Abstract
Background
There is a need to identify sensitive biomarkers of early tobacco‐related cardiovascular disease. We examined the association of smoking status, burden, time since quitting, and intensity, with markers of inflammation and subclinical atherosclerosis.
Methods and Results
We studied 14 103 participants without clinical cardiovascular disease in ELSA‐Brasil (Brazilian Longitudinal Study of Adult Health). We evaluated baseline cross‐sectional associations between smoking parameters and inflammation (high‐sensitivity C‐reactive protein [hsCRP]) and measures of subclinical atherosclerosis (carotid intima–media thickness, ankle‐brachial index, and coronary artery calcium [CAC]). The cohort included 1844 current smokers, 4121 former smokers, and 8138 never smokers. Mean age was 51.7±8.9 years; 44.8% were male. After multivariable adjustment, compared with never smokers, current smokers had significantly higher levels of hsCRP (β=0.24, 0.19–0.29 mg/L; P<0.001) and carotid intima–media thickness (β=0.03, 0.02–0.04 mm; P<0.001) and odds of ankle‐brachial index ≤1.0 (odds ratio: 2.52; 95% confidence interval, 2.06–3.08; P<0.001) and CAC >0 (odds ratio: 1.83; 95% confidence interval, 1.46–2.30; P<0.001). Among former and current smokers, pack‐years of smoking (burden) were significantly associated with hsCRP (P<0.001 and P=0.006, respectively) and CAC (P<0.001 and P=0.002, respectively). Among former smokers, hsCRP and carotid intima–media thickness levels and odds of ankle‐brachial index ≤1.0 and CAC >0 were lower with increasing time since quitting (P<0.01). Among current smokers, number of cigarettes per day (intensity) was positively associated with hsCRP (P<0.001) and CAC >0 (P=0.03) after adjusting for duration of smoking.
Conclusions
Strong associations were observed between smoking status, burden, and intensity with inflammation (hsCRP) and subclinical atherosclerosis (carotid intima–media thickness, ankle‐brachial index, CAC). These markers of early cardiovascular disease injury may be used for the further study and regulation of traditional and novel tobacco products.
Keywords: atherosclerosis, inflammation, smoking, subclinical atherosclerosis risk factor, tobacco products
Subject Categories: Imaging, Ultrasound, Inflammation, Atherosclerosis
Clinical Perspective
What Is New?
We found significant associations between multiple measures of smoking behavior and abnormal markers of inflammation and atherosclerosis including high‐sensitivity C‐reactive protein, carotid intima–media thickness, ankle‐brachial index, and coronary artery calcium.
Results were consistently significant for the association between secondhand smoking and higher high‐sensitivity C‐reactive protein levels.
Consistent with prior studies, high‐sensitivity C‐reactive protein may be a sensitive marker of cardiovascular injury associated with tobacco exposure.
What Are the Clinical Implications?
The US Food and Drug Administration's new tobacco “Deeming Rule” mandates regulation of all novel tobacco products and performance of research on their safety.
These studied markers of inflammation and atherosclerosis can be used for the further study and regulation of traditional and novel tobacco products.
Introduction
Tobacco smoking, including secondhand exposure, is a leading cause of death and disability in the world.1, 2 Annually, ≈6.2 million deaths worldwide and 200 000 deaths per year in Brazil have been attributed to smoking,2, 3 with almost one third of coronary heart disease deaths reported to be caused by smoking. Although the rates of smoking have declined since the 1990s, ≈12.5% to 15.5% of the Brazilian population were current smokers in 2012, comparable to the 15% to 16.5% smoking prevalence in the United States for the same year.4
Deaths due to smoking‐related cardiovascular disease (CVD) are generally preceded by subclinical cardiovascular injury that may be detected early in the disease process. Interest is rising in finding biomarkers of CVD‐related injury in asymptomatic persons,5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21 and there is a need to identify sensitive biomarkers of smoking‐related early subclinical cardiovascular damage. Such biomarkers may assist in risk identification and stratification among smokers. In addition, such biomarkers could help regulatory agencies identify and quantify potential cardiovascular health hazards of exposure to novel tobacco products, such as e‐cigarettes, years before data on CVD events would be available due to the long latency between tobacco exposure and clinically evident CVD.22
Recent findings from MESA (Multi‐Ethnic Study of Atherosclerosis) in the United States demonstrated that tobacco smoking is associated with early markers of cardiovascular injury, with current smokers having higher levels of high‐sensitivity C‐reactive protein (hsCRP), carotid intima–media thickness (cIMT) and lower coronary artery calcium (CAC) score and ankle‐brachial index (ABI) compared with former smokers.23, 24 These findings require validation in a large, independent, multiethnic cohort. In addition, little is known about these associations in Latin American and Caribbean populations such as that of Brazil.25
The present study aimed to examine the associations between cigarette smoking and inflammation and subclinical atherosclerosis in ELSA‐Brasil (Brazilian Longitudinal Study of Adult Health). We hypothesized that cigarette smoking, as assessed by status (current or former), burden (pack‐years), time since quitting, and intensity of smoking (cigarettes per day), is associated with inflammation and subclinical atherosclerosis.
Methods
ELSA‐Brasil is a multicenter prospective cohort study enrolling 15 105 civil servants aged 35 to 74 years (54% women) from 6 cities of Brazil (Belo Horizonte, Porto Alegre, Rio de Janeiro, Salvador, São Paulo, and Vitoria). The objective of ELSA‐Brasil is to determine the role of individual and environmental risk factors in predicting future CVD events and diabetes mellitus. The study design, methods, and recruitment of participants have been described previously.26 The study protocol was approved at all 6 centers of Brazil by their respective institutional review boards, and written informed consent was obtained from all participants.
Briefly, the participants were active or retired employees aged 35 to 74 years. The first (baseline) examination was conducted during 2008–2010, and data were collected through interviews and examinations at participants' job sites and study clinics. Race was defined by participants' self‐declared skin color or race, which included white, brown, black, Asian, and indigenous. Brown race was considered an ethnic mixture of white, black, and, to a lesser extent, indigenous ancestry.
Of 15 105 participants who were enrolled after baseline examination in the cohort and had available data regarding smoking, inflammation, and subclinical atherosclerosis, we excluded 1002 participants who had prevalent diseases including self‐reported medical diagnosis of myocardial infarction, congestive heart failure, stroke, and coronary revascularization (coronary artery bypass grafting or percutaneous coronary intervention). Our final analytic sample included 14 103 participants free of known CVD.
Ascertainment of Smoking Exposure
Smoking habits were ascertained during a phase 1 interview through self‐report including both past and current cigarette smoking habits. The primary smoking variables were smoking status (never, former, current), smoking burden (pack‐years of smoking), time since quitting (among former smokers), and smoking intensity (number of cigarettes smoked per day among current smokers). Never smoking status was defined as lifetime consumption of <100 cigarettes. Former smokers were participants with past history of smoking who had not smoked cigarettes within the previous 30 days. Current smoking was defined as consumption of cigarettes within the previous 30 days. Ever smokers included both former and current smokers. Pack‐years of smoking were defined as the average number of packs of cigarettes smoked per day multiplied by the duration of smoking in years. Time since quitting among former smokers was calculated by the difference in current age and age when quitting smoking. We created a multilevel variable ranging from 0 to 3 if participants reported no (0) or yes in 1, 2, or all 3 variables of secondhand smoke exposure at home, transportation, or work.
Ascertainment of Subclinical Markers of Atherosclerosis
Measurement of hsCRP
Blood samples were collected at baseline after a 12‐hour overnight fast, and analyses were performed at the core laboratory at the São Paulo site. Participants were asked to abstain from smoking 12 hours before examinations. The inflammatory biomarker hsCRP was measured via immunochemistry (nephelometry). Data on hsCRP were available for 14 000 participants in the final analytic sample.
Measurement of cIMT
The detailed protocol for cIMT measurements was published previously.27, 28, 29 The cIMT was measured at all centers using a Toshiba (Aplio XG) ultrasound device with a 7.5‐MHz linear transducer in the outer wall of a predefined carotid segment of 1 cm in length from 1 cm below carotid bifurcation, during 3 cardiac cycles. The carotid images were sent to the centralized core reading center in São Paulo from all participating centers. Acquired images were considered valid if clearly visualized on (1) both the left and right sides of the anatomic guides for the common carotid arteries, (2) interfaces between the lumen and the vessel far wall, and (3) interfaces between the media and the adventitia layers of the far vessel wall. MIA software was used to standardize the reading and interpretation of carotid scans. Data on cIMT were available for 10 225 (72.5%) participants in the final analytic sample.
Measurement of ABI
ABI is a noninvasive test recommended for assessment of peripheral arterial disease.30 In the current study, ABI was measured with an automated device rather than the mercury column.28 Three systolic blood pressure measurements were taken with the participant in the supine position at each ankle, alternating between right and left ankles every 2 minutes. Next, 3 BP measures were taken at the right arm every 2 minutes (HEM‐705CPINT; Omron, Kyoto, Japan). The total time for all measurements was 20 to 25 minutes. We calculated the ABI by dividing the lowest of the 4 systolic blood pressure measurements of the leg by the highest systolic blood pressure measurements of the arm, as this method was shown to better estimate CVD risk for preventive purposes.31 ABI ≤1 versus >1 was used to discriminate between normal and diseased peripheral arteries. ABI data were available for 14 038 participants in the final analytic sample.
