Abstract
Background
Gut microbiota is emerging as a novel risk factor for atherothrombosis, but the predictive role of gut‐derived lipopolysaccharide (LPS) is unknown. We analyzed (1) the association between LPS and major adverse cardiovascular events (MACE) in atrial fibrillation (AF) and (2) its relationship with adherence to a Mediterranean diet (Med‐diet).
Methods and Results
This was a prospective single‐center study including 912 AF patients treated with vitamin K antagonists (3716 patient‐years). The primary end point was a composite of MACE. Baseline serum LPS, adherence to Med‐diet (n=704), and urinary excretion of 11‐dehydro‐thromboxane B2 (TxB2, n=852) were investigated. Mean age was 73.5 years; 42.9% were women. A total of 187 MACE (5.0% per year) occurred: 54, 59, and 74 in the first, second, and third tertile of LPS, respectively (log‐rank test P=0.004). Log‐LPS (hazard ratio 1.194, P=0.009), age (hazard ratio 1.083, P<0.001), and previous cerebrovascular (hazard ratio 1.634, P=0.004) and cardiac events (hazard ratio 1.822, P<0.001) were predictors of MACE. In the whole cohort, AF (versus sinus rhythm) (β 0.087, P=0.014) and low‐density lipoprotein cholesterol (β 0.069, P=0.049) were associated with circulating LPS. Furthermore, Med‐diet score (β −0.137, P<0.001) was predictive of log‐LPS, with fruits (β −0.083, P=0.030) and legumes (β −0.120, P=0.002) negatively associated with log‐LPS levels. Log‐LPS and log‐TxB2 were highly correlated (r=0.598, P<0.001). Log‐LPS (β 0.574, P<0.001) and Med‐diet score (β −0.218, P<0.001) were significantly associated with baseline urinary excretion of TxB2.
Conclusions
In this cohort of AF patients, LPS levels were predictive of MACE and negatively affected by high adherence to Med‐diet. LPS may contribute to MACE incidence in AF by increasing platelet activation.
Keywords: atrial fibrillation, cardiovascular events, lipopolysaccharide, Mediterranean diet, thromboxane
Subject Categories: Arrhythmias, Cardiovascular Disease, Risk Factors, Cerebrovascular Disease/Stroke, Coronary Artery Disease
Clinical Perspective
What Is New?
In this observational prospective study we found a significant association between circulating LPS levels and risk of MACE in a large cohort of atrial fibrillation patients treated with vitamin K antagonists; in particular, patients in the highest tertile of LPS (>100 pg/mL) had the highest risk of MACE.
Circulating LPS levels were inversely associated with adherence to a Mediterranean diet (Med‐diet), in particular with fruit and legume intake.
What Are the Clinical Implications?
The inverse association between adherence to Med‐diet and circulating LPS suggests the need to explore the effect of an ad hoc nutritional or pharmacologic interventional study aimed at lowering LPS in atrial fibrillation patients.
Introduction
There is mounting evidence to indicate that gut microbiota is responsible for systemic inflammation and contributes to different pathologies such as diabetes mellitus, obesity, hypertension, and chronic inflammatory gut disease.1 Recent data are also in favor of the hypothesis that gut microbiota may represent a novel risk factor for atherosclerosis and thrombosis. Experimental and clinical studies discovered that intestinal microbiota produces molecules such as trimethylamine N‐oxide (TMAO), which may be implicated in both processes.2, 3 TMAO stems from microbial conversion of dietary nutrients containing choline, phosphatidylcholine, and L‐carnitine to trimethylamine, which is absorbed by the intestinal tube and converted to TMAO by a hepatic flavin monooxygenase 3.4 Studies in animals have demonstrated that TMAO possesses proatherogenic property, and clinical studies have demonstrated that circulating levels of TMAO independently predicted cardiovascular events such as myocardial infarction and cardiovascular death.5 More recent studies have also documented that TMAO possesses prothrombotic effect by activating human platelets via increased release of Ca2+ from intracellular stores.6
Other products of gut microbiota, such as lipopolysaccharide (LPS), may also be involved in the atherothrombosis process. LPS is a potentially interesting molecule that, once it crosses the gut mucosa and enters into the systemic circulation, may lead to atherosclerosis, possibly via chronic inflammation and thrombosis.7 About 100 trillion gut bacteria contribute to an enteric reservoir of >1 g LPS, which travels in the circulation of healthy subjects in a range of ≈10 to 200 pg/mL.8 LPS is translocated to human circulation with chylomicrons and, therefore, increases after a meal, particularly in the case of a fatty meal, suggesting a prominent role of diet in modulating circulating LPS.9, 10 In addition, in some clinical settings such as hypertension, LPS may enter the systemic circulation as a consequence of increased gut permeability due to an impairment of tight junctions.11, 12 Experimental studies have showed that LPS is proatherogenic in vivo as its injection in mice and rabbits accelerates the formation of plaque.13 In humans, a relationship between LPS levels and carotid atherosclerosis has been documented,14 and circulating LPS has been found to be increased in several conditions associated with atherosclerosis, such as type 2 diabetes mellitus and obesity.8, 15 However, so far, no data regarding LPS and clinical outcomes have been reported.
Atrial fibrillation (AF) is the most common cardiac arrhythmia and is complicated by cardiovascular events of thromboembolic and atherothrombotic origin such as ischemic stroke, peripheral embolism, and myocardial infarction.16 Taking into account that LPS may play a role not only in the atherosclerotic process but also in clotting and platelet activation, we tested the hypothesis that LPS could be associated with cardiovascular events in AF patients. For this purpose, we performed a prospective, observational study in AF to assess (1) the relationship between circulating LPS and cardiovascular outcomes during a follow‐up of about 3 years and (2) the association between LPS and adherence to Med‐diet and consumption of its components.
Methods
Study Design
This prospective single‐center study included patients with nonvalvular AF who were referred to the center for monitoring and management of antithrombotic therapies by the Department of Internal Medicine and Medical Specialties of Sapienza‐University of Rome.
