Abstract
Background
Disopyramide is effective in ameliorating symptoms in patients with hypertrophic cardiomyopathy; however, its potential for proarrhythmic effect has raised concerns about its use in the ambulatory setting. The risk of initiating disopyramide in this manner has never been evaluated.
Methods and Results
All charts of patients seen in the outpatient hypertrophic cardiomyopathy clinic between 2010 and 2014 were screened for initiation of disopyramide and data were extracted. Disopyramide in our clinic is usually initiated at a dose of 300 mg daily and titrated during follow‐up. A total of 2015 patients were seen in the clinic, including 168 who were started on disopyramide. There were no cardiac events within 3 months of disopyramide initiation. During long‐term follow‐up (255 patient‐years; mean, 447 days; interquartile range, 201–779), only 2 patients developed cardiac events (syncope of unknown cause in both). Thirty‐eight patients (23%) developed side effects of disopyramide and 18 (11%) stopped the drug because of these side effects. Of the patients continuing disopyramide long term, 63% remained free of septal reduction interventions at end of follow‐up. Disopyramide at a dose of 300 mg prolonged the mean QTc interval by 19±23 ms; however, increasing the dose to 600 mg had no further significant effect.
Conclusions
Initiation of disopyramide in the outpatient setting is safe and the risk of subsequent sudden cardiac death is low. Because of its QT‐prolonging effect, precautions may be necessary in patients at higher risk of torsades de pointes.
Keywords: acquired long QT syndrome, disopyramide, hypertrophic cardiomyopathy, sudden cardiac death
Subject Categories: Cardiomyopathy, Sudden Cardiac Death
Introduction
Hypertrophic cardiomyopathy (HCM) is the most common genetic cardiac disorder with an estimated prevalence of 1:500.1 Typical findings in HCM include asymmetrical septal hypertrophy and systolic anterior motion of the mitral valve, which may cause left ventricular outflow tract obstruction (LVOTO). LVOTO, found in up to two thirds of patients,2 may result in symptoms such as exertional shortness of breath, presyncope, and syncope. Fortunately, treatment effective in ameliorating LVOTO‐related symptoms is available. Drugs with a negative inotropic effect are most commonly used as first line and include beta‐blockers (BB) and/or nondihydropyridine calcium‐channel blockers (CCB; primarily verapamil).3 However, a substantial portion of patients (40% in 1 large study)4 suffer from symptoms that are refractory to these agents. In such patients, an interventional approach for reduction of septal myocardial mass by either surgery (myectomy) or catheterization (alcohol septal ablation) may be recommended.5, 6 Nevertheless, before referral for an intervention, a third drug is available in our arsenal and may be considered.
Disopyramide is a class Ia antiarrhythmic drug; however, it is now rarely used to control arrhythmias. In 1982, our center demonstrated, for the first time, that, because of its negative inotropic properties, it is effective in reducing the pressure gradient created by LVOTO.7 Since then, several studies4, 8, 9 have demonstrated its effectiveness in reducing LVOT gradients and related symptoms. Indeed, for relief of resistant symptoms, the European Society of Cardiology5 and American Heart Association/American College of cardiology6 guidelines rate disopyramide with a class I and IIa recommendation, respectively. However, disopyramide's antiarrhythmic background continues to haunt it. Because of its capability to block the rapid delayed rectifier cardiac potassium current (IKr), it has a significant QT‐prolonging effect and, as a corollary, may induce torsades de pointes (TdP).10 This potential for proarrhythmia is the main reason for the recommendation to initiate disopyramide treatment during hospitalization. Nevertheless, over the years, this recommendation has been practiced by some HCM centers, but not by others.9 Furthermore, whereas the American guidelines for treatment of HCM6 clearly state that treatment with disopyramide should be initiated in the hospital setting, the European guidelines give no such recommendation.5 In our institution, disopyramide has been initiated in the outpatient clinic for more than 2 decades. The purpose of this study was to review our experience and examine the safety of this approach.
Methods
Population Selection
The study subjects were identified through the database of the multidisciplinary HCM clinic at the Toronto General Hospital, which serves as a large tertiary referral center. All charts of patients seen in the HCM clinic between January 2010 and December 2014 were screened for initiation of disopyramide and were mined for relevant clinical data. Exclusion criteria included initiation of disopyramide elsewhere or preceding the period studied and patients referred only for consultation without planned follow‐up in our clinic.
