Abstract
Background
We aimed to determine the risk conferred by metabolic syndrome (METS) and diabetes mellitus (DM) to recurrent stroke in patients with minor ischemic stroke or transient ischemic attack from the CHANCE (Clopidogrel in High‐risk patients with Acute Non‐disabling Cerebrovascular Events) trial.
Methods and Results
In total, 3044 patients were included. Patients were stratified into 4 groups: neither, METS only, DM only, or both. METS was defined using the Chinese Diabetes Society (CDS) and International Diabetes Foundation (IDF) definitions. The primary outcome was new stroke (including ischemic and hemorrhagic) at 90 days. A multivariable Cox regression model was used to assess the relationship of METS and DM status to the risk of recurrent stroke adjusted for potential covariates. Using the CDS criteria of METS, 53.2%, 17.2%, 19.8%, and 9.8% of patients were diagnosed as neither, METS only, DM only, and both, respectively. After 90 days of follow‐up, there were 299 new strokes (293 ischemic, 6 hemorrhagic). Patients with DM only (16.1% versus 6.8%; adjusted hazard ratio 2.50, 95% CI 1.89–3.39) and both (17.1% versus 6.8%; adjusted hazard ratio 2.76, 95% CI 1.98–3.86) had significantly increased rates of recurrent stroke. No interaction effect of antiplatelet therapy by different METS or DM status for the risk of recurrent stroke (P=0.82 for interaction in the fully adjusted model of CDS) was observed. Using the METS (IDF) criteria demonstrated similar results.
Conclusions
Concurrent METS and DM was associated with an increased risk of recurrent stroke in patients with minor stroke and transient ischemic attack.
Keywords: diabetes mellitus, metabolic syndrome, prognosis, stroke
Subject Categories: Ischemic Stroke
Introduction
The metabolic syndrome (METS) refers to a cluster of highly interrelated metabolic risk factors.1 Regardless of the details of several criteria by different organizations for its diagnosis,2, 3 it is generally accepted that the prevalence of METS in diverse racial populations is increasing (between 10% and 84%).4, 5 Previous prospective studies showed that the presence of METS identifies persons at an elevated risk for ischemic stroke or transient ischemic attack (TIA).6, 7 There are limited data, however, on the relationship between METS and the risk of stroke recurrence. Previous studies found that METS may not be predictive for stroke recurrence in patients with general ischemic stroke,8, 9 whereas another study demonstrated that METS was associated with higher risk of stroke recurrence in patients with ischemic stroke.10 The relationship between METS and recurrence of stroke after a stroke or TIA remains controversial.
METS was defined for use in persons without diabetes mellitus (DM), but the definition has developed in recent decades to include those with DM.11, 12 Given a synergistic relationship among these components, the collective entity of METS provides better stroke risk estimates. Nevertheless, this integration of each component of METS made it difficult to understand the effect of DM or other components on stroke recurrence compared with the role of METS as an independent risk factor.
Minor ischemic stroke and TIA account for ≈65% of all acute ischemic cerebrovascular events13 and lead to a risk of 10% to 15% stroke occurrence within 90 days.14, 15 Factors associated with a high risk of recurrence in patients with TIA or minor stroke were different from those of general stroke16; however, data from previous studies were derived from trials or cohorts in which patients were recruited weeks or months after their initial event and underestimated early recurrence, especially for minor stroke or TIA.8, 9, 17 Consequently, the risks of recurrent stroke caused by METS and DM in patients with a minor stroke or TIA should be further examined.
We compared the risk of recurrent stroke in patients with different METS and DM status and minor stroke or TIA from the CHANCE (Clopidogrel in High‐risk patients with Acute Non‐disabling Cerebrovascular Events) trial. Our hypothesis was that METS and DM were associated with an increased risk of recurrent stroke after a minor stroke or TIA.
Methods
Study Patients
The CHANCE trial was a randomized, double‐blind, controlled trial that enrolled 5170 patients within 24 hours after onset of minor stroke (National Institutes of Health Stroke Scale [NIHSS] ≤3) or high‐risk TIA (ABCD2 ≥4) from 114 clinical centers in China.18, 19, 20 In total, 73 (64%) of 114 participating hospitals voluntarily participated in the serum biomarker substudy in the CHANCE trial. The triglyceride and high‐density lipoprotein levels, which are the significant items for the diagnosis of METS, were analyzed in the laboratory using collected serum. A total of 3044 patients in these 73 centers with available triglyceride and high‐density lipoprotein levels were included in this analysis.
Standard Protocol Approvals, Registrations, and Patient Consents
The CHANCE trial is registered at ClinicalTrials.gov (identifier NCT00979589). The protocol and data collection of the CHANCE trial were approved by the ethics committee of Beijing Tiantan Hospital and all other study centers. All participants or his or her representatives provided written informed consent before being entered into the study.
Measurements
Baseline demographics and clinical characteristics, including age, sex, medical history of ischemic stroke, TIA, myocardial infarction, angina, congestive heart failure, known atrial fibrillation or flutter, valvular heart disease, hypertension, DM, hypercholesterolemia, and baseline NIHSS and ABCD2 scores were collected through face‐to‐face interviews by neurologists from clinical centers. Body mass index (BMI) was calculated as the weight in kilograms divided by the height in meters squared (kg/m2). Blood pressure was measured in the left arm of supine patients using a mercury or electronic sphygmomanometer. Venous blood was drawn from fasting patients 24±12 hours after randomization in 3044 patients of these 73 centers.21 Plasma glucose after overnight fasting was then analyzed. The serum specimens were collected and shipped on ice by overnight courier from each participating hospital to Beijing Tiantan Hospital (China), where all data analyses were performed. The triglyceride and high‐density lipoprotein levels were analyzed in the laboratory using collected serum by testing personnel blinded to clinical data in Beijing Tiantan Hospital. Data were analyzed with a Roche Modular P800 system.
DM was defined as a fasting glucose level ≥7.0 mmol/L (126 mg/dL), self‐reported history of DM, or receiving treatment for DM. METS was defined using the definitions of the Chinese Diabetes Society (CDS),3 which is the only official recommendation for the diagnosis of METS in the Chinese population, and International Diabetes Foundation (IDF).2 We excluded patients with DM or fasting plasma glucose ≥7.0 mmol/L in both definitions of METS. Patients in our study were stratified into 4 groups: neither METS or DM, METS only, DM only, or both.
METS (CDS) was determined by the presence of ≥3 of the following metabolic risk factors: overweight or obesity (BMI ≥25); fasting plasma glucose of 6.1 to 6.9 mmol/L (110–125 mg/dL), 2‐hour plasma glucose ≥7.8 mmol/L (140 mg/dL), or a history of DM with antidiabetic medication; elevated blood pressure (≥140/≥90 mm Hg) or a history of hypertension with antihypertensive medication; and dyslipidemia, which includes increased triglyceride levels (≥1.7 mmol/L [150 mg/dL]) or reduced high‐density lipoprotein levels (<0.9 mmol/L [35 mg/dL] in men and <0.9 mmol/L [39 mg/dL] in women).3
METS (IDF) was defined by abdominal visceral obesity (increased waist circumference [WC], ≥90 cm in men and ≥80 cm in women for an Asian population) plus any ≥2 of the following factors: triglyceride level (≥150 mg/dL) or history of hyperlipemia with antihyperlipemia medication; reduced high‐density lipoprotein level (<40 mg/dL in men and <50 mg/dL in women); elevated blood pressure (≥130/≥85 mm Hg) or a history of hypertension with antihypertensive medication; fasting plasma glucose of 100 to 125 mg/dL.2 BMI ≥25 was used as a proxy for abdominal obesity because WC data are not available in the CHANCE study. METS (IDF) was used to perform sensitivity analysis.