All patients were treated with vitamin K antagonists after appropriate thrombotic risk stratification.17 International normalized ratio values were maintained in an intended range between 2.0 to 3.0, and time in therapeutic range was calculated to assess the quality of anticoagulation.18 Exclusion criteria were prosthetic heart valves or the presence of any severe valvulopathies, severe cognitive impairment, chronic infections (human immunodeficiency virus infection, hepatitis C virus, hepatitis B virus), or autoimmune systemic disease. Subjects were also excluded from the study if they had active cancer or liver insufficiency (eg, cirrhosis).
At baseline, each patient provided written informed consent, the patient's medical history was recorded, and cardiovascular risk factors, such as arterial hypertension,19 diabetes mellitus,20 and heart failure21 were defined according to international guidelines.
Major Adverse Cardiovascular Events
The primary outcome of the study was a combined end point of major adverse cardiovascular events (MACE) including fatal/nonfatal myocardial infarction and ischemic stroke, cardiac revascularization (stent or coronary artery bypass surgery), cardiovascular death, and transient ischemic attack. Diagnosis of myocardial infarction was made according to the definition proposed by the Joint ESC/ACCF/AHA/WHF Task Force.22 Ischemic stroke was determined on clinical manifestations and confirmed by radiological findings; a transient ischemic attack was defined according to the Classification of Cerebrovascular Diseases III.23 If a patient died within 4 weeks of myocardial infarction or ischemic stroke, this event was recorded as fatal myocardial infarction or fatal ischemic stroke, respectively. Death was classified as vascular unless the central adjudication committee (see below) confirmed an unequivocal noncardiovascular cause of death. Cardiovascular death included death due to peripheral artery disease, hemorrhagic stroke, sudden death, progressive congestive heart failure, and procedure‐related death. Data on MACE were prospectively collected, and only the first event that occurred during follow‐up was used in the analysis. Details on MACE were registered, and death certificates, hospital discharge letter, or copy of the medical records of hospitalization and other clinical documentation (ie, radiology and laboratory data) were also obtained from patients or, in case of death, from relatives of patients or from a general practitioner. All patients provided a written informed consent at baseline. The study protocol was approved by the local ethical board of Sapienza‐University of Rome (n° 1306/2007) and was conducted according to principles of the Declaration of Helsinki.24
Laboratory Analysis
At baseline, a lipid profile including total cholesterol (mg/dL), high‐density lipoprotein (mg/dL), and triglycerides (mg/dL) was obtained. Low‐density lipoprotein (mg/dL) cholesterol was calculated by the validated Friedwald formula, and very low‐density lipoprotein (mg/dL) cholesterol as triglycerides/5.
Serum Lipopolysaccharide
Lipopolysaccharide serum levels were measured using a commercial ELISA kit (Cusabio, Wuhan, China). Standards of LPS, purified from Escherichia coli, and blood samples were plated for 2 hours at room temperature onto a microplate precoated with the antibody specific for LPS. After incubation, samples were read at 450 nm. Values were expressed as picograms per milliliter; intra‐assay and interassay coefficients of variation were 8% and 10%, respectively.
Urinary 11‐dehydro‐thromboxane B2
The excretion of urinary 11‐dehydro‐thromboxane B2 (TxB2) was measured by an enzyme‐linked immunosorbent assay commercial kit (Cayman Chemical, Ann Arbor, MI), as previously described.25 Data are expressed as nanograms per milligram creatinine. Intra‐assay and interassay coefficients of variation were 4.0% and 3.6%, respectively.
Adherence to Mediterranean Diet
In a subgroup of AF patients, we investigated the relationship between adherence to Med‐diet and circulating LPS. Adherence to Med‐diet was assessed by a validated short questionnaire, as previously described.26
Statistical Analyses
Categorical variables were reported as counts (percentage), and Pearson chi‐squared test was used to compare proportions. Continuous variables were expressed as mean±standard deviation or median and interquartile range, depending on their distribution, which was assessed by the Kolmogorov‐Smirnov test. Bivariate analysis was performed with Pearson linear correlation. Appropriate nonparametric tests (Mann‐Whitney U test and Spearman rank correlation test) were employed for all bivariate analyses with nonnormal variables. Continuous variables with nonnormal distribution were log‐transformed for multivariate analysis. After dividing the cohort into tertiles according to LPS values, the cumulative risk for MACE was estimated using a Kaplan‐Meier method. The survival curves were then formally compared using the log‐rank test. Cox proportional hazards analysis was used to calculate the adjusted relative hazards of MACE by each clinical variable. For multivariable analyses, LPS values were log transformed. Multivariable linear regression analyses were used to determine factors associated with serum log‐LPS and urinary TxB2 levels.
In a subgroup of 704 AF patients who completed the Med‐diet questionnaire, we evaluated the adherence to Med‐diet. We divided the cohort according to the median value of the Med‐diet score to compare biochemical characteristics of patients with low (0‐5 points) versus high (6‐9 points) adherence to Med‐diet. We built 2 separate models of multivariable linear regression analyses, 1 with Med‐diet score as a continuous variable and another with single foods. All tests were 2‐tailed, and analyses were performed using computer software packages (SPSS‐18.0, IBM, Armonk, NY). Only P values <0.05 were considered as statistically significant.
Results
Study Population Characteristics
Of the 998 patients initially included, 37 were excluded because of missing biological samples, and 49 were lost to follow‐up; thus, 912 AF patients composed the prospective study cohort. Baseline characteristic of the whole cohort are described in Table 1. Mean age was 73.5±8.3 years, and 42.9% were women. Most patients were hypertensive (89.7%), 20.1% were diabetic, and 16.1% had heart failure. A previous cardiac or cerebrovascular event was present in 24.8% and 14.5%, respectively. Median CHA2DS2‐VASc score was 4.0 (3.0‐4.0) points.
Table 1.