The clinical diagnosis of HCM was based on the 2‐dimensional echocardiographic finding of unexplained left ventricular hypertrophy (LVH) with a maximal wall thickness ≥15 mm and in the absence of other causes of LVH. In selected patients, the diagnosis was made with a maximal wall thickness ≥13 mm in the presence of family history of HCM and other findings suggestive of the diagnosis.
The study was approved by the institutional research ethics board with the requirement for informed consent waived.
Disopyramide Protocol
Disopyramide is initiated in our clinic in HCM patients with LVOTO (gradient ≥30 mm Hg) and related symptoms which are refractory to maximally tolerated doses of BB or CCB and do not have other indications for cardiovascular surgery (eg, coronary artery disease, severe valvular disease). The routine initial dose is 100 mg short‐acting disopyramide 3 times daily. At the day of disopyramide initiation, an electrocardiogram (ECG) and echocardiogram are performed. These are repeated on the next follow‐up visit, which is usually scheduled 2 months after. At that time, and according to the clinical response, increasing the disopyramide dose, discontinuation of the drug, or referral for a septal reduction intervention (as per guideline indications)5, 6 is considered. Pyridostigmine, which has been demonstrated to attenuate the anticholinergic side effects of disopyramide,4 is also considered if patients develop such side effects.
Absolute contraindications for disopyramide include reduced left ventricular (LV) systolic function, congenital long QT syndrome or history of TdP and pregnancy. Relative contraindications for disopyramide include urinary retention, prostatism, glaucoma, and myasthenia gravis, which may be exacerbated by the anticholinergic effect of disopyramide.
Caution is utilized when disopyramide is prescribed to patients with a prolonged QT at baseline, and efforts are made to avoid or discontinue concomitant administration of drugs that prolong the QT interval. Caution is also used in patients with renal or hepatic impairment attributed to potential effects on drug clearance and history of significant electrolyte imbalance (especially hypokalemia and hypomagnesemia) attributed to increased risk of proarrhythmia. Caution is also utilized in patients with atrial fibrillation and atrial flutter because of the potential for disopyramide‐induced augmentation of atrioventricular conduction and increased ventricular rate and because of increased risk of TdP postconversion to sinus rhythm or after a long pause. The decision to initiate disopyramide therapy is taken by the treating physician as is the tailoring of the above‐mentioned protocol to the individual patient.
Risk stratification of sudden cardiac death was routinely performed in all patients, and insertion of implantable cardioverter‐defibrillators (ICD) was recommended if this risk was high.
Follow‐up
Short‐term follow‐up was defined as 3 months postdisopyramide initiation whereas long‐term follow‐up was defined as >3 months. Information on adverse events (mortality, cardiac arrest, syncope, heart failure, anticholinergic side effects, and other side effects possibly associated with disopyramide) and referral to septal reduction procedures was gathered. The follow‐up period was censored at date of last follow‐up. Patients were defined as lost to follow‐up if they failed to arrive for a scheduled follow‐up visit for an unknown reason.
ECG Measurements
ECGs recorded on the day of disopyramide initiation and on the first visit postinitiation were analyzed. PR, QRS, and QT intervals were measured manually by a single investigator experienced in ECG interpretation (A.A.). QT intervals were measured using the tangents method using lead II. If measurement in lead II was impossible because of technical limitations, leads V5 or V2 were used in this order. Correction for heart rate was performed using Fridericia's formula. Although Bazett's formula is the most commonly used, it is less accurate in relative tachycardia or bradycardia and therefore less reliable when comparing ECGs with different heart rates. Accordingly, Fridericia's formula is recommended over Bazetts’ for evaluation of drug‐induced QT prolongation (http://www.fda.gov/downloads/drugs/guidancecomplianceregulatoryinformation/guidances/ucm073153.pdf).11 Patients who were ventricularly paced were excluded from ECG analysis.
Statistical Analysis
Continuous variables are described as mean±SD except for follow‐up period, which is described as median+interquartile range. Categorical data are described as frequency (percent). Paired 2‐sided t test was used for comparison between variables pre‐ and postdisopyramide. An unpaired t test was used for comparison of QT changes between men and women. Statistical analysis was performed using GraphPad Prism software (GraphPad Software Inc., La Jolla, CA, USA).