Efficacy Outcomes
The primary efficacy outcome was a new stroke (ischemic or hemorrhagic) within 90 days.18 Recurrent stroke was defined by the presence of a sudden new symptomatic neurological deficit on a background of stability or improvement after the presenting event.22 Secondary efficacy outcome contained composite events (ischemic stroke, hemorrhagic stroke, myocardial infarction, or vascular death).18 All events were evaluated and confirmed by a central adjudication committee that was blinded to the study group assignments.
Statistical Analysis
Continuous variables of baseline characteristics were presented as medians with interquartile ranges and categorical variables as proportions. Baseline variables between patients included in and excluded from this analysis were compared with the Wilcoxon rank sum test for continuous variables and the χ2 test for categorical variables. Baseline variables among different METS and DM status (both, METS only, DM only, or neither) were compared with the Kruskal–Wallis test for continuous variables and the χ2 test for categorical variables.
The interaction effect of METS and DM status with antiplatelet therapy group assignment was examined using METS/DM status by treatment group assignment in multivariable Cox models. We further assessed the relationship between METS and DM status and outcomes of minor stroke or TIA using multivariable Cox regression models with the neither group as reference. Adjusted hazards ratios (HRs) and their 95% CIs were calculated. All potential confounding variables were adjusted. The proportional hazards assumption for the Cox models was examined by adding a time‐dependent covariate with interaction of METS/DM status and a logarithmic function of survival time in the model.
In sensitivity analyses, the relationship between METS/DM status and patient outcomes was assessed by propensity score methods. The generalized propensity score for each METS/DM category was estimated using a nonparsimonious multivariable multinomial logistic regression model. All baseline variables were included to calculate the generalized propensity score. Then, HRs with their CIs were estimated by Cox regression models with adjustment of propensity score or weighting of inverse probability of METS/DM category.23 We also performed a similar analysis using METS (IDF) criteria in a sensitivity analysis.
A 2‐sided P<0.05 was considered to be statistically significant. All analyses were performed with SAS software version 9.4 (SAS Institute Inc).
Results
Baseline Characteristics
Among 5170 patients, a total of 3044 patients (59%) with minor stroke or TIA were included from these 73 centers, and 2126 patients (41%) were excluded because of missing data of triglyceride and high‐density lipoprotein levels. The patients included in and excluded from the study were well balanced, except for a slightly higher proportion of DM and TIA and less severity in symptom presentation in excluded patients (Table 1). For the included patients, the baseline characteristics in the clopidogrel–aspirin and aspirin‐alone groups were well balanced (Table 2).
Table 1.
Baseline Characteristics of the Patients Included in and Excluded From This Analysis
| Characteristic | Included (n=3044) | Excluded (n=2126) | P Value |
|---|---|---|---|
| Age (y), median (IQR) | 62.2 (54.7–71.2) | 62.3 (54.6–71.3) | 0.79 |
| Female, n (%) | 1017 (33.4) | 733 (34.5) | 0.42 |
| Medical history, n (%) | |||
| Ischemic stroke | 582 (19.1) | 451 (21.2) | 0.06 |
| TIA | 95 (3.1) | 79 (3.7) | 0.24 |
| Myocardial infarction | 55 (1.8) | 41 (1.9) | 0.75 |
| Angina | 95 (3.1) | 89 (4.2) | 0.04 |
| Congestive heart failure | 54 (1.8) | 26 (1.2) | 0.11 |
| Known atrial fibrillation or flutter | 57 (1.9) | 39 (1.8) | 0.92 |
| Valvular heart disease | 10 (0.3) | 4 (0.2) | 0.34 |
| Hypertension | 1984 (65.2) | 1415 (66.6) | 0.30 |
| Diabetes mellitus | 613 (20.1) | 480 (22.6) | 0.03 |
| Hypercholesterolemia | 318 (10.5) | 255 (12.0) | 0.08 |
| Smoking status, n (%) | 0.96 | ||
| Never | 1739 (57.1) | 1210 (56.9) | |
| Current | 301 (9.9) | 215 (10.1) | |
| Previous | 1004 (33.0) | 701 (33.0) | |
| Index event, n (%) | 0.03 | ||
| Minor stroke | 2227 (73.2) | 1498 (70.5) | |
| TIA | 817 (26.8) | 628 (29.5) | |
| NIHSS score on admission, median (IQR) | 2 (0–2) | 1 (0–2) | 0.04 |
| Mean time to randomization, h | 12.0 (6.5–19.4) | 12.0 (6.3–19.6) | 0.80 |
| Time to randomization, n (%) | 0.91 | ||
| <12 h | 1513 (49.7) | 1060 (49.9) | |
| ≥12 h | 1531 (50.3) | 1066 (50.1) | |
| Antiplatelet therapy, n (%) | |||
| Aspirin only | 1526 (50.1) | 1060 (49.9) | 0.85 |
| Clopidogrel plus aspirin | 1518 (49.9) | 1066 (50.1) | |
| Primary end points (stroke) at 90 days | 299 (9.8) | 216 (10.2) | 0.69 |
IQR indicates interquartile range; NIHSS, National Institutes of Health Stroke Scale; TIA, transient ischemic attack.
Table 2.
Baseline Characteristics of the Patients Included in the Analysis by Treatment Group
| Characteristic | Clopidogrel Plus Aspirin (n=1518) | Aspirin Only (n=1526) | P Value |
|---|---|---|---|
| Age (y), median (IQR) | 62.4 (54.8–71.3) | 62.2 (54.6–71.0) | 0.65 |
| Female, n (%) | 493 (32.5) | 524 (34.3) | 0.28 |
| Medical history, n (%) | |||
| Ischemic stroke | 295 (19.4) | 287 (18.8) | 0.66 |
| TIA | 49 (3.2) | 46 (3.0) | 0.73 |
| Myocardial infarction | 23 (1.5) | 32 (2.1) | 0.23 |
| Angina | 54 (3.6) | 41 (2.7) | 0.17 |
| Congestive heart failure | 30 (2.0) | 24 (1.6) | 0.40 |
| Known atrial fibrillation or flutter | 28 (1.8) | 29 (1.9) | 0.91 |
| Valvular heart disease | 4 (0.3) | 6 (0.4) | 0.76 |
| Hypertension | 996 (65.6) | 988 (64.7) | 0.62 |
| Diabetes mellitus | 304 (20.0) | 309 (20.2) | 0.88 |
| Hypercholesterolemia | 165 (10.9) | 153 (10.0) | 0.45 |
| Smoking status, n (%) | 0.16 | ||
| Never | 850 (56.0) | 889 (58.3) | |
| Current | 143 (9.4) | 158 (10.4) | |
| Previous | 525 (34.6) | 479 (31.4) | |
| Index event, n (%) | 0.66 | ||
| Minor stroke | 1116 (73.5) | 1111 (72.8) | |
| TIA | 402 (26.5) | 415 (27.2) | |
| NIHSS score on admission, median (IQR) | 2 (0–2) | 2 (0–2) | 0.04 |
| Mean time to randomization, h | 11.6 (6.2–19.4) | 12.0 (6.5–19.5) | 0.37 |
| Time to randomization, n (%) | 0.40 | ||
| <12 h | 766 (50.5) | 747 (49.0) | |
| ≥12 h | 752 (49.5) | 779 (51.0) | |
IQR indicates interquartile range; NIHSS, National Institutes of Health Stroke Scale; TIA, transient ischemic attack.
The baseline characteristics of 3044 included patients are listed in Table 3. Of the 3044 participants, the average age was 62.2 years, and 1017 (33.4%) were female. Using the CDS definition of METS, 53.2%, 17.2%, 19.8%, and 9.8% of the patients met criteria for neither, METS only, DM only, and both conditions, respectively. Using the IDF criteria for METS, 44.6%, 20.8%, 18.8%, and 10.8% of the patients were diagnosed as neither, METS only, DM only, and both, respectively. Compared with patients with neither condition, patients with METS or DM had more vascular risk factors (such as higher BMI, history of ischemic stroke, TIA, hypertension, hypercholesterolemia) and higher NIHSS. Patients with DM had more history of myocardial infarction and higher ABCD2 score (for TIAs only). Patients with DM only were more likely to be female and less likely to be current smokers, and those with METS only were younger (Tables 3 and 4).