Baseline Characteristics of AF Patients According to the Occurrence of MACE
| Overall | MACE | P Value | ||
|---|---|---|---|---|
| (n=912) | No (n=728) | Yes (n=184) | ||
| Age, y | 73.5±8.3 | 72.7±8.4 | 76.7±7.1 | <0.001 |
| Female sex, % | 42.9 | 43.3 | 41.2 | 0.620 |
| AF (persistent/permanent AF) vs sinus rhythm, % | 53.1 | 52.0 | 57.2 | 0.218 |
| Current smoking, % | 9.5 | 9.5 | 9.6 | 0.964 |
| Body mass index, kg/m2 | 27.5±4.7 | 27.5±4.9 | 27.2±3.8 | 0.315 |
| Arterial hypertension, % | 89.7 | 89.0 | 92.5 | 0.096 |
| Previous cardiac events, % | 24.8 | 20.6 | 41.2 | <0.001 |
| Diabetes mellitus, % | 20.1 | 18.3 | 26.7 | 0.014 |
| Heart failure, % | 16.1 | 13.5 | 26.2 | <0.001 |
| Previous cerebrovascular events, % | 14.5 | 11.6 | 25.7 | <0.001 |
| Antiplatelet drugs, % | 19.7 | 18.1 | 26.2 | 0.017 |
| Statins, % | 41.4 | 41.9 | 39.6 | 0.618 |
| Total cholesterol, mg/dL | 177.5±39.7 | 178.2±37.8 | 174.8±47.0 | 0.297 |
| HDL cholesterol, mg/dL | 47.1±13.8 | 47.6±13.7 | 45.2±14.0 | 0.037 |
| LDL cholesterol, mg/dL | 106.5±32.4 | 106.9±30.9 | 105.1±37.5 | 0.528 |
| Triglycerides, mg/dL | 118.2±54.0 | 117.2±52.2 | 121.8±60.2 | 0.298 |
| VLDL cholesterol, mg/dL | 23.6±10.8 | 23.4±10.4 | 24.4±12.0 | 0.339 |
| LPS, pg/mL | 50.0 (15.0‐108.0) | 50.0 (15.0‐103.0) | 55.0 (20.0‐151.0) | 0.021 |
| Urinary 11‐dehydro‐thromboxane B2 (n=852) | 120.0 (70.0‐196.5) | 115.0 (68.0‐184.0) | 149.5 (90.0‐274.0) | <0.001 |
AF indicates atrial fibrillation; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; LPS, lipopolysaccharide; MACE, major adverse cardiovascular events; VLDL, very low‐density lipoprotein.
LPS and MACE
Median follow‐up was 40.0 (20.5‐68.0) months, yielding 3716 patient‐years of observation. During follow‐up, 187 (5.0% per year) MACE were registered: 139 cardiac outcomes (3.7% per year; 41 fatal/nonfatal myocardial infarctions, 20 cardiac revascularizations, 78 cardiovascular deaths) and 48 cerebrovascular events (1.3% per year; 41 fatal/nonfatal ischemic strokes and 7 transient ischemic attacks). Compared with those without, patients with a MACE were older and had a higher prevalence of diabetes mellitus, HF, history of ischemic events, and lower high‐density lipoprotein cholesterol (Table 1).
In the whole cohort, median LPS was 50.0 (15.0‐108.0) pg/mL. Median value of LPS was 10.0 (7.0‐15.0) pg/mL in the first tertile, 50.0 (33.0‐65.0) in the second, and 145.0 (108.0‐180.0) in the third tertile. Survival analysis demonstrated a significant difference in risk of MACE across tertiles of LPS, with 54, 59, and 74 MACE in the first, second, and third tertiles, respectively (log‐rank test P=0.004, Figure). In particular, as compared to the first, we found a nonsignificantly increased risk of MACE in the second tertile of LPS (hazard ratio 1.304, 95%CI 0.901‐1.888, P=0.159), while the third tertile, conferred the highest risk of MACE (hazard ratio 1.795, 95%CI 1.263‐2.552, P=0.001). Multivariable Cox regression analysis is reported in Table 2; we found that log‐LPS, age, and previous cerebrovascular and cardiac events were independent predictors of MACE (Table 2). We obtained similar risks for log‐LPS (hazard ratio 1.382, 95%CI 1.115‐1.712, P=0.003) after adjustment for time in therapeutic range (hazard ratio 0.988, 95%CI 0.977‐0.999, P=0.029).
Figure 1.
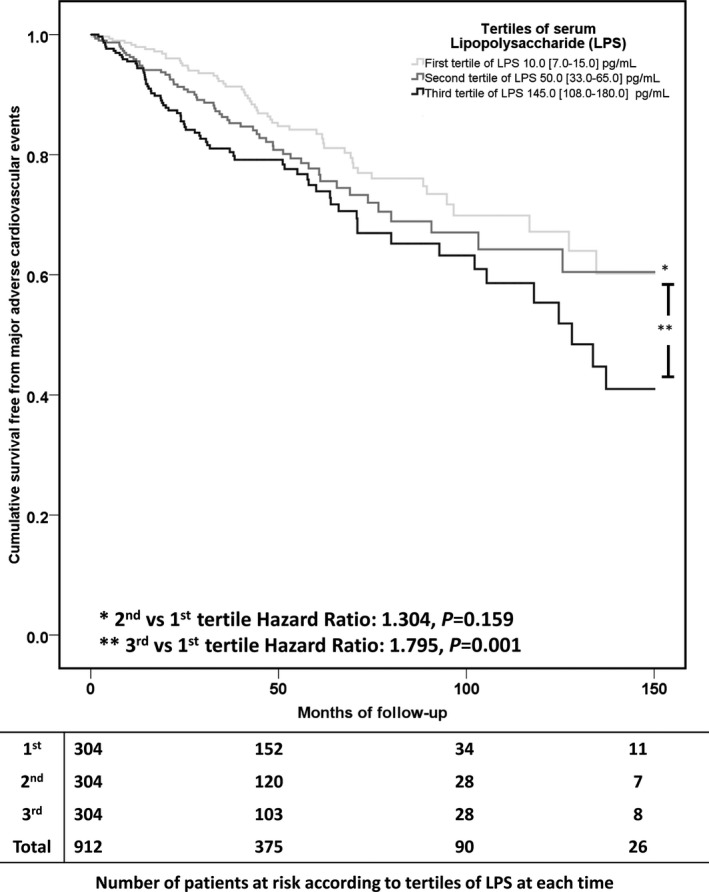
Kaplan‐Meier curve estimates of survival free from major adverse cardiovascular events according to tertiles of lipopolysaccharide (LPS).
Table 2.