Results
Between January 2010 and December 2014, 2015 patients with HCM or suspected HCM were seen in the HCM clinic. In total, 323 of these patients were treated with disopyramide, of which 168 started disopyramide in our clinic during the study period and were included in the study. The basic characteristics of these patients are detailed in Table 1. Patients taking potentially QT‐prolonging drugs at time of disopyramide initiation included 16 who were treated with antidepressants, 3 with antihistamines, and 1 with tacrolimus.
Table 1.
Patients’ Baseline Characteristics (n=168)
| Age, mean±SD | 59±14 |
| Female sex, n (%) | 70 (42) |
| ICDa, n (%) | 9 (5) |
| Hx of syncope, n (%) | 14 (8) |
| Atrial fibrillation, n (%) | 19 (11) |
| Paroxysmal, n (%) | 17 (10) |
| Persistent/permanent, n (%) | 2 (1) |
| Medications | |
| BB, n (%) | 134 (80) |
| CCB, n (%) | 12 (7) |
| BB+CCB, n (%) | 7 (4) |
| QT prolonging drugs, n (%) | 20 (12) |
| Diuretics, n (%) | 28 (17) |
BB indicates beta‐blockers; CCB, calcium‐channel blockers; ICD, implantable cardioverter‐defibrillator.
All for primary prevention.
Initial disopyramide dose was 302±44 mg. In the vast majority of patients (148), the initial dose was 300 mg. In 44 patients, the disopyramide dose was changed during follow‐up with a final mean dose of 350±107 mg.
Disopyramide Side Effects
Of the 168 patients started on 300 mg of disopyramide daily, 33 (20%) developed side effects. These included anticholinergic effects in 27 patients, weakness or fatigue possibly associated with disopyramide in 5, and nausea in 1. Five additional patients developed side effects after the disopyramide dose was increased to 400 to 600 mg daily (3 developed anticholinergic side effects and 2 developed dizziness). In all but 1 patient, side effects developed within the first month of treatment or increase in disopyramide dose. In 1 patient, the side effect was noticed only 6 months after disopyramide initiation. In total, 18 patients (11%) discontinued dispoyramide because of the side effects.
Electrocardiographic Analysis
ECGs before and after initiation of disopyramide at the 300‐mg dose were available for 100 patients (Table 2). Heart rate slightly increased after disopyramide initiation. PR, QRS, QT, and corrected QT (QTc) intervals were all prolonged with disopyramide. The mean change in the QTc interval was 19 ms and was not significantly different between men and women (18±23 vs 21±21; P=0.4). The QTc was prolonged by <10 ms in 37 patients, between 10 and 20 ms in 16, and by more than 20 ms in 47. The number of patients with QTc prolongation (defined as ≥460 ms) more than doubled from 16% before drug prescription to 33% after its initiation. Of these, 64% had mild prolongation of less than 480 ms, with only 1 patient having QTc intervals longer than 500 ms before disopyramide and 4 after its initiation (Table 2). The longest QTc on 300 mg of disopyramide was 520 ms. Typical QT prolongation and T‐wave morphology changes associated with disopyramide are presented in Figure.
Table 2.
Electrocardiographic Characteristics Before and After Initiation of Disopyramide (n=100)
| Pre‐Disopyramide | Post‐Disopyramide (300 mg) | Δ | P Value | |
|---|---|---|---|---|
| Heart rate, bpm ±SD | 61±9 | 65±11 | 4±9.8 | 0.016 |
| PR, ms ±SD | 168±32 | 181±27 | 13±28 | 0.001 |
| QRS, ms ±SD | 105±19 | 109±22 | 4±10 | 0.224 |
| QT, ms ±SD | 424±37 | 435±37 | 10±28 | 0.038 |
| QTcB, ms ±SD | 426±33 | 450±32 | 24±27 | 0.0001 |
| QTcF, ms ±SD | 425±31 | 445±29 | 19±23 | 0.0001 |
| QTcF | ||||
| ≥460 ms | 16 (16) | 33 (33) | ||
| 460 to 479 ms | 12 (12) | 21 (21) | ||
| 480 to 499 ms | 3 (3) | 8 (8) | ||
| ≥500 ms, n (%) | 1 (1) | 4 (4) | ||
QTcB indicates corrected QT interval, Bazett's formula; QTcF, corrected QT interval, Fridericia's formula.
