Abstract
Background
Pulse pressure (PP) is related to cardiac function, arterial stiffness, fluid status, and vascular events. This study aimed to explore the prognostic role of PP upon admission in patients with acute ischemic stroke (AIS) based on a nation‐wide stroke registry.
Methods and Results
We evaluated the association between PP upon admission and outcomes 3 months after a stroke in patients who had an AIS registered in the Taiwan Stroke Registry, including 56 academic and community hospitals between 2006 and 2013. Three months after the stroke, unfavorable outcomes were defined using a modified Rankin scale of 3 to 6. Of 33 530 patients (female, 40.6%; mean age, 68.8±13.3 years) who had an AIS, PP upon admission had a reverse J‐curve association with an unfavorable outcome. After adjusting for clinical variables, including AIS subtypes, initial National Institutes of Health Stroke Scale, and systolic and diastolic blood pressure upon admission, a PP of <50 mm Hg was associated with unfavorable outcomes (P<0.0001). Compared with patients with a PP of 50 to 69 mm Hg, the odds ratios for unfavorable outcomes were 1.24 (95% CI, 1.14–1.36) with a PP of 30 to 49 mm Hg and 1.85 (95% CI, 1.50–2.28) with a PP of <30 mm Hg. Moreover, the prognostic impact of PP upon admission was similar across all AIS subtypes.
Conclusions
Low PP upon admission was associated with unfavorable patient outcomes in AIS.
Keywords: blood pressure, ischemic stroke, outcome, pulse pressure, stroke registry
Subject Categories: Ischemic Stroke, Blood Pressure, Prognosis
Clinical Perspective
What is New?
The present study demonstrated a reverse J‐curve association of admission pulse pressure with an unfavorable outcome in patients with acute ischemic stroke based on a nation‐wide stroke registry.
What are the Clinical Implications?
Admission pulse pressure is associated with poststroke functional outcomes in patients with acute ischemic stroke.
Introduction
Stroke is a life‐threatening disease, leading cause of death, and severe long‐term disability worldwide.1 Among several factors that may increase the risk of stroke, hypertension is the single most important one.2 Uncontrolled blood pressure (BP) would lead to atherosclerosis and, in turn, harden and weaken the vessels in the brain, heart, and limbs, causing end‐organ damage.3 However, the occurrence of stroke may directly lead to an acute increase in BP.4, 5 Although the role of BP management after a stroke remains controversial, systolic, mean, or diastolic BP levels upon hospital arrival have demonstrated a U‐ or J‐curve association with poststroke mortality and functional dependency.6, 7, 8, 9, 10, 11, 12
In addition to the steady components of systolic and diastolic values, BP is also characterized by its pulsatile nature and is estimated using pulse pressure (PP).13, 14 PP is the difference between the systolic and diastolic BP; can be conceptualized as being proportional to the stroke volume; and is inversely related to aortic compliance.15, 16, 17 However, it remained unclear whether a relationship between admission PP and clinical outcomes among patients with acute stroke existed.18, 19, 20, 21 Thus, the present study aimed to explore the prognostic role of admission PP in patients with acute ischemic stroke (AIS) based on a nation‐wide stroke registry.
Methods
Taiwan Stroke Registry
Since 2006, the Taiwan Stroke Registry (TSR) has been a nation‐wide hospital‐based prospective study engaging 56 academic and community hospitals and has 4 steps of quality control to ensure the reliability of the stroke database.22 The registry enrolls patients who had a stroke and who present to the hospital within 10 days of symptom onset. Study protocols were approved by the institutional review boards of all participating hospitals. Patients who signed the informed consent obtained follow‐up 3 months after stroke onset during outpatient clinic visits and/or a telephone interview conducted by trained nurses who served as stroke case managers. Patients were excluded if they were aged <18 years, had a final diagnosis involving a condition other than a stroke, or were lost to follow‐up. For patients who had multiple admissions because of a stroke, only the first stroke admission was included for analysis. In the present study, we retrieved the TSR registration data recorded between August 1, 2006 and August 31, 2013 that contained a total of 83 666 patients who had a stroke.