Predictors of MACE (Multivariable Cox Proportional Hazard Analysis)
| Hazard Ratio (95%CI) | P Value | |
|---|---|---|
| AF vs sinus rhythm | 0.840 (0.620‐1.138) | 0.260 |
| Log‐LPS | 1.194 (1.045‐1.364) | 0.009 |
| Female sex | 0.800 (0.589‐1.086) | 0.153 |
| Age | 1.083 (1.060‐1.107) | <0.001 |
| Arterial hypertension | 1.022 (0.583‐1.790) | 0.940 |
| Diabetes mellitus | 1.348 (0.964‐1.884) | 0.081 |
| Current smoking | 1.527 (0.922‐2.530) | 0.100 |
| Heart failure | 1.383 (0.971‐1.969) | 0.072 |
| Previous cerebrovascular events | 1.634 (1.166‐2.290) | 0.004 |
| Previous cardiac events | 1.822 (1.317‐2.521) | <0.001 |
| Antiplatelet drugs | 1.009 (0.723‐1.410) | 0.957 |
| Statins | 1.061 (0.777‐1.450) | 0.709 |
| Body mass index | 1.015 (0.979‐1.051) | 0.428 |
Statins have been used instead of lipid profile for the model. AF indicates atrial fibrillation; LPS, lipopolysaccharide.
Determinants of LPS
We performed a multivariable linear regression analysis in the whole cohort to assess factors associated with circulating log‐LPS. We found that AF (versus sinus rhythm) (β 0.087, P=0.014) and low‐density lipoprotein cholesterol (β 0.069, P=0.049) were associated with circulating log‐LPS (Table 3, model A).
Table 3.
Multivariable Linear Regression Analysis of Factors Affecting Circulating Log‐LPS
| Unstandardized Coefficients | Standardized Coefficients | P Value | 95.0%CI | |||
|---|---|---|---|---|---|---|
| B | SE | β | Lower | Upper | ||
| Model A | ||||||
| AF vs sinus rhythm | 0.193 | 0.079 | 0.087 | 0.014 | 0.038 | 0.347 |
| Female sex | −0.067 | 0.079 | −0.030 | 0.394 | −0.223 | 0.088 |
| Age | 0.003 | 0.005 | 0.022 | 0.547 | −0.007 | 0.013 |
| Body mass index | 0.002 | 0.008 | 0.007 | 0.857 | −0.015 | 0.018 |
| Arterial hypertension | 0.060 | 0.127 | 0.017 | 0.634 | −0.188 | 0.309 |
| Diabetes mellitus | 0.084 | 0.097 | 0.030 | 0.389 | −0.107 | 0.275 |
| Current smoking | −0.249 | 0.131 | −0.066 | 0.058 | −0.506 | 0.008 |
| Heart failure | 0.077 | 0.107 | 0.025 | 0.475 | −0.134 | 0.287 |
| Previous cerebrovascular events | 0.028 | 0.110 | 0.009 | 0.797 | −0.187 | 0.243 |
| Previous cardiac events | 0.077 | 0.096 | 0.030 | 0.425 | −0.112 | 0.266 |
| Antiplatelet drugs | −0.174 | 0.100 | −0.063 | 0.082 | −0.369 | 0.022 |
| Triglycerides | −0.001 | 0.001 | −0.038 | 0.274 | −0.002 | 0.001 |
| LDL cholesterol | 0.002 | 0.001 | 0.069 | 0.049 | 0.000 | 0.005 |
| Model B | ||||||
| AF vs sinus rhythm | 0.242 | 0.085 | 0.113 | 0.005 | 0.075 | 0.410 |
| Female sex | −0.164 | 0.087 | −0.075 | 0.060 | −0.334 | 0.007 |
| Age | 0.006 | 0.006 | 0.044 | 0.299 | −0.005 | 0.017 |
| Body mass index | 0.001 | 0.009 | 0.003 | 0.940 | −0.017 | 0.019 |
| Arterial hypertension | 0.103 | 0.145 | 0.028 | 0.475 | −0.181 | 0.388 |
| Diabetes mellitus | 0.111 | 0.109 | 0.041 | 0.307 | −0.102 | 0.324 |
| Current smoking | −0.195 | 0.144 | −0.053 | 0.177 | −0.478 | 0.088 |
| Heart failure | 0.003 | 0.123 | 0.001 | 0.983 | −0.239 | 0.245 |
| Previous cerebrovascular events | 0.003 | 0.127 | 0.001 | 0.981 | −0.247 | 0.253 |
| Previous cardiac events | −0.014 | 0.113 | −0.005 | 0.899 | −0.237 | 0.208 |
| Antiplatelet drugs | −0.193 | 0.118 | −0.067 | 0.102 | −0.425 | 0.038 |
| Triglycerides | 0.000 | 0.001 | −0.005 | 0.901 | −0.002 | 0.002 |
| LDL cholesterol | 0.002 | 0.001 | 0.072 | 0.067 | 0.000 | 0.005 |
| Med‐diet score | −0.098 | 0.028 | −0.137 | <0.001 | −0.152 | −0.043 |
Statin use, total, HDL, and VLDL cholesterol were excluded from the model for collinearity. AF indicates atrial fibrillation; HDL, high‐density lipoprotein; LDL, low‐density lipoprotein; SE, standard error; VLDL, very low‐density lipoprotein.
Model A, whole cohort; model B, subgroup of patients with Mediterranean diet score.
In 704 of 912 AF patients we assessed the adherence to Med‐diet: median Med‐diet score was 5.0 (4.0‐6.0). Med‐diet score and log‐LPS levels were significantly and inversely correlated (r=−0.144, P<0.001). A second model of multivariable linear regression analysis adjusting for Med‐diet score showed that AF (versus sinus rhythm) and Med‐diet score were predictors of circulating log‐LPS, whereas association between low‐density lipoprotein cholesterol and log‐LPS was attenuated (Table 3, model B). According to the median value of Med‐diet score, we did not find any difference regarding lipid profile (Table 4).
Table 4.