Measurement of CAC
A total 4278 (30.3%) ELSA‐Brasil participants underwent noncontrast cardiac‐gated computed tomography at the São Paulo site. CAC scoring was performed with a 64‐detector computed tomographic scanner (Brilliance 64; Philips Healthcare, Best, The Netherlands).32, 33 An ECG‐gated prospective scan acquisition with a tube potential of 120 kV and a tube current adjusted to body habitus was used on each participant after scout images. Images were reconstructed with 2.5‐mm slice thickness using standard filtered back projection. The CAC score was calculated using a threshold of 130 Housfield units, and results were expressed as Agatston units.34
Ascertainment of Covariates
Sociodemographic characteristics such as age, sex, race/ethnicity, educational history, and other health and medical history were ascertained through self‐report in interviews during both phases 1 and 2. Family history of myocardial infarction was similarly obtained during the phase 1 interview. Information on alcohol consumption (usual type, frequency of intake, and drinking patterns) and medication use was assessed during the phase 2 interview. Other covariates were assessed through a comprehensive set of examinations and measurements. The anthropometric parameters, such as weight and height, were measured using standard equipment and techniques. Resting blood pressure and heart rate were measured 3 times with 1‐minute intervals in the seated position after 5 minutes rest; the average of the second and third measurements was used in the analyses. Hypertension was defined as systolic blood pressure ≥140 mm Hg, diastolic blood pressure ≥90 mm Hg, or the use of antihypertensive medication. Diabetes mellitus was defined as either previous medical diagnosis of diabetes mellitus, the use of hypoglycemic medications, or fulfillment of the diagnostic criteria for diagnosis based on fasting ≥126 mg/dL or 2‐hour plasma glucose levels obtained as part of a 75‐g oral glucose tolerance test ≥200 or the glycated hemoglobin test (HbA1c) ≥6.5%. Total cholesterol, high‐density lipoprotein cholesterol, low‐density lipoprotein cholesterol (LDL‐C), and triglycerides were measured in the baseline blood samples following a 12‐hour overnight fast. Aliquots for the test performance were stored in freezers at −80°C until they were analyzed by an ADVIA 1200 (Siemens, Munich, Germany) system. LDL‐C was estimated using the Friedewald equation when the triglyceride level was <400 mg/dL. For those with a triglyceride level ≥400 mg/dL, LDL‐C was measured directly.
Modeling of Smoking and Outcome Measures
Smoking status was modeled as a categorical variable (with indicator terms) for former and current smokers versus never smokers. Pack‐years and time‐since‐quitting years were each modeled as a continuous variable expressed per 5‐U increase. Smoking intensity was represented as a continuous variable expressed per 10 cigarettes per day among current smokers.
Outcomes were modeled as:
Inflammation (hsCRP): continuous (natural log‐transformed because of nonnormal distribution) and binary (≥2 versus <2 mg/L).
cIMT: continuous (untransformed).
ABI: binary (≤1.0 versus >1.0).
CAC: binary (>0 versus =0).
Statistical Methods and Analyses
Descriptive analyses were performed using 1‐way ANOVA or Kruskal–Wallis tests for continuous covariates to compare means and medians, respectively, across categories of smoking status. We performed χ2 or Fisher exact tests to study the baseline characteristics of categorical covariates across categories of smoking status.
The associations between smoking exposure and subclinical inflammation and measures of atherosclerosis were cross‐sectionally assessed, and a complete case analysis method was used for primary results where missing observations for covariates were excluded from the analyses. The associations of smoking variables with hsCRP and cIMT were performed using multivariable linear regression models with the output of β‐coefficients indicating absolute difference in hsCRP or cIMT between former and current versus never smokers. Associations for ABI ≤1.0 and CAC >0 were performed using multivariable logistic regression models and expressed as prevalence odds ratios (ORs). We adjusted for age, sex, race, education, study site, alcohol use, family history of myocardial infarction, body mass index, systolic blood pressure, LDL‐C, high‐density lipoprotein cholesterol, triglycerides, hypertension status, diabetes mellitus, and statin use for all models. Several ways of modeling systolic blood pressure and hypertension status to account for multicollinearity showed no changes in results; therefore, we included full models with multiple blood pressure variables in final analyses.
We also tested for effect modification of smoking exposure variables with age, sex, race/ethnicity, and education by testing interaction terms in the fully adjusted multivariable model, with Wald P value of <0.05 considered significant. If the interaction terms were found to be significant, then we further conducted stratified analyses according to the levels of the effect modifiers to get stratum‐specific estimates. Consistent with a prior study by McEvoy et al,23 we also studied the potential interaction of hsCRP ≥2 and <2 mg/L and smoking for their association with CAC >0.
We secondarily evaluated the association between secondhand smoking among smokers and nonsmokers and subclinical inflammation and atherosclerosis with and without adjusting for smoking status and burden. In sensitivity analyses, we performed Monte Carlo multiple imputation for missing observations of continuous covariates and missing indicator terms for categorical covariates.35 We also repeated the analyses defining the ABI outcome as ABI ≤1.0 or ≥1.4. Analyses were repeated for the association of smoking and different categories of CAC (100 and 400 Agatston scores). We used multinomial logistic regression models to compare categories of CAC against CAC 0 as the reference. We also evaluated the association between all measures of smoking behavior and CAC as a continuous variable, defined as naturally log‐transformed (ln‐transformed) CAC among participants who had CAC >0. Because the amount of smoking may affect time since quitting, we repeated the analyses by adjusting for pack‐years of smoking among former smokers and assessed for any change in results for the association between time since quitting and markers of subclinical atherosclerosis and inflammation.
Analyses were performed with STATA (version 13.0) and SAS University Edition (SAS Institute Inc, Cary, NC), and a P value of <0.05 was considered significant (2‐sided).
Results
Baseline Characteristics
Mean age of the study cohort was 51.7±8.9 years, 44.8% were men, and 51.7% were white, followed by brown (27.9%) and black (15.8%) races. Of the cohort participants, 8138 were never smokers (57.7%), 4121 were former smokers (29.2%), and 1844 were current smokers (13.1%). Table 1 represents characteristics of never, former, and current smokers. Of specific interest, current and former smokers were older than never smokers (P<0.001) and were more likely to be men (P<0.001). The highest prevalence of current smoking was among black participants (14.7%) and lowest in Asian/indigenous participants (10.0%); prevalence in whites and brown participants was 12.3% and 13.5%, respectively. The number of cigarettes per day smoked was lower in current smokers compared with former smokers (12.7±9.7 versus 15.5±13.6; P<0.001). Mean pack‐years were higher among current smokers compared with former smokers (20.4±18.6 versus 15.7±19.0 pack‐years; P<0.001) despite being younger on average compared with former smokers. The average time since quitting smoking among former smokers was 17.1±10.9 years.
Table 1.
Baseline Characteristics of the Study Population Free of Prevalent CVD (N=14 103) by Categories of Smoking Status
| Variable | Smoking Status | ||||
|---|---|---|---|---|---|
| Total Population (N=14 103) | Never Smokers (n=8138) | Former Smokers (n=4121) | Current Smokers (n=1844) | P Value | |
| Age, y | 51.7±8.9 | 50.5±9.3 | 54.0±8.3 | 51.6±7.7 | <0.001 |
| Male, % | 44.8 | 39.9 | 52.6 | 49.0 | <0.001 |
| Racea, % | <0.001 | ||||
| White | 51.7 | 51.0 | 54.2 | 48.7 | |
| Brown (mixed) | 27.9 | 28.4 | 26.6 | 28.7 | |
| Black | 15.8 | 16.1 | 14.3 | 17.7 | |
| Asian/other | 3.5 | 3.5 | 3.5 | 3.2 | |
| Cigarettes per d | 6.2±10.9 | 0±0 | 15.5±13.6 | 12.7±9.7 | <0.001 |
| Duration of smoking, y | 8.9±13.1 | 0±0 | 17.2±11.0 | 29.8±10.7 | <0.001 |
| Pack‐years of smoking | 7.2±14.9 | 0±0 | 15.7±19.0 | 20.4±18.6 | <0.001 |
| Y since quittinga | ··· | ··· | 17.1±10.9 | ··· | ··· |
| Body mass index, kg/m2 | 26.9±4.7 | 26.7±4.8 | 27.6±4.6 | 26.3±4.7 | <0.001 |
| Systolic blood pressure, mm Hg | 120.8±17.0 | 120.1±16.7 | 122.5±17.0 | 120.1±18.3 | <0.001 |
| Diastolic blood pressure, mm Hg | 76.2±10.7 | 75.9±10.5 | 77.0±10.6 | 75.7±11.6 | <0.001 |
| Antihypertensive medication use, % | 25.5 | 23.7 | 31.2 | 20.8 | <0.001 |
| Heart ratea, bpm | 66.5±9.5 | 66.8±9.5 | 66.1±9.5 | 66.3±9.8 | <0.001 |
| Diabetes mellitus, % | 18.5 | 15.7 | 23.2 | 19.9 | <0.001 |
| Lipid‐lowering drugs, % | 11.4 | 10.4 | 14.7 | 8.5 | <0.001 |
| Total cholesterol, mg/dL | 215.3±41.9 | 213.3±40.6 | 217.4±43.4 | 219.7±43.4 | <0.001 |
| Triglycerides, mg/dL | 137.8±104.3 | 126.6±81.6 | 152.5±132.2 | 154.9±115.4 | <0.001 |
| LDL‐C, mg/dL | 131.6±34.8 | 130.9±34.3 | 131.5±34.8 | 134.9±36.6 | <0.001 |
| HDL‐C, mg/dL | 56.9±14.6 | 57.5±14.4 | 56.4±14.8 | 55.2±14.7 | <0.001 |
| Alcohol use, % | <0.001 | ||||
| Never | 10.6 | 14.6 | 5.1 | 4.8 | |
| Former | 19.7 | 19.3 | 22.5 | 14.9 | |
| Current | 69.6 | 65.9 | 72.3 | 80.0 | |
| Family history of MIa, % | 12.8 | 12.4 | 12.9 | 14.2 | 0.09 |
Continuous variables presented as means (standard deviations), and categorical variables are presented as percentages. P value for continuous variables was calculated using 1‐way ANOVA and for categorical variables using the χ2 test among never, former, and current smokers. HDL‐C indicates high‐density lipoprotein cholesterol; LDL‐C, low‐density lipoprotein cholesterol; MI, myocardial infarction.