Table 3.
Baseline Characteristics of Patients According to METS (Chinese Diabetes Society) Status
| Characteristic | Total (n=3044) | Neither (n=1618) | METS Only (n=525) | DM Only (n=602) | Both (n=299) | P Value |
|---|---|---|---|---|---|---|
| Age (y), median (IQR) | 62.2 (54.7–71.2) | 63.0 (55.2–71.9) | 58.7 (51.4–66.7) | 64.2 (56.8–72.7) | 60.2 (53.6–69.4) | <0.001 |
| Female, n (%) | 1017 (33.4) | 534 (33.0) | 137 (26.1) | 250 (41.5) | 96 (32.1) | <0.001 |
| BMI, median (IQR) | 24.5 (22.8–26.6) | 23.7 (22.0–25.1) | 26.7 (25.5–28.2) | 24.0 (22.6–25.7) | 26.7 (25.7–28.7) | <0.001 |
| Medical history, n (%) | ||||||
| Ischemic stroke | 582 (19.1) | 277 (17.1) | 111 (21.1) | 134 (22.3) | 60 (20.1) | 0.02 |
| TIA | 95 (3.1) | 37 (2.3) | 21 (4.0) | 25 (4.2) | 12 (4.0) | 0.047 |
| Myocardial infarction | 55 (1.8) | 19 (1.2) | 6 (1.1) | 17 (2.8) | 13 (4.4) | <0.001 |
| Angina | 95 (3.1) | 41 (2.5) | 18 (3.4) | 25 (4.2) | 11 (3.7) | 0.22 |
| Congestive heart failure | 54 (1.8) | 25 (1.6) | 9 (1.7) | 14 (2.3) | 6 (2.0) | 0.65 |
| Known atrial fibrillation or flutter | 57 (1.9) | 37 (2.3) | 4 (0.8) | 12 (2.0) | 4 (1.3) | 0.14 |
| Valvular heart disease | 10 (0.3) | 8 (0.5) | 0 (0.0) | 1 (0.2) | 1 (0.3) | 0.31 |
| Hypertension | 1984 (65.2) | 950 (58.7) | 393 (74.9) | 397 (66.0) | 244 (81.6) | <0.001 |
| DM | 613 (20.1) | 0 (0.00) | 0 (0.00) | 403 (67.0) | 210 (70.2) | <0.001 |
| Hypercholesterolemia | 318 (10.5) | 132 (8.2) | 71 (13.5) | 67 (11.1) | 48 (16.1) | <0.001 |
| Smoking status, n (%) | <0.001 | |||||
| Never | 1739 (57.1) | 910 (56.2) | 264 (50.3) | 396 (65.8) | 169 (56.52) | |
| Current | 301 (9. 9) | 164 (10.1) | 44 (8.4) | 61 (10.1) | 32 (10.70) | |
| Previous | 1004 (33.0) | 544 (33.6) | 217 (41.3) | 145 (24.1) | 98 (32.78) | |
| Index event, n (%) | 0.16 | |||||
| Minor stroke | 2227 (73.2) | 1179 (72.9) | 375 (71.4) | 461 (76.6) | 212 (70.9) | |
| TIA | 817 (26.8) | 439 (27.1) | 150 (28.6) | 141 (23.4) | 87 (29.1) | |
| NIHSS score on admission, median (IQR) | 2 (0–2) | 1 (0–2) | 2 (0–2) | 2 (1–3) | 2 (0–2) | 0.005 |
| ABCD2 score on admission, median (IQR)a | 4 (4–5) | 4 (4–5) | 4 (4–5) | 5 (4–6) | 5 (4–6) | <0.001 |
| Mean time to randomization, h | 12.0 (6.5–19.4) | 11.8 (6.5–19.0) | 11.8 (6.3–19.7) | 12.2 (6.50–19.8) | 12.0 (6.3–18.6) | 0.82 |
| Time to randomization, n (%) | 0.84 | |||||
| <12 h | 1513 (49.7) | 812 (50.2) | 264 (50.3) | 290 (48.2) | 147 (49.2) | |
| ≥12 h | 1531 (50.3) | 806 (49.8) | 261 (49.7) | 312 (51.8) | 152 (50.8) | |
| Antiplatelet therapy, n (%) | 0.46 | |||||
| Aspirin only | 1526 (50.1) | 793 (49.0) | 278 (53.0) | 302 (50.2) | 153 (51.2) | |
| Clopidogrel plus aspirin | 1518 (49.9) | 825 (51.0) | 247 (47.0) | 300 (49.8) | 146 (48.8) | |
BMI indicates body mass index; DM, diabetes mellitus; IQR, interquartile range; METS, metabolic syndrome; NIHSS, National Institutes of Health Stroke Scale; TIA, transient ischemic attack.
ABCD2 stroke risk scores range from 0 to 7, with higher scores indicating higher risk. Data are provided only for the group of 817 patients for whom TIA was the qualifying event for inclusion in the trial.
Table 4.
Baseline Characteristics of Patients According to METS (International Diabetes Foundation) Status
| Characteristic | Neither (n=1511) | METS Only (n=632) | DM Only (n=573) | Both (n=328) | P Value |
|---|---|---|---|---|---|
| Age (y), median (IQR) | 62.9 (55.28–71.95) | 59.7 (51.63–67.94) | 64.2 (56.6–72.7) | 60.4 (54.0–69.8) | <0.001 |
| Female, n (%) | 472 (31.2) | 199 (31.5) | 225 (39.3) | 121 (36.9) | 0.002 |
| BMI, median (IQR) | 23.4 (21.8–24.5) | 27.0 (26.0–28.4) | 23.7 (22.5–24.8) | 27.0 (26.0–28.9) | <0.001 |
| Medical history, n (%) | |||||
| Ischemic stroke | 264 (17.5) | 124 (19.6) | 124 (21.6) | 70 (21.3) | 0.10 |
| TIA | 32 (2.1) | 26 (4.1) | 21 (3.7) | 16 (4.9) | 0.01 |
| Myocardial infarction | 19 (1.3) | 6 (1.0) | 16 (2.8) | 14 (4.3) | <0.001 |
| Angina | 38 (2.5) | 21 (3.3) | 21 (3.7) | 15 (4.6) | 0.19 |
| Congestive heart failure | 21 (1.4) | 13 (2.1) | 11 (1.9) | 9 (2.7) | 0.33 |
| Known atrial fibrillation or flutter | 32 (2.1) | 9 (1.4) | 13 (2.3) | 3 (0.9) | 0.35 |
| Valvular heart disease | 8 (0.5) | 0 (0.0) | 1 (0.2) | 1 (0.3) | 0.22 |
| Hypertension | 899 (59.5) | 444 (70.3) | 375 (65.5) | 266 (81.1) | <0.001 |
| Diabetes mellitus | 0 (0.0) | 0 (0.0) | 384 (67.0) | 229 (69.8) | <0.001 |
| Hypercholesterolemia | 124 (8.2) | 79 (12.5) | 61 (10.7) | 54 (16.5) | <0.001 |
| Smoking status, n (%) | <0.001 | ||||
| Never | 826 (54.7) | 348 (55.1) | 374 (65.3) | 191 (58.2) | |
| Current | 153 (10.1) | 55 (8.7) | 55 (9.6) | 38 (11.6) | |
| Previous | 532 (35.2) | 229 (36.2) | 144 (25.1) | 99 (30.2) | |
| Index event, n (%) | 0.12 | ||||
| Minor stroke | 1099 (72.7) | 455 (72.0) | 441 (77.0) | 232 (70.7) | |
| TIA | 412 (27.3) | 177 (28.0) | 132 (23.0) | 96 (29.3) | |
| NIHSS score on admission, median (IQR) | 1 (0–2) | 2 (0–2) | 2 (1–3) | 2 (0–2) | <0.001 |
| ABCD2 score on admission, median (IQR)a | 4 (4–5) | 4 (4–5) | 5 (4–6) | 5 (4–6) | <0.001 |
| Mean time to randomization, h | 12.0 (6.5–19.4) | 11.0 (6.2–19.5) | 12.0 (6.3–19.5) | 12.0 (6.5–18.7) | 0.64 |
| Time to randomization, n (%) | 0.53 | ||||
| <12 h | 746 (49.4) | 330 (52.2) | 279 (48.7) | 158 (48.2) | |
| ≥12 h | 765 (50.6) | 302 (47.8) | 294 (51.3) | 170 (51.8) | |
| Antiplatelet therapy, n (%) | 0.88 | ||||
| Aspirin only | 748 (49.5) | 323 (51.1) | 292 (51.0) | 163 (49.7) | |
| Clopidogrel plus aspirin | 763 (50.5) | 309 (48.9) | 281 (49.0) | 165 (50.3) | |
BMI indicates body mass index; DM, diabetes mellitus; IQR, interquartile range; METS, metabolic syndrome; NIHSS, National Institutes of Health Stroke Scale; TIA, transient ischemic attack.
