy in only symptomatic BrS patients.
Methods
Study Population
All patients diagnosed with BrS in Queen Sirikit Heart Center of The Northeast and Srinagarind Hospital, Khon Kaen University, Thailand, between 2008 and 2011 were enrolled. The diagnosis of BrS was made clinically in patients presenting with SCA or unexplained syncope with 1 of the following criteria: evidence of documented ventricular tachycardia or VF; a family history of sudden cardiac death at <45 years of age; a similar type ECG in family members; history of nocturnal agonal respiration with spontaneous type 1 Brugada ECG; or showing type 1 Brugada ECG after provocation by using high intercostal ECG lead detection or after administration of flecainide. Type 1 ECG was defined as a prominent coved ST segment elevation >2 mm followed by a negative T wave.2
All patients received cardiac workups, including echocardiograms and coronary angiograms, to exclude structural heart diseases. All workups of structural heart disease were required to be normal. The study protocol was approved by an institutional review committee, Khon Kaen University, and conformed to the Declaration of Helsinki. All patients provided informed consent before study enrollment.
The following clinical data were captured and documented: age at diagnosis; sex; birthplace; family history of SCA; type of Brugada ECG at diagnosis; results of pharmacological testing or high intercostal lead detection for unmasking the coved type ECG pattern; circumstances of diagnosis (survival of SCA, syncope); indication for ICD implantation; genetic study with SCN5A genotyping; and ICD follow‐up data. High intercostal ECGs were obtained by placing the precordial V1 to V2 leads in the fourth intercostal space, V3 to V4 in the third intercostal space, and V5 to V6 in the second intercostal space. V1, V3, and V5 were placed on the right side, with V2, V4, and V6 on the left side. The diagnosis of BrS was made by evidence of coved‐type ECG in at least 1 of the high intercostal leads.12
SCN5A Genotyping
Ten milliliters of peripheral venous blood were obtained from patients using standard blood sampling techniques. Genomic DNA of each patient was isolated from peripheral leukocytes. All 28 exons of SCN5A were determined by amplifying using specific primers.13 The polymerase chain reaction products were sequenced using the BigDye Terminator v3.1 Cycle Sequencing Kit and run on the automated ABI Prism Model 3100 Avant Genetic Analyzer (Applied Biosystems, Foster City, CA).
Outcomes
All patients were followed at the cardiac device clinic every 3 to 6 months. ICD data of each patient were collected and interpreted at each follow‐up. All patients were categorized as BrS with or without recurrent appropriate ICD shock. Appropriate ICD shock is defined as having non‐pacing‐associated ICD shock after the occurrence of ventricular tachycardia or VF.14 Those patients who had inappropriate ICD shocks, such as sinus tachycardia, atrial fibrillation, or lead fractures, were excluded.
Statistical Analysis
The Wilcoxon rank‐sum test was used for comparison of medians of continuous variables between BrS patients with or without appropriate shock. Fisher's exact test was used to compare categorical variables between the 2 groups.
Univariate Cox regression analysis was applied to calculate the crude hazard ratios of individual variables for having appropriate ICD shocks over times. All variables with P<0.20 in univariate analysis or that were clinically significant were included in subsequent multivariate Cox regression analysis. Clinically significant factors were previously reported potential confounding factors associated with appropriate ICD shock, such as family history of SCA. The final model was composed of potential confounding factors associated with appropriate ICD shock as follows: family history of cardiac arrest; PR interval; QRS duration; and SCN5A‐R1193Q. Analytical results are presented as adjusted hazard ratios and 95% CIs. The significant factors were plotted for the ICD shock‐free times and time to the first ICD shock by Kaplan–Meier analysis.
Results
During the study period, 40 patients met the study inclusion criteria and received single‐chamber ICD treatment programed for a VF zone of >188 beats/min. The median (range) age of all patients was 43 years (22–66). The median (range) follow‐up time was 24 months (13–52). There were 29 patients (72.5%) who had spontaneous Brugada ECG type 1; others (11 patients; 27.5%) were unmasked by high intercostal lead placement.