The proportion of missing observations for these variables was ≥1%. Missing sample sizes were race (n=162, 1.1%), years since quitting (n=164, 1.2%), heart rate (n=816, 5.8%), and family history of myocardial infarction (n=141, 1.0%).
Smoking and Inflammation Measured by hsCRP
A total 14 000 (99.9%) participants were included for the analysis. Median hsCRP levels were significantly higher in current smokers (1.86 mg/L; interquartile range [IQR]: 0.89–3.81 mg/L) and former smokers (1.51 mg/L; IQR: 0.75–3.27 mg/L) compared with never smokers (1.35 mg/L; IQR: 0.67–3.15 mg/L; P<0.001; Table 2). After full multivariable adjustment for age, sex, race, education, study site, alcohol use, family history of myocardial infarction, body mass index, systolic blood pressure, LDL‐C, high‐density lipoprotein cholesterol, triglycerides, hypertension status, diabetes mellitus, and statin use, current smokers had significantly higher ln‐transformed hsCRP levels (β=0.24; 95% confidence interval [CI], 0.19–0.29; P<0.001) compared with never smokers, although results were not significant for former smoking (β=0.01; 95% CI, −0.02 to 0.05; P=0.46) (Table 3). Similarly, current smokers had 70% higher odds of having hsCRP ≥2 mg/L compared with never smokers (OR: 1.70; 95% CI, 1.51–1.91; P<0.001), with no association in former smokers (OR: 1.03; 95% CI, 0.95–1.13; P=0.44; Table S1).
Table 2.
Baseline Distributions of the Inflammatory Biomarker (hs‐CRP) and Measures of Subclinical Atherosclerosis (cIMT, ABI, and CAC)
| Outcomes | Total Population (N=14 103) | Smoking Status | P Value | ||
|---|---|---|---|---|---|
| Never Smokers (n=8138) | Former Smokers (n=4121) | Current Smokers (n=1844) | |||
| Median hsCRP, mg/L | 1.45 (0.72–3.28) | 1.35 (0.67–3.15) | 1.51 (0.75–3.27) | 1.86 (0.89–3.81) | <0.001 |
| hsCRP ≥2 mg/L, % (n) | 39.7 (5554) | 37.4 (3019) | 40.4 (1650) | 48.3 (885) | <0.001 |
| Mean cIMTa, mm | 0.75±0.16 | 0.73±0.15 | 0.78±0.17 | 0.77±0.18 | <0.001 |
| Mean ABI | 1.24±0.10 | 1.24±0.10 | 1.24±0.10 | 1.21±0.11 | <0.001 |
| ABI ≤1.0, % (n) | 5.94 (893) | 4.36 (353) | 5.78 (237) | 9.80 (180) | <0.001 |
| Mean CACb score | 46.2±194.3 | 31.9±156.7 | 63.9±226.4 | 60.6±234.7 | <0.001 |
| CAC >0, % (n) | 27.4 (1174) | 22.9 (527) | 32.2 (417) | 33.5 (230) | <0.001 |
P value for continuous variables was calculated using 1‐way ANOVA (means) or Kruskal–Wallis Test (medians) and for categorical variables using the χ2 test for never, former, and current smokers. ABI indicates ankle‐brachial index; CAC, coronary artery calcium; cIMT, carotid intima–media thickness; hsCRP, high‐sensitivity C‐reactive protein.
10 225 (72.5%) of participants underwent cIMT measurements and had adequate image quality for analysis of both common carotid arteries.
4278 (30.3%) of participants underwent CAC scoring because it was conducted at 1 site only (São Paulo, Brazil).
Table 3.
Baseline Mean Absolute Difference With 95% CIs in Levels of Biomarkers of hsCRP and cIMT and ORs for ABI ≤1.0 and CAC >0 by Smoking Status, Burden, Years Since Quitting, and Intensity
| Exposure | Ln hsCRP | cIMTa | ABI ≤1.0 | CAC (Agatston)b >0 | ||||
|---|---|---|---|---|---|---|---|---|
| β Coefficient (95% CI) | P Value | β Coefficient (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Smoking status | ||||||||
| Never | Reference | Reference | Reference | Reference | ||||
| Former | 0.01 (−0.02 to 0.05) | 0.46 | 0.01 (0.01–0.02) | <0.001 | 1.32 (1.11–1.58) | 0.002 | 1.12 (0.93–1.35) | 0.22 |
| Current | 0.24 (0.19–0.29) | <0.001 | 0.03 (0.02–0.04) | <0.001 | 2.52 (2.06–3.08) | <0.001 | 1.83 (1.46–2.30) | <0.001 |
| Smoking burden (pack‐years of smoking per 5‐unit increase) | ||||||||
| Ever smokersc | 0.02 (0.01–0.03) | <0.001 | 0.003 (0.002–0.004) | <0.001 | 1.06 (1.04–1.09) | <0.001 | 1.10 (1.07–1.14) | <0.001 |
| Former smokers | 0.01 (0.00–0.02) | 0.006 | 0.003 (0.002–0.005) | <0.001 | 1.04 (1.01–1.07) | 0.01 | 1.10 (1.06–1.14) | <0.001 |
| Current smokers | 0.03 (0.01–0.04) | <0.001 | 0.002 (−0.000 to 0.004) | 0.09 | 1.08 (1.04–1.12) | <0.001 | 1.09 (1.03–1.16) | 0.002 |
| Y since quitting smoking among former smokers | ||||||||
| Per 5‐y increase | −0.03 (−0.05 to −0.02) | <0.001 | −0.007 (−0.010 to −0.004) | <0.001 | 0.90 (0.84–0.96) | 0.003 | 0.87 (0.81–0.93) | <0.001 |
| Smoking intensity among current smokersd | ||||||||
| Per 10 cigarettes/d increase | 0.10 (0.05–0.15) | <0.001 | 0.003 (0.006–0.012) | 0.52 | 1.30 (1.10–1.52) | 0.002 | 1.26 (1.02–1.56) | 0.03 |
Linear regression models were used for hsCRP and cIMT and logistic regression for ABI and CAC. This was a complete‐case analysis. Models were adjusted for age, sex, race, education, study site, alcohol use, family history of myocardial infection, body mass index, systolic blood pressure, low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol, triglycerides, hypertension status, diabetes mellitus, and statin use. ABI indicates ankle‐brachial index; CAC, coronary artery calcium; CI, confidence interval; cIMT, carotid intima‐media thickness; hsCRP, high‐sensitivity C‐reactive protein; OR, odds ratio.
10 225 (72.5%) of participants underwent cIMT measurements and had adequate image quality for analysis of both common carotid arteries.
4278 (30.3%) of participants underwent CAC scoring because it was conducted at 1 site only (São Paulo, Brazil).
Includes both former and current smokers.
Also adjusted for duration of smoking in years.
Among ever smokers, after full multivariable adjustment, each 5‐year increase in pack‐years was associated with higher levels of ln‐transformed hsCRP (β=0.02; 95% CI, 0.01–0.03; P<0.001; Table 3). The findings were similar when further stratified according to former or current smokers, although the magnitude of effect was stronger for current smokers. Each 5‐year increase in time since quitting smoking was associated with lower ln‐transformed hsCRP among former smokers (β=−0.03; 95% CI, −0.05 to −0.02; P<0.001; Figure 1A). After full multivariable adjustment, smoking intensity (per 10‐cigarette/day increase in smoking), adjusted for duration of smoking, was associated with higher natural ln‐transformed hsCRP among current smokers (β=0.10; 95% CI, 0.05–0.15; P<0.001). All findings were similar when using the dichotomous variable of hsCRP ≥2 mg/L (Table S1).