ABCD2 stroke risk scores range from 0 to 7, with higher scores indicating higher risk. Data are provided only for the group of 817 patients for whom TIA was the qualifying event for inclusion in the trial.
As shown in Table 5, hypertension was the most prevalent metabolic component of METS (CDS), detected in 99.1% of the nondiabetic patients, followed by dyslipidemia (89.9%) and obesity (88.2%), whereas elevated fasting glucose was relatively uncommon (33.1%).
Table 5.
Distributions of Metabolic Factors in Patients With or Without METS (Chinese Diabetes Society)
| METS Category | Total | Non‐METS | METS | P Value |
|---|---|---|---|---|
| Elevated blood pressure | 1916 (89.4) | 1396 (86.3) | 520 (99.1) | <0.001 |
| Elevated fasting glucose | 241 (11.3) | 67 (4.1) | 174 (33.1) | <0.001 |
| BMI ≥25 | 871 (40.6) | 408 (25.2) | 463 (88.2) | <0.001 |
| Dyslipidemia | 902 (42.1) | 430 (26.6) | 472 (89.9) | <0.001 |
BMI indicates body mass index; METS, metabolic syndrome.
Association of METS Status With Risk of Recurrent Stroke
After 3 months of follow‐up, there were 299 recurrent strokes, of which 293 (98.0%) were ischemic and 6 (2.0%) were hemorrhagic. There was no interaction effect of antiplatelet therapy by different METS (both criteria) and DM status for the risk of recurrent stroke (P=0.82 for interaction in the fully adjusted model of CDS criteria and P=0.97 for IDF criteria) (Tables 6 and 7).
Table 6.
Risk of Stroke at 3 Months for Clopidogrel–Aspirin Combined Therapy Comparing With Aspirin Alone by METS (Chinese Diabetes Society) Status
| METS Status | Aspirin | Clopidogrel–Aspirin | Model 1a | Model 2b | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Events, n (%) | n | Events, n (%) | Adjusted HR (95% CI) | P Value | P Value for Interaction | Adjusted HR (95% CI) | P Value | P Value for Interaction | |
| Neither | 793 | 63 (7.9) | 825 | 47 (5.7) | 0.71 (0.49–1.04) | 0.08 | 0.87 | 0.70 (0.48–1.02) | 0.07 | 0.82 |
| METS only | 278 | 28 (10.1) | 247 | 13 (5.3) | 0.52 (0.27–1.00) | 0.050 | 0.48 (0.24–0.93) | 0.03 | ||
| DM only | 302 | 59 (19.5) | 300 | 38 (12.7) | 0.63 (0.42–0.94) | 0.03 | 0.60 (0.40–0.90) | 0.01 | ||
| Both | 153 | 31 (20.3) | 146 | 20 (13.7) | 0.67 (0.38–1.18) | 0.16 | 0.71 (0.40–1.27) | 0.25 | ||
DM indicates diabetes mellitus; HR, hazard ratio; METS, metabolic syndrome.
Model 1: adjusted for age and sex.
Model 2: adjusted for age, sex, history of ischemic stroke, transient ischemic attack, myocardial infarction, angina, congestive heart failure, known atrial fibrillation or flutter, valvular heart disease, smoking status, index event and National Institutes of Health Stroke Scale on admission, and time to randomization.
Table 7.
Risk of Stroke at 3 Months for Clopidogrel–Aspirin Combined Therapy Comparing With Aspirin Alone by METS (International Diabetes Foundation) Status
| METS Status | Aspirin | Clopidogrel–Aspirin | Model 1a | Model 2b | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| n | Events, n (%) | N | Events, n (%) | Adjusted HR (95% CI) | P Value | P Value for Interaction | Adjusted HR (95% CI) | P Value | P Value for Interaction | |
| Neither | 748 | 59 (7.9) | 763 | 39 (5.1) | 0.65 (0.43–0.97) | 0.04 | 0.98 | 0.66 (0.44–0.98) | 0.04 | 0.97 |
| METS only | 323 | 32 (9.9) | 309 | 21 (6.8) | 0.67 (0.39–1.17) | 0.16 | 0.63 (0.36–1.10) | 0.11 | ||
| DM only | 292 | 56 (19.2) | 281 | 34 (12.1) | 0.60 (0.39–0.92) | 0.02 | 0.58 (0.38–0.90) | 0.01 | ||
| Both | 163 | 34 (20.9) | 165 | 24 (14.5) | 0.66 (0.39–1.12) | 0.13 | 0.67 (0.39–1.15) | 0.14 | ||
DM indicates diabetes mellitus; HR, hazard ratio; METS, metabolic syndrome.
Model 1: adjusted for age and sex.
Model 2: adjusted for age, sex, history of ischemic stroke, transient ischemic attack, myocardial infarction, angina, congestive heart failure, known atrial fibrillation or flutter, valvular heart disease, smoking status, index event and National Institutes of Health Stroke Scale on admission, and time to randomization.
Using the CDS criteria of METS, patients with DM only (16.1% versus 6.8%; adjusted HR 2.50, 95% CI 1.89–3.39) and both (17.1% versus 6.8%; adjusted HR 2.76, 95% CI 1.98–3.86) were associated with increased risk of recurrent stroke (FigureA). Using the IDF criteria of METS, patients with DM only (15.7% versus 6.5%; adjusted HR 2.53, 95% CI 1.89–3.37) and both (17.7% versus 6.5%; adjusted HR 3.08, 95% CI 2.22–4.28) were also associated with increased risk of recurrent stroke (FigureB). METS only did not show an increased risk of recurrent stroke in both definitions (adjusted HR 1.18, 95% CI 0.82–1.69 for CDS criteria; adjusted HR 1.34, 95% CI 0.96–1.87 for IDF criteria). All proportional hazards assumptions for the Cox models were met (P=0.11 for CDS criteria and P=0.23 for IDF criteria). In sensitivity analyses, we observed similar results using the propensity score method (Table 8). Similar results were observed in the secondary outcomes of composite events and ischemic stroke in both criteria.
Figure 1.