Of 28 exons of SCN5A genotyping, only H558R (Exon‐12, c. 1673 A>G, rs1805124) and R1193Q (Exon 20, c.3578G>A, rs41261344) were identified in 9 (22.5%) and 6 (15.0%) patients, with allele frequencies of 0.113 and 0.075. The Hardy–Weinberg equilibrium was tested to check whether the included individuals were in equilibrium for the frequencies of genotype. The genotypes of these 2 single‐nucleotide polymorphisms of SCN5A (H558R and R1193Q) followed the Hardy–Weinberg equilibrium, which implies that the included individuals are likely to represent the population.
Among them, there were 16 patients (40%) who had the appropriate ICD shock therapy after ICD treatment; 10 of these patients with a history of SCA. The average time to first ICD shock was 8.50±4.43 months. Almost all patients were male (97.5%), and SCA was the most common indication for ICD treatment (72.5%). A significantly higher percentage of patients with SCN5A‐R1193Q was observed in the group with appropriate ICD shock (31.25% versus 4.35%; P=0.029). However, the frequency of patients who carried SCN5A‐H558R was not significantly different between these 2 groups as shown in Table 1.
Table 1.
Characteristics of Symptomatic BrS Patients Treated With an ICD Categorized by Occurrence of Recurrent ICD Shock
| Factors | No ICD Shock (n=24) | ICD Shock (n=16) | P Value |
|---|---|---|---|
| Median age (range), y | 45 (22–60) | 43 (31–66) | 0.934 |
| Male sex | 23 (95.83) | 16 (100) | 0.999 |
| Sudden cardiac arrest | 16 (66.67) | 13 (81.25) | 0.473 |
| Family history of sudden death | 7 (29.17) | 7 (43.75) | 0.343 |
| Spontaneuos Brugada ECG type 1 | 16 (66.67) | 13 (81.25) | 0.312 |
| Median PR interval, ms | 167 (148–224) | 177 (154–220) | 0.082 |
| QRS duration, ms | 134 (100–152) | 139 (124–162) | 0.292 |
| SCN5A genotype | |||
| H558R+ | 5 (20.83) | 4 (25.00) | 0.999 |
| R1193Q+ | 1 (4.35) | 5 (31.25) | 0.029 |
| H558R+/R1193Q+ | 1 (4.17) | 1 (6.25) | 0.999 |
| H558R+/R1193Q− | 4 (16.67) | 3 (18.75) | 0.999 |
| H558R−/R1193Q+ | 0 | 4 (25.00) | 0.020 |
| H558R−/R1193Q− | 19 (79.17) | 8 (50.00) | 0.086 |
Data presented as number (percentage), unless indicated otherwise; H558R+: patients who carried mutation at 558 from H to R; R1193Q+: patients who carried mutation at 1193 from R to Q; H558R+/R1193Q−: patients who carried mutation at 558 from H to R, but no mutation at 1193; H558R−/R1193Q+: patients who carried mutation at 1193 from R to Q, but no mutation at 558; H558R−/R1193Q−: patients without mutation at both positions. ICD indicates implantable cardioverter defibrillator.
Of 40 eligible symptomatic BrS patients, 9 (22.5%) had SCN5A‐H558R and/or R1193Q. Two (5%) BrS patients had both SCN5A‐H558R and R1193Q, whereas 7 (18%) and 4 (10%) BrS patients had only SCN5A‐H558R and R1193Q (Table 1). The association between the SCN5A polymorphisms and electrocardiographic findings are shown in Tables 2, 3 through 4. SCN5A‐R1193Q was associated with a longer PR interval (201 [196–212] versus 168 [162–176] ms; P=0.002) and a widening of the QRS duration (145 [140–155] versus 133 [128–142] ms; P=0.023) as shown in Table 3 and Figure 1.
Table 2.