Data Collection and Measurements
Patient characteristics relevant to acute stroke, including stroke type, neurological deficit severity defined by the National Institute of Health Stroke Scale (NIHSS), systolic and diastolic BP upon admission, medical history, pre‐existing comorbidities, and demographic data, were collected according to a predefined system. The definition of AIS in the TSR was acute onset of neurological deficit with signs or symptoms persisting longer than 24 hours with or without acute ischemic lesion(s) observed on brain computed tomogram or magnetic resonance imaging scans. Hypertension was considered if subjects were administered antihypertensive medicine before admission, documented to have hypertension in previous clinic visits, or had an average systolic BP of ≥140 mm Hg or diastolic BP of ≥90 mm Hg. Diabetes mellitus was considered if subjects were prescribed oral hypoglycemic agents or insulin for diabetes mellitus; if diabetes mellitus was documented in previous clinic visits or hospital admissions; or if patients had fasting glucose levels of ≥126 mg/dL. Past cerebrovascular events, including a stroke or transient ischemic attack, and a history of cardiovascular diseases, including coronary artery disease, atrial fibrillation, or congestive heart failure, were confirmed based on previous medical records during admission and at clinic visits.
Ischemic stroke was classified into 5 major subtypes according to the TOAST (Trial of ORG 10172 in Acute Stroke Treatment) criteria into large artery atherosclerosis, small vessel occlusion, cardioembolism, other specific etiologies, and undetermined etiology.23 As for the 3‐month follow‐up, patients were assessed upon admission and discharge, during follow‐up clinic visits, or through phone calls. The main outcomes were modified Rankin Scale 3 months after stroke onset. Unfavorable outcomes were defined as a modified Rankin Scale of 3 to 6.
Admission BP was obtained by nurses when patients were transferred to wards. Before the study, all designated nurses at the participating hospitals had obtained training on standardized procedures for BP measurement and TSR data entry. After the patients had lain down for 5 minutes, a BP monitor was used to measure systolic and diastolic BP from the arms of each enrolled patient. PP was defined as systolic BP minus diastolic BP.
Statistical Analysis
All descriptive data are expressed as number of patients, percentage, and mean value with SD. Prognostic factors and poor outcome were determined using univariable and multivariable analyses. In the univariable analysis, unfavorable outcome rates for ischemic stroke in patients with different characteristics are expressed as percentages on a histogram. Distributions in age, sex, NIHSS level, and chronic medical conditions were also assessed using a chi‐square or independent t test. In the multivariable analysis, a logistic regression method was used to adjust for known prognostic factors with univariable P values of <0.10, including age, sex, hypertension, diabetes mellitus, previous stroke, coronary artery disease, atrial fibrillation, dyslipidemia, current smoker, carotid stenosis, receiving thrombolyric therapy, initial NIHSS, and continuous systolic and diastolic BP at admission. Spearman's correlation coefficient was use for the correlation between PP and systolic BP, and PP and diastolic BP. Data were analyzed using SAS software (version 9.2; SAS Institute Inc, Cary, NC). Statistical significance was considered at a P value of <0.05.
Results
Study Subject Demographics
Among 83 666 patients who had an acute stroke in the TSR, 23 487 had a hemorrhagic stroke; 18 865 were lost during the 3‐month follow‐up; and 7784 who had other specified conditions were excluded (Figure 1). The present study included 33 530 patients with AIS (mean age, 68.8±13.3 years; 59.4% males). Detailed demographic and clinical characteristics for this cohort are presented in Table 1. Mean values for systolic BP, diastolic BP, and PP upon admission were 160.2±31.1, 87.9±19.5, and 72.4±23.8 mm Hg, respectively. By the way, PP was strongly correlated with systolic BP (r=0.78; P<0.0001), but only very weakly correlated with diastolic BP (r=0.02; P<0.0001). The percentages of mortality and unfavorable outcomes 3 months poststroke were 8.7% and 46.8%, respectively. In addition, all clinical variables in Table 1 were significant between patients who had AIS with favorable and unfavorable outcomes in the univariable analysis. Besides, we compared subjects included in the present study with those who were excluded because of missing information on 3‐month outcome (n=18 865). There were similar age and stroke severity (NIHSS at admission) between included (N=33 530) and excluded (N=18 865) populations. But there were different of sex and stroke risk factors containing coronary artery disease, atrial fibrillation, dyslipidemia, and current stenosis.
Figure 1.