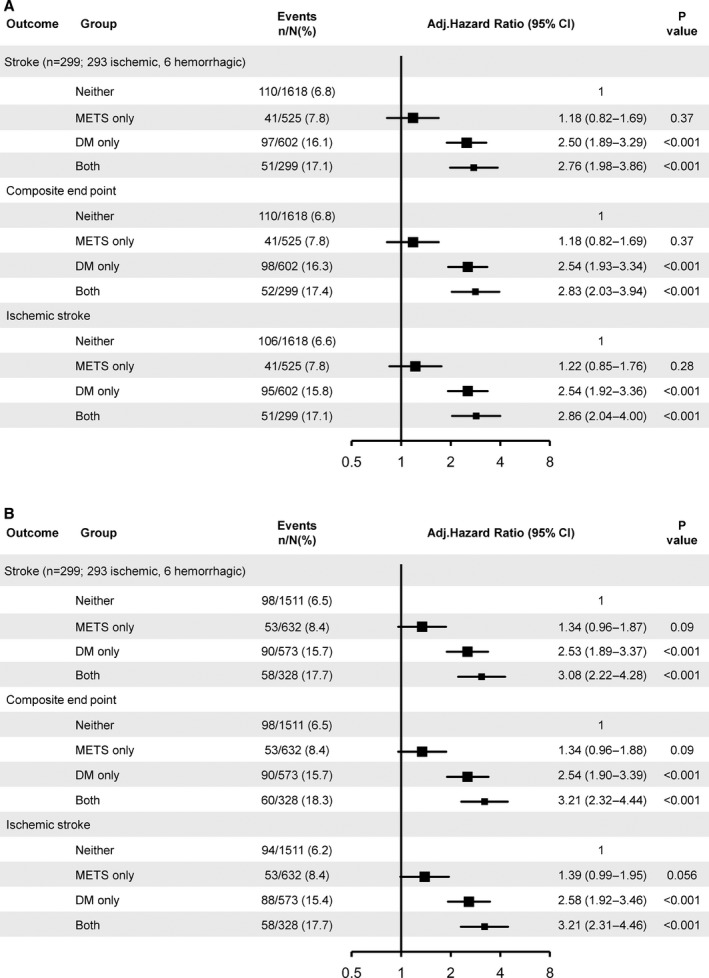
Adjusted hazard ratios of stroke recurrence according to METS (CDS and IDF) and DM status. A, METS defined by CDS. B, METS defined by IDF. Adjusted for age, sex, history of ischemic stroke, transient ischemic attack, myocardial infarction, angina, congestive heart failure, known atrial fibrillation or flutter, valvular heart disease, smoking status, index event and National Institutes of Health Stroke Scale on admission, time to randomization, and antiplatelet therapy. CDS indicates Chinese Diabetes Society; DM, diabetes mellitus; IDF, International Diabetes Foundation; METS, metabolic syndrome.
Table 8.
Sensitivity Analysis of Hazard Ratios Estimated by Propensity Score Method
| METS Definition | Outcome | METS Status | Propensity Score Regression Adjustment | Propensity Score Weighting | ||
|---|---|---|---|---|---|---|
| HR (95% CI) | P Value | HR (95% CI) | P Value | |||
| CDS | Stroke | Neither | 1 | 1 | ||
| METS only | 0.99 (0.65–1.52) | 0.97 | 1.02 (0.70–1.49) | 0.92 | ||
| DM only | 3.66 (2.61–5.14) | <0.001 | 3.09 (2.40–3.99) | <0.001 | ||
| Both | 3.69 (2.32–5.86) | <0.001 | 2.88 (2.09–3.97) | <0.001 | ||
| Composite end point | Neither | 1 | 1 | |||
| METS only | 0.99 (0.65–1.52) | 0.99 | 1.02 (0.70–1.49) | 0.92 | ||
| DM only | 3.70 (2.64–5.18) | <0.001 | 3.10 (2.41–4.00) | <0.001 | ||
| Both | 3.87 (2.45–6.12) | <0.001 | 2.96 (2.15–4.07) | <0.001 | ||
| Ischemic stroke | Neither | 1 | 1 | |||
| METS only | 1.01 (0.66–1.55) | 0.95 | 1.05 (0.72–1.53) | 0.80 | ||
| DM only | 3.74 (2.66–5.27) | <0.001 | 3.14 (2.43–4.06) | <0.001 | ||
| Both | 3.82 (2.40–6.08) | <0.001 | 2.96 (2.14–4.08) | <0.001 | ||
| IDF | Stroke | Neither | 1 | 1 | ||
| METS only | 1.21 (0.76–1.94) | 0.42 | 0.91 (0.61–1.36) | 0.64 | ||
| DM only | 3.80 (2.68–5.38) | <0.001 | 2.54 (2.09–3.10) | <0.001 | ||
| Both | 4.76 (3.04–7.46) | <0.001 | 2.53 (1.87–3.44) | <0.001 | ||
| Composite end point | Neither | 1 | 1 | |||
| METS only | 1.21 (0.76–1.94) | 0.42 | 0.91 (0.61–1.36) | 0.64 | ||
| DM only | 3.80 (2.68–5.38) | <0.001 | 2.54 (2.09–3.10) | <0.001 | ||
| Both | 5.02 (3.23–7.82) | <0.001 | 2.61 (1.93–3.54) | <0.001 | ||
| Ischemic stroke | Neither | 1 | 1 | |||
| METS only | 1.25 (0.78–2.00) | 0.36 | 0.92 (0.62–1.38) | 0.69 | ||
| DM only | 3.88 (2.73–5.52) | <0.001 | 2.35 (1.91–2.88) | <0.001 | ||
| Both | 4.96 (3.16–7.79) | <0.001 | 2.57 (1.89–3.49) | <0.001 | ||
CDS indicates Chinese Diabetes Society; DM, diabetes mellitus; HR, hazard ratio; IDF, International Diabetes Foundation; METS, metabolic syndrome.
Discussion
In this post hoc analysis of the CHANCE study, patients with DM only or concurrent METS and DM had higher recurrent stroke risk than those with neither condition; however, METS only was not associated with stroke recurrence in patients with minor stroke or TIA. There was no difference in the effect of antiplatelet treatment in reducing these events in patients with or without METS or DM.
As we hypothesized, DM only and concurrent METS and DM were significant risk factors of recurrent stroke in patients with minor stroke or TIA in present study. Different from what we expected, METS showed only a trend of increased risk of recurrent stroke and did not reach statistical significance. Even with medical intervention, minor stroke and TIA still led to a high risk of recurrence that could raise the disability rate.14, 15 METS was frequently found in patients with minor stroke and TIA.5 It was reported that the prevalence of METS in different racial populations may be between 10% and 84%.4, 5 Our study added to the evidence that concurrent METS and DM was associated with an increased risk of new stroke in patients with minor stroke and TIA. It may be important to highlight early identification of patients at high risk of developing DM (eg, patients with METS) to predict the prognosis of patients with minor stroke or TIAs.
Patients with METS only did not have significantly higher risk of any recurrent stroke than those with neither condition. This finding might be associated with the fact that the definition of METS in our study did not include patients with DM. A previous study showed that METS likely played a crucial role in the development of recurrent ischemic stroke in patients with ischemic stroke or TIA.10 The definition of METS in this study included DM; however, results of substudies of the SPARCL (Secondary Analysis of the Stroke Prevention by Aggressive Reduction in Cholesterol Levels) trial and the SPS3 (Secondary Prevention of Small Subcortical Strokes) study demonstrated that patients with METS only were not at an increased risk of recurrent stroke.9, 17 Unlike previous studies, the definition of METS in these 2 studies did not include DM, similar to our present study. Consequently, the definition of METS including DM or not might be a factor that influenced the relationship between METS and risk of stroke recurrence.