Association Between SCN5A Polymorphism and Electrocardiographic Findings
| SCN5A + (n=9) | SCN5A − (n=31) | P Value | |
|---|---|---|---|
| PR interval, ms | 190 (188–194) | 166 (162–176) | 0.006 |
| QRS duration, ms | 142 (138–146) | 132 (128–142) | 0.017 |
Positive SCN5A indicated presence of H558R and/or R1193Q. Data presented as median (25th quartile, 75th quartile).
Table 3.
Association Between R1193Q and Electrocardiographic Findings
| R1193Q+ (N=4) | R1193Q− (N=34) | P Value | |
|---|---|---|---|
| PR interval, ms | 201 (196–212) | 168 (162–176) | 0.002 |
| QRS duration, ms | 145 (140–155) | 133 (128–142) | 0.023 |
Data presented as median (25th quartile, 75th quartile).
Table 4.
Association Between H558R and Electrocardiographic Findings
| Factors | H558R+ (n=7) | H558R− (n=31) | P Value |
|---|---|---|---|
| PR interval, ms | 188 (168–192) | 166 (162–176) | 0.042 |
| QRS duration, ms | 140 (138–144) | 132 (128–142) | 0.082 |
Data presented as median (25th quartile, 75th quartile).
Figure 1.
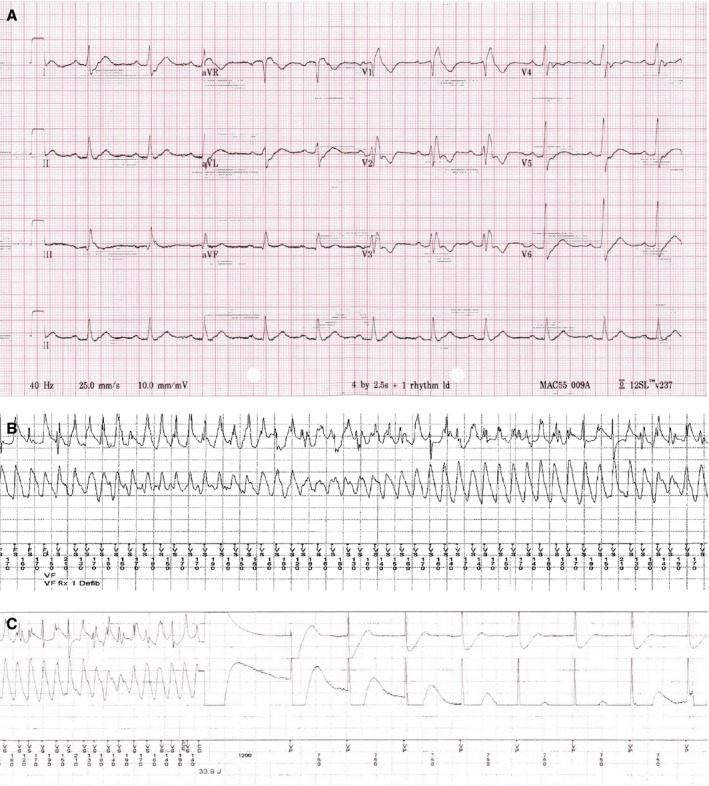
ECGs of an SCN5A‐R1193Q carrier who had recurrent ventricular fibrillation (VF) with appropriate implantable cardioverter defibrillator (ICD) treatment. A, ECG shows widening of QRS duration (152 ms) and prolongation of PR interval (200 ms). B, ICD record with appropriate VF detection. C, Successful ICD shock from VF.
Four potential confounding factors were included in the Cox proportional model to identify factors associated with appropriate ICD therapy, including family history of cardiac arrest, PR interval, QRS duration, and SCN5A‐R1193Q as shown in Table 5. Unadjusted hazard ratio of SCN5A‐R1193Q was highest at 10.629 (Table 5). For the multivariate model, only SCN5A‐R1193Q was significantly associated with appropriate ICD shock therapy in symptomatic BrS patients who received ICD treatment as secondary prevention with an adjusted hazard ratio of 10.550 (95% CI, 1.631–68.232). Those patients with a positive SCN5A‐R1193Q had shorter times to the ICD shock‐free time compared with those patients with a negative SCN5A‐R1193Q (Figure 2).