Biochemical Characteristics of Atrial Fibrillation Patients According to the Median Value of the Med‐Diet
| Adherence to Med‐Dieta | Mean Value | Standard Deviation | P Value | |
|---|---|---|---|---|
| Total cholesterol | Low | 178.3 | 36.3 | 0.902 |
| High | 177.9 | 40.9 | ||
| HDL cholesterol | Low | 47.6 | 13.7 | 0.681 |
| High | 48.1 | 13.8 | ||
| Triglycerides | Low | 117.3 | 54.7 | 0.922 |
| High | 117.7 | 51.9 | ||
| LDL cholesterol | Low | 107.3 | 30.9 | 0.561 |
| High | 105.8 | 33.3 | ||
| VLDL cholesterol | Low | 23.5 | 10.9 | 0.922 |
| High | 23.5 | 10.4 | ||
| Log‐LPS | Low | 3.9 | 1.0 | <0.001 |
| High | 3.5 | 1.1 | ||
| Log‐thromboxane B2 | Low | 4.9 | 0.6 | <0.001 |
| High | 4.5 | 0.8 |
HDL indicates high‐density lipoprotein; LDL, low‐density lipoprotein; LPS, lipopolysaccharide; VLDL, very low‐density lipoprotein.
Low adherence, 0‐5 points of Med‐diet; high adherence, 6‐9 points of Med‐diet.
Then, we investigated LPS levels according to the intake of single foods included in the Med‐diet questionnaire (Table 5) and found significantly lower LPS levels in patients with a high intake of fruit and legumes; a trend was also evident for low intake of meat (Table 5). The negative association between log‐LPS and intakes of fruit (β −0.083, P=0.030) and legumes (β −0.120, P=0.002) remained significant at multivariable linear regression analysis (after adjustment for variables listed in Table 3, model B).
Table 5.
Circulating LPS Levels According to Single Foods
| Foods | Intake | Median LPS | P Value | |
|---|---|---|---|---|
| 1. | Olive oil (≥1 tablespoon/d) | Yes | 50.0 (15.0‐101.0) | 0.273 |
| No | 58.0 (22.0‐112.0) | |||
| 2. | Fruit (≥1 serving/d) | Yes | 48.0 (15.0‐100.0) | 0.009 |
| No | 73.0 (26.0‐120.6) | |||
| 3. | Vegetables or salad (≥1 serving/d) | Yes | 49.0 (15.0‐100.0) | 0.195 |
| No | 56.0 (18.0‐112.0) | |||
| 4. | Fruit (≥1 serving/d) and vegetables or salad (≥1 serving/d) | Yes | 48.0 (14.0‐100.0) | 0.164 |
| No | 55.0 (20.0‐110.0) | |||
| 5. | Legumes (≥2 servings/w) | Yes | 45.0 (11.0‐96.0) | 0.005 |
| No | 56.0 (20.0‐108.0) | |||
| 6. | Fish (≥3 servings/w) | Yes | 45.5 (12.0‐105.0) | 0.402 |
| No | 52.5 (17.0‐105.0) | |||
| 7. | Wine (≥1 glass/d) | Yes | 49.0 (12.0‐104.0) | 0.186 |
| No | 51.0 (20.0‐105.0) | |||
| 8. | Meat (<1 serving/d) | Yes | 50.0 (14.5‐100.0) | 0.085 |
| No | 56.0 (20.0‐120.0) | |||
| 9. | Bread (both white bread [<1/d] and rice [<1/w]) or whole‐grain bread (>5/w) | Yes | 48.5 (15.0‐95.0) | 0.382 |
| No | 53.0 (18.0‐105.0) |
LPS indicates lipopolysaccharide.
LPS and Platelet Activation
Median 11‐dehydro‐TxB2 was 120.0 (70.0‐196.5) ng/mg creatinine (n=852). Patients experiencing a MACE (n=184) disclosed significantly higher levels of urinary 11‐dehydro‐TxB2 compared with those who did not (149.5 [90.0‐274.0] versus 115.0 [68.0‐184.0]ng/mg, P<0.001).
Log‐LPS and log‐TxB2 were highly correlated (r=0.598, P<0.001); Med‐diet and log‐TxB2 were inversely correlated (r=−0.310, P<0.001). A multivariable linear regression analysis showed that log‐LPS (β 0.574, P<0.001) and Med‐diet score (β −0.218, P<0.001) were significantly associated with urinary excretion of 11‐dehydro‐TxB2 (after adjustment for variables listed in Table 3, model B).
Discussion
This is the first prospective study reporting a significant association between circulating LPS and MACE occurrence in a large cohort of AF patients. Circulating levels of LPS were higher in patients with AF compared with those with sinus rhythm, and were not associated with classical cardiovascular risk factors. After controlling for Med‐diet adherence, we found an inverse association between LPS and Med‐diet score, in particular with fruit and legume intake.
In the present study, we found a rate of MACE of 5% per year, which is consistent with other reports.27, 28 This is in accordance with Gallego et al, who found in a cohort of 529 AF patients a rate of thrombotic/cardiovascular events of 5.17% per year in a median follow‐up of 28 months28. Similar findings have been recently reported in a study including 754 AF patients restarting treatment with warfarin after gastric ulcer bleeding. In the subgroup of 458 patients treated with warfarin, the rate of MACE was 5.4% per year.27 Our results are also in keeping with a prespecified analysis from the ROCKET‐AF trial, which showed that AF patients with prior cardiac events are at higher risk of MACE than cardiovascular‐free patients.29
The novel finding of the present study is that patients experiencing MACE disclosed significantly higher values of LPS, as compared with patients free from events during follow‐up. In particular, we found that patients in the third tertile of LPS (>100 pg/mL), as compared with the first one, had the highest risk of MACE.
Our findings provide support and extend current knowledge on the role of gut microbiota and its products in many cardiovascular diseases, suggesting that they may not only have an important pathogenetic role in human atherosclerosis but may also have an important prognostic value.