Figure 1.
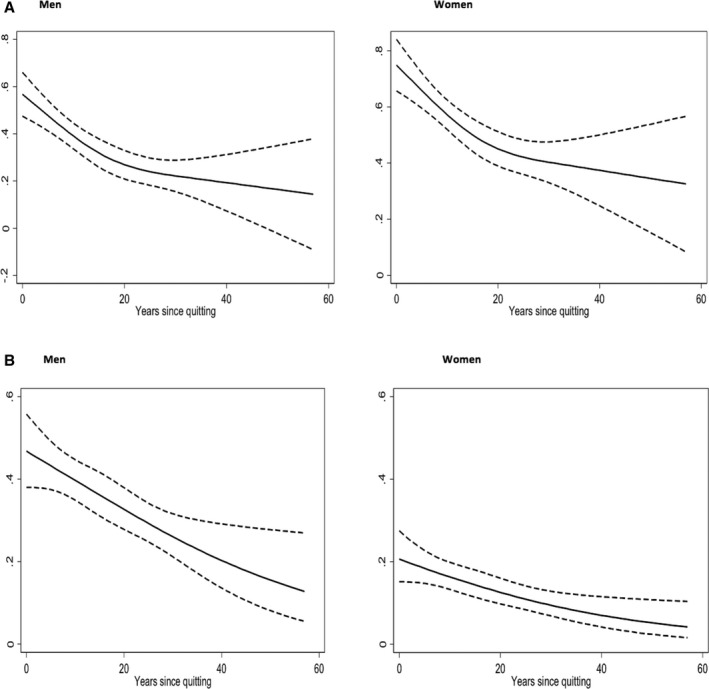
Age‐ and sex‐adjusted associations of (A) log‐transformed high‐sensitivity C‐reactive protein and (B) coronary artery calcium (CAC) >0 with years since quitting smoking among former smokers in men and women.
Significant interaction was observed for current versus never smokers with the effect modifiers of age, sex, race, and education (P<0.05 for each; Table S2). The change in ln‐transformed hsCRP was more pronounced among older adults (aged ≥50 years), men, nonwhite participants, and participants with lower education (Table 4, Table S3). A similar interaction with these effect modifiers was observed for the association of pack‐years with ln‐transformed hsCRP, whereas smoking intensity had interaction with sex only (Figure 2).
Table 4.
Stratified Analyses for by Age, Sex, Race and Education for Biomarkers of Subclinical Inflammation (hsCRP) and Atherosclerosis (cIMT, ABI, and CAC) for Interaction With Smoking Status
| Ln hsCRP | cIMTa | ABI ≤1.0 | CAC >0b | |||||
|---|---|---|---|---|---|---|---|---|
| Former (n=4087) | Current (n=1832) | Former (n=2973) | Current (n=1357) | Former (n=4097) | Current (n=1836) | Former (n=1294) | Current (n=686) | |
| β Coefficient (95% CI) | β Coefficient (95% CI) | β Coefficient (95% CI) | β Coefficient (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | OR (95% CI) | |
| Overall | 0.01 (−0.02 to 0.05) | 0.24 (0.19–0.29) | 0.01 (0.01–0.02) | 0.03 (0.02–0.04) | 1.32 (1.11–1.58) | 2.52 (2.06–3.08) | 1.12 (0.93–1.35) | 1.83 (1.46–2.30) |
| Male (n=6315) | 0.05 (−0.01 to 0.10) | 0.39 (0.31–0.46) | 0.02 (0.01–0.03) | 0.04 (0.03–0.06) | NS | NS | 0.99 (0.78–1.26) | 1.52 (1.12–2.06) |
| Female (n=7788) | −0.01 (−0.06 to 0.05) | 0.09 (0.01–0.16) | 0.007 (−0.000 to 0.015) | 0.02 (0.01–0.03) | NS | NS | 1.42 (1.07–1.90) | 2.41 (1.71–3.40) |
| Age ≥50 (n=7836), y | 0.03 (−0.02 to 0.08) | 0.31 (0.24–0.37) | 0.02 (0.01–0.03) | 0.03 (0.01–0.04) | 1.80 (1.43–2.25) | 3.80 (2.94–4.91) | NS | NS |
| Age <50 (n=6267), y | −0.01 (−0.08 to 0.05) | 0.15 (0.06–0.23) | 0.02 (0.01–0.02) | 0.03 (0.02–0.04) | 0.89 (0.64–1.24) | 1.35 (0.93–1.93) | NS | NS |
| High school or less (n=6573) | 0.01 (−0.05 to 0.07) | 0.27 (0.20–0.34) | 0.02 (0.01–0.03) | 0.03 (0.02–0.05) | NS | NS | NS | NS |
| College (n=7530) | 0.02 (−0.04 to 0.07) | 0.18 (0.09–0.26) | 0.01 (0.00–0.02) | 0.02 (0.01–0.03) | NS | NS | NS | NS |
| White (n=7287) | −0.01 (−0.06 to 0.05) | 0.20 (0.13–0.28) | 0.01 (0.00–0.02) | 0.02 (0.01–0.03) | NS | NS | NS | NS |
| Nonwhite (n=6655) | 0.04 (−0.02 to 0.10) | 0.28 (0.20–0.36) | 0.02 (0.01–0.03) | 0.04 (0.03–0.05) | NS | NS | NS | NS |
| Brown (n=3939) | 0.02 (−0.06 to 0.09) | 0.25 (0.15–0.36) | 0.02 (0.01–0.03) | 0.04 (0.02–0.06) | NS | NS | NS | NS |
| Nonbrown (n=10 002) | 0.01 (−0.03 to 0.06) | 0.24 (0.17–0.30) | 0.01 (0.00–0.02) | 0.02 (0.01–0.03) | NS | NS | NS | NS |
| Black (n=2224) | 0.02 (−0.09 to 0.12) | 0.24 (0.10–0.37) | 0.01 (−0.01 to 0.03) | 0.04 (0.02–0.06) | NS | NS | NS | NS |
| Nonblack (n=11 717) | 0.01 (−0.03 to 0.06) | 0.24 (0.18–0.30) | 0.01 (0.01–0.02) | 0.02 (0.02–0.03) | NS | NS | NS | NS |
NS: P interaction was >0.05, and no stratified analyses were conducted. ABI indicates ankle‐brachial index; CAC, coronary artery calcium; CI, confidence interval; cIMT, carotid intima‐media thickness; hsCRP, high‐sensitivity C‐reactive protein; ln, naturally log transformed.
10 225 (72.5%) of participants underwent cIMT measurements and had adequate image quality for analysis of both common carotid arteries.
4278 (30.3%) of participants underwent CAC scoring because it was conducted at 1 site only (São Paulo, Brazil).
Figure 2.
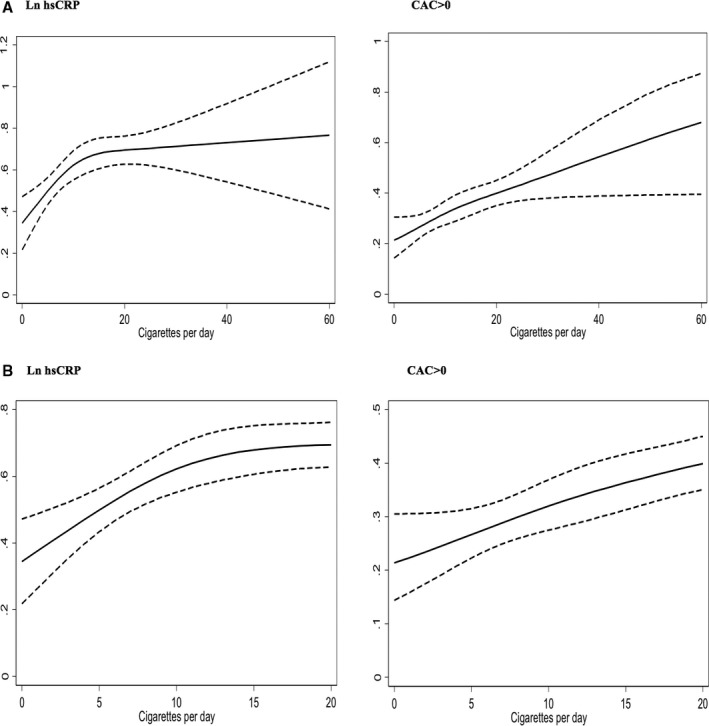
A, Dose‐response restricted cubic spline graphs for the association of mean log‐transformed high‐sensitivity C‐reactive protein (ln hs‐CRP) levels and coronary artery calcium (CAC) >0 with smoking intensity. B, Graphs restricted to smoking ≤20 cigarettes per day.