Figure 1.
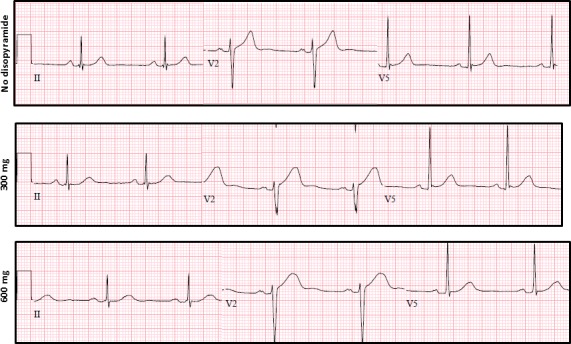
Disopyramide‐induced electrocardiographic changes. Electrocardiograms of a 53‐year‐old man with hypertrophic cardiomyopathy and left ventricular outflow tract gradients of up to 90 mm Hg. He suffered from exertional shortness of breath despite trials of beta‐blockers and calcium‐channel blockers. After initiation of disopyramide and increase in dose to 600 mg daily, his symptoms resolved and gradients diminished to 15 mm Hg. Electrocardiograms were recorded before disopyramide and while on 300 and 600 mg daily. Heart rate and QRS interval showed no significant change after disopyramide initiation. The PR interval was 180 ms at baseline, prolonged minimally on 300 mg and to 200 ms on 600 mg. The corrected QT interval was 378 ms at baseline, prolonged to 426 ms on 300 mg and remained without significant change (419 ms) after dose increase to 600 mg. Note the change in T‐wave morphology with a more‐rounded peak on disopyramide.
In 10 patients, ECGs were available before and after increasing disopyramide from 300 to 600 mg. In the majority of these patients (70%), no significant change in QTc interval (ΔQTc <5 ms) was observed. However, in 3 (30%) patients, ΔQTc was greater than 20 ms. Only in 1 patient on 600 mg of disopyramide was the QTc >500 ms.
In the entire cohort, irrespective of initial disopyramide dose, 22 patients (14%) had QTc intervals ≥460 ms before disopyramide initiation. Of these, 5 (3%) had QTc ≥480 but <500 ms and 3 (2%) had a QTc between 500 and 510 ms. Maximal QTc on disopyramide, irrespective of dose, was ≥460 in 45 patients (35%). Of these, 11 (8%) had QTc ≥480 but <500 ms and 10 (8%) had a QTc ≥500. Maximal QTc on disopyramide at any dose was 525 ms. A single patient, excluded from this analysis, developed a QTc interval of 600 ms in the setting of stress‐induced cardiomyopathy (see next section).
Follow‐up
No patients were lost to short‐term follow‐up. Median long‐term follow‐up was 447 days (IQR, 201–779). Fifteen patients (9%) who were lost to long‐term follow‐up had a median follow‐up period of 263 days (IQR, 147–491) before loss of contact.
During short‐term follow‐up, a single patient had syncope 2 months after dispoyramide initiation. However, the circumstances of this event were typical of a vasovagal episode. A second patient had seizures 10 weeks after starting disopyramide; however, he suffered similar episodes before this drug's prescription and the event was not thought to be associated with disopyramide. During long‐term follow‐up (255 patient‐years), 2 patients (1%) suffered syncopal episodes of uncertain cause 7 and 39 months after disopyramide was started. The first patient had a baseline QTc interval of 445 ms. On 400 and 600 mg of disopyramide, his QTc intervals were 475 and 455 ms, respectively. The second patient had a baseline QTc of 410 ms. He then developed stress‐induced cardiomyopathy with deep inverted T waves and a QTc of 600 ms. His disopyramide was stopped and he went on to have a successful myectomy. No patients suffered cardiac arrest or sudden cardiac death and no appropriate ICD therapies were delivered.
Disopyramide was discontinued by 74 patients (44%) during follow‐up. Reasons for discontinuation included side effects in 18 (11% of patients started on disopyramide) and lack of clinical improvement in 56 (33%).
Septal reduction interventions were performed in 55 patients (8 alcohol septal ablations and 47 myectomies). Thus, of the 150 patients who did not discontinue disopyramide because of side effects, 37% underwent interventions and 63% remained intervention free during follow‐up.