Table 5.
Factors Associated With an Appropriate ICD Shock Therapy in Symptomatic BrS Patients
| Factors | Unadjusted Hazard Ratio (95% CI) | Adjusted Hazard Ratio (95% CI) |
|---|---|---|
| Family history of cardiac arrest | 1.290 (0.428–3.891) | 1.054 (0.271–4.098) |
| PR interval | 1.015 (0.988–1.043) | 0.980 (0.888–1.081) |
| QRS duration | 1.027 (0.977–1.081) | 1.058 (0.881–1.271) |
| SCN5A‐R1193Q | 10.629 (1.807–62.508) | 10.550 (1.631–68.232) |
ICD indicates implantable cardioverter defibrillator.
Figure 2.
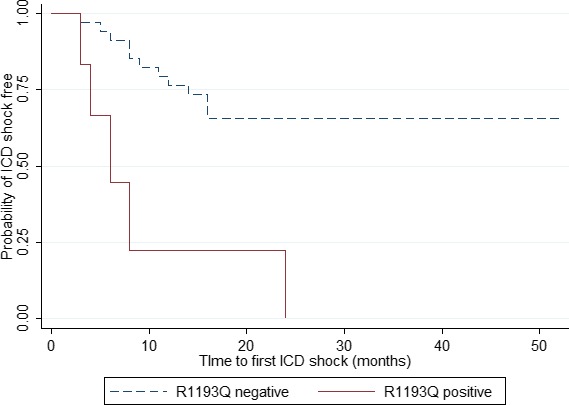
Kaplan–Meier survival curve of symptomatic Brugada Syndrome patients treated with an implantable cardioverter defibrillator (ICD) showed probabilities of ICD shock free and time to the first ICD shock categorized by presence of R1193Q polymorphism.
Discussion
Only 2 known SCN5A polymorphisms were identified in these 40 symptomatic BrS patients—H558R and R1193Q in 9 (22.5%) and 6 (15.0%) patients, with allele frequencies of 0.113 and 0.075. As in a previous report, the global frequency of R1193Q (OMIM: 600163.0023) is 0.0124. R1193Q is mostly found in Asian, such as Chinese (0.05–0.08), Vietnamese (0.08), and Japanese (0.02), whereas it is defined as a mutation in other populations, such as white (0.00) and black (0.00). The frequency of R1193Q in this study is higher than that in the Thai general population (0.075 versus 0.0395), because only symptomatic BrS cases (majority with history of SCA) are included in the present study.15
Moreover, the R1193Q in the present study is associated with cardiac conduction delay with evidence of a longer PR, a wider QRS duration, and a significantly higher frequency of R1193Q was observed in the BrS patients receiving appropriate ICD shock therapy more than those who did not receive the ICD shock therapy (31.25% versus 4.35%; P=0.029), as shown in Table 1. Ohkubo et al also reported prolonged QRS duration of more than 120 ms, as measured on a standard ECG, that is associated with life‐threatening ventricular arrhythmia in BrS.9 These findings occur because of abnormal slow and fragmented epicardial electrograms from reduction in gap junction expression at right ventricular outflow tract evidenced by autopsies.11 These abnormal electrograms caused by cardiac conduction delay may lead to generate reentry of ventricular arrhythmia, as shown in this study, by appropriate ICD therapy in BrS patients (Table 1). This study also confirms the importance of QRS duration as a risk of ventricular arrhythmia.
Previous reports also showed that R1193Q polymorphism could accelerate the inactivation of the sodium‐channel current, leading to a cardiac conduction defect and endocardial and epicardial repolarization abnormalities resulting in arrhythmogenesis.16, 17, 18 The PR prolongation could differentiate between SCN5A and non‐SCN5A BrS patients.19, 20 The study herein also showed longer PR intervals in BrS patients with either R1193Q or H558R SCN5A polymorphisms (Tables 2, 3 through 4).