Smoking and Subclinical Atherosclerosis
Carotid intima–media thickness
In total, 10 225 (72.5%) participants were included for analysis. After full multivariable adjustment, cIMT was significantly higher among former smokers (β=0.01; 95% CI, 0.01–0.02; P<0.001) and current smokers (β=0.03; 95% CI, 0.02–0.04; P<0.001) compared with never smokers (Table 3). Each 5‐pack‐year increase in smoking was associated with higher cIMT among ever smokers (β=0.003; 95% CI, 0.002–0.004; P<0.001). Results were significant for increasing pack‐years among former smokers, with a trend toward increase among current smokers (β=0.002; 95% CI, −0.000 to 0.004; P=0.09). Each 5‐year period after quitting smoking was associated with lower cIMT (β=−0.007; 95% CI, −0.010 to −0.004; P<0.001). Among current smokers, however, smoking intensity was not associated with cIMT (β=0.003; 95% CI, −0.006 to 0.012; P=0.52).
There was evidence of significant interaction of former and current versus never smokers with age, sex, race, and education for cIMT, such that those who were older than 50 years, male, nonwhite, and less educated had a more pronounced association between current smoking status and higher cIMT levels (Table S2). Pack‐years also had significant interaction with sex and education in similar fashion (Table 4, Table S4).
Ankle‐brachial index
In total, 13 268 (99.4%) participants were included in the analysis. Prevalence of ABI ≤1.0 among current smokers (9.8%) was higher compared with former smokers (5.8%) and never smokers (4.4%; P<0.001). After full multivariable adjustment, compared with never smokers, former and current smokers had higher odds of ABI ≤1.0 (former: OR: 1.32; 95% CI, 1.11–1.58; P=0.02; current: OR: 2.52; 95% CI, 2.06–3.08; P<0.001; Table 3). Among ever smokers, each 5‐pack‐year increase was associated with higher odds of ABI ≤1.0 (OR: 1.06; 95% CI, 1.01–1.10; P=0.02). Each 5‐year lapsed since quitting was associated with lower odds of ABI ≤1.0 (OR: 0.90; 95% CI, 0.84–0.96; P=0.003). Smoking intensity was also associated with ABI ≤1.0 (OR: 1.30; 95% CI, 1.10–1.52; P=0.002).
There was an interaction between smoking status and age such that participants older than 50 years had a more pronounced association between current smoking status and ABI ≤1.0 (Table 4, Tables S2 and S3). No interaction was found for sex, race, and education.
Coronary artery calcium
In total, 4278 (30.3%) participants were included in the analysis. Prevalence of CAC was significantly higher (P<0.001) among former smokers (32.2%) and current smokers (33.5%) than never smokers (22.9%). After full multivariable adjustment, compared with never smokers, current smokers had higher odds of CAC >0 (OR: 1.83; 95% CI, 1.46–2.30; P<0.001), although no association was found among former smokers (OR: 1.12; 95% CI, 0.93–1.35; P=0.22; Table 3). Each 5‐pack‐year increase was associated with 10% higher odds of CAC >0 among ever smokers, and the relationship held after stratifying further among former and current smokers. Each 5‐year period passed since quitting smoking was associated with 13% lower odds of CAC >0 among former smokers (Figure 1B). Smoking intensity was also associated with CAC >0 (OR: 1.26; 95% CI, 1.02–1.56; P=0.03).
For CAC >0, we found significant interaction of current and former smoking by sex but not race and of smoking intensity with age and race (Table S2) but not sex (Figure 2). Associations were more pronounced among women compared with men, among older adults (age ≥50), and among blacks versus nonblack participants (Table S3). There was no evidence of interaction of any of the smoking exposure variables with hsCRP ≥2 and <2 mg/L for CAC >0 (Table S5).
Secondary and Sensitivity Analyses
Secondary analysis of the association of secondhand smoking among the whole cohort (including nonsmokers and former and current smokers) with subclinical inflammation and atherosclerosis revealed significant positive associations for hsCRP and cIMT. Results were consistent among nonsmokers. When adjusted for smoking status and pack‐years of smoking, results were significant only for hsCRP (Table 5). Increasing number of sites related to secondhand exposure to smoking (home, transportation, and work) was significantly associated only with increased hsCRP levels among the whole cohort but not when analyses were restricted to nonsmokers (Table S6).
Table 5.
Baseline Mean Absolute Difference With 95% CIs in Levels of hsCRP and cIMT, and ORs for ABI ≤1.0 and CAC >0 by Secondhand Smoking Status
| Exposure | Ln hsCRP | cIMTa | ABI ≤1.0 | CAC (Agatston)b >0 | ||||
|---|---|---|---|---|---|---|---|---|
| Secondhand Smoke (Yes vs No) | β Coefficient (95% CI) | P Value | β Coefficient (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value |
| Model 1 | 0.09 (0.05–0.14) | <0.001c | 0.02 (0.01–0.02) | <0.001c | 1.16 (0.99–1.36) | 0.07 | 1.07 (0.91–1.27) | 0.41 |
| Model 2 | 0.06 (0.02–0.10) | <0.001c | 0.01 (0.00–0.02) | 0.01c | 1.12 (0.95–1.32) | 0.18 | 1.03 (0.87–1.23) | 0.71 |
| Model 3 | 0.04 (0.00–0.08) | 0.04c | 0.00 (−0.00 to 0.01) | 0.09 | 1.02 (0.86–1.21) | 0.83 | 0.97 (0.81–1.16) | 0.73 |
| Model 4 | 0.05 (0.01–0.09) | <0.01c | 0.01 (−0.00 to 0.01) | 0.06 | 1.07 (0.90–1.26) | 0.45 | 0.98 (0.82–1.17) | 0.79 |
Model 1 is adjusted for age, sex, race, and education. Model 2 is adjusted for model 1 plus study site, family history of myocardial infarction, body mass index, alcohol use, systolic blood pressure, low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol, triglycerides, hypertension status, diabetes mellitus, and statin use. Model 3 is adjusted for model 2 plus self‐reported smoking status (never, former, current). Model 4 is adjusted for model 2 plus pack‐years of smoking. ABI indicates ankle‐brachial index; CAC, coronary artery calcium; CI, confidence interval; cIMT, carotid intima–media thickness; hsCRP, high‐sensitivity C‐reactive protein; ln, naturally log transformed; OR, odds ratio.
10 225 (72.5%) of participants underwent cIMT measurements and had adequate image quality for analysis of both common carotid arteries.
4278 (30.3%) of participants underwent CAC scoring because it was conducted at 1 site only (São Paulo, Brazil).
Significant results.
Sensitivity analyses demonstrated similar results to the complete‐case analysis after performing multiple imputation for missing observations of continuous covariates and use of missing indicators for categorical covariates. Excluding the body mass index variable from the model and adjusting for obesity (defined as body mass index ≥30) and physical activity did not change the results. Repeating the analyses for participants with ABI ≤1.0 or ≥1.4 and with different cut points for CAC showed consistent results. The ORs and relative risk ratios of having abnormal CAC showed a robust and consistent increase for both smoking status and number of cigarettes per day using higher CAC thresholds (Table 6 and Table S7). Among participants with present CAC, current and former smoking status, pack‐years of smoking among ever and current smokers, and number of cigarettes per day were all associated with ln‐transformed CAC (P<0.05), but pack‐years of smoking and time since quitting among former smokers were not. We found no significant associations between secondhand smoking status and ln‐transformed CAC. Our results remained robust and consistent with added adjustment for pack‐years of smoking in models demonstrating the association between time since quitting and markers of subclinical inflammation and atherosclerosis.
Table 6.
Baseline Mean Absolute Difference With 95% CIs in the ORs for Categories of CAC Using Logistic Regression Models Among 4278 Participants in ELSA‐Brasil
| Exposure | CAC (Agatston) >0 | CAC (Agatston) ≥100 | CAC (Agatston) ≥400 | |||
|---|---|---|---|---|---|---|
| OR (95% CI) | P Value | OR (95% CI) | P Value | OR (95% CI) | P Value | |
| Smoking status | ||||||
| Never | Reference | Reference | Reference | |||
| Former | 1.12 (0.93–1.35) | 0.22 | 1.30 (0.99–1.69) | 0.058 | 1.97 (1.28–3.04) | 0.002 |
| Current | 1.83 (1.46–2.30) | <0.001 | 2.12 (1.52–1.69) | <0.001 | 2.39 (1.32–4.32) | 0.004 |
| Smoking burden (pack‐years of smoking per 5‐unit increase) | ||||||
| Ever smokersa | 1.10 (1.07–1.14) | <0.001 | 1.10 (1.06–1.14) | <0.001 | 1.10 (1.04–1.15) | <0.001 |
| Former smokers | 1.10 (1.06–1.14) | <0.001 | 1.09 (1.04–1.13) | <0.001 | 1.09 (1.03–1.15) | 0.004 |
| Current smokers | 1.09 (1.03–1.16) | 0.002 | 1.12 (1.05–1.21) | 0.001 | 1.13 (1.02–1.26) | 0.025 |
| Y since quitting smoking among former smokers | ||||||
| Per 5‐y increase | 0.87 (0.81–0.93) | <0.001 | 0.93 (0.85–1.02) | 0.118 | 0.88 (0.77–1.00) | 0.043 |
| Smoking intensity among current smokersb | ||||||
| Per 10 cigarettes/d increase | 1.26 (1.02–1.56) | 0.03 | 1.69 (1.28–2.24) | <0.001 | 1.82 (1.14–2.89) | 0.012 |
This was a complete‐case analysis. Models were adjusted for age, sex, race, education, study site, alcohol use, family history of myocardial infection, body mass index, systolic blood pressure, low‐density lipoprotein cholesterol, high‐density lipoprotein cholesterol, triglycerides, hypertension status, diabetes mellitus, and statin use. CAC, coronary artery calcium; CI, confidence interval; OR, odds ratio.