Several organizations formulated different criteria for METS diagnosis, but all showed 4 main categories of metabolic abnormalities: atherogenic dyslipidemia, increased blood pressure, abnormal glucose regulation, and obesity.2, 3 Among these factors, abnormal glucose regulation was an established risk factors of recurrent stroke in patients with stroke or TIA.24 The physiology underlying the elevated risk of recurrent ischemic stroke in diabetic METS may be that it was a recognized risk factor of intracranial atherosclerosis.25, 26 Previous studies also reported that diabetic METS was associated with recurrent ischemic stroke in patients with large‐vessel infarction or lacunar stroke,10, 17 which we were not able to examine in our study. Patients enrolled in the CHANCE trial primarily were minor stroke patients with stroke subtypes of large‐artery atherosclerosis and small‐vessel occlusion.18, 20 Therefore, patients with concurrent METS and DM had higher risk of recurrent stroke than those with nondiabetic METS (METS only) and neither in our study.
In this post hoc analysis, we applied BMI rather than WC for definition of abdominal fat based on a previous study that showed WC and BMI can both be used in the prediction of abdominal visceral obesity for Chinese adults,1 and the BMI cutoff for abdominal obesity was ≥25.27, 28, 29 Even so, this still might be a bit inaccurate for the diagnosis of METS (IDF). Nevertheless, it is unlikely that using BMI as a proxy would alter the results because similar patients were diagnosed with METS regardless of whether BMI or WC was used.30 Large epidemiologic studies have shown a high correlation between BMI and WC.31 Furthermore, the association between obesity and insulin resistance was similar regardless of whether WC or BMI was used for obesity diagnosis.32 Using METS with the IDF definition in a sensitivity analysis in our study, the results also demonstrated similar results.
Our study has some limitations. First, data on 2‐hour plasma glucose, which is an item for assessment of METS (CDS), were not available in the CHANCE trial; therefore, we may have missed some patients who could be diagnosed as having METS. Second, only Chinese patients were enrolled in our study; further evaluation of METS in other races might be required. Third, the characteristics of minor stroke and TIA patients enrolled in this study were different from those of a typical minor stroke or TIA sample from population‐based cohorts. This study enrolled only minor stroke patients with noncardiogenic embolism and high‐risk TIA patients (ABCD2 scores ≥4), which may have resulted in high events rates. Furthermore, large‐scale population‐based cohorts assessing the association of METS and recurrent stroke are needed to confirm this finding. Finally, duration of DM was not recorded in the CHANCE trial. The duration of DM might be associated with the prognosis of patients with stroke.33
Conclusions
The results from our study showed that patients with DM only or concurrent METS and DM were associated with an elevated risk of stroke among patients with minor ischemic stroke and TIA. Nondiabetic METS (METS only) was not observed to be associated with stroke recurrence in patients with minor stroke or TIA.
Appendix
The CHANCE Investigators
Yongjun Wang (Beijing Tiantan Hospital, Principal Investigator); S. Claiborne Johnston (Departments of Neurology and Epidemiology, University of California, San Francisco, USA, Co‐Principal Investigator); Yilong Wang (Beijing Tiantan Hospital, Executive Committee); Xingquan Zhao (Beijing Tiantan Hospital, Site Investigator); Zhimin Wang (Taizhou First People's Hospital, Site Investigator); Haiqin Xia (Taiyuan Iron And Steel [Group] Co, Ltd, General Hospital, Site Investigator); Bin Li (Dagang Oilfield Gengeal Hospital, Site Investigator); Guiru Zhang (Penglai People's Hospital, Site Investigator); Xudong Ren (The Third People's Hospital of Datong, Site Investigator); Chunling Ji (The Fourth Central Hospital of Tianjin, Site Investigator); Guohua Zhang (The Second Hospital of Hebei Medical University, Site Investigator); Jianhua Li (The First Hospital of Fangshan District, Beijing, Site Investigator); Bohua Lu (Beijing Puren Hospital, Site Investigator); Liping Wang (Tianjin Ninghe District Hospital, Site Investigator); Shutao Feng (The People's Hospital of Zhengzhou, Site Investigator); Dali Wang (Affiliated Hospital of North China Coal Medical College, Site Investigator); Weiguo Tang (Zhejiang Zhoushan Hospital, Site Investigator); Juntao Li (Han Dan Central Hospital, Site Investigator); Hongtian Zhang (Zhecheng People's Hospital, Site Investigator); Guanglai Li (Shanxi Medical University Second Hospital, Site Investigator); Baojun Wang (Baotou Central Hospital, Site Investigator); Yuhua Chen (The General Hospital of Changjiang River Shipping, Site Investigator); Ying Lian (Dalian Economic And Technological Development Zone Hospital, Site Investigator); Bin Liu (First Neurology Department, Affiliated Hospital of North China Coal Medical College, Site Investigator); Junfang Teng (The First Affiliated Hospital of Zhengzhou University, Site Investigator); Rubo Sui (First Affiliated Hospital of Liaoning Medical, Site Investigator); Lejun Li (Lianyungang Municipal Hospital of TCM, Site Investigator); Zhiling Yuan (Central Hospital In Qiu County, Site Investigator); Dawei Zang (Tianjin First Center Hospital, Site Investigator); Zuneng Lu (Renmin Hospital of Wuhan University, Site Investigator); Li Sun (Qingdao Central Hospital, Site Investigator); Dong Wang (Baogang Hospital, Site Investigator); Liying Hou (Changzhi City People's Hospital of Shanxi Province, Site Investigator); Dongcai Yuan (HaLixun International Peace Hospital, Site Investigator); Yongliang Cao (People's Hospital of Linzi District, Zibo, Site Investigator); Hui Li (Yantai City Yantai Mountain Hospital, Site Investigator); Xiuge Tan (Beijing Pinggu District Hospital, Site Investigator); Huicong Wang (Taiyuan Central Hospital, Site Investigator); Haisong Du (Chengde Central Hospital, Site Investigator); Mingyi Liu (Shijiazhuang Central Hospital, Site Investigator); Suping Wang (First Neurology Department, Dalian Municipal Central Hospital, Site Investigator); Qiuwu Liu (Xian 141 Hospital, Site Investigator); Zhong Zhang (Chengdu Third Municipal People's Hospital, Site Investigator); Qifu Cui (Affiliated Hospital of Chifeng University, Site Investigator); Runqing Wang (Zhengzhou Central Hospital, Site Investigator); Jialin Zhao (Ningbo City, Zhejiang Province Lihuili Hospital Medical Center, Site Investigator); Jiewen Zhang (Henan Provincial People's Hospital, Site Investigator); Jianping Zhao (Jinzhong City Second Hospital, Site Investigator); Qi Bi (Beijing Anzhen Hospital, Capital Medical University, Site Investigator); Xiyou Qi (Beijing Huairou District Chinese Medicine Hospital, Site Investigator); Junyan Liu (Hebei Medical University Third Hospital, Site Investigator); Changxin Li (First Affiliated Hospital Shanxi Medical Unversity, Site Investigator); Ling Li (Hebei Provincial People's Hospital, Site Investigator); Xiaoping Pan (Guangzhou First Municipal Peoples Hospital, Site Investigator); Junling Zhang (Central