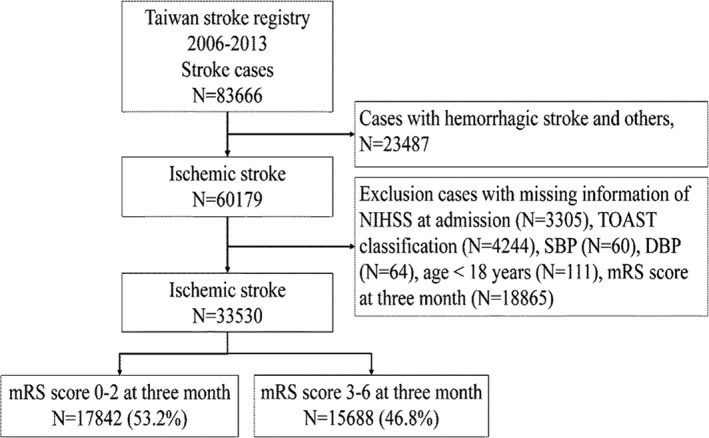
Flow chart of the study subjects. DBP indicates diastolic blood pressure; mRS, modified Rankin Scale; NIHSS, National Institutes of Health Stroke Scale; SBP, systolic blood pressure; TOAST, Trial of ORG 10172 in Acute Stroke Treatment.
Table 1.
Three‐Month Outcome of Acute Ischemic Stroke Patients
| Total (N=33 530) | Modified Rankin Scale | P Value | ||
|---|---|---|---|---|
| 0 to 2 (N=17 842) | 3 to 6 (N=15 688) | |||
| Mean age, y | 68.8±13.3 | 65.1±12.9 | 73.0±12.4 | <0.0001 |
| Age ≥65 y | 21 425 (63.9%) | 9423 (52.8%) | 12 002 (76.5%) | <0.0001 |
| Female sex | 13 622 (40.6%) | 6156 (34.5%) | 7466 (47.6%) | <0.0001 |
| Stroke risk factors | ||||
| Hypertension | 24 640 (73.5%) | 12 911 (72.4%) | 11 729 (74.8%) | <0.0001 |
| Diabetes mellitus | 12 293 (36.7%) | 6282 (35.2%) | 6011 (38.3%) | <0.0001 |
| Previous stroke or TIA | 8782 (26.2%) | 4090 (22.9%) | 4692 (29.9%) | <0.0001 |
| Coronary artery disease | 4507 (13.4%) | 1998 (11.2%) | 2509 (16.0%) | <0.0001 |
| Atrial fibrillation | 4363 (13.0%) | 1505 (8.44%) | 2858 (18.2%) | <0.0001 |
| Dyslipidemia | 14 484 (43.2%) | 7959 (44.6%) | 6525 (41.6%) | <0.0001 |
| Current smoker | 8047 (24.0%) | 4716 (26.4%) | 3331 (21.2%) | <0.0001 |
| Carotid stenosis | 2937 (8.8%) | 1287 (7.2%) | 1650 (10.5%) | <0.0001 |
| TOAST classification | <0.0001 | |||
| Large artery atherosclerosis | 9315 (27.8%) | 4376 (24.5%) | 4939 (31.5%) | |
| Small vessel occlusion | 12 747 (38.0%) | 8759 (49.1%) | 3988 (25.4%) | |
| Cardioembolism | 4002 (11.9%) | 1571 (8.8%) | 2431 (15.5%) | |
| Specific etiology | 574 (1.7%) | 257 (1.4%) | 317 (2.0%) | |
| Undetermined etiology | 6892 (20.6%) | 2879 (16.1%) | 4013 (25.6%) | |
| Characteristics on admission | ||||
| NIHSS | 6.2±8.1 | 4.6±6.7 | 7.9±9.1 | <0.0001 |
| Systolic blood pressure, mm Hg | 160.2±31.1 | 160.8±30.1 | 159.6±32.1 | 0.0005 |
| Diastolic blood pressure, mm Hg | 87.9±19.5 | 89.0±18.9 | 86.6±20.1 | <0.0001 |
| PP, mm Hg | 72.4±23.8 | 71.8±22.8 | 73.0±24.8 | <0.0001 |
| Thrombolyric therapy | 1070 (3.2%) | 427 (2.4%) | 643 (4.1%) | <0.0001 |
Values are number (percentage) or mean (SD). NIHSS indicates National Institutes of Health Stroke Scale; PP, pulse pressure; TIA, transient ischemic attack; TOAST, Trial of ORG 10172 in Acute Stroke Treatment.
Association of Admission PP With Outcome
As shown in Figure 2, there is a reverse J‐curve association between PP upon admission and unfavorable outcomes. After adjusting for clinical variables, including AIS subtypes, initial NIHSS, and systolic BP and diastolic BP upon admission, PP of <50 mm Hg remained a factor for unfavorable outcomes 3 months poststroke (P<0.0001). Compared with patients with a PP of 50 to 69 mm Hg, the adjusted odds ratio for unfavorable outcomes increased gradually with 1.24 (95% CI, 1.14–1.36) for PP of 30 to 49 mm Hg and 1.85 (95% CI, 1.50–2.28) for PP of <30 mm Hg (Table 2).
