Abstract
Background
China has gaps in the quality of care provided to patients with ST‐elevation myocardial infarction, but little is known about how quality varies between hospitals.
Methods and Results
Using nationally representative data from the China PEACE‐Retrospective AMI Study, we characterized the quality of care for ST‐elevation myocardial infarction at the hospital level and examined variation between hospitals. Two summary measures were used to describe the overall quality of care at each hospital and to characterize variations in quality between hospitals in 2001, 2006, and 2011. The composite rate measured the proportion of opportunities a hospital had to deliver 6 guideline‐recommended treatments for ST‐elevation myocardial infarction that were successfully met, while the defect‐free rate measured the proportion of patients at each hospital receiving all guideline‐recommended treatments for which they were eligible. Risk‐standardized mortality rates were calculated. Our analysis included 12 108 patients treated for ST‐elevation myocardial infarction at 162 hospitals. The median composite rate increased from 56.8% (interquartile range [IQR], 45.9–72.0) in 2001 to 80.5% (IQR, 74.7–84.8) in 2011; however, substantial variation remained in 2011 with defect‐free rates ranging from 0.0% to 76.9%. The median risk‐standardized mortality rate increased from 9.9% (IQR, 9.1–11.7) in 2001 to 12.6% (IQR, 10.9–14.6) in 2006 before falling to 10.4% (IQR, 9.1–12.4) in 2011.
Conclusions
Higher rates of guideline‐recommended care and a decline in variation between hospitals are indicative of an improvement in quality. Although some variation persisted in 2011, very top‐performing hospitals missed few opportunities to provide guideline‐recommended care. Quality improvement initiatives should focus on eliminating residual variation as well as measuring and improving outcomes.
Clinical Trial Registration
URL: https://www.clinicaltrials.gov. Unique identifier: NCT01624883.
Keywords: China, hospital performance, quality improvement, quality measurement, variation
Subject Categories: Health Services, Quality and Outcomes, Revascularization, Percutaneous Coronary Intervention, Myocardial Infarction
Clinical Perspectives
What is New?
This nationally representative study provides a comprehensive overview of the quality of care provided by hospitals in China to patients experiencing a ST‐segment myocardial infarction.
There was significant variation in the quality of care provided by individual hospitals: some hospitals matched the performance of top US hospitals and consistently provided care of the highest quality, whereas other hospitals missed many opportunities to provide guideline‐recommended care.
The proportion of patients receiving reperfusion (ie, thrombolysis or percutaneous coronary intervention), even after excluding those ineligible for such an intervention, at the “average” hospital was just above 50%.
What are the Clinical Implications?
Measuring variation in the quality of care between hospitals means that quality improvement efforts can be tailored to individual institutions.
Reducing variation in the quality of care between hospitals has the potential to yield significant improvements in performance.
There is an opportunity to expand the use of reperfusion to maximize the proportion of patients eligible for these potentially life‐saving therapies who are treated with thrombolysis using evidence‐based agents and percutaneous coronary intervention.
Introduction
Little is known about the quality of care provided by Chinese hospitals. Given that care for life‐threatening conditions, such as ST‐elevation myocardial infarction (STEMI), is provided by hospitals, understanding the performance of these organizations can inform quality improvement efforts. A handful of studies of Chinese patients have revealed gaps in the care provided to individual patients with acute coronary syndromes.1, 2 Although these studies have provided an important foundation for quality improvement efforts, patient‐level analyses pool data across hospitals, which means that variation in performance among individual institutions cannot be detected.
Measuring quality at the hospital level is a fundamentally different approach. Such hospital‐level assessments allow comparison of the performance of individual institutions and enables variation in quality between hospitals to be identified. It is important to understand the range of hospital performance—from best to worst—because average results, such as those computed in patient‐level analyses, may obscure marked heterogeneity. Another objective of hospital‐level analyses is to describe variations in care so that poorer performing sites can learn from better performing sites.
STEMI represents an ideal starting point to evaluate hospital performance in China for 3 reasons. First, China is experiencing an epidemic of cardiovascular disease, with the rate of hospital admission for STEMI quadrupling from 2001 to 2011.2 Second, there are established guidelines in China and internationally that recommend several evidence‐based interventions for patients with STEMI,3, 4 thereby enabling the use of process measures as actionable metrics of hospital performance. Third, outcomes can be compared across hospitals using an established measure of risk‐standardized mortality after AMI (acute myocardial infarction) that accounts for differences in patients presenting to each hospital for care.5
As a foundation for quality improvement in China and to provide insight into the heterogeneity of performance, we sought to better understand the quality of STEMI care and outcomes, and variations across hospitals. To do this, we used data collected in the China PEACE‐Retrospective AMI Study, a nationally representative study of STEMI patients in 2001, 2006, and 2011.6 Our previous reports from this study describe the aggregate mortality rate across the entire country and the corresponding rates with which certain guideline‐recommended treatments are applied. By aggregating data to the hospital level, we are able to characterize hospital performance and variation across institutions using process measures and risk‐standardized mortality rates.