Hospital In Cangzhou, Site Investigator); Derang Jiao (The Chinese People's Armed Police Force Medical School Affiliated Hospital, Site Investigator); Zhao Han (Zhejiang Wenzhou Medical College First Affiliated Hospital, Site Investigator); Dawei Qian (Jilin Central Hospital, Site Investigator); Jin Xiao (Anhui Maanshan Central Hospital, Site Investigator); Yan Xing (Beijing Aviation Industry Central Hospital, Site Investigator); Huishan Du (Luhe Hospital, Tongzhou District, Beijing, Site Investigator); Guang Huang (Beijing Fuxing Hospital, Capital Medical University, Site Investigator); Yongqiang Cui (The 306th Hospital of P.L.A, Site Investigator); Yan Li (The First Affiliated Hospital of Tianjin University of Chinese Medicine, Site Investigator); Lianyuan Feng (Baiqiuen International Peace Hospital of People's Liberation Army, Site Investigator); Lianbo Gao (Fourth Affiliated Hospital of China Medical University, Site Investigator); Bo Xiao (Xiangya Hospital Central‐South University, Site Investigator); Yibin Cao (Tangshan Worker's Hospital, Site Investigator); Yiping Wu (The 1st Hospital In Handan, Site Investigator); Jinfeng Liu (Yangquan Coal (Group) Co, Ltd General Hospital, Site Investigator); Zhiming Zhang (Tianjin Tianhe Hospital, Site Investigator); Zhengxie Dong (Nantong First People's Hospital, Site Investigator); Limin Wang (The 1st Hospital of Zhangjiakou City, Site Investigator); Li He (West China Hospital, Sichuan University, Site Investigator); Xinchen Wang (The Second Affiliated Hospital of Shandong University of TCM, Site Investigator); Xueying Guo (Fenyang Hospital of Shanxi Province, Site Investigator); Ming Wang (Zhejiang Zhoushan Putuo District People's Hospital, Site Investigator); Xiaosha Wang (Xiyuan Hospital of China Academy of Chinese Traditional Medicine, Site Investigator); Jiandong Jiang (No. 2 People's Hospital East In Lianyungang City, Site Investigator); Renliang Zhao (Affiliated Hospital of Qingdao University Medical College, Site Investigator); Shengnian Zhou (Qilu Hospital of Shandong University, Site Investigator); Hao Hu (Zibo Hospital of Traditional Chinese Medicine, Site Investigator); Maolin He (Beijing Shijitan Hospital, Site Investigator); Fengchun Yu (Beijing Haidian Hospital, Site Investigator); Quping Ouyang (Beijing Shunyi District Hospital, Site Investigator); Jingbo Zhang (Dalian Third Municipal Hospital, Site Investigator); Anding Xu (The First Affliated Hospital of Jinan University, Site Investigator); Xiaokun Qi (Navy Genaral Hospital of P.L.A, Site Investigator); Lei Wang (Beijing Second Artillery General Hospital, Site Investigator); Fuming Shi (Beijing Daxing District Hospital, Site Investigator); Fuqiang Guo (Sichuan Province People's Hospital, Site Investigator); Jianfeng Wang (Dalian Municipal Central Hospital, Site Investigator); Fengli Zhao (The Second Hospital In Baoding, Site Investigator); Ronghua Dou (The Hospital Combine Traditional Chinese And Western Medicine In Cang zhou, Site Investigator); Dongning Wei (The 309th Hospital of P.L.A, Site Investigator); Qingwei Meng (Liangxiang Hospital of Fangshan District, Beijing, Site Investigator); Yilu Xia (HuaXin Hospital First Hospital of Tsinghua University, Site Investigator); Shimin Wang (TianjinHuanhu Hospital, Site Investigator); Zhangcang Xue (Shijiazhuang First Hospital, Site Investigator); Yuming Xu (The First Affiliated Hospital of Zhengzhou University, Site Investigator); Liping Ma (Xinzhou City People's Hospital, Site Investigator); Chun Wang (Sichuan Province People's Hospital of Deyang City, Site Investigator); Jiang Wu (First Hospital, Jilin University, Site Investigator); Yifeng Du (Shandong Provincial Hospital, Site Investigator); Yinzhou Wang (Fujian Province Hospital, Site Investigator); Lijun Xiao (Liaoyang City Third People's Hospital, Site Investigator); Fucong Song (Handan City Center Hospital, Site Investigator); Wenli Hu (Beijing Chaoyang Hospital, Capital Medical University, Site Investigator); Zhigang Chen (Beijing University of Chinese Medicine East Hospital, Site Investigator); Qingrui Liu (Hebei Medical University Fourth Hospital, Site Investigator); Jiemin Zhang (The Fourth Affiliated Hospital of Soochow University, Site Investigator); Mei Chen (Zhejiang University of Chinese Medicine Affiliated First Hospital, Site Investigator); Xiaodong Yuan (Affiliated Hospital of Kailuan Company Ltd, Site Investigator); Zhihui Liu (Affiliated Hospital of Weifang Medical University, Site Investigator); Guozhong Li (The First Hospital of Harbin Medical University, Site Investigator); Xiaohong Li (Dalian Friendship Hospital, Site Investigator); Tingchen Tian (Tianjin Dagang Hospital, Site Investigator).
Sources of Funding
This study was supported by grants from National Key Technology Research and Development Program of the Ministry of Science and Technology of the People's Republic of China (2013BAI09B03, 2015BAI12B04 and 2015BAI12B02), a grant from Beijing Municipal Science & Technology Commission (D151100002015001), a grant from Beijing Institute for Brain Disorders (1152130306) and a grant from the National Natural Science Foundation of China (No. 81322019).
Disclosures
None.
Acknowledgments
We appreciated Haipeng Shen, PhD (University of Hong Kong, China) and Jiming Fang, PhD (Institute for Clinical Evaluative Sciences, Canada) for offering us valuable comments on statistical analysis. We also sincerely thank all the patients who participated in the CHANCE trial.
(J Am Heart Assoc. 2017;6:e005446 DOI: 10.1161/JAHA.116.005446.)28572281
Contributor Information
Yilong Wang, Email: yilong528@gmail.com.
Yongjun Wang, Email: yongjunwang1962@gmail.com.
References
- 1. Ovbiagele B, Saver JL, Lynn MJ, Chimowitz M; WASID Study Group . Impact of metabolic syndrome on prognosis of symptomatic intracranial atherostenosis. Neurology. 2006;66:1344–1349. [DOI] [PubMed] [Google Scholar]
- 2. Grundy SM, Cleeman JI, Daniels SR, Donato KA, Eckel RH, Franklin BA, Gordon DJ, Krauss RM, Savage PJ, Smith SC Jr, Spertus JA, Costa F; American Heart Association; National Heart, Lung, and Blood Institute . Diagnosis and management of the metabolic syndrome: an American Heart Association/National Heart, Lung, and Blood Institute Scientific Statement. Circulation. 2005;112:2735–2752. [DOI] [PubMed] [Google Scholar]
- 3. Xiang K, Ji L, Xiang H, Yang W, Jia W, Qian R, Weng J. Diagnosis and management of the metabolic syndrome: a Chinese Diabetes Society Scientific Statement. Chin J Diabetes. 2004;12:156–161 (in Chinese). [Google Scholar]
- 4. Ford ES, Giles WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. JAMA. 2002;287:356–359. [DOI] [PubMed] [Google Scholar]
- 5. O'Neill S, O'Driscoll L. Metabolic syndrome: a closer look at the growing epidemic and its associated pathologies. Obes Rev. 2015;16:1–12. [DOI] [PubMed] [Google Scholar]
- 6. Rodriguez‐Colon SM, Mo J, Duan Y, Liu J, Caulfield JE, Jin X, Liao D. Metabolic syndrome clusters and the risk of incident stroke: the Atherosclerosis Risk in Communities (ARIC) study. Stroke. 2009;40:200–205. [DOI] [PubMed] [Google Scholar]
- 7. Chen HJ, Bai CH, Yeh WT, Chiu HC, Pan WH. Influence of metabolic syndrome and general obesity on the risk of ischemic stroke. Stroke. 2006;37:1060–1064. [DOI] [PubMed] [Google Scholar]