Figure 2.
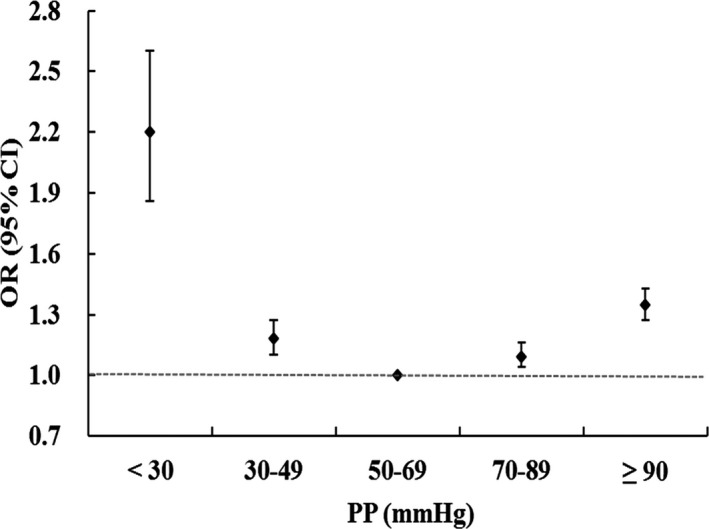
Relationship between admission pulse pressure (PP) and poor outcome at 3 months after acute ischemic stroke reveals a “reversed J‐curve” association using logistic regression.
Table 2.
Relation Between Admission PP Levels and Poor Outcome in Acute Ischemic Stroke Patients
| PP, mm Hg | mRS at 3 Months | mRS 3 to 6 vs 0 to 2 | |||||
|---|---|---|---|---|---|---|---|
| 0 to 2 (N=17 842) | 3 to 6 (N=15 688) | Odds Ratio (95% CIs) | |||||
| n | % | n | % | Crude | Adjusteda | Adjustedb | |
| <30 | 232 | 1.3 | 393 | 2.5 | 2.20 (1.86–2.60) | 1.94 (1.62–2.34) | 1.85 (1.50–2.28) |
| 30 to 49 | 2496 | 14.0 | 2274 | 14.5 | 1.18 (1.10–1.27) | 1.27 (1.17–1.37) | 1.24 (1.14–1.36) |
| 50 to 69 | 6106 | 34.2 | 4709 | 30.0 | 1.00 | 1.00 | 1.00 |
| 70 to 89 | 5353 | 30.0 | 4513 | 28.8 | 1.09 (1.04–1.16) | 0.99 (0.93–1.05) | 1.01 (0.93–1.09) |
| ≥90 | 3655 | 20.5 | 3799 | 24.2 | 1.35 (1.27–1.43) | 1.08 (1.01–1.16) | 1.14 (0.99–1.31) |
PP of 50 to 69 mm Hg as a reference group. mRS indicates modified Rankin Scale; PP, pulse pressure.
Adjusted for age, sex, hypertension, diabetes mellitus, previous stroke, coronary artery disease, atrial fibrillation, dyslipidemia, current smoker, carotid stenosis, thrombolyric therapy, and National Institutes of Health Stroke Scale at admission.
Adjusted for age, sex, hypertension, diabetes mellitus, previous stroke, coronary artery disease, atrial fibrillation, dyslipidemia, current smoker, carotid stenosis, thrombolyric therapy, National Institutes of Health Stroke Scale at admission, and systolic and diastolic blood pressure.
Furthermore, regarding ischemic stroke subtypes based on the TOAST classification, the prognostic impact of admission PP on unfavorable outcomes 3 months poststroke are shown in Table 3. There were significantly more unfavorable outcomes if the admission PP was <30 mm Hg across all ischemic stroke subtypes, and if the admission PP of 30 to 49 mm Hg in large artery atherosclerosis and other specific and undetermined etiologies.
Table 3.