To explore potential mechanisms underlying the association between LPS and MACE, we measured the urinary excretion of 11‐dehydro‐TxB2, which is a validated marker of platelet activation.30 We previously had demonstrated that in AF platelet function is increased and significantly associated with MACE incidence.25 A step forward in the knowledge of platelet function in AF is provided by the present study, suggesting that LPS may represent an important trigger for platelet activation; consistent with this hypothesis is the significant correlation between LPS levels and 11‐dehydro‐TxB2 excretion. Previous in vitro study also demonstrated that LPS amplifies platelet response to common agonists in a range of concentration similar to that detected in our AF population.31
However, other mechanisms may account for the association between LPS and MACE. Thus, animal models showed that long‐term exposure to LPS was associated with endothelial dysfunction32 and atherosclerotic plaque destabilization,13 and in vitro LPS stimulation was associated with increased monocyte‐derived thrombin generation.33
Although there was no association between circulating LPS and classic cardiovascular risk factors, we found a significant inverse association between adherence to Med‐diet and LPS. Med‐diet represents a healthy dietary model recognized worldwide for its favorable effect in the primary and secondary prevention of cardiovascular events.34 In the context of AF, we previously demonstrated that adherence to Med‐diet had several beneficial effects, including a reduction of systemic oxidative stress26 and platelet activation,35 which were in turn associated with a lower rate of MACE. The novel finding of the present study is in the inverse association between adherence to Med‐diet and circulating LPS, which provides a novel insight into the putative anti‐inflammatory effect exerted by Med‐diet.31 It is interesting to note that, among the Med‐diet nutrients, fruits and legumes were those showing a striking association with lower LPS, indicating a relevant role for low‐fat‐content food in modulating circulating LPS.36
The study has clinical implications. The fact that LPS is an independent predictor of MACE suggests that gut microbiota may also favor cardiovascular disease in the setting of AF, but the observational design of the study does not make it possible to establish a cause‐effect relationship. Indirect evidence in support of this is provided by the inverse association between Med‐diet and circulating LPS. Only an ad‐hoc nutritional or pharmacologic interventional study aimed at lowering LPS may clarify this issue. The inclusion of only white patients and data collected by a single center are other limitations of the study. Finally, we cannot exclude that LPS may contribute to the increased MACE incidence through other not yet identified mechanisms.
In conclusion, we provided further support to the role of gut microbiota as a factor potentially implicated in cardiovascular disease by demonstrating that circulating LPS is associated with MACE in AF patients during a long‐term follow‐up.
Disclosures
None.
(J Am Heart Assoc. 2017;6:e005784 DOI: 10.1161/JAHA.117.005784.)28584074
References
- 1. Jonsson AL, Backhed F. Role of gut microbiota in atherosclerosis. Nat Rev Cardiol. 2017;14:79–87. [DOI] [PubMed] [Google Scholar]
- 2. Tang WH, Hazen SL. Microbiome, trimethylamine N‐oxide, and cardiometabolic disease. Transl Res. 2017;179:108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Randrianarisoa E, Lehn‐Stefan A, Wang X, Hoene M, Peter A, Heinzmann SS, Zhao X, Konigsrainer I, Konigsrainer A, Balletshofer B, Machann J, Schick F, Fritsche A, Haring HU, Xu G, Lehmann R, Stefan N. Relationship of serum trimethylamine N‐oxide (TMAO) levels with early atherosclerosis in humans. Sci Rep. 2016;6:26745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Tang WH, Wang Z, Levison BS, Koeth RA, Britt EB, Fu X, Wu Y, Hazen SL. Intestinal microbial metabolism of phosphatidylcholine and cardiovascular risk. N Engl J Med. 2013;368:1575–1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Li XS, Obeid S, Klingenberg R, Gencer B, Mach F, Raber L, Windecker S, Rodondi N, Nanchen D, Muller O, Miranda MX, Matter CM, Wu Y, Li L, Wang Z, Alamri HS, Gogonea V, Chung YM, Tang WH, Hazen SL, Luscher TF. Gut microbiota‐dependent trimethylamine N‐oxide in acute coronary syndromes: a prognostic marker for incident cardiovascular events beyond traditional risk factors. Eur Heart J. 2017;38:814–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Zhu W, Gregory JC, Org E, Buffa JA, Gupta N, Wang Z, Li L, Fu X, Wu Y, Mehrabian M, Sartor RB, McIntyre TM, Silverstein RL, Tang WH, Di Donato JA, Brown JM, Lusis AJ, Hazen SL. Gut microbial metabolite TMAO enhances platelet hyperreactivity and thrombosis risk. Cell. 2016;165:111–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Michelsen KS, Wong MH, Shah PK, Zhang W, Yano J, Doherty TM, Akira S, Rajavashisth TB, Arditi M. Lack of Toll‐like receptor 4 or myeloid differentiation factor 88 reduces atherosclerosis and alters plaque phenotype in mice deficient in apolipoprotein e. Proc Natl Acad Sci USA. 2004;101:10679–10684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Saad MJ, Santos A, Prada PO. Linking gut microbiota and inflammation to obesity and insulin resistance. Physiology. 2016;31:283–293. [DOI] [PubMed] [Google Scholar]