- 8. Mi D, Jia Q, Zheng H, Hoff K, Zhao X, Wang C, Liu G, Wang Y, Liu L, Wang X , Wang Y; On behalf of the investigators for the survey on Abnormal Glucose Regulation in Patients with Acute Stroke Across China ACROSS‐China . Metabolic syndrome and stroke recurrence in Chinese ischemic stroke patients—the ACROSS‐China study. PLoS One. 2012;7:e51406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Callahan A, Amarenco P, Goldstein LB, Sillesen H, Messig M, Samsa GP, Altafullah I, Ledbetter LY, MacLeod MJ, Scott R, Hennerici M, Zivin JA, Welch KM; SPARCL Investigators . Risk of stroke and cardiovascular events after ischemic stroke or transient ischemic attack in patients with type 2 diabetes or metabolic syndrome: secondary analysis of the Stroke Prevention by Aggressive Reduction in Cholesterol Levels (SPARCL) trial. Arch Neurol. 2011;68:1245–1251. [DOI] [PubMed] [Google Scholar]
- 10. Liou CW, Tan TY, Lin TK, Wang PW, Yip HK. Metabolic syndrome and three of its components as risk factors for recurrent ischaemic stroke presenting as large‐vessel infarction. Eur J Neurol. 2008;15:802–809. [DOI] [PubMed] [Google Scholar]
- 11. Najarian RM, Sullivan LM, Kannel WB, Wilson PW, D'Agostino RB, Wolf PA. Metabolic syndrome compared with type 2 diabetes mellitus as a risk factor for stroke: the Framingham Offspring Study. Arch Intern Med. 2006;166:106–111. [DOI] [PubMed] [Google Scholar]
- 12. Cull CA, Jensen CC, Retnakaran R, Holman RR. Impact of the metabolic syndrome on macrovascular and microvascular outcomes in type 2 diabetes mellitus: United Kingdom Prospective Diabetes Study 78. Circulation. 2007;116:2119–2126. [DOI] [PubMed] [Google Scholar]
- 13. von Weitzel‐Mudersbach P, Andersen G, Hundborg HH, Johnsen SP. Transient ischemic attack and minor stroke are the most common manifestations of acute cerebrovascular disease: a prospective, population‐based study—the Aarhus TIA study. Neuroepidemiology. 2013;40:50–55. [DOI] [PubMed] [Google Scholar]
- 14. Coull AJ, Lovett JK, Rothwell PM; Oxford Vascular Study . Population based study of early risk of stroke after transient ischaemic attack or minor stroke: implications for public education and organisation of services. BMJ. 2004;328:326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Giles MF, Rothwell PM. Risk of stroke early after transient ischaemic attack: a systematic review and meta‐analysis. Lancet Neurol. 2007;6:1063–1072. [DOI] [PubMed] [Google Scholar]
- 16. Ois A, Gomis M, Rodriguez‐Campello A, Cuadrado‐Godia E, Jimenez‐Conde J, Pont‐Sunyer C, Cuccurella G, Roquer J. Factors associated with a high risk of recurrence in patients with transient ischemic attack or minor stroke. Stroke. 2008;39:1717–1721. [DOI] [PubMed] [Google Scholar]
- 17. Zhu S, McClure LA, Lau H, Romero JR, White CL, Babikian V, Nguyen T, Benavente OR, Kase CS, Pikula A. Recurrent vascular events in lacunar stroke patients with metabolic syndrome and/or diabetes. Neurology. 2015;85:935–941. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Wang Y, Wang Y, Zhao X, Liu L, Wang D, Wang C, Wang C, Li H, Meng X, Cui L, Jia J, Dong Q, Xu A, Zeng J, Li Y, Wang Z, Xia H, Johnston SC; CHANCE Investigators . Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. 2013;369:11–19. [DOI] [PubMed] [Google Scholar]
- 19. Wang Y, Pan Y, Zhao X, Li H, Wang D, Johnston SC, Liu L, Meng X, Wang A, Wang C, Wang Y; CHANCE Investigators . Clopidogrel with aspirin in acute minor stroke or transient ischemic attack (CHANCE) trial: one‐year outcomes. Circulation. 2015;132:40–46. [DOI] [PubMed] [Google Scholar]
- 20. Wang Y, Johnston SC; CHANCE Investigators . Rationale and design of a randomized, double‐blind trial comparing the effects of a 3‐month clopidogrel‐aspirin regimen versus aspirin alone for the treatment of high‐risk patients with acute nondisabling cerebrovascular event. Am Heart J. 2010;160:380–386.e381. [DOI] [PubMed] [Google Scholar]
- 21. Li J, Wang Y, Wang D, Lin J, Wang A, Zhao X, Liu L, Wang C, Wang Y; CHANCE Investigators . Glycated albumin predicts the effect of dual and single antiplatelet therapy on recurrent stroke. Neurology. 2015;84:1330–1336. [DOI] [PubMed] [Google Scholar]
- 22. Rothwell PM, Giles MF, Chandratheva A, Marquardt L, Geraghty O, Redgrave JN, Lovelock CE, Binney LE, Bull LM, Cuthbertson FC, Welch SJ, Bosch S, Alexander FC, Silver LE, Gutnikov SA, Mehta Z; Early use of Existing Preventive Strategies for Stroke (EXPRESS) study . Effect of urgent treatment of transient ischaemic attack and minor stroke on early recurrent stroke (EXPRESS study): a prospective population‐based sequential comparison. Lancet. 2007;370:1432–1442. [DOI] [PubMed] [Google Scholar]
- 23. Feng P, Zhou XH, Zou QM, Fan MY, Li XS. Generalized propensity score for estimating the average treatment effect of multiple treatments. Stat Med. 2012;31:681–697. [DOI] [PubMed] [Google Scholar]
- 24. Fonville S, Zandbergen AA, Koudstaal PJ, den Hertog HM. Prediabetes in patients with stroke or transient ischemic attack: prevalence, risk and clinical management. Cerebrovasc Dis. 2014;37:393–400. [DOI] [PubMed] [Google Scholar]
- 25. Park JH, Kwon HM, Roh JK. Metabolic syndrome is more associated with intracranial atherosclerosis than extracranial atherosclerosis. Eur J Neurol. 2007;14:379–386. [DOI] [PubMed] [Google Scholar]
- 26. Bang OY, Kim JW, Lee JH, Lee MA, Lee PH, Joo IS, Huh K. Association of the metabolic syndrome with intracranial atherosclerotic stroke. Neurology. 2005;65:296–298. [DOI] [PubMed] [Google Scholar]
- 27. Jia WP, Lu JX, Xiang KS, Bao YQ, Lu HJ, Chen L. Prediction of abdominal visceral obesity from body mass index, waist circumference and waist‐hip ratio in Chinese adults: receiver operating characteristic curves analysis. Biomed Environ Sci. 2003;16:206–211. [PubMed] [Google Scholar]
- 28. Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus provisional report of a WHO consultation. Diabet Med. 1998;15:539–553. [DOI] [PubMed] [Google Scholar]
- 29. Jia WP, Xiang KS, Chen L, Lu JX, Wu YM. Epidemiological study on obesity and its comorbidities in urban Chinese older than 20 years of age in Shanghai, China. Obes Rev. 2002;3:157–165. [DOI] [PubMed] [Google Scholar]
- 30. Einhorn D, Reaven GM, Cobin RH, Ford E, Ganda OP, Handelsman Y, Hellman R, Jellinger PS, Kendall D, Krauss RM, Neufeld ND, Petak SM, Rodbard HW, Seibel JA, Smith DA, Wilson PW. American College of Endocrinology position statement on the insulin resistance syndrome. Endocr Pract. 2003;9:237–252. [PubMed] [Google Scholar]
- 31. Ford ES, Mokdad AH, Giles WH. Trends in waist circumference among U.S. adults. Obes Res. 2003;11:1223–1231. [DOI] [PubMed] [Google Scholar]
- 32. Ferrannini E, Natali A, Bell P, Cavallo‐Perin P, Lalic N, Mingrone G. Insulin resistance and hypersecretion in obesity. European Group for the Study of Insulin Resistance (EGIR). J Clin Invest. 1997;100:1166–1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Banerjee C, Moon YP, Paik MC, Rundek T, Mora‐McLaughlin C, Vieira JR, Sacco RL, Elkind MS. Duration of diabetes and risk of ischemic stroke: the Northern Manhattan Study. Stroke. 2012;43:1212–1217. [DOI] [PMC free article] [PubMed] [Google Scholar]