Methods
Sample Construction and Hospital Characteristics
The design of the China PEACE‐Retrospective AMI Study has been previously described in detail.6 In brief, we created a nationally representative sample of Chinese hospitals in 2011, using simple random sampling procedures to identify hospitals from 5 predefined regions (eastern rural, central rural, western rural, eastern urban, and central–western urban), and traced this group backward to produce the 2001 and 2006 samples. Hospitals were classified according to their government‐defined level in 2011: Secondary hospitals have at least 100 inpatient beds and the capacity to treat local populations of at least 100 000, whereas tertiary hospitals are large referral centers that provide more‐advanced care. In addition, hospitals were classified as either “established” if they operated in all 3 years of the study period, or “new” if they first provided care after 2001. Hospitals were also classified by teaching status and percutaneous coronary intervention (PCI) capability. The central ethics committee at the China National Center for Cardiovascular Diseases approved the China PEACE‐Retrospective AMI Study, which is registered at www.clinicaltrials.gov (NCT01624883).
Data Abstraction
Patients with AMI were identified by searching hospitals' patient databases using International Classification of Disease, Clinical Modification codes (version 9: 410.xx; version 10: I21.xx) and written hospitalization logs, if necessary. Using systematic random sampling procedures, we produced a sample of AMI patients for each hospital in each study year. The mean proportion of cases sampled at a hospital was ≈60% (range, 7–100). The medical records associated with sampled patients were copied and sent to the study coordinating center in Beijing where trained individuals abstracted information about patient demographics, cardiovascular risk factors, medical history, clinical characteristics at admission, in‐hospital procedures, treatments, and outcomes. Quality of the abstraction was monitored by randomly auditing 5% of each batch of abstracted charts, with the overall accuracy of the abstraction exceeding 98%.6
In this analysis, we limited our sample to patients with a definite STEMI using discharge diagnosis terms and electrocardiographic findings. An independent cardiologist who was not involved in the study randomly audited 300 cases to confirm the diagnosis; the agreement rate between the electrocardiographic findings and the discharge diagnosis was 94.7%. All incoming and outgoing transfers were excluded from this analysis.
Process Measures
Process measures, which reflect China's guidelines for the treatment of STEMI,3, 7 were calculated for 6 treatments: (1) reperfusion with thrombolysis or PCI; (2) aspirin within 24 hours of admission; (3) clopidogrel within 24 hours of admission; (4) beta‐blockers during hospitalization; (5) angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers during hospitalization; and (6) statins during hospitalization (for comparison of process measures to the 2001 and 2010 Chinese guidelines, see Table S1). We considered these processes only among “ideal” patients, defined as those for whom each treatment was available and for which there was no documented contraindication. For example, only patients without defined contraindications to beta‐blockers, namely drug allergy, cardiogenic shock, acute heart failure, second‐ or third‐degree heart block, systolic blood pressure lower than 100 mm Hg, heart rate of less than 60 bpm, or another documented contraindication, were considered to represent ideal patients for the calculation of the beta‐blocker measure (for details of definitions of ideal patients, see Data S1).
Outcome Measures
The composite of in‐hospital mortality and withdrawal from treatment attributed to terminal status was used to analyze outcomes. Because terminally ill patients often seek to return home before their death in China, “withdrawal from treatment,” namely those patients for whom treatment was stopped because of their expected terminal status at discharge, is an essential component of the outcome. Indeed, the Chinese government includes withdrawal from treatment in its measure of in‐hospital mortality.8 To compare outcomes between hospitals, we calculated risk‐standardized mortality rates (RSMRs), including withdrawal from treatment, for each hospital using an established method that adjusts for patient mix.5
Statistical Analysis
All analyses were performed at the hospital level. Descriptive statistics were used to summarize hospital characteristics. The demographic and clinical characteristics of patients treated at each hospital were first summarized using frequencies for categorical variables and the median for continuous variables, and subsequently descriptive statistics were used to understand hospital‐level differences. Next, the mini‐Global Registry of Acute Coronary Events risk score, a modified version of the Global Registry of Acute Coronary Events risk score, which includes age, systolic blood pressure, ST‐segment deviation, cardiac arrest at admission, elevated cardiac enzyme levels, and heart rate, was calculated for each patient as a measure of STEMI severity9 and the data aggregated to the hospital level.
Process measures for each of the 6 guideline‐recommended treatments were calculated for each hospital by determining the proportion of ideal patients who received each therapy, excluding those patients with a length of stay of less than 24 hours, regardless of outcome, to reflect the possibility that there was insufficient time for comprehensive treatment. We summarized hospitals' performance across these process measures by calculating 2 summary measures: the “composite” rate and “defect‐free” rate. The composite rate was calculated by dividing the number of times each hospital successfully delivered each of the guideline‐recommended care processes to an ideal patient by the total number of opportunities that the hospital had to deliver such interventions. The defect‐free rate was defined as the proportion of patients at each hospital who received all treatments for which they were considered ideal. Because clopidogrel was not widely endorsed in the guidelines until 2010, the clopidogrel measure was not included in calculations of the composite and defect‐free rate in 2001 and 2006. The 6 process measures and the 2 summary measures were computed for each hospital in each year, and, after excluding those hospitals with fewer than 5 ideal patients for a given measure in a specific year, descriptive statistics were used to summarize hospital performance and the associated variation. A similar analytic approach was used to describe RMSRs. Analyses of composite and defect‐free rates as well as RSMRs were stratified by 4 key hospital characteristics: level (secondary, tertiary); ability to perform PCI; maturity (established, new); and quartile of hospital STEMI volume.