Relation Between Admission PP Levels and Poor Outcome in Acute Ischemic Stroke Patients by Stroke Subtypes
| Odds Ratio (95% CIs) of mRS 3 to 6 vs 0 to 2 | ||||||||
|---|---|---|---|---|---|---|---|---|
| Large Artery Atherosclerosis | P Value | Small Vessel Occlusion | P Value | Cardioembolism | P Value | Others | P Value | |
| PP, mm Hg | ||||||||
| <30 | 1.55 (1.04–2.32) | 0.03 | 1.71 (1.12–2.60) | 0.01 | 1.72 (1.03–2.87) | 0.04 | 2.59 (1.71–3.92) | <0.0001 |
| 30 to 49 | 1.35 (1.14–1.60) | 0.0006 | 1.14 (0.97–1.33) | 0.11 | 1.16 (0.90–1.50) | 0.25 | 1.38 (1.14–1.66) | 0.0009 |
| 50 to 69 | 1.00 | 1.00 | 1.00 | 1.00 | ||||
| 70 to 89 | 1.12 (0.97–1.29) | 0.14 | 1.01 (0.88–1.15) | 0.90 | 0.82 (0.65–1.06) | 0.11 | 0.95 (0.80–1.12) | 0.52 |
| ≥90 | 1.18 (0.91–1.53) | 0.21 | 1.18 (0.93–1.49) | 0.18 | 0.92 (0.59–1.44) | 0.72 | 1.07 (0.79–1.47) | 0.66 |
The values are adjusted by age, sex, hypertension, diabetes mellitus, previous stroke, coronary artery disease, atrial fibrillation, dyslipidemia, current smoker, carotid stenosis, thrombolyric therapy, National Institute of Health Stroke Scale, and systolic and diastolic blood pressure at admission. mRS indicates modified Rankin Scale; PP, pulse pressure.
Discussion
The present study is a large nation‐wide prospective registry of AIS patients who presented with wide‐ranging BP levels that were used to derive the pathophysiological significance of PP upon admission. This study had 3 key findings. First, there is a nonlinear reverse J‐curve association between the admission PP level and 3‐month poststroke functional outcomes. Second, the impact of a low PP on unfavorable outcomes was persistent even after adjusting for some well‐known outcome parameters, including systolic and diastolic BP. Third, the findings fit all AIS subtypes.
Previously, the relationship between early‐stage PP level and stroke outcomes had been investigated only in a few studies. One study containing a total of 2178 patients who had AIS showed that admission PP was not associated with mortality during hospitalization or dependency upon discharge.18 Another study including 339 patients with first‐ever acute stroke (20.6% intracerebral hemorrhage) underwent 24‐hour BP monitoring during the first 24 hours of a stroke.19 The result showed that elevated 24‐hour PP levels, but not systolic or diastolic BP, significantly associated with a high risk of long‐term recurrence. Aslanyan et al analyzed 1455 cases of AIS with mostly moderate severity.20 An elevated weighted‐average PP during the first 60 hours was associated with a poor stroke outcome at 3 months. Recently, Tien et al reported on 136 ischemic stroke patients with no >50% culprit artery stenosis.21 This study demonstrated that an elevated PP 24 hours after an emergency department visit for an acute stroke is independently associated with unfavorable 3‐month poststroke outcomes. Overall, controversy exists regarding the prognostic role of PP in acute stroke from previous studies.
Theoretically, PP is determined by both cardiac components (stroke volume, heart rate, and left ventricular ejection rate) and arterial circulation properties, such as aortic distensibility and peripheral vascular tone.24, 25 Thus, an increase in PP may occur in patients with major artery stiffness, aortic regurgitation, old age, increased systolic hypertension, or decreased diastolic hypertension. In contrast, a decreased PP may be found with hypovolemia, cardiac failure, cardiac arrhythmia, valvular heart disease, an aortic dissection, or a low BP. Apparently, all the above‐mentioned factors affecting PP can precipitate the occurrence of AIS; however, this may increase the risk of stroke in‐evolution and poor outcomes in patients with AIS.26
Importantly, our study clearly demonstrated a detrimental effect on prognosis in patients with AIS who had a low PP, even after adjusting for systolic and diastolic BP. For every 20 mm Hg decrease in PP to the nadir, the hazard ratio for poor functional outcomes gradually increased to a maximum of around 200%, as compared with 50 to 69 mm Hg. These findings highlight the role of the pulsatile component of BP and the importance of maintaining adequate perfusion and cardiac function on poststroke outcomes. During the acute stage of an ischemic stroke, a low PP may reduce cerebral perfusion by disrupting the autoregulation of cerebral blood flow.27 Furthermore, concomitant severe cardiac disease may also be an important determinant. Upon review of previous medical literature, a low PP has been shown as an indicator of decreased cardiac function and poor outcomes in patients with myocardiac infarction and a predictor of cardiovascular death in patients with mild to advanced heart failure.28
This study had several limitations. First, we did not have BP data that were recorded at multiple time points during the acute phase of a stroke. The present study was based on a single BP measurement upon admission. Fluctuations in BP following admission could also have a significant impact on the short‐term mortality rate, but information on this important aspect was not available for the present study. Second, data on cardiac function and echocardiographic findings, including valvular dysfunction, which could have arthropometric effects on PP amplification that could impact stroke mortality, were also not available. Third, those with no consent or lost to follow‐up were not included. This may likely bias the association of admission PP with outcome. Nevertheless, the novel finding of a reverse J‐curve relationship between admission PP levels and poststroke outcomes drives the need for further studies.