Includes both former and current smokers.
Also adjusted for duration of smoking in years.
Discussion
In this study of asymptomatic individuals free of known CVD, we demonstrated that current smoking status, smoking burden (pack‐years), and smoking intensity (number of cigarettes per day) were all positively associated with higher levels of the inflammatory biomarker hsCRP and less favorable measures of subclinical atherosclerosis, including ABI and CAC score, whereas time since quitting was inversely related to these measures. Results regarding secondhand smoking demonstrated that after adjusting for other indices of exposure, hsCRP levels were elevated. These data suggest that hsCRP, ABI, and CAC may be useful biomarkers of smoking‐induced CVD.
Cigarette smoking has been linked to both acute and chronic cardiovascular risk, which may necessitate different measures of exposures and biomarkers.36 Given the long latency period between tobacco exposure and cardiovascular events, the identification of sensitive biomarkers of vascular injury is important for the assessment of potential cardiovascular harm from both traditional and novel tobacco products such as Electronic Nicotine Delivery Systems (ENDS) and smokeless tobacco.37 The use of noncigarette tobacco product is rising among adults and teenagers in the United States.38 Considering the novelty of these products and the lack of longitudinal studies, little is known about the potential harm of ENDS, such as e‐cigarettes, with respect to cardiovascular health and disease. The US Food and Drug Administration's (FDA's) new tobacco “Deeming Rule” necessitates regulation of all novel tobacco products and performing research on their safety.38 This study, which is funded by the FDA, introduces sensitive biomarkers of cardiovascular injury that can be used by regulatory agencies for assessing the hazardous effects of current and future tobacco products.
Different measures of smoking behavior can help evaluate the severity of smoking exposure while considering time as an important factor. Although status and burden of smoking are associated with long‐term chronic exposure, time since quitting and intensity reflect nearer term exposure. In the analyses for time since quitting and intensity, we adjusted for pack‐years and duration of smoking, respectively. This allowed us to assess proximal associations of measures of smoking behavior with subclinical inflammation and atherosclerosis regardless of the effect of burden and longevity of smoking.39 Smoking burden as an indicator of chronic tobacco exposure was related to abnormalities in all markers of inflammation and subclinical atherosclerosis in both former and current smokers. These biomarkers can be used to detect the presence and extent of inflammation and atherosclerosis following chronic tobacco exposure during and after smoking cessation. Attenuated associations with the increase in time since quitting showed the beneficial cardiovascular effects of smoking cessation and demonstrated the potential utility of biomarkers, in particular, hsCRP, for monitoring tobacco abstinence–related risk attenuation among former smokers. Smoking intensity was positively associated with hsCRP independent of the duration of exposure. This suggests that acute tobacco exposure is associated with subclinical vascular damage and can be quantified via serum hsCRP.
In our study, cIMT, ABI, and CAC were not associated with smoking intensity among younger participants, potentially due to the shorter duration of smoking exposure. On the contrary, hsCRP levels were well reflective of acuteness of exposure, as they were positively associated with higher smoking intensity regardless of age and duration of smoking. As a marker in the inflammation cascade, hsCRP is detectable before manifest clinical inflammation appears. In addition, hsCRP is associated with atherosclerotic processes40 and thus may also be directly involved in cardiovascular injury. The high sensitivity of this test, as indicated by significant differences via secondhand smoking exposure and consistent results for different indices of tobacco exposure in ELSA‐Brasil and across other studies,5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15, 41 highlights hsCRP as a favorable marker for monitoring hazardous health effects of tobacco exposure.
We showed that measures of tobacco exposure were associated with subclinical atherosclerosis, as demonstrated by the presence of CAC, although results were not significant for ABI ≤1 and cIMT. These disparate results may indicate site‐specific atherosclerotic processes following smoking exposure or differences in sensitivities of tests for measuring subclinical atherosclerosis. Previous studies have shown high sensitivity and reliability of cardiac‐gated computed tomography scanning as a tool to detect asymptomatic coronary atherosclerosis that heralds evident CVD.42 Our results regarding the association of smoking burden and intensity with CAC >0 provide additional detailed information for tobacco exposure and regulation sciences about detrimental cardiovascular health effects of smoking. Moreover, our findings contribute insight into the mechanisms through which cigarette smoking leads to atherosclerotic CVD events and thus generate hypotheses for future studies investigating pathways through which particular hazardous constituents may be involved in inflammation cascade or atherosclerotic processes. Our data indicate that hsCRP and CAC should be considered in studies aimed at assessing the cardiovascular impact of new tobacco products.
Limited studies have been conducted to assess associations of smoking or smoking cessation on markers of inflammation such as hsCRP,5, 6, 7, 8, 9, 10, 11, 12, 13, 14, 15 and even fewer studies have assessed measures of subclinical atherosclerosis.16, 17, 18, 19, 20, 21 These studies have generally shown higher levels of hsCRP among current and former smokers compared with nonsmokers5, 6, 9, 10, 12, 13, 14, 15 and decreased levels after smoking cessation, albeit slower than systolic blood pressure and total cholesterol and LDL‐C.8, 11, 12 Calculating the probability of CAC onset following smoking exposure in the Heinz Nixdorf Recall study also demonstrated that onset of CAC was expedited by 10 years among current smokers and 5 years among former smokers compared with never smokers.16 Our findings agree with the recent findings from MESA that found smoking to be associated with early markers of CVD injury, with current smokers having higher levels of hsCRP, cIMT, and CAC score and lower levels of ABI compared with former smokers.23
We did not find any associations between secondhand smoking and CAC, but results were significant for hsCRP and cIMT. Although hsCRP is a downstream inflammatory marker, it has been shown to be indicative of inflammatory processes that have a proximal role in processes involved in atherosclerosis.24 Consequently, it may be easier to demonstrate elevated hsCRP levels before accumulated subclinical coronary atherosclerosis is visible by imaging. In addition, cIMT has no zero values; therefore, it is easier to observe minimal changes at the lower ends of the scales.
This is the first large‐scale study of association between smoking and measures of subclinical CVD injury in the understudied Latin American and Caribbean population, which has a prevalence of smoking similar to that in the United States.3 This study is unique, given the social and ethnic diversity of the ELSA‐Brasil cohort, which includes middle‐ to high‐income participants from 6 major Brazilian cities. The large sample size of ELSA‐Brasil allowed assessment of effect modification by age, sex, race, and education for the associations of the smoking exposure variables with each of the outcomes.
This study has also limitations. This was a cross‐sectional study at baseline, so causal relations and the temporality of smoking exposure and outcomes could not be ascertained. Similarly, lower levels of markers of inflammation and atherosclerosis with increasing time since quitting does not mean that they were reduced over time; therefore, “reduction” of these markers can potentially be explained by smoking cessation among former smokers prior to increases in levels of these markers. The self‐report method for collecting data related to smoking status, burden, intensity, and secondhand smoking, although consistent with previous cohort studies, may have introduced potential misclassification. A previous MESA analysis showed that 1.2% of participants who identified themselves as never or former smokers had high cotinine levels and thus were classified as current smokers.43 This misclassification, however, is likely to be nondifferential and would have only attenuated the associations toward the null. Classification may have been improved with an objective measure of smoking like urinary cotinine, which was not measured at baseline. In future ELSA‐Brasil examinations, measuring cotinine levels may help validate self‐reported smoking status among this Latin American population as well. Overall, cIMT and CAC were measured for ≈70% and 30% of participants, respectively, for budgetary reasons and not patient characteristics; therefore, it is unlikely that this approach caused any biases in results.
In conclusion, in Brazilian men and women who are free of known CVD, tobacco smoking is associated with higher levels of the inflammatory biomarker hsCRP and worse measures of subclinical atherosclerosis. The magnitude of the results is consistent with that reported in MESA—a multiethnic cohort from the United States. The marker hsCRP was the only one in our study that changed following secondhand exposure—supportive of its utility as a sensitive monitoring test. According to the FDA new tobacco Deeming Rule, it is important to evaluate the safety of all tobacco products including ENDS. This study has distinct regulatory implications and adds to a growing body of data indicating that hsCRP and measures of subclinical atherosclerosis are robust candidate markers for the potential CVD toxicity of new and traditional tobacco products. Although our results provide explanations for the association between traditional smoking and CVD, they may be used for the regulation of novel tobacco products, such as electronic cigarettes, the prevalence of which is rising remarkably in the United States.22 Further studies may similarly lend insight into potential vascular harms of new tobacco products decades before clinical event studies.