To describe variation between hospitals, we computed the median odds ratio (OR) for the process and outcome measures. When applied to multilevel data, such as information about patients treated at different hospitals, the median OR quantifies the average difference in treatment rates or outcomes across hospitals. The interpretation of the median OR is the average difference of a statistically identical patient being treated or dying at a given hospital as compared with another. As a result, a high median OR indicates substantial between‐hospital variation in the relevant treatment rate or outcome, whereas a low median OR suggests that hospital performance is consistent across institutions. To calculate the median OR, we used hierarchical logistic regression to model the 6 process measures and the RSMR in 2001, 2006, and 2011 as a function of patient characteristics as well as a hospital‐level random effect to measure between hospital variance.10 Last, we compared the composite rate of providing guideline‐recommended treatments with 3 medications (aspirin, angiotensin‐converting enzyme inhibitor, and beta‐blocker) among Chinese hospitals in 2006 and 2011 to the corresponding rates among Medicare‐certified hospitals in the United States. To do this, we calculated the composite rate in China using the approach described above, but for the United States we used the corresponding publicly reported quality measures (for complete methods, see Data S2). Chi‐square and Wilcoxon tests were used to assess the strength of observed associations. All statistical analyses were performed using SAS software (version 9.2; SAS Institute Inc, Cary, NC).
Results
Hospital Characteristics
There were 162 unique hospitals that provided treatment for STEMI during the study period: 133 in 2001, 151 in 2006, and 161 in 2011. The location of these hospitals generally reflects China's demographics (Table). Notably, the proportion of hospitals with PCI capability grew from 17.3% (23 of 133) in 2001 to 44.1% (71 of 161) in 2011 (P<0.001); other hospital characteristics were unchanged over time (P>0.6 for all comparisons).
Table 1.
Hospital Characteristics
| 2001 (n=133) | 2006 (n=151) | 2011 (n=161) | P for Difference | |
|---|---|---|---|---|
| Level, no. (%) | 0.90 | |||
| Secondary | 76 (57.1) | 87 (57.6) | 96 (59.6) | |
| Tertiary | 57 (42.9) | 64 (42.4) | 65 (40.4) | |
| Region, no. (%) | 0.98 | |||
| Eastern | 55 (41.4) | 60 (39.7) | 64 (39.8) | |
| Central | 42 (31.6) | 48 (31.8) | 48 (29.8) | |
| Western | 36 (27.1) | 43 (28.5) | 49 (30.4) | |
| Location, no. (%) | 0.99 | |||
| Rural | 80 (60.2) | 92 (60.9) | 98 (60.9) | |
| Urban | 53 (39.8) | 59 (39.1) | 63 (39.1) | |
| Teaching hospital, no. (%) | 0.66 | |||
| No | 47 (35.3) | 56 (37.1) | 65 (40.4) | |
| Yes | 86 (64.7) | 95 (62.9) | 96 (59.6) | |
| PCI‐capable, no. (%) | <0.001 | |||
| No | 110 (82.7) | 105 (69.5) | 90 (55.9) | |
| Yes | 23 (17.3) | 46 (30.5) | 71 (44.1) | |
| Maturity (%) | <0.001 | |||
| Established* | 133 (100) | 132 (87.4) | 132 (82.0) | |
| New | 0 (0.0) | 19 (12.6) | 29 (18.0) |
*One established hospital did not treat at least 5 patients with acute myocardial infarction in all study years and was correspondingly excluded from the analysis in 2006 and 2011 per the study's inclusion criteria. PCI indicates percutaneous coronary intervention.
After excluding all transfers and patients without a definitive diagnosis of STEMI, 12 108 patients who were treated for STEMI were sampled from these 162 hospitals during the study period. Patient characteristics varied between hospitals. For example, in 2011 the median proportion of female patients treated at each hospital was 30.0% (interquartile range [IQR], 21.5–36.8), whereas average age also varied (for complete details of patient characteristics at the hospital level, see Table S2). Most hospitals treated patients with comparable disease severity (median hospital‐level mini Global Registry of Acute Coronary Events score in 2011, 143; IQR, 137–151).
Process Measures
There was significant variation in the 6 process measures, although the magnitude of these variations decreased over the study period (Figure 1). For example, whereas the hospital‐level reperfusion rate fell slightly from 2001 to 2011, variation among hospitals appeared to decrease: the median OR for reperfusion rate was 3.4 in 2001 (95% CI, 2.4–4.4; IQR of the reperfusion rate, 40.0–80.0), compared with 1.8 in 2006 (95% CI, 1.5–2.2; IQR of the reperfusion rate, 46.4–64.3) and 2.3 in 2011 (95% CI, 1.9–2.6; IQR of the reperfusion rate, 43.8–71.1). Rates of statin, clopidogrel, and aspirin therapy rose, whereas those of beta‐blocker and angiotensin‐converting enzyme inhibitor use were steady. In all cases, variation was decreased; however, significant interhospital variation persisted in 2011.
Figure 1.