This study shows that the rate of appropriate ICD therapy was 34.5% in the aborted SCA group. These rates were comparable with a previous study, which reported discharge rates for appropriate ICD therapy of 44% in the aborted SCA group during a 7‐year follow‐up period.21 Forty symptomatic BrS patients receiving ICD treatment were examined in this study. R1193Q was the only factor that may be associated with appropriate ICD shock therapy after ICD treatment by both univariate and multivariate Cox regression for survival analysis (Table 5). This finding may be explained by the genetic heterogeneous character of BrS in different ethnicities. R1193Q was associated with cardiac conduction delays that may modulate arrhythmogenic substrates, leading to ventricular arrhythmia and recurrent appropriate ICD therapy.22
R1193Q, a G to A mutation at position 3578, which causes the substitution of arginine for glutamine at position 1193, appeared to be a genetic factor associated with appropriate ICD shock therapy in symptomatic BrS patients with the ICD treatment. Therefore, cardiologists should carefully follow up with an awareness of recurrent ventricular tachycardia and VF in symptomatic BrS patients who have this polymorphism by avoiding modulating factors, such as psychotropic drugs, fever, class I antiarrhythmic drugs, and electrolyte abnormalities.23
H558R, an A to G mutation at position 1673, which causes the substitution of histidine for arginine at position 558, may be associated with sodium‐channel inactivation, but not as strong as R1193Q.24 The results of this study supported this hypothesis, evidenced by the prolongation of PR interval, but not significant QRS widening, in BrS patients with the presence of H558R (Table 4). And, H558R was not associated with appropriate ICD shocks in symptomatic BrS patients (Table 1).
The family history of SCA has been proposed as 1 risk predictor in the past.4 Recent evidence, however, has clearly demonstrated that family history has not been a reliable marker for high risk in patients affected by BrS.2 Moreover, the value of a family history of sudden death in BrS patients has been extensively investigated, and results in that family history of SCA are not predictive for future arrhythmic events.25, 26 Consistent with these studies, present results have also shown that family history of SCA was not associated with recurrent ICD shocks in Thai symptomatic BrS.
BrS is an uncommon genetic disease resulting in a small sample size. Presence of R1193Q polymorphism are categorical data. Because of small study populations and categorical data, the CI of R1193Q polymorphism is large. This study is in a relatively isolated population because most of the patients were originally from northeastern Thailand, and the findings may not be inferred for other populations. Another limitation of this study is that only 2 polymorphisms of SCN5A were identified in this study. If the BrS patients have other mutations in addition to R1193Q, risks for ventricular arrhythmias may be higher. Further large studies are needed to confirm the results of this study. But, the strength and uniqueness of this study is that patients enrolled in the study were all symptomatic BrS patients and mostly with documented SCA. In this study, the diagnosis of BrS was made clinically. We retrospectively traced back all patients in this study with the 2016 diagnostic criteria for BrS.27 All patients met with the definite criteria for BrS by the median Shanghai score of 6.5 (range, 4.0–7.5).
In conclusion, SCN5A‐R1193Q polymorphism is associated with a cardiac conduction disturbance and may be a genetic factor associated with ventricular arrhythmia, leading to appropriate ICD shock therapy in symptomatic BrS patients with ICD treatment. This study is needed to be confirmed in other ethnicities and with a larger sample size in the future.
Sources of Funding
The Thailand Research Fund (TRF; IRG 5780016), the TRF Senior Research Scholar Grant from the Thailand Research Fund (TRF Grant No. RTA5880001), and the Higher Education Research Promotion and National Research University Project of Thailand, Office of the Higher Education Commission, Thailand, through the Health Cluster (SHeP‐GMS), Khon Kaen University; and grant of Faculty of Medicine, Khon Kaen University, Thailand (Grant No. RG59301).
Disclosures
None.
Acknowledgments
The authors would like to thank James A. Will (University of Wisconsin, USA) for his kind manuscript English editing via Publication Clinic KKU, Thailand.