Conclusion
The present study that was based on a large TSR cohort of ischemic stroke demonstrated that admission PP was associated with poststroke functional outcomes in patients with AIS.
Sources of Funding
This study is supported, in part, by Taiwan Ministry of Health and Welfare Clinical Trial and Research Center of Excellence (MOHW105‐TDU‐B‐212‐133019), China Medical University Hospital, Academia Sinica Taiwan Biobank Stroke Biosignature Project (BM10501010037), NRPB Stroke Clinical Trial Consortium (MOST 105‐2325‐B‐039‐003), Tseng‐Lien Lin Foundation, Taichung, Taiwan, Taiwan Brain Disease Foundation, Taipei, Taiwan, and Katsuzo and Kiyo Aoshima Memorial Funds, Japan.
Disclosures
None.
Supporting information
Appendix S1. Taiwan Stroke Registry Investigators.
(J Am Heart Assoc. 2017;6:e005113 DOI: 10.1161/JAHA.116.005113.)28642220
References
- 1. Lozano R, Naghavi M, Foreman K, Lim S, Shibuya K, Aboyans V, Abraham J, Adair T, Aggarwal R, Ahn SY, Alvarado M, Anderson HR, Anderson LM, Andrews KG, Atkinson C, Baddour LM, Barker‐Collo S, Bartels DH, Bell ML, Benjamin EJ, Bennett D, Bhalla K, Bikbov B, Bin Abdulhak A, Birbeck G, Blyth F, Bolliger I, Boufous S, Bucello C, Burch M, Burney P, Carapetis J, Chen H, Chou D, Chugh SS, Coffeng LE, Colan SD, Colquhoun S, Colson KE, Condon J, Connor MD, Cooper LT, Corriere M, Cortinovis M, de Vaccaro KC, Couser W, Cowie BC, Criqui MH, Cross M, Dabhadkar KC, Dahodwala N, De Leo D, Degenhardt L, Delossantos A, Denenberg J, Des Jarlais DC, Dharmaratne SD, Dorsey ER, Driscoll T, Duber H, Ebel B, Erwin PJ, Espindola P, Ezzati M, Feigin V, Flaxman AD, Forouzanfar MH, Fowkes FG, Franklin R, Fransen M, Freeman MK, Gabriel SE, Gakidou E, Gaspari F, Gillum RF, Gonzalez‐Medina D, Halasa YA, Haring D, Harrison JE, Havmoeller R, Hay RJ, Hoen B, Hotez PJ, Hoy D, Jacobsen KH, James SL, Jasrasaria R, Jayaraman S, Johns N, Karthikeyan G, Kassebaum N, Keren A, Khoo JP, Knowlton LM, Kobusingye O, Koranteng A, Krishnamurthi R, Lipnick M, Lipshultz SE, Ohno SL, Mabweijano J, MacIntyre MF, Mallinger L, March L, Marks GB, Marks R, Matsumori A, Matzopoulos R, Mayosi BM, McAnulty JH, McDermott MM, McGrath J, Mensah GA, Merriman TR, Michaud C, Miller M, Miller TR, Mock C, Mocumbi AO, Mokdad AA, Moran A, Mulholland K, Nair MN, Naldi L, Narayan KM, Nasseri K, Norman P, O'Donnell M, Omer SB, Ortblad K, Osborne R, Ozgediz D, Pahari B, Pandian JD, Rivero AP, Padilla RP, Perez‐Ruiz F, Perico N, Phillips D, Pierce K, Pope CA III, Porrini E, Pourmalek F, Raju M, Ranganathan D, Rehm JT, Rein DB, Remuzzi G, Rivara FP, Roberts T, De Leon FR, Rosenfeld LC, Rushton L, Sacco RL, Salomon JA, Sampson U, Sanman E, Schwebel DC, Segui‐Gomez M, Shepard DS, Singh D, Singleton J, Sliwa K, Smith E, Steer A, Taylor JA, Thomas B, Tleyjeh IM, Towbin JA, Truelsen T, Undurraga EA, Venketasubramanian N, Vijayakumar L, Vos T, Wagner GR, Wang M, Wang W, Watt K, Weinstock MA, Weintraub R, Wilkinson JD, Woolf AD, Wulf S, Yeh PH, Yip P, Zabetian A, Zheng ZJ, Lopez AD, Murray CJ, AlMazroa MA, Memish ZA. Global and regional mortality from 235 causes of death for 20 age groups in 1990 and 2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2095–2128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Feigin VL, Roth GA, Naghavi M, Parmar P, Krishnamurthi R, Chugh S, Mensah GA, Norrving B, Shiue I, Ng M, Estep K, Cercy K, Murray CJ, Forouzanfar MH. Global burden of stroke and risk factors in 188 countries, during 1990–2013: a systematic analysis for the Global Burden of Disease Study 2013. Lancet Neurol. 2016;15:913–924. [DOI] [PubMed] [Google Scholar]