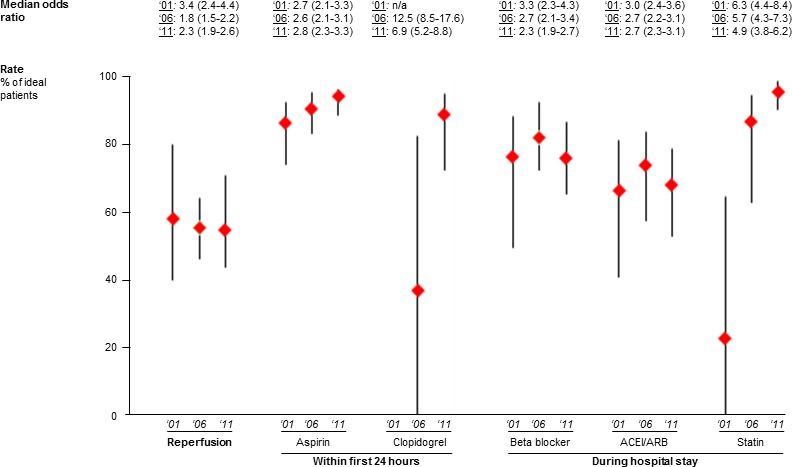
Variation (median odds ratio, median, and interquartile range) in rates of 6 process measures for ST‐elevation myocardial infarction in 2001, 2006, and 2011. ACEi indicates angiotensin converter enzyme inhibitor; ARB, angiotensin receptor blockers; n/a, not applicable.
The median composite rate rose steadily from 56.8% (IQR, 45.9–72.0) in 2001 to 78.6% (IQR, 68.0–85.1) in 2006 to 80.5% (IQR, 74.7–84.8) in 2011 (P for trend, <0.001). Although variation in the composite rate appeared to decline over the study period (Figure 2A), there were still significant outliers in 2011, with the composite rate ranging from 4.5% to 95.1%. Similarly, the median defect‐free rate rose from 14.3% (IQR, 2.7–30.0) in 2001 to 46.2% (IQR, 31.7–59.5) in 2006 before stabilizing to 42.0% (IQR, 30.0–53.8) in 2011 (P for trend, <0.001). The best performing hospital in 2001 had a defect‐free rate of 36.4% compared with 75.0% in 2006 and 76.9% in 2011 (Figure 2B). After stratifying by hospital characteristics, the composite and defect‐free rates were higher at tertiary hospitals and PCI‐capable hospitals than at secondary hospitals and non‐PCI‐capable hospitals, respectively (P<0.001 for both comparisons; Figures S1 and S2). In addition, defect‐free rate differed according to hospital volume, with lower volume hospitals tending to have lower defect‐free rates.
Figure 2.
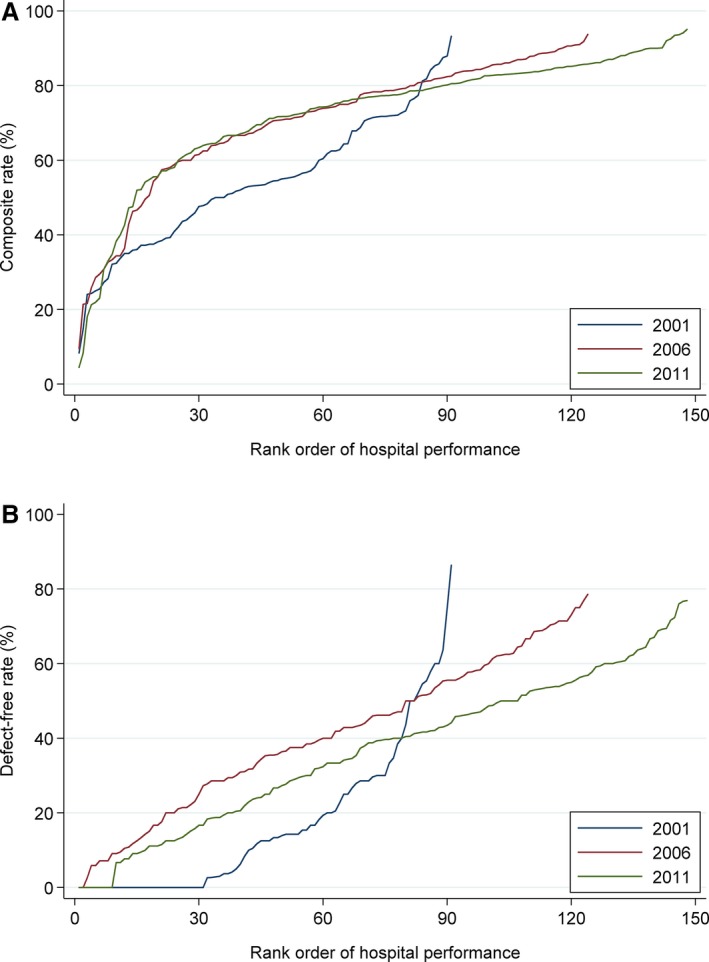
Distribution of composite (A) and defect‐free (B) rates for ST‐elevation myocardial infarction in 2001, 2006, and 2011. **The composite rate was calculated by dividing the number of times each hospital successfully delivered each of the guideline‐recommended care processes to an ideal patient by the total number of opportunities that the hospital had to deliver such interventions. The defect‐free rate was defined as the proportion of patients at each hospital who received all treatments for which they were considered ideal.