(J Am Heart Assoc. 2017;6:e005009 DOI: 10.1161/JAHA.116.005009.)28584071
References
- 1. Priori SG, Wilde AA, Horie M, Cho Y, Behr ER, Berul C, Blom N, Brugada J, Chiang CE, Huikuri H, Kannankeril P, Krahn A, Leenhardt A, Moss A, Schwartz PJ, Shimizu W, Tomaselli G, Tracy C. HRS/EHRA/APHRS expert consensus statement on the diagnosis and management of patients with inherited primary arrhythmia syndromes: document endorsed by HRS, EHRA, and APHRS in May 2013 and by ACCF, AHA, PACES, and AEPC in June 2013. Heart Rhythm. 2013;10:1932–1963. [DOI] [PubMed] [Google Scholar]
- 2. Berne P, Brugada J. Brugada syndrome 2012. Circ J. 2012;76:1563–1571. [DOI] [PubMed] [Google Scholar]
- 3. Nademanee K, Veerakul G, Nimmannit S, Chaowakul V, Bhuripanyo K, Likittanasombat K, Tunsanga K, Kuasirikul S, Malasit P, Tansupasawadikul S, Tatsanavivat P. Arrhythmogenic marker for the sudden unexplained death syndrome in Thai men. Circulation. 1997;96:2595–2600. [DOI] [PubMed] [Google Scholar]
- 4. Tatsanavivat P, Chirawatkul A, Klungboonkrong V, Chaisiri S, Jarerntanyaruk L, Munger RG, Saowakontha S. Sudden and unexplained deaths in sleep (Lai Tai) of young men in rural northeastern Thailand. Int J Epidemiol. 1992;21:904–910. [DOI] [PubMed] [Google Scholar]
- 5. Brugada J, Brugada R, Brugada P. Pharmacological and device approach to therapy of inherited cardiac disease associated with cardiac arrhythmias and sudden death. J Electrocardiol. 2000;33:41–47. [DOI] [PubMed] [Google Scholar]
- 6. Nademanee K, Veerakul G, Mower M, Likittanasombat K, Krittayapong R, Bhuripanyo K, Sitthisook S, Chaothawee L, Lai MY, Azen SP. Defibrillator versus beta blockers for unexplained death in Thailand (DEBUT): a randomized clinical trial. Circulation. 2003;107:2221–2226. [DOI] [PubMed] [Google Scholar]
- 7. Ackerman M, Splawski I, Makielski J, Tester DJ, Will ML, Timothy KW, Keating MT, Jones G, Chadha M, Burrow CR, Stephens JC, Xu C, Judson R, Curran ME. Spectrum and prevalence of cardiac sodium channel variants among black, white, Asian and Hispanic individuals: implication for arrhythmogenic susceptibility and Brugada/long QT syndrome genetic testing. Heart Rhythm. 2004;5:600–607. [DOI] [PubMed] [Google Scholar]
- 8. Kapplinger JD, Tester DJ, Alders M, Benito B, Berthet M, Brugada J, Brugada P, Fressart V, Guerchicoff A, Harris‐Kerr C, Kamakura S, Kyndt F, Koopmann TT, Miyamoto Y, Pfeiffer R, Pollevick GD, Probst V, Zumhagen S, Vatta M, Towbin JA, Shimizu W, Schulze‐Bahr E, Antzelevitch C, Salisbury BA, Guicheney P, Wilde AA, Brugada R, Schott JJ, Ackerman MJ. An international compendium of mutations in the SCN5A ‐encoded cardiac sodium channel in patients referred for Brugada syndrome genetic testing. Heart Rhythm. 2010;7:33–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ohkubo K, Watanabe I, Okumura Y, Ashino S, Kofune M, Nagashima K, Kofune T, Nakai T, Kunimoto S, Kasamaki Y, Hirayama A. Prolonged QRS duration in lead V2 and risk of life‐threatening ventricular arrhythmia in patients with Brugada syndrome. Int Heart J. 2011;52:98–102. [DOI] [PubMed] [Google Scholar]