- 3. Calhoun DA, Jones D, Textor S, Goff DC, Murphy TP, Toto RD, White A, Cushman WC, White W, Sica D, Ferdinand K, Giles TD, Falkner B, Carey RM. Resistant hypertension: diagnosis, evaluation, and treatment. A scientific statement from the American Heart Association Professional Education Committee of the Council for High Blood Pressure Research. Hypertension. 2008;51:1403–1419. [DOI] [PubMed] [Google Scholar]
- 4. Idicula TT, Waje‐Andreassen U, Brogger J, Naess H, Lundstadsveen MT, Thomassen L. The effect of physiologic derangement in patients with stroke treated with thrombolysis. J Stroke Cerebrovasc Dis. 2008;17:141–146. [DOI] [PubMed] [Google Scholar]
- 5. Ahmed N, Wahlgren N, Brainin M, Castillo J, Ford GA, Kaste M, Lees KR, Toni D. Relationship of blood pressure, antihypertensive therapy, and outcome in ischemic stroke treated with intravenous thrombolysis: retrospective analysis from Safe Implementation of Thrombolysis in Stroke‐International Stroke Thrombolysis Register (SITS‐ISTR). Stroke. 2009;40:2442–2449. [DOI] [PubMed] [Google Scholar]
- 6. Malyszko J, Muntner P, Rysz J, Banach M. Blood pressure levels and stroke: J‐curve phenomenon? Curr Hypertens Rep. 2013;15:575–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Banach M, Michalska M, Kjeldsen SE, Malyszko J, Mikhailidis DP, Rysz J. What should be the optimal levels of blood pressure: does the J‐curve phenomenon really exist? Expert Opin Pharmacother. 2011;12:1835–1844. [DOI] [PubMed] [Google Scholar]
- 8. Farnett L, Mulrow CD, Linn WD, Lucey CR, Tuley MR. The J‐curve phenomenon and the treatment of hypertension. Is there a point beyond which pressure reduction is dangerous? JAMA. 1991;265:489–495. [PubMed] [Google Scholar]
- 9. Ishitsuka K, Kamouchi M, Hata J, Fukuda K, Matsuo R, Kuroda J, Ago T, Kuwashiro T, Sugimori H, Nakane H, Kitazono T. High blood pressure after acute ischemic stroke is associated with poor clinical outcomes: Fukuoka Stroke Registry. Hypertension. 2014;63:54–60. [DOI] [PubMed] [Google Scholar]
- 10. Vemmos KN, Tsivgoulis G, Spengos K, Zakopoulos N, Synetos A, Manios E, Konstantopoulou P, Mavrikakis M. U‐shaped relationship between mortality and admission blood pressure in patients with acute stroke. J Intern Med. 2004;255:257–265. [DOI] [PubMed] [Google Scholar]
- 11. Leonardi‐Bee J, Bath PM, Phillips SJ, Sandercock PA. Blood pressure and clinical outcomes in the International Stroke Trial. Stroke. 2002;33:1315–1320. [DOI] [PubMed] [Google Scholar]
- 12. Lin MP, Ovbiagele B, Markovic D, Towfighi A. Systolic blood pressure and mortality after stroke: too low, no go? Stroke. 2015;46:1307–1313. [DOI] [PubMed] [Google Scholar]
- 13. Sesso HD, Stampfer MJ, Rosner B, Hennekens CH, Gaziano JM, Manson JE, Glynn RJ. Systolic and diastolic blood pressure, pulse pressure, and mean arterial pressure as predictors of cardiovascular disease risk in men. Hypertension. 2000;36:801–807. [DOI] [PubMed] [Google Scholar]
- 14. Glasser SP, Halberg DL, Sands C, Gamboa CM, Muntner P, Safford M. Is pulse pressure an independent risk factor for incident acute coronary heart disease events? The REGARDS study. Am J Hypertens. 2014;27:555–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Millar JA, Lever AF. Implications of pulse pressure as a predictor of cardiac risk in patients with hypertension. Hypertension. 2000;36:907–911. [DOI] [PubMed] [Google Scholar]