Sources of Funding
This analysis was supported by funding from the American Heart Association Tobacco Regulation and Addiction Center (A‐TRAC), NIH (1 P50 HL120163‐01), a member of the FDA Tobacco Centers of Regulatory Science for Research Relevant to the Family Smoking Prevention and Tobacco Control Act (P50). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the FDA. The ELSA‐Brasil baseline study was supported by the Brazilian Ministry of Health (Science and Technology Department) and the Brazilian Ministry of Science and Technology and National Counsel of Technological and Scientific Development (CNPq) ‐ National Research Council (grants # 01 06 0010.00 RS, 01 06 0212.00 BA, 01 06 0300.00 ES, 01 06 0278.00 MG, 01 06 0115.00 SP, 01 06 0071.00 RJ).
Disclosures
Dr Lotufo receives honoraria (modest) from AbbVie Brazil. The remaining authors have no disclosures to report.
Supporting information
Table S1. Odds Ratios With 95% Confidence Intervals for the Levels of Biomarkers of Cardiovascular Injury (High‐Sensitivity C‐Reactive Protein ≥2 Versus <2 mg/L) by Smoking Status, Burden, Years Since Quitting, and Intensity
Table S2. β‐Coefficients or Odds Ratios and Interaction ‐ Values for Age, Sex, Race, and Education in Model 2 by Smoking Status, Burden, Years Since Quitting, and Intensity
Table S3. Stratified Analyses by Age, Sex, Race, and Education for Naturally Log‐Transformed High‐Sensitivity C‐Reactive Protein for Which Interaction P Values Were <0.05 by Smoking Status, Burden, Years Since Quitting, and Intensity
Table S4. Stratified Analyses by Age, Sex, Race and Education for Carotid Intima–Media Thickness for Which Interaction P Values Were <0.05 by Smoking Status and Burden
Table S5. Stratified Analyses by High‐Sensitivity C‐Reactive Protein Levels for Coronary Artery Calcium >0 Outcome (Interaction P>0.05) by Smoking Status, Burden, Years Since Quitting, and Intensity
Table S6. Baseline Mean Absolute Difference (95% Confidence Interval) in Levels of Biomarkers of Cardiovascular Injury (High‐Sensitivity C‐Reactive Protein and Carotid Intima–Media Thickness) and Odds Ratios for Measures of Subclinical Atherosclerosis (Ankle‐Brachial Index <1.0 and Coronary Artery Calcium >0) by Secondhand Smoking Status
Table S7. Baseline Relative Risk Ratios (RRR) With 95% Confidence Intervals (CI) for Categories of Coronary Artery Calcium (CAC) Using Multinomial Logistic Regression Models Among 4278 Participants in ELSA‐Brasil
Acknowledgments
We thank the other investigators, staff, and participants of ELSA‐Brasil for their valuable contributions.
(J Am Heart Assoc. 2017;6:e005088 DOI: 10.1161/JAHA.116.005088.)28647689
This work was orally presented at the American Heart Association Scientific Sessions, November 12–16, 2016, in New Orleans, LA.
Contributor Information
Paulo A. Lotufo, Email: palotufo@usp.br.
Michael J. Blaha, Email: mblaha1@jhmi.edu.
References
- 1. GBD 2013 Risk Factors Collaborators . Global, regional, and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 188 countries, 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet. 2015;386:2287–2323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brugha TS, Murray C. Global, regional and national comparative risk assessment of 79 behavioural, environmental and occupational, and metabolic risks or clusters of risks in 195 countries, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet. 2016;388:1659–1724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Ribeiro ALP, Duncan BB, Brant LC, Lotufo PA, Mill JG, Barreto SM. Cardiovascular health in Brazil trends and perspectives. Circulation. 2016;133:422–433. [DOI] [PubMed] [Google Scholar]
- 4. Ng M, Freeman MK, Fleming TD, Robinson M, Dwyer‐Lindgren L, Thomson B, Wollum A, Sanman E, Wulf S, Lopez AD. Smoking prevalence and cigarette consumption in 187 countries, 1980–2012. JAMA. 2014;311:183–192. [DOI] [PubMed] [Google Scholar]
- 5. Marano KM, Kathman SJ, Jones BA, Nordskog BK, Brown BG, Borgerding MF. Study of cardiovascular disease biomarkers among tobacco consumers. Part 3: evaluation and comparison with the US National Health and Nutrition Examination Survey. Inhal Toxicol. 2015;27:167–173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Golzarand M, Toolabi K, Ebrahimi‐Mameghani M, Aliasgarzadeh A, Arefhosseini S. Association between modifiable lifestyle factors and inflammatory markers in patients with metabolic syndrome. East Mediterr Health J. 2012;18:735–741. [PubMed] [Google Scholar]
- 7. Zatu MC, Van Rooyen JM, Schutte AE. Smoking and vascular dysfunction in Africans and Caucasians from South Africa. Cardiovasc J Afr. 2011;22:18–24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Reichert V, Xue X, Bartscherer D, Jacobsen D, Fardellone C, Folan P, Kohn N, Talwar A, Metz CN. A pilot study to examine the effects of smoking cessation on serum markers of inflammation in women at risk for cardiovascular disease. Chest. 2009;136:212–219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lao XQ, Jiang CQ, Zhang WS, Adab P, Lam TH, Cheng KK, Thomas GN. Smoking, smoking cessation and inflammatory markers in older Chinese men: the Guangzhou Biobank Cohort Study. Atherosclerosis. 2009;203:304–310. [DOI] [PubMed] [Google Scholar]
- 10. Madsen C, Nafstad P, Eikvar L, Schwarze PE, Ronningen KS, Haaheim LL. Association between tobacco smoke exposure and levels of C‐reactive protein in the Oslo II Study. Eur J Epidemiol. 2007;22:311–317. [DOI] [PubMed] [Google Scholar]
- 11. Bakhru A, Erlinger TP. Smoking cessation and cardiovascular disease risk factors: results from the Third National Health and Nutrition Examination Survey. PLoS Med. 2005;2:e160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Ohsawa M, Okayama A, Nakamura M, Onoda T, Kato K, Itai K, Yoshida Y, Ogawa A, Kawamura K, Hiramori K. CRP levels are elevated in smokers but unrelated to the number of cigarettes and are decreased by long‐term smoking cessation in male smokers. Prev Med. 2005;41:651–656. [DOI] [PubMed] [Google Scholar]
- 13. Bazzano LA, He J, Muntner P, Vupputuri S, Whelton PK. Relationship between cigarette smoking and novel risk factors for cardiovascular disease in the United States. Ann Intern Med. 2003;138:891–897. [DOI] [PubMed] [Google Scholar]
- 14. Bermudez EA, Rifai N, Buring JE, Manson JE, Ridker PM. Relation between markers of systemic vascular inflammation and smoking in women. Am J Cardiol. 2002;89:1117–1119. [DOI] [PubMed] [Google Scholar]
- 15. Hastie CE, Haw S, Pell JP. Impact of smoking cessation and lifetime exposure on C‐reactive protein. Nicotine Tob Res. 2008;10:637–642. [DOI] [PubMed] [Google Scholar]
- 16. Lehmann N, Mohlenkamp S, Mahabadi AA, Schmermund A, Roggenbuck U, Seibel R, Gronemeyer D, Budde T, Dragano N, Stang A, Mann K, Moebus S, Erbel R, Jockel KH. Effect of smoking and other traditional risk factors on the onset of coronary artery calcification: results of the Heinz Nixdorf Recall Study. Atherosclerosis. 2014;232:339–345. [DOI] [PubMed] [Google Scholar]
- 17. Jockel KH, Lehmann N, Jaeger BR, Moebus S, Mohlenkamp S, Schmermund A, Dragano N, Stang A, Gronemeyer D, Seibel R, Mann K, Volbracht L, Siegrist J, Erbel R. Smoking cessation and subclinical atherosclerosis–results from the Heinz Nixdorf Recall Study. Atherosclerosis. 2009;203:221–227. [DOI] [PubMed] [Google Scholar]
- 18. Hansen K, Ostling G, Persson M, Nilsson PM, Melander O, Engstrom G, Hedblad B, Rosvall M. The effect of smoking on carotid intima‐media thickness progression rate and rate of lumen diameter reduction. Eur J Intern Med. 2016;28:74–79. [DOI] [PubMed] [Google Scholar]
- 19. Baldassarre D, Castelnuovo S, Frigerio B, Amato M, Werba JP, De Jong A, Ravani AL, Tremoli E, Sirtori CR. Effects of timing and extent of smoking, type of cigarettes, and concomitant risk factors on the association between smoking and subclinical atherosclerosis. Stroke. 2009;40:1991–1998. [DOI] [PubMed] [Google Scholar]