- 10. Nishii N, Ogawa M, Morita H, Nakamura K, Banba K, Miura D, Kumagai N, Matsunaga A, Kawamura H, Urakawa S, Miyaji K, Nagai M, Satoh K, Nakagawa K, Tanaka M, Hiramatsu S, Tada T, Murakami M, Nagase S, Kohno K, Kusano KF, Saku K, Ohe T, Ito H. SCN5A mutation is associated with early and frequent recurrence of ventricular fibrillation in patients with Brugada syndrome. Circ J. 2010;74:2572–2578. [DOI] [PubMed] [Google Scholar]
- 11. Nademanee K, Raju H, de Noronha SV, Papadakis M, Robinson L, Rothery S, Makita N, Kowase S, Boonmee N, Vitayakritsirikul V, Ratanarapee S, Sharma S, van der Wal AC, Christiansen M, Tan HL, Wilde AA, Nogami A, Sheppard MN, Veerakul G, Behr ER. Fibrosis, connexin‐43, and conduction abnormalities in the Brugada syndrome. J Am Coll Cardiol. 2015;66:1976–1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Sangwatanaroj S, Prechawat S, Sunsaneewitayakul B, Sitthisook S, Tosukhowong P, Tungsanga K. New electrocardiographic leads and the procainamide test for the detection of the Brugada sign in sudden unexplained death syndrome survivors and their relatives. Eur Heart J. 2001;22:2290–2296. [DOI] [PubMed] [Google Scholar]
- 13. Koo SH, Teo WS, Ching CK, Chan SH, Lee EJ. Mutation screening in KCNQ1, HERG, KCNE1, KCNE2 and SCN5A genes in a long QT syndrome family. Ann Acad Med Singapore. 2007;36:394–398. [PubMed] [Google Scholar]
- 14. Sweeney MO, Ruetz LL, Belk P, Mullen TJ, Johnson JW, Sheldon T. Bradycardia pacing‐induced short‐long‐short sequences at the onset of ventricular tachyarrhythmias: a possible mechanism of proarrhythmia? J Am Coll Cardiol. 2007;50:614–622. [DOI] [PubMed] [Google Scholar]
- 15. Matsusue A, Yuasa I, Umetsu K, Nakayashiki N, Dewa K, Nishimukai H, Kashiwagi M, Hara K, Waters B, Takayama M, Ikematsu N, Kubo S. The global distribution of the p.R1193Q polymorphism in the SCN5A gene. Leg Med (Tokyo). 2016;19:72–76. [DOI] [PubMed] [Google Scholar]
- 16. Matsusue A, Kashiwagi M, Hara K, Waters B, Sugimura T, Kubo S. An autopsy case of sudden unexpected nocturnal death syndrome with R1193Q polymorphism in SCN5A gene. Leg Med (Tokyo). 2012;14:317–319. [DOI] [PubMed] [Google Scholar]
- 17. Huang H, Zhao J, Barrane FZ, Champagne J, Chahine M. Nav1.5/R1193Q polymorphism is associated with both long QT and Brugada syndromes. Can J Cardiol. 2006;22:309–313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Probst V, Allouis M, Sacher F, Pattier S, Babuty D, Mabo P, Mansourati J, Victor J, Nguyen JM, Schott JJ, Boisseau P, Escande D, Le Marec H. Progressive cardiac conduction defect is the prevailing phenotype in carriers of a Brugada syndrome SCN5A mutation. J Cardiovasc Electrophysiol. 2006;17:270–275. [DOI] [PubMed] [Google Scholar]
- 19. Smits JP, Eckardt L, Probst V, Bezzina CR, Schott JJ, Remme CA, Haverkamp W, Breithardt G, Escande D, Schulze‐Bahr E, LeMarec H, Wilde AA. Genotype‐phenotype relationship in Brugada syndrome: electrocardiographic features differentiate SCN5A‐related patients from non‐SCN5A‐related patients. J Am Coll Cardiol. 2002;40:350–356. [DOI] [PubMed] [Google Scholar]