Discussion
Initiation of any drug in the hospital setting offers obvious benefits—acute reactions and side effects may be monitored and treated, the drug's effect evaluated, and rapid titration achieved. Nevertheless, the shortcomings of such an approach are also apparent with considerable hospital cost and patients’ inconvenience being chief among these.12 Furthermore, a recommendation for hospital monitoring for initiation of a drug is likely to limit its utilization significantly. In the case of disopyramide, underutilization may lead to unnecessary interventions given that therapy with this drug may abolish the need for septal reduction procedures in most patients with LVOTO‐related symptoms.4, 9
In this setting, our findings regarding the safety of disopyramide initiated in the outpatient setting are of special importance. During the 5 years of the study period, disopyramide was started in 168 patients with no cardiac events occurring in the first 3 months of therapy. This safety profile remained high (no cardiac events) in the first 3 months after increase in disopyramide dose. The long‐term safety of disopyramide was also found to be high. Only 2 patients had cardiac events (syncope in both) during 225 patient‐years of treatment. This high safety profile is in concordance with previous publications demonstrating no increase in sudden cardiac death risk in HCM patients treated with higher mean doses (430–500 mg) of this drug.4, 9 Nevertheless, disopyramide does have significant electrophysiological effects. Evaluation of ECGs before and after disopyramide initiation demonstrated an increase in heart rate and PR and QRS intervals. Most important, the QTc interval was prolonged by a mean of 19 ms. To put this into perspective, drugs prolonging the QTc by 5 ms are associated with minimal risk of TdP.11 Those with an effect in the range of 5 to 20 ms (eg, moxifloxacin) are of uncertain significance, but some of these drugs have been associated with increased risk. Those with a larger effect are regarded as having a substantial likelihood of proarrhythmia. Thus, disopyramide's effect in HCM patients puts it on the border of the “dangerous drugs zone.” Interestingly, increasing the dose from 300 to 600 mg had very little effect on the mean QTc. This may actually be expected because disopyramide's QT‐prolonging effect is thought to be reversely dose dependent.13 It is hypothesized that with increased dose, the sodium‐channel–blocking effect of disopyramide increases, opposing its potassium channel blockade. Given that the block of the potassium current is responsible for prolongation of repolarization, QT interval, and triggering of TdP, lower doses of disopyramide may actually be more torsadogenic than higher ones.13
Clinical Implication
The US Food and Drug Administration drug information package regarding disopyramide includes the following sentence: “Initiation of Disopyramide treatment, as with other antiarrhythmic agents used to treat life‐threatening arrhythmias, should be carried out in the hospital” (https://www.drugs.com/pro/disopyramide.html). Nevertheless, HCM patients are a unique group and their indication for disopyramide is not antiarrhythmic. Therefore, the requirement for initiation of therapy with disopyramide during hospitalization is not directly pertinent and applicable for this patient population. The main reason for initiation of disopyramide in the hospital setting is to evaluate its effect on the QT interval and monitor for development of arrhythmias, mainly TdP. The rationale behind such a recommendation stems from 2 main points: (1) Excessive prolongation of the QT interval can be diagnosed and the drug discontinued if required, and (2) most arrhythmias occur in the first few days of drug therapy. Our study suggests that both of these points can, with appropriate precautions, be addressed without the need for hospital monitoring.
First, the maximal QTc on 300 mg in our cohort was 525 ms. Although this is a significantly prolonged QTc interval, its prognostic value in HCM patients is unclear, and, to our knowledge, no studies have delineated a cut‐off point portending increased risk in this setting. According to a protocol for disopyramide initiation in the hospital setting published by Sherrid and Arabadjian, disopyramide should be discontinued if the QTc exceeds 525 or 550 ms in the presence of a baseline wide QRS.14 Using this mark, all of our patients would continue on disopyramide throughout and after the initial hospitalization. According to the European guidelines, the dose should be reduced if the QTc exceeds 480 ms.5 In our cohort, this would involve 18% of patients; however, this recommendation is probably derived from data on non‐HCM patients and because it has not been substantiated by experience either at our institution, or elsewhere, it is not our practice to reduce the dose routinely in these cases.