- 20. Fan M, Raitakari OT, Kahonen M, Juonala M, Hutri‐Kahonen N, Porsti I, Viikari J, Lehtimaki T. The association between cigarette smoking and carotid intima‐media thickness is influenced by the ‐930A/G CYBA gene polymorphism: the Cardiovascular Risk in Young Finns Study. Am J Hypertens. 2009;22:281–287. [DOI] [PubMed] [Google Scholar]
- 21. Hisamatsu T, Miura K, Arima H, Kadota A, Kadowaki S, Torii S, Suzuki S, Miyagawa N, Sato A, Yamazoe M. Smoking, smoking cessation, and measures of subclinical atherosclerosis in multiple vascular beds in Japanese men. J Am Heart Assoc. 2016;5:e003738 DOI: 10.1161/JAHA.116.003738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bhatnagar A. E‐cigarettes and cardiovascular disease risk: evaluation of evidence, policy implications, and recommendations. Curr Cardiovasc Risk Rep. 2016;10:24. [Google Scholar]
- 23. McEvoy JW, Nasir K, DeFilippis AP, Lima JA, Bluemke DA, Hundley WG, Barr RG, Budoff MJ, Szklo M, Navas‐Acien A, Polak JF, Blumenthal RS, Post WS, Blaha MJ. Relationship of cigarette smoking with inflammation and subclinical vascular disease: the Multi‐Ethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2015;35:1002–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. McEvoy JW, Blaha MJ, DeFilippis AP, Lima JA, Bluemke DA, Hundley WG, Min JK, Shaw LJ, Lloyd‐Jones DM, Barr RG. Cigarette smoking and cardiovascular events role of inflammation and subclinical atherosclerosis from the Multiethnic Study of Atherosclerosis. Arterioscler Thromb Vasc Biol. 2015;35:700–709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Lotufo PA. Smoking and cancer: Brazil and the Global Burden of Disease initiative. Sao Paulo Med J. 2015;133:385–387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Schmidt MI, Duncan BB, Mill JG, Lotufo PA, Chor D, Barreto SM, Aquino EM, Passos VMA, Matos SM, Maria del Carmen BM. Cohort profile: longitudinal study of adult health (ELSA‐Brasil). Int J Epidemiol. 2015;44:68–75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Santos IS, Bittencourt MS, Oliveira IR, Souza AG, Meireles DP, Rundek T, Foppa M, Bezerra DC, Freire CM, Roelke LH, Carrilho S, Bensenor IM, Lotufo PA. Carotid intima‐media thickness value distributions in the Brazilian Longitudinal Study of Adult Health (ELSA‐Brasil). Atherosclerosis. 2014;237:227–235. [DOI] [PubMed] [Google Scholar]
- 28. Mill JG, Pinto K, Griep RH, Goulart A, Foppa M, Lotufo PA, Maestri MK, Ribeiro AL, Andreao RV, Dantas EM, Oliveira I, Fuchs SC, Cunha Rde S, Bensenor IM. [Medical assessments and measurements in ELSA‐Brasil]. Rev Saude Publica. 2013;47:54–62. [DOI] [PubMed] [Google Scholar]
- 29. Santos IS, Alencar AP, Rundek T, Goulart AC, Barreto SM, Pereira AC, Bensenor IM, Lotufo PA. Low impact of traditional risk factors on carotid intima‐media thickness: the ELSA‐Brasil cohort. Arterioscler Thromb Vasc Biol. 2015;35:2054–2059. [DOI] [PubMed] [Google Scholar]
- 30. Sacks D, Bakal CW, Beatty PT, Becker GJ, Cardella JF, Raabe RD, Wiener HM, Lewis CA. Position statement on the use of the ankle‐brachial index in the evaluation of patients with peripheral vascular disease: a consensus statement developed by the standards division of the society of cardiovascular & interventional radiology. J Vasc Interv Radiol. 2002;13:353. [DOI] [PubMed] [Google Scholar]
- 31. Miname M, Bensenor IM, Lotufo PA. Different methods of calculating ankle‐brachial index in mid‐elderly men and women: the Brazilian Longitudinal Study of Adult Health (ELSA‐Brasil). Braz J Med Biol Res. 2016;49:e5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bensenor IM, Goulart AC, Santos IS, Bittencourt MS, Pereira AC, Santos RD, Nasir K, Blankstein R, Lotufo PA. Association between a healthy cardiovascular risk factor profile and coronary artery calcium score: results from the Brazilian Longitudinal Study of Adult Health (ELSA‐Brasil). Am Heart J. 2016;174:51–59. [DOI] [PubMed] [Google Scholar]
- 33. Pereira AC, Gomez LM, Bittencourt MS, Staniak HL, Sharovsky R, Foppa M, Blaha MJ, Bensenor IM, Lotufo PA. Age, gender, and race‐based coronary artery calcium score percentiles in the Brazilian Longitudinal Study of Adult Health (ELSA‐Brasil). Clin Cardiol. 2016;39:352–359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr, Detrano R. Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol. 1990;15:827–832. [DOI] [PubMed] [Google Scholar]
- 35. Roth PL, Switzer FS, Switzer DM. Missing data in multiple item scales: a Monte Carlo analysis of missing data techniques. Organ Res Methods. 1999;2:211–232. [Google Scholar]
- 36. Pell JP, Haw S, Cobbe S, Newby DE, Pell AC, Fischbacher C, McConnachie A, Pringle S, Murdoch D, Dunn F. Smoke‐free legislation and hospitalizations for acute coronary syndrome. N Engl J Med. 2008;359:482–491. [DOI] [PubMed] [Google Scholar]
- 37. Benowitz NL. Emerging nicotine delivery products. Implications for public health. Ann Am Thorac Soc. 2014;11:231–235. [DOI] [PubMed] [Google Scholar]
- 38. Barnett TE, Soule EK, Forrest JR, Porter L, Tomar SL. Adolescent electronic cigarette use: associations with conventional cigarette and hookah smoking. Am J Prev Med. 2015;49:199–206. [DOI] [PubMed] [Google Scholar]
- 39. Nance R, Delaney J, McEvoy JW, Blaha MJ, Burke GL, Navas‐Acien A, Kaufman JD, Oelsner EC, McClelland RL. Smoking intensity (pack/day) is a better measure than pack‐years or smoking status for modeling cardiovascular disease outcomes. J Clin Epidemiol. 2016;S0895–4356:30483–30488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Libby P, Ridker PM. Inflammation and atherosclerosis: role of C‐reactive protein in risk assessment. Am J Med. 2004;116:9–16. [DOI] [PubMed] [Google Scholar]
- 41. Jones MR, Magid HS, Al‐Rifai M, McEvoy JW, Kaufman JD, Hinckley Stukovsky KD, Barr G, Szklo M, Polak J, Burke G, Post WS, Blaha MJ, Navas‐Acien A. Secondhand smoke exposure and subclinical cardiovascular disease: the Multi‐Ethnic Study of Atheroslcerosis. J Am Heart Assoc. 2016;5:e002965 DOI: 10.1161/JAHA.115.002965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Blaha MJ, Budoff MJ, DeFilippis AP, Blankstein R, Rivera JJ, Agatston A, O'Leary DH, Lima J, Blumenthal RS, Nasir K. Associations between C‐reactive protein, coronary artery calcium, and cardiovascular events: implications for the JUPITER population from MESA, a population‐based cohort study. Lancet. 2011;378:684–692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Keith RJ, Al Rifai M, Carruba C, De Jarnett N, McEvoy JW, Bhatnagar A, Blaha MJ, Defilippis AP. Tobacco use, insulin resistance, and risk of type 2 diabetes: results from the Multi‐Ethnic Study of Atherosclerosis. PLoS One. 2016;11:e0157592. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Odds Ratios With 95% Confidence Intervals for the Levels of Biomarkers of Cardiovascular Injury (High‐Sensitivity C‐Reactive Protein ≥2 Versus <2 mg/L) by Smoking Status, Burden, Years Since Quitting, and Intensity
Table S2. β‐Coefficients or Odds Ratios and Interaction ‐ Values for Age, Sex, Race, and Education in Model 2 by Smoking Status, Burden, Years Since Quitting, and Intensity
Table S3. Stratified Analyses by Age, Sex, Race, and Education for Naturally Log‐Transformed High‐Sensitivity C‐Reactive Protein for Which Interaction P Values Were <0.05 by Smoking Status, Burden, Years Since Quitting, and Intensity
Table S4. Stratified Analyses by Age, Sex, Race and Education for Carotid Intima–Media Thickness for Which Interaction P Values Were <0.05 by Smoking Status and Burden
Table S5. Stratified Analyses by High‐Sensitivity C‐Reactive Protein Levels for Coronary Artery Calcium >0 Outcome (Interaction P>0.05) by Smoking Status, Burden, Years Since Quitting, and Intensity
Table S6. Baseline Mean Absolute Difference (95% Confidence Interval) in Levels of Biomarkers of Cardiovascular Injury (High‐Sensitivity C‐Reactive Protein and Carotid Intima–Media Thickness) and Odds Ratios for Measures of Subclinical Atherosclerosis (Ankle‐Brachial Index <1.0 and Coronary Artery Calcium >0) by Secondhand Smoking Status
Table S7. Baseline Relative Risk Ratios (RRR) With 95% Confidence Intervals (CI) for Categories of Coronary Artery Calcium (CAC) Using Multinomial Logistic Regression Models Among 4278 Participants in ELSA‐Brasil