In the international comparison of the composite rate of early aspirin use and in‐hospital angiotensin‐converting enzyme inhibitor and beta‐blocker use, hospitals in the United States delivered these treatments more frequently than hospitals in China in both 2006 and 2011 (Figure 3). In addition, there was notable improvement across hospitals in the United States between 2006 and 2011, whereas composite rates in hospitals in China were largely unchanged. In 2006, the median composite rate across hospitals in the United States was 94.3% (IQR, 89.0–97.5) compared with 80.9% (IQR, 69.4–88.8) in hospitals in China, and in 2011, it was 99.4% (IQR, 98.0–100.0) in the United States compared with 80.0% (IQR, 68.6–85.9) in China. The best‐performing hospitals in China matched near‐perfect rates achieved by the majority of US hospitals on this composite measure of 3 guideline‐recommended care processes.
Figure 3.
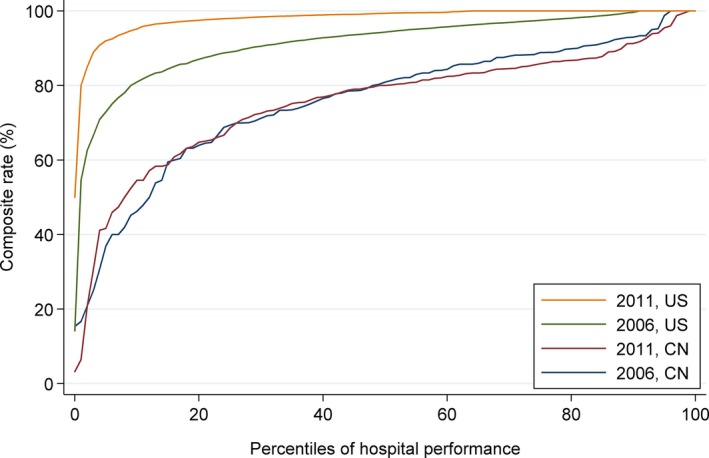
Comparison of composite rate of aspirin, ACE inhibitor and beta‐blocker therapy for patients with ST‐elevation myocardial infarction treated in hospitals in China (CN) and the United States in 2006 and 2011. ACEi indicates angiotensin converter enzyme.
Outcomes
Median hospital‐level RSMRs increased from 9.9% (IQR, 9.1–11.7) in 2001 to 12.6% (IQR, 10.9–14.6) in 2006 before falling to 10.4% (IQR, 9.1–12.4) in 2011 (Figure 4). In addition, there was notable hospital‐level variation in this outcome: The median OR in 2001 was 1.5 (95% CI: 1.1–1.9), 1.6 (95% CI 1.2–1.8) in 2006 and 1.6 (95% CI 1.4–1.8) in 2011. In the stratified analysis, there was no difference in RSMR between secondary and tertiary hospitals in any year. In 2011, RSMRs achieved by established hospitals were significantly lower than those of new hospitals (P for difference, <0.001). RSMRs were lower at PCI‐capable hospitals than non‐PCI‐capable hospitals (P for difference, <0.001). Last, differences in RSMR in 2011 emerged after stratifying by quartile of STEMI volume (P for difference, <0.001).
Figure 4.
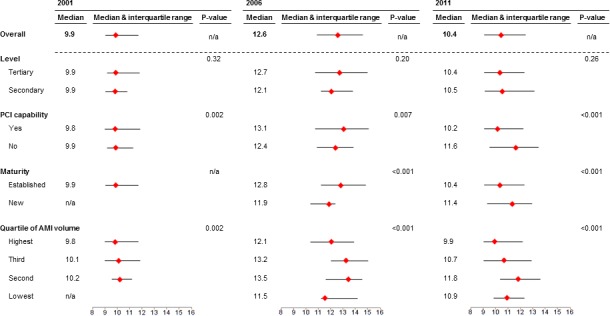
Risk‐standardized mortality rates for ST‐elevation myocardial infarction in 2001, 2006, and 2011, overall and stratified by hospital characteristics. AMI indicates acute myocardial infarction; n/a, not applicable; PCI, percutaneous coronary intervention.
Discussion
This study, which represents the first nationally representative evaluation of hospital quality in China, found marked variation in performance by individual hospitals, a heterogeneity that is not evident from average system performance. The perspective provided by hospital‐level analysis is distinct from the average changes in practices and outcomes from patient‐level analysis that cannot convey information about heterogeneity in performance across hospitals. In this study, high median ORs, which indicate significant variation in quality between hospitals, were observed throughout the decade‐long study period despite improvements in the overall rates of adherence to the various process measures. As an example, even though hospitals provided statin therapy, on average, to more than 90% of their patients in 2011, the median odds of a statistically identical ideal patient receiving statin therapy at 1 randomly chosen hospital compared with another was 4.9. Although we identified significant variation in all quality measures, there was evidence that the magnitude of variation in quality between hospitals was reduced during the study period. In addition, we found exemplary performance at some hospitals. Top‐performing hospitals in China delivered appropriate treatment for 95% of opportunities and provided defect‐free care to three quarters of their patients. The path toward improving care and outcomes in China might begin with a focus on poorly performing hospitals and the development of systems that enable more uniform and high performance by all hospitals.