- 9. Vors C, Pineau G, Drai J, Meugnier E, Pesenti S, Laville M, Laugerette F, Malpuech‐Brugere C, Vidal H, Michalski MC. Postprandial endotoxemia linked with chylomicrons and lipopolysaccharides handling in obese versus lean men: a lipid dose‐effect trial. J Clin Endocrinol Metab. 2015;100:3427–3435. [DOI] [PubMed] [Google Scholar]
- 10. Laugerette F, Alligier M, Bastard JP, Drai J, Chanseaume E, Lambert‐Porcheron S, Laville M, Morio B, Vidal H, Michalski MC. Overfeeding increases postprandial endotoxemia in men: inflammatory outcome may depend on LPS transporters LBP and sCD14. Mol Nutr Food Res. 2014;58:1513–1518. [DOI] [PubMed] [Google Scholar]
- 11. Li J, Zhao F, Wang Y, Chen J, Tao J, Tian G, Wu S, Liu W, Cui Q, Geng B, Zhang W, Weldon R, Auguste K, Yang L, Liu X, Chen L, Yang X, Zhu B, Cai J. Gut microbiota dysbiosis contributes to the development of hypertension. Microbiome. 2017;5:14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Moreira AP, Texeira TF, Ferreira AB, Peluzio MdC, Alfenas RdC. Influence of a high‐fat diet on gut microbiota, intestinal permeability and metabolic endotoxaemia. Br J Nutr. 2012;108:801–809. [DOI] [PubMed] [Google Scholar]
- 13. Jaw JE, Tsuruta M, Oh Y, Schipilow J, Hirano Y, Ngan DA, Suda K, Li Y, Oh JY, Moritani K, Tam S, Ford N, van Eeden S, Wright JL, Man SF, Sin DD. Lung exposure to lipopolysaccharide causes atherosclerotic plaque destabilisation. Eur Respir J. 2016;48:205–215. [DOI] [PubMed] [Google Scholar]
- 14. Serrano M, Moreno‐Navarrete JM, Puig J, Moreno M, Guerra E, Ortega F, Xifra G, Ricart W, Fernandez‐Real JM. Serum lipopolysaccharide‐binding protein as a marker of atherosclerosis. Atherosclerosis. 2013;230:223–227. [DOI] [PubMed] [Google Scholar]
- 15. Cox AJ, Zhang P, Bowden DW, Devereaux B, Davoren PM, Cripps AW, West NP. Increased intestinal permeability as a risk factor for type 2 diabetes. Diabetes Metab. 2017;43:163–166. [DOI] [PubMed] [Google Scholar]
- 16. Violi F, Soliman EZ, Pignatelli P, Pastori D. Atrial fibrillation and myocardial infarction: a systematic review and appraisal of pathophysiologic mechanisms. J Am Heart Assoc. 2016;5:e003347 DOI: 10.1161/JAHA.116.003347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. European Heart Rhythm Association; European Association for Cardio‐Thoracic Surgery , Camm AJ, Kirchhof P, Lip GY, Schotten U, Savelieva I, Ernst S, Van Gelder IC, Al‐Attar N, Hindricks G, Prendergast B, Heidbuchel H, Alfieri O, Angelini A, Atar D, Colonna P, De Caterina R, De Sutter J, Goette A, Gorenek B, Heldal M, Hohloser SH, Kolh P, Le Heuzey JY, Ponikowski P, Rutten FH. Guidelines for the management of atrial fibrillation: the task force for the management of atrial fibrillation of the European Society of Cardiology (ESC). Eur Heart J. 2010; 31:2369–2429. [DOI] [PubMed] [Google Scholar]
- 18. Poller L, Keown M, Ibrahim S, Lowe G, Moia M, Turpie AG, Roberts C, van den Besselaar AM, van der Meer FJ, Tripodi A, Palareti G, Jespersen J. A multicentre randomised clinical endpoint study of parma 5 computer‐assisted oral anticoagulant dosage. Br J Haematol. 2008;143:274–283. [DOI] [PubMed] [Google Scholar]
- 19. Mancia G, Fagard R, Narkiewicz K, Redan J, Zanchetti A, Bohm M, Christiaens T, Cifkova R, De Backer G, Dominiczak A, Galderisi M, Grobbee DE, Jaarsma T, Kirchof P, Kjeldsen SE, Laurent S, Manolis AJ, Nilsson PM, Ruilope LM, Schmieder RE, Sirnes PA, Sleight P, Viigimaa M, Waeber B, Zannad F; ESH/ESC Task Force for the Management of Arterial Hypertension . 2013 Practice guidelines for the management of arterial hypertension of the European Society of Hypertension (ESH) and the European Society of Cardiology (ESC): ESH/ESC task force for the management of arterial hypertension. J Hypertens. 2013;2013:1925–1938. [DOI] [PubMed] [Google Scholar]
- 20. Authors/Task Force Members , Rydén L, Grant PJ, Anker SD, Berne C, Cosentino F, Danchin N, Deaton C, Escaned J, Hammes HP, Huikuri H, Marre M, Marx N, Mellbin L, Ostergren J, Patrono C, Seferovic P, Uva MS, Taskinen MR, Tendera M, Tuomilehto J, Valensi P, Zamorano JL, ESC Committee for Practice Guitelines , Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, Document Reviewers , De Backer G, Sirnes PA, Ezquerra EA, Avogaro A, Badimon L, Baranova E, Baumgartner H, Betteridge J, Ceriello A, Fagard R, Funck‐Brentano C, Gulba DC, Hasdai D, Hoes AW, Kjekshus JK, Knuuti J, Kolh P, Lev E, Mueller C, Neyses L, Nilsson PM, Perk J, Ponikowski P, Reiner Z, Sattar N, Schachinger V, Scheen A, Schirmer H, Stromberg A, Sudzhaeva S, Tamargo JL, Viigimaa M, Vlachopoulos C, Xuereb RG. ESC guidelines on diabetes, pre‐diabetes, and cardiovascular diseases developed in collaboration with the EASD: the task force on diabetes, pre‐diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD). Eur Heart J. 2013; 34:3035–3087. [DOI] [PubMed] [Google Scholar]