- 20. Gehi AK, Duong TD, Metz LD, Gomes JA, Mehta D. Risk stratification of individuals with the Brugada electrocardiogram: a meta‐analysis. J Cardiovasc Electrophysiol. 2006;17:577–583. [DOI] [PubMed] [Google Scholar]
- 21. Conte G, Sieira J, Ciconte G, de Asmundis C, Chierchia GB, Baltogiannis G, Di Giovanni G, La Meir M, Wellens F, Czapla J, Wauters K, Levinstein M, Saitoh Y, Irfan G, Julià J, Pappaert G, Brugada P. A 20 year single center experience Implantable cardioverter‐defibrillator therapy in Brugada syndrome: a 20‐year single‐center experience. J Am Coll Cardiol. 2015;65:879–888. [DOI] [PubMed] [Google Scholar]
- 22. Probst V, Wilde AA, Barc J, Sacher F, Babuty D, Mabo P, Mansourati J, Le Scouarnec S, Kyndt F, Le Caignec C, Guicheney P, Gouas L, Albuisson J, Meregalli PG, Le Marec H, Tan HL, Schott JJ. SCN5A mutations and the role of genetic background in the pathophysiology of Brugada syndrome. Circ Cardiovasc Genet. 2009;2:552–557. [DOI] [PubMed] [Google Scholar]
- 23. Sacher F, Probst V, Iesaka Y, Jacon P, Laborderie J, Mizon‐Gérard F, Mabo P, Reuter S, Lamaison D, Takahashi Y, O'Neill MD, Garrigue S, Pierre B, Jaïs P, Pasquié JL, Hocini M, Salvador‐Mazenq M, Nogami A, Amiel A, Defaye P, Bordachar P, Boveda S, Maury P, Klug D, Babuty D, Haïssaguerre M, Mansourati J, Clémenty J, Le Marec H. Outcome after implantation of a cardioverter‐defibrillator in patients with Brugada syndrome: a multicenter study. Circulation. 2006;114:2317–2324. [DOI] [PubMed] [Google Scholar]
- 24. Poelzing S, Forleo C, Samodell M, Dudash L, Sorrentino S, Anaclerio M, Troccoli R, Iacoviello M, Romito R, Guida P, Chahine M, Pitzalis M, Deschênes I. SCN5A polymorphism restores trafficking of a Brugada syndrome mutation on a separate gene. Circulation. 2006;114:368–376. [DOI] [PubMed] [Google Scholar]
- 25. Priori SG, Gasparini M, Napolitano C, Della Bella P, Ottonelli AG, Sassone B, Giordano U, Pappone C, Mascioli G, Rossetti G, De Nardis R, Colombo M. Risk stratification in Brugada syndrome: results of the PRELUDE (PRogrammed ELectrical stimUlation preDictive valuE) registry. J Am Coll Cardiol. 2012;59:37–45. [DOI] [PubMed] [Google Scholar]
- 26. Sarkozy A, Sorgente A, Boussy T, Casado R, Paparella G, Capulzini L, Chierchia GB, Yazaki Y, De Asmundis C, Coomans D, Brugada J, Brugada P. The value of a family history of sudden death in patients with diagnostic type I Brugada ECG pattern. Eur Heart J. 2011;32:2153–2160. [DOI] [PubMed] [Google Scholar]
- 27. Antzelevitch C, Yan GX, Ackerman MJ, Borggrefe M, Corrado D, Guo J, Gussak I, Hasdemir C, Horie M, Huikuri H, Ma C, Morita H, Nam GB, Sacher F, Shimizu W, Viskin S, Wilde AA. J‐wave syndromes expert consensus conference report: emerging concepts and gaps in knowledge. Heart Rhythm. 2016;13:e295–e324. [DOI] [PMC free article] [PubMed] [Google Scholar]