It is instructive to view the findings in the context of the experience of the United States. Whereas the very top hospitals in China performed similarly to hospitals in the United States, the vast majority performed at much lower levels: On average, the composite rate of 3 important care processes was 15% lower at hospitals in China compared with those in the United States. Improvements in hospital performance in the United States eliminated most variation by 2011,11 which suggests that it may be possible to rapidly improve hospital performance in China. The progress in the United States was driven by a series of measurement initiatives that encouraged hospitals to improve their performance.
In recent years, China's National Commission for Health and Family Planning (formerly the Ministry of Health) has sought to establish a panel of quality measures to characterize hospital performance for secondary hospitals.12 In addition, the launch of the Hospital Quality Monitoring System, which intends to collect mortality data from all tertiary hospitals,13 has the potential to provide an infrastructure of ongoing quality assessment that can support hospital improvement. Although quality measurement may stimulate some improvement, it will likely be inadequate by itself. The mixed results of the second phase of the Clinical Pathways in Acute Coronary Syndromes study, which randomized 75 hospitals in China to implementation of clinical pathways for the management of acute coronary syndromes or observation,14 highlighted the challenge of improving hospital performance. Importantly, subsequent qualitative research revealed several barriers, including lack of leadership support, out‐of‐pocket costs, and variation in capacity and resources, which limited the utility of the interevention.14 These results highlight that measurement must be complemented by efforts to identify effective strategies for improving performance and accountability at the hospital level. In the United States, the identification of practices associated with low door‐to‐balloon times,15 and the ensuing national initiative,16 were key contributors to improvements in the timeliness of primary PCI. If quality improvement efforts are to be successful in China, barriers must be identified and overcome at both the hospital and system level.
Despite improvement in delivering guideline‐recommended treatments and a meaningful reduction in variation, risk‐standardized mortality rates increased between 2001 and 2006, but decreased between 2006 and 2011. There are several possible explanations for this finding. Our study was conducted at a time when PCI was being adopted rapidly across China.17 Early outcomes associated with the use of this new technology could have been suboptimal as operators gained experience, thus providing a possible explanation for the higher RSMRs observed at PCI‐capable hospitals in 2006 before their outcomes improved by 2011. Despite the benefits of PCI, equal proportion of patients in 2011 received PCI and fibrinolysis, and it has been shown that urokinase, a drug for which there are little data demonstrating effectiveness in STEMI, is the preferred fibrinolytic agent in China.18 In addition to the possibility of suboptimal use of reperfusion, there may be other care processes that we did not measure in this analyses, such as the use of traditional Chinese medicines in 70% of STEMI patients,2 contributing to outcomes. Nonetheless, the relationship between hospitals' performance on process measures and risk‐standardized outcomes is known to be complex.19, 20 If quality improvement efforts are to improve outcomes, they must do more than simply focus on process measures. Indeed, hospitals' structural characteristics, such as the availability of primary PCI, staffing models, and organizational culture, likely influence outcomes. In order to be successful, hospital quality improvement efforts in China ought to address these domains as well.
Our study should be interpreted in the context of several potential limitations. First, we relied on information documented in medical charts, so there may be hospital‐ or provider‐level variation in the quality of documentation. Second, the retrospective design precluded the measurement of outcomes after hospitalization, meaning that this study necessarily focused on short‐term in‐hospital outcomes. Third, biomarker testing, a key component of the definition of AMI, was not performed for all patients and may bias our sample; however, this should not affect the evaluation of quality measures across hospitals in China unless the rates of biomarker use varied dramatically between hospitals, which might lead to underestimation of the variation between hospitals. Fourth, our process measures describe the rates with which ideal patients were treated and do not reflect the inappropriate use of therapies among patients for whom they are not indicated. Fifth, the method used to compute RSMRs was developed for administrative claims data in the United States and here it is applied to clinical data in China. Although this application has not been validated, comparisons between hospitals are still valid because any miscalibration would apply equally to all hospitals. Sixth, although our study was retrospective, there were concomitant government quality improvement initiatives and so a Hawthorne effect may have contributed to some of the improvements observed during the study period. Last, although our study was nationally representative and the results are illustrative of the range of performance achieved by the ≈3000 Chinese hospitals that provide inpatient care for STEMI, we focused on a single condition; it is unknown whether our findings can be extrapolated to other conditions.
This analysis of hospital performance using data from the nationally representative China PEACE‐Retrospective AMI Study reveals substantial, but decreasing, variation between hospitals in quality of care provided to patients with STEMI, which means that patients are likely to be treated differently, and have different outcomes, depending on where they present for care. The persistent variation in the quality of care highlights an opportunity for China to improve the hospital quality by elevating all hospitals to level of performance that has been achieved by top‐performing hospitals.
Sources of Funding
This project was supported by the Research Special Fund for Public Welfare Industry of Health (201202025, 201502009) from the National Health and Family Planning Commission of China, the National Key Technology R&D Program (2013BAI09B01, 2015BAI12B01, and 2015BAI12B02) from the Ministry of Science and Technology of China and the 111 Project (B16005). Dr Dharmarajan is supported by grant K23AG048331 from the National Institute on Aging and American Federation for Aging Research through the Paul B. Beeson Career Development Award Program. The funders were not involved in the design of the study, conduct of the work, or the development and submittal of the work for publication.