- 21. McMurray JJ, Adamopoulos S, Anker SD, Auricchio A, Bohm M, Dickstein K, Falk V, Filippatos G, Fonseca C, Gomez‐Sanchez MA, Jaarsma T, Kober L, Lip GY, Maggioni AP, Parkhomenko A, Pieske BM, Popescu BA, Ronnevik PK, Rutten FH, Schwitter J, Seferovic P, Stepinska J, Trindade PT, Voors AA, Zannad F, Zeiher A; Task Force for the Diagnosis and Treatment of Acute and Chronic Heart Failure 2012 of the European Society of Cardiology , Bax JJ, Baumgartner H, Ceconi C, Dean V, Deaton C, Fagard R, Funck‐Brentano C, Hasdai D, Hoes A, Kirchhof P, Knuuti J, Kolh P, McDonagh T, Moulin C, Popescu BA, Reiner Z, Sechtem U, Sirnes PA, Tendera M, Torbicki A, Vahanian A, Windecker S, McDonagh T, Sechtem U, Bonet LA, Avraamides P, Ben Lamin HA, Brignole M, Coca A, Cowburn P, Dargie H, Elliott P, Flachskampf FA, Guida GF, Hardman S, Iung B, Merkely B, Mueller C, Nanas JN, Nielsen OW, Orn S, Parissis JT, Ponikowski P; Guidelines ESCCfP . ESC guidelines for the diagnosis and treatment of acute and chronic heart failure 2012: the task force for the diagnosis and treatment of acute and chronic heart failure 2012 of the European Society of Cardiology developed in collaboration with the Heart Failure Association (HFA) of the ESC. Eur J Heart Fail. 2012; 14:803–869. [DOI] [PubMed] [Google Scholar]
- 22. Thygesen K, Alpert JS, White HD; Joint ESC/ACCF/AHA/WHF Task Force for the Redefinition of Myocardial Infarction , Jaffe AS, Apple FS, Galvani M, Katus HA, Newby LK, Ravkilde J, Chaitman B, Clemmensen PM, Dellborg M, Hod H, Porela P, Underwood R, Bax JJ, Beller GA, Bonow R, Van der Wall EE, Bassand JP, Wijns W, Ferguson TB, Steg PG, Uretsky BF, Williams DO, Armstrong PW, Antman EM, Fox KA, Hamm CW, Ohman EM, Simoons ML, Poole‐Wilson PA, Gurfinkel EP, Lopez‐Sendon JL, Pais P, Mendis S, Zhu JR, Wallentin LC, Fernandez‐Aviles F, Fox KM, Parkhomenko AN, Priori SG, Tendera M, Voipio‐Pulkki LM, Vahanian A, Camm AJ, De Caterina R, Dean V, Dickstein K, Filippatos G, Funck‐Brentano C, Hellemans I, Kristensen SD, McGregor K, Sechtem U, Silber S, Tendera M, Widimsky P, Zamorano JL, Morais J, Brener S, Harrington R, Morrow D, Lim M, Martinez‐Rios MA, Steinhubl S, Levine GN, Gibler WB, Goff D, Tubaro M, Dudek D, Al‐Attar N. Universal definition of myocardial infarction. Circulation. 2007; 116:2634–2653. [DOI] [PubMed] [Google Scholar]
- 23. Special report from the National Institute of Neurological Disorders and Stroke. Classification of cerebrovascular diseases III. Stroke. 1990; 21:637–676. [DOI] [PubMed] [Google Scholar]
- 24. World Medical Association . World Medical Association Declaration of Helsinki. Ethical principles for medical research involving human subjects. Bull World Health Org. 2001; 79:373–374. [PMC free article] [PubMed] [Google Scholar]
- 25. Pastori D, Pignatelli P, Farcomeni A, Cangemi R, Hiatt WR, Bartimoccia S, Nocella C, Vicario T, Bucci T, Carnevale R, Lip GY, Violi F. Urinary 11‐dehydro‐thromboxane B2 is associated with cardiovascular events and mortality in patients with atrial fibrillation. Am Heart J. 2015;170:e491. [DOI] [PubMed] [Google Scholar]
- 26. Pastori D, Carnevale R, Bartimoccia S, Nocella C, Tanzilli G, Cangemi R, Vicario T, Catena M, Violi F, Pignatelli P. Does Mediterranean diet reduce cardiovascular events and oxidative stress in atrial fibrillation? Antioxid Redox Signal. 2015;23:682–687. [DOI] [PubMed] [Google Scholar]
- 27. Lee SJ, Sung JH, Kim JB, Ahn MS, Lee HY, Uhm JS, Pak HN, Lee MH, Kim JY, Joung B. The safety and efficacy of vitamin K antagonist in atrial fibrillation patients with previous ulcer bleeding: long‐term results from a multicenter study. Medicine. 2016;95:e5467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Gallego P, Roldan V, Marin F, Romera M, Valdes M, Vicente V, Lip GY. Cessation of oral anticoagulation in relation to mortality and the risk of thrombotic events in patients with atrial fibrillation. Thromb Haemost. 2013;110:1189–1198. [DOI] [PubMed] [Google Scholar]
- 29. Mahaffey KW, Stevens SR, White HD, Nessel CC, Goodman SG, Piccini JP, Patel MR, Becker RC, Halperin JL, Hacke W, Singer DE, Hankey GJ, Califf RM, Fox KA, Breithardt G; ROCKET AF Investigators . Ischaemic cardiac outcomes in patients with atrial fibrillation treated with vitamin K antagonism or factor Xa inhibition: results from the ROCKET AF trial. Eur Heart J. 2014;35:233–241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Catella F, Healy D, Lawson JA, FitzGerald GA. 11‐Dehydrothromboxane B2: a quantitative index of thromboxane A2 formation in the human circulation. Proc Natl Acad Sci USA. 1986;83:5861–5865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Raparelli V, Basili S, Carnevale R, Napoleone L, Del Ben M, Nocella C, Bartimoccia S, Lucidi C, Talerico G, Riggio O, Violi F. Low‐grade endotoxemia and platelet activation in cirrhosis. Hepatology. 2017;65:571–581. [DOI] [PubMed] [Google Scholar]
- 32. Suda K, Eom J, Jaw JE, Mui T, Bai N, Or C, Ngan D, Li Y, Wang X, Tsuruta M, Tam S, Man SP, Van Eeden S, Sin DD. Endotoxin‐induced cardiovascular dysfunction in mice: effect of simvastatin. J Appl Physiol. 1985;2011:1118–1124. [DOI] [PubMed] [Google Scholar]
- 33. Ferro D, Basili S, Alessandri C, Cara D, Violi F. Inhibition of tissue‐factor‐mediated thrombin generation by simvastatin. Atherosclerosis. 2000;149:111–116. [DOI] [PubMed] [Google Scholar]
- 34. Sofi F, Cesari F, Abbate R, Gensini GF, Casini A. Adherence to Mediterranean diet and health status: meta‐analysis. BMJ. 2008;337:a1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Pignatelli P, Pastori D, Farcomeni A, Nocella C, Bartimoccia S, Vicario T, Bucci T, Carnevale R, Violi F. Mediterranean diet reduces thromboxane A2 production in atrial fibrillation patients. Clin Nutr. 2015;34:899–903. [DOI] [PubMed] [Google Scholar]
- 36. Lyte JM, Gabler NK, Hollis JH. Postprandial serum endotoxin in healthy humans is modulated by dietary fat in a randomized, controlled, cross‐over study. Lipids Health Dis. 2016;15:186. [DOI] [PMC free article] [PubMed] [Google Scholar]