- 16. Millar JA, Lever AF, Burke V. Pulse pressure as a risk factor for cardiovascular events in the MRC Mild Hypertension Trial. J Hypertens. 1999;17:1065–1072. [DOI] [PubMed] [Google Scholar]
- 17. Blacher J, Staessen JA, Girerd X, Gasowski J, Thijs L, Liu L, Wang JG, Fagard RH, Safar ME. Pulse pressure not mean pressure determines cardiovascular risk in older hypertensive patients. Arch Intern Med. 2000;160:1085–1089. [DOI] [PubMed] [Google Scholar]
- 18. Ju Z, Zhang H, Tong W, Xu T, Zhang Y, Wang N, Zhang Y. Relationship between admission pulse pressure and clinical outcome during hospitalization among acute stroke patients. Acta Neurol Belg. 2009;109:18–23. [PubMed] [Google Scholar]
- 19. Zakopoulos NA, Ikonomidis I, Vemmos KN, Manios E, Spiliopoulou I, Tsivgoulis G, Spengos K, Psaltopoulou D, Mavrikakis M, Moulopoulos SD. Twenty‐four‐hour heart rate and blood pressure are additive markers of left ventricular mass in hypertensive subjects. Am J Hypertens. 2006;19:170–177. [DOI] [PubMed] [Google Scholar]
- 20. Aslanyan S, Weir CJ, Lees KR. Elevated pulse pressure during the acute period of ischemic stroke is associated with poor stroke outcome. Stroke. 2004;35:e153–e155. [DOI] [PubMed] [Google Scholar]
- 21. Tien YT, Chang MH, Lee YS, Liaw YF, Chen PL. Pulse blood pressure correlates with late outcome in acute ischemic stroke without significant culprit artery stenosis. J Stroke Cerebrovasc Dis. 2016;25:1229–1234. [DOI] [PubMed] [Google Scholar]
- 22. Hsieh FI, Lien LM, Chen ST, Bai CH, Sun MC, Tseng HP, Chen YW, Chen CH, Jeng JS, Tsai SY, Lin HJ, Liu CH, Lo YK, Chen HJ, Chiu HC, Lai ML, Lin RT, Sun MH, Yip BS, Chiou HY, Hsu CY. Get with the guidelines‐stroke performance indicators: surveillance of stroke care in the Taiwan Stroke Registry: Get With the Guidelines‐Stroke in Taiwan. Circulation. 2010;122:1116–1123. [DOI] [PubMed] [Google Scholar]
- 23. Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, Marsh EE III. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. Stroke. 1993;24:35–41. [DOI] [PubMed] [Google Scholar]
- 24. Malone AF, Reddan DN. Pulse pressure. Why is it important? Perit Dial Int. 2010;30:265–268. [DOI] [PubMed] [Google Scholar]
- 25. Liu FD, Shen XL, Zhao R, Tao XX, Wang S, Zhou JJ, Zheng B, Zhang QT, Yao Q, Zhao Y, Zhang X, Wang XM, Liu HQ, Shu L, Liu JR. Pulse pressure as an independent predictor of stroke: a systematic review and a meta‐analysis. Clin Res Cardiol. 2016;105:677–686. [DOI] [PubMed] [Google Scholar]
- 26. Jauch EC, Saver JL, Adams HP Jr, Bruno A, Connors JJ, Demaerschalk BM, Khatri P, McMullan PW Jr, Qureshi AI, Rosenfield K, Scott PA, Summers DR, Wang DZ, Wintermark M, Yonas H. Guidelines for the early management of patients with acute ischemic stroke: a guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke. 2013;44:870–947. [DOI] [PubMed] [Google Scholar]
- 27. Muller M, van der Graaf Y, Visseren FL, Vlek AL, Mali WP, Geerlings MI. Blood pressure, cerebral blood flow, and brain volumes. The SMART‐MRI study. J Hypertens. 2010;28:1498–1505. [DOI] [PubMed] [Google Scholar]
- 28. El‐Menyar A, Zubaid M, Almahmeed W, Alanbaei M, Rashed W, Al Qahtani A, Singh R, Zubair S, Al Suwaidi J. Initial hospital pulse pressure and cardiovascular outcomes in acute coronary syndrome. Arch Cardiovasc Dis. 2011;104:435–443. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Appendix S1. Taiwan Stroke Registry Investigators.