Disclosures
Dr Krumholz is a recipient of research agreements from Medtronic and from Johnson & Johnson (Janssen), through Yale, to develop methods of clinical trial data sharing; is the recipient of a grant from the US Food and Drug Administration and Medtronic, through Yale, to develop methods for postmarket surveillance of medical devices; works under contract with the Centers for Medicare & Medicaid Services to develop and maintain performance measures; chairs a cardiac scientific advisory board for UnitedHealth; is a participant/participant representative of the IBM Watson Health Life Sciences Board; and is a member of the advisory board for Element Science and the physician advisory board for Aetna; and is the founder of Hugo, a personal health information platform. Dr Masoudi has a contract with the American College of Cardiology as the Senior Medical Officer of the National Cardiovascular Data Registry. Dr Spertus has received grants from Gilead, Genentech, Lilly, Abbott Vascular, and Amorcyte; provides consulting services to Amgen, Novartis, Janssen, and Regeneron; owns the copyright to the Seattle Angina Questionnaire, Kansas City Cardiomyopathy Questionnaire, and Peripheral Artery Questionnaire; and has an equity interest in Health Outcomes Sciences. Dr Dharmarajan is a consultant and scientific advisory board member for Clover Health; he also works under contract with the Centers for Medicare & Medicaid Services to develop and maintain performance measures. The other authors do not have relationships to disclose.
Supporting information
Appendix S1. China PEACE‐Retrospective AMI Study Site Investigators by Hospital.
Data S1. Definition of ideal patients used to compute process measures.
Data S2. For the international comparison, we first determined whether there were publicly available quality measures for hospitals in the United States that corresponded to the 6 process measures used to characterize the performance of hospitals in China. To do this, we reviewed process measures for US hospitals posted on the Hospital Compare website and found corresponding measures for 3 of the 6 processes described for hospitals in China.
Table S1. Comparison of China's 2001 and 2010 Guidelines for STEMI and Process Measures Used in this Study
Table S2. Patient Characteristics at the Hospital Level
Figure S1. Composite rate in 2001, 2006, and 2011 stratified by hospital characteristics.
Figure S2. Defect‐free rate in 2001, 2006, and 2011 stratified by hospital characteristics.
Acknowledgments
Data access, responsibility and analysis: Mr Yongfei Wang had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
(J Am Heart Assoc. 2017;6:e005040 DOI: 10.1161/JAHA.116.005040.)28645937
References
- 1. Bi Y, Gao R, Patel A, Su S, Gao W, Hu D, Huang D, Kong L, Qi W, Wu Y, Yang Y, Turnbull F. Evidence‐based medication use among Chinese patients with acute coronary syndromes at the time of hospital discharge and 1 year after hospitalization: results from the Clinical Pathways for Acute Coronary Syndromes in China (CPACS) study. Am Heart J. 2009;157(509–516):e501. [DOI] [PubMed] [Google Scholar]
- 2. Li J, Li X, Wang Q, Hu S, Wang Y, Masoudi FA, Spertus JA, Krumholz HM, Jiang L. ST‐segment elevation myocardial infarction in China from 2001 to 2011 (the China PEACE‐Retrospective Acute Myocardial Infarction Study): a retrospective analysis of hospital data. Lancet. 2015;385:441–451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. China Society of Cardiology of Chinese Medical Association Editorial Board of Chinese Journal of Cardiology . Guideline for diagnosis and treatment of patients with ST‐elevation myocardial infarction. Zhonghua Xin Xue Guan Bing Za Zhi. 2010;38:675–690. [PubMed] [Google Scholar]
- 4. O'Gara PT, Kushner FG, Ascheim DD, Casey DE, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, Granger CB, Krumholz HM, Linderbaum JA, Morrow DA, Newby LK, Ornato JP, Ou N, Radford MJ, Tamis‐Holland JE, Tommaso CL, Tracy CM, Woo YJ, Zhao DX. 2013 ACCF/AHA guideline for the management of ST‐elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J Am Coll Cardiol. 2013;61:e78–e140. [DOI] [PubMed] [Google Scholar]
- 5. Krumholz HM, Wang Y, Mattera JA, Wang Y, Han LF, Ingber MJ, Roman S, Normand S‐LT. An administrative claims model suitable for profiling hospital performance based on 30‐day mortality rates among patients with an acute myocardial infarction. Circulation. 2006;113:1683–1692. [DOI] [PubMed] [Google Scholar]
- 6. Dharmarajan K, Li J, Li X, Lin Z, Krumholz HM, Jiang L. The China Patient‐Centered Evaluative Assessment of Cardiac Events (China PEACE) retrospective study of acute myocardial infarction: study design. Circ Cardiovasc Qual Outcomes. 2013;6:732–740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. China Society of Cardiology of Chinese Medical Association Editorial Board of Chinese Journal of Cardiology . Guideline for diagnosis and treatment of patients with acute myocardial infarction. Zhongguo Xun Huan Za Zhi. 2001;16:407–422. [Google Scholar]
- 8. Ministry of Health of the People's Republic of China . The qualification standard of secondary general hospital (version 2012). 2012. Available at: http://www.nhfpc.gov.cn/yzygj/s3586q/201201/b8dda05b1d23413c94150b5c17b5cc6f.shtml. Accessed June 16, 2016.