Second, it is reasonable to assume that the proarrhythmic effect of AADs will manifest in the first few days of treatment; however, studies supporting this hypothesis included mainly patients treated for atrial tachycardias.15, 16 This is of importance given that the acute changes in RR interval in patients with atrial fibrillation or postconversion to sinus rhythm may augment the torsadogenic effect. Furthermore, in a study examining the timing to TdP in patients with drug‐induced long QT syndrome, the majority of patients developed this complication more than 3 days after drug initiation.17 Thus, a short admission for disopyramide initiation can be expected to pick up only the minority of patients with a rare adverse event (TdP). Indeed, in our cohort, no cardiac events occurred during the first 3 months of therapy. However, our study, the largest, to our knowledge, examining the ECG effects of disopyramide, did demonstrate that the drug has a substantial influence on several ECG parameters. Therefore, some prudence is required when initiating therapy with it. Specifically, periodic outpatient ECG and electrolyte monitoring and caution with prescription of other QT‐prolonging drugs is recommended. Additional precautions may be required in patients who are at higher risk, such as elderly patients, those with a prolonged baseline QT interval, those on necessary QT prolonging drugs, patients with atrial fibrillation or cardiac conduction abnormalities, and those with history of electrolyte abnormalities or taking potassium‐depleting diuretics. On a case‐by‐case basis in these patients, initiation of therapy in hospital is a reasonable clinical judgment.
Limitations
Although not the primary goal of our study, the long‐term follow‐up analysis is limited by 9% of the patients being lost to follow‐up. Despite it being customary for treating physicians to inform us of significant adverse events, we could not rule out the possibility that some of these patients did suffer such outcomes.
Our results regarding the influence of increasing the dose of disopyramide from 300 to 600 mg on the ECG are in line with the expected electrophysiological effect of the drug; however, they are based on a small number of patients. Indeed, in some patients, considerable QTc interval prolongation (>30 ms) was noted after dose increase. Accordingly, the lack of significant QT prolongation after prescription of 300 mg should not be regarded as warranty when increasing the dose and appropriate precautions should be maintained.
The current study was not designed and could not answer questions regarding frequency of ECG monitoring and QT‐interval thresholds that portend too high a risk. Accordingly, such decisions remain based on expert opinion.
In our institution, short‐acting disopyramide is used while, in many other countries, a long‐acting formulation is available. Although the difference between formulations is unlikely to have a major effect on safety and efficacy, some effect is possible and could not be ruled out.
Conclusions
The current study demonstrates the safety of initiating disopyramide in the outpatient HCM clinic. This may encourage a more‐widespread use of this drug that was shown to be effective4, 9 in ameliorating LVOTO‐related symptoms in patients with HCM without the need for septal‐reduction interventions.
Sources of Funding
Adler and Fourey were funded by the Richard and Edith Strauss Canada Foundation and the Halpern HCM Research Fund.
Disclosures
None.
(J Am Heart Assoc. 2017;6:e005152 DOI: 10.1161/JAHA.116.005152.)28550094
References
- 1. Maron BJ, Gardin JM, Flack JM, Gidding SS, Kurosaki TT, Bild DE. Prevalence of hypertrophic cardiomyopathy in a general population of young adults. Echocardiographic analysis of 4111 subjects in the CARDIA Study. Coronary Artery Risk Development in (Young) Adults. Circulation. 1995;92:785–789. [DOI] [PubMed] [Google Scholar]