- 9. Simms AD, Reynolds S, Pieper K, Baxter PD, Cattle BA, Batin PD, Wilson JI, Deanfield JE, West RM, Fox KAA, Hall AS, Gale CP. Evaluation of the NICE mini‐GRACE risk scores for acute myocardial infarction using the Myocardial Ischaemia National Audit Project (MINAP) 2003–2009: National Institute for Cardiovascular Outcomes Research (NICOR). Heart. 2013;99:35–40. [DOI] [PubMed] [Google Scholar]
- 10. Merlo J, Chaix B, Ohlsson H, Beckman A, Johnell K, Hjerpe P, Råstam L, Larsen K. A brief conceptual tutorial of multilevel analysis in social epidemiology: using measures of clustering in multilevel logistic regression to investigate contextual phenomena. J Epidemiol Community Health. 2006;60:290–297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Nuti SV, Wang Y, Masoudi FA, Bratzler DW, Bernheim SM, Murugiah K, Krumholz HM. Improvements in the distribution of hospital performance for the care of patients with acute myocardial infarction, heart failure, and pneumonia, 2006–2011. Med Care. 2015;53:485–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Medical Administration Department of the National Commission for Health and Family Planning of the People's Republic of China . Notification on accreditation standards for secondary general hospitals. 2012. Available at: http://www.nhfpc.gov.cn/yzygj/s3586q/201201/b8dda05b1d23413c94150b5c17b5cc6f.shtml. Accessed July 11, 2016.
- 13. Ministry of Health of the People's Republic of China . Hospital quality monitoring system (HQMS). 2009. Available at: http://www.nhfpc.gov.cn/yzygj/s3585u/200912/ac5407234912449e92e7318ed3603170.shtml. Accessed September 6, 2016.
- 14. Du X, Gao R, Turnbull F, Wu Y, Rong Y, Lo S, Billot L, Hao Z, Ranasinghe I, Iedema R, Kong L, Hu D, Lin S, Shen W, Huang D, Yang Y, Ge J, Han Y, Lv S, Ma A, Gao W, Patel A. Hospital quality improvement initiative for patients with acute coronary syndromes in China: a cluster randomized controlled trial. Circ Cardiovasc Qual Outcomes. 2014;7:217–226. [DOI] [PubMed] [Google Scholar]
- 15. Bradley EH, Herrin J, Wang Y, Barton BA, Webster TR, Mattera JA, Roumanis SA, Curtis JP, Nallamothu BK, Magid DJ, McNamara RL, Parkosewich J, Loeb JM, Krumholz HM. Strategies for reducing the door‐to‐balloon time in acute myocardial infarction. New Engl J Med. 2006;355:2308–2320. [DOI] [PubMed] [Google Scholar]
- 16. Krumholz HM, Bradley EH, Nallamothu BK, Ting HH, Batchelor WB, Kline‐Rogers E, Stern AF, Byrd JR, Brush JE Jr. A campaign to improve the timeliness of primary percutaneous coronary intervention: door‐to‐balloon: an alliance for quality. JACC Cardiovasc Interv. 2008;1:97–104. [DOI] [PubMed] [Google Scholar]
- 17. Zheng X, Curtis JP, Hu S, Wang Y, Yang Y, Masoudi FA, Spertus JA, Li X, Li J, Dharmarajan K, Downing NS, Krumholz HM, Jiang L; China PEACE Collaborative Group . Coronary catheterization and percutaneous coronary intervention in china: 10‐year results from the china peace‐retrospective cathpci study. JAMA Intern Med. 2016;176:512–521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Li J, Li X, Ross JS, Wang Q, Wang Y, Desai NR, Xu X, Nuti SV, Masoudi FA, Spertus JA, Krumholz HM, Jiang L; China PCG . Fibrinolytic therapy in hospitals without percutaneous coronary intervention capabilities in China from 2001 to 2011: China PEACE‐retrospective AMI study. Eur Heart J Acute Cardiovasc Care. 2016;6:232–243. [DOI] [PubMed] [Google Scholar]
- 19. Peterson ED, Roe MT, Mulgund J, DeLong ER, Lytle BL, Brindis RG, Smith SC Jr, Pollack CV Jr, Newby LK, Harrington RA, Gibler WB, Ohman EM. Association between hospital process performance and outcomes among patients with acute coronary syndromes. JAMA. 2006;295:1912–1920. [DOI] [PubMed] [Google Scholar]
- 20. Bradley EHHJ, Elbel B, McNamara RL, Magid DJ, Nallamothu BK, Wang Y, Normand SL, Spertus JA, Krumholz HM. Hospital quality for acute myocardial infarction: correlation among process measures and relationship with short‐term mortality. JAMA. 2006;296:72–78. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. China PEACE‐Retrospective AMI Study Site Investigators by Hospital.
Data S1. Definition of ideal patients used to compute process measures.
Data S2. For the international comparison, we first determined whether there were publicly available quality measures for hospitals in the United States that corresponded to the 6 process measures used to characterize the performance of hospitals in China. To do this, we reviewed process measures for US hospitals posted on the Hospital Compare website and found corresponding measures for 3 of the 6 processes described for hospitals in China.
Table S1. Comparison of China's 2001 and 2010 Guidelines for STEMI and Process Measures Used in this Study
Table S2. Patient Characteristics at the Hospital Level
Figure S1. Composite rate in 2001, 2006, and 2011 stratified by hospital characteristics.
Figure S2. Defect‐free rate in 2001, 2006, and 2011 stratified by hospital characteristics.