- 2. Shah JS, Esteban MT, Thaman R, Sharma R, Mist B, Pantazis A, Ward D, Kohli SK, Page SP, Demetrescu C, Sevdalis E, Keren A, Pellerin D, McKenna WJ, Elliott PM. Prevalence of exercise‐induced left ventricular outflow tract obstruction in symptomatic patients with non‐obstructive hypertrophic cardiomyopathy. Heart. 2008;94:1288–1294. [DOI] [PubMed] [Google Scholar]
- 3. Maron BJ, Maron MS. Hypertrophic cardiomyopathy. Lancet. 2013;381:242–255. [DOI] [PubMed] [Google Scholar]
- 4. Sherrid MV, Shetty A, Winson G, Kim B, Musat D, Alviar CL, Homel P, Balaram SK, Swistel DG. Treatment of obstructive hypertrophic cardiomyopathy symptoms and gradient resistant to first‐line therapy with beta‐blockade or verapamil. Circ Heart Fail. 2013;6:694–702. [DOI] [PubMed] [Google Scholar]
- 5. Elliott PM, Anastasakis A, Borger MA, Borggrefe M, Cecchi F, Charron P, Hagege AA, Lafont A, Limongelli G, Mahrholdt H, McKenna WJ, Mogensen J, Nihoyannopoulos P, Nistri S, Pieper PG, Pieske B, Rapezzi C, Rutten FH, Tillmanns C, Watkins H. 2014 ESC guidelines on diagnosis and management of hypertrophic cardiomyopathy: the Task Force for the Diagnosis and Management of Hypertrophic Cardiomyopathy of the European Society of Cardiology (ESC). Eur Heart J. 2014;35:2733–2779. [DOI] [PubMed] [Google Scholar]
- 6. Gersh BJ, Maron BJ, Bonow RO, Dearani JA, Fifer MA, Link MS, Naidu SS, Nishimura RA, Ommen SR, Rakowski H, Seidman CE, Towbin JA, Udelson JE, Yancy CW. 2011 ACCF/AHA guideline for the diagnosis and treatment of hypertrophic cardiomyopathy: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Developed in collaboration with the American Association for Thoracic Surgery, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. J Am Coll Cardiol. 2011;58:e212–e260. [DOI] [PubMed] [Google Scholar]
- 7. Pollick C. Muscular subaortic stenosis: hemodynamic and clinical improvement after disopyramide. N Engl J Med. 1982;307:997–999. [DOI] [PubMed] [Google Scholar]
- 8. Pollick C. Disopyramide in hypertrophic cardiomyopathy. II. Noninvasive assessment after oral administration. Am J Cardiol. 1988;62:1252–1255. [DOI] [PubMed] [Google Scholar]
- 9. Sherrid MV, Barac I, McKenna WJ, Elliott PM, Dickie S, Chojnowska L, Casey S, Maron BJ. Multicenter study of the efficacy and safety of disopyramide in obstructive hypertrophic cardiomyopathy. J Am Coll Cardiol. 2005;45:1251–1258. [DOI] [PubMed] [Google Scholar]
- 10. Tzivoni D, Keren A, Stern S, Gottlieb S. Disopyramide‐induced Torsade de Pointes. Arch Intern Med. 1981;141:946–947. [PubMed] [Google Scholar]
- 11. Morganroth J. Cardiac repolarization and the safety of new drugs defined by electrocardiography. Clin Pharmacol Ther. 2007;81:108–113. [DOI] [PubMed] [Google Scholar]
- 12. Kim MH, Klingman D, Lin J, Pathak P, Battleman DS. Cost of hospital admission for antiarrhythmic drug initiation in atrial fibrillation. Ann Pharmacother. 2009;43:840–848. [DOI] [PubMed] [Google Scholar]
- 13. Wyse KR, Ye V, Campbell TJ. Action potential prolongation exhibits simple dose‐dependence for sotalol, but reverse dose‐dependence for quinidine and disopyramide: implications for proarrhythmia due to triggered activity. J Cardiovasc Pharmacol. 1993;21:316–322. [DOI] [PubMed] [Google Scholar]
- 14. Sherrid MV, Arabadjian M. A primer of disopyramide treatment of obstructive hypertrophic cardiomyopathy. Prog Cardiovasc Dis. 2012;54:483–492. [DOI] [PubMed] [Google Scholar]
- 15. Prystowsky EN. Inpatient versus outpatient initiation of antiarrhythmic drug therapy for patients with supraventricular tachycardia. Clin Cardiol. 1994;17:II7–II10. [DOI] [PubMed] [Google Scholar]
- 16. Chung MK, Schweikert RA, Wilkoff BL, Niebauer MJ, Pinski SL, Trohman RG, Kidwell GA, Jaeger FJ, Morant VA, Miller DP, Tchou PJ. Is hospital admission for initiation of antiarrhythmic therapy with sotalol for atrial arrhythmias required? Yield of in‐hospital monitoring and prediction of risk for significant arrhythmia complications. J Am Coll Cardiol. 1998;32:169–176. [DOI] [PubMed] [Google Scholar]
- 17. Zeltser D, Justo D, Halkin A, Prokhorov V, Heller K, Viskin S. Torsade de pointes due to noncardiac drugs: most patients have easily identifiable risk factors. Medicine (Baltimore). 2003;82:282–290. [DOI] [PubMed] [Google Scholar]


