Abstract
Background
We sought to determine whether right ventricular stunning could be detected after supply (during coronary balloon occlusion [BO]) and supply/demand ischemia (induced by rapid pacing [RP] during transcatheter aortic valve replacement) in humans.
Methods and Results
Ten subjects with single‐vessel right coronary artery disease undergoing percutaneous coronary intervention with normal ventricular function were studied in the BO group. Ten subjects undergoing transfemoral transcatheter aortic valve replacement were studied in the RP group. In both, a conductance catheter was placed into the right ventricle, and pressure volume loops were recorded at baseline and for intervals over 15 minutes after a low‐pressure BO for 1 minute or a cumulative duration of RP for up to 1 minute. Ischemia‐induced diastolic dysfunction was seen 1 minute after RP (end‐diastolic pressure [mm Hg]: 8.1±4.2 versus 12.1±4.1, P<0.001) and BO (end‐diastolic pressure [mm Hg]: 8.1±4.0 versus 8.7±4.0, P=0.03). Impairment of systolic and diastolic function after BO remained at 15‐minutes recovery (ejection fraction [%]: 55.7±9.0 versus 47.8±6.3, P<0.01; end‐diastolic pressure [mm Hg]: 8.1±4.0 versus 9.2±3.9, P<0.01). Persistent diastolic dysfunction was also evident in the RP group at 15‐minutes recovery (end‐diastolic pressure [mm Hg]: 8.1±4.1 versus 9.9±4.4, P=0.03) and there was also sustained impairment of load‐independent indices of systolic function at 15 minutes after RP (end‐systolic elastance and ventriculo‐arterial coupling [mm Hg/mL]: 1.25±0.31 versus 0.85±0.43, P<0.01).
Conclusions
RP and right coronary artery balloon occlusion both cause ischemic right ventricular dysfunction with stunning observed later during the procedure. This may have intraoperative implications in patients without right ventricular functional reserve.
Keywords: myocardial, percutaneous coronary intervention, rapid pacing, right ventricular dysfunction, stunning, transcatheter aortic valve implantation
Subject Categories: Physiology, Percutaneous Coronary Intervention, Aortic Valve Replacement/Transcather Aortic Valve Implantation
Introduction
The importance of the right ventricle (RV) in the pathophysiology of heart disease is of increasing clinical relevance.1 Involvement of the RV in inferior myocardial infarction increases the risk of cardiogenic shock and increases mortality, even when treated with primary percutaneous coronary intervention (PCI).2 Pre‐existing RV failure portends a poor prognosis in several conditions,3 and perioperative RV dilatation and dysfunction are independent prognostic risk factors after transcatheter aortic valve replacement (TAVR).4, 5 Acute deterioration in RV function often has important hemodynamic and clinical consequences.
The blood supply to the RV depends upon the coronary anatomy. In a right‐dominant system (80%), the right coronary artery (RCA) supplies most of the right ventricle.6 The RV is believed to be relatively resistant to ischemia compared with the left ventricle (LV), as propelling blood into a low‐resistance pulmonary circulation requires less work and the RV has thinner, less muscular walls with a lower energetic demand and a lower nutrient/oxygen requirement as a result.7 Coronary balloon inflation during PCI provides a model of supply ischemia. Brief coronary balloon occlusion (BO) of the RCA reduces RV stroke volume and stroke work.8 However, the response of the RV after reperfusion is unknown. Studies of brief coronary occlusion in the left ventricle suggest that, after a transient improvement in function resulting from reactive hyperemia, residual ventricular dysfunction is revealed (stunning) when coronary flow normalizes sometime after reperfusion.9
Rapid pacing (RP) during TAVR has been reported as safe10 but nevertheless induces a combination of ventricular supply and demand ischemia.11 RP increases myocardial oxygen demand as the ventricular rate is increased while there is also a reduction in coronary flow because of the decreased diastolic time during extreme tachycardia.12 The immediate intraoperative effect of RP‐induced RV ischemia has not been studied invasively during TAVR in humans.
Studies investigating the effect of TAVR on RV function have often utilized noninvasive imaging days to weeks following TAVR.13, 14 Echo studies have provided inconsistent findings on the effect of TAVR on RV function, ranging from RV dysfunction4 to improved RV function compared with surgical aortic valve replacement.13 Conventional 2‐dimensional echo assessment of RV function is challenging because of the complex geometry of the RV, and this may explain the discrepancies between studies.15 Greater understanding of RV hemodynamics assessed invasively in the minutes following TAVR may provide more accurate insight into the acute changes of RV function that potentially impact on periprocedural outcome.
This study aims to investigate the impact of brief right coronary BO and RP on RV function assessed using the “gold standard” conductance catheter technique.16, 17, 18 These data enable comparison of the magnitude of ischemic RV dysfunction and track recovery that may inform potential changes to procedural protocols, to minimize hemodynamic disturbance during interventional procedures.
Methods
Study Population
Twenty patients were recruited into the study in 2 groups. Ten patients with severe RCA disease awaiting single‐vessel elective PCI and normal RV function assessed by echocardiography were recruited and 10 patients with severe aortic stenosis undergoing transfemoral TAVR, with normal RV function were recruited. Patients were excluded if they had suffered a myocardial infarction in the preceding 3 months, had a pacemaker, or were not in sinus rhythm. All patients gave written informed consent before study inclusion. The study was approved by the local ethics committee (REC 12/EE/0085 and 12/EE/0473), and complied with the guidelines set out in the Declaration of Helsinki. The study was registered on Clinicaltrials.gov NCT02236299 and the trial ID was UKCRN14028.
Pre‐Study Protocol
Variables that could alter coronary or ventricular hemodynamics were minimized. Patients were asked to abstain from consuming caffeine, alcohol, nicotine, as well as nicorandil and oral/sublingual nitrates in the 24 hours leading up to the procedure. All subjects were fasted for 6 hours, and received aspirin 300 mg and clopidogrel 300 mg before the procedure. Patients were anticoagulated with unfractionated heparin 70 to 100 IU/kg. An activated coagulation time was maintained >250 s throughout the procedure.
Cardiac Catheterization
In those undergoing PCI, a 6F sheath was placed in the right radial artery and a 7F sheath was placed in the right femoral vein under local anesthetic. Nitrates could be administered as needed in the radial artery, but not in the coronary arteries. In those undergoing TAVR, a 6F sheath was placed in the right femoral artery, a 7F sheath was placed in the right femoral vein, and a 10F sheath was placed in the left femoral vein.
A 6F multipurpose catheter was positioned in the pulmonary artery and then right atrium to measure mean pressures and obtain mixed venous blood gas saturations for determination of indirect Fick cardiac output. Blood was also sampled to measure blood resistivity. A 7F 8‐electrode conductance catheter (Millar Instruments, Houston, TX) was then connected to an MPVS Ultra (Millar Instruments) signal‐conditioning unit in series with an ADInstruments PowerLab 16/30 Series (ADInstruments, New South Wales, Australia) 16‐channel amplifier. The conductance catheter was submersed in a bath of saline and the pressure transducer zeroed before insertion through the 7F femoral venous sheath, and it was positioned apically along the long axis of the RV under fluoroscopic guidance (Figure 1).
Figure 1.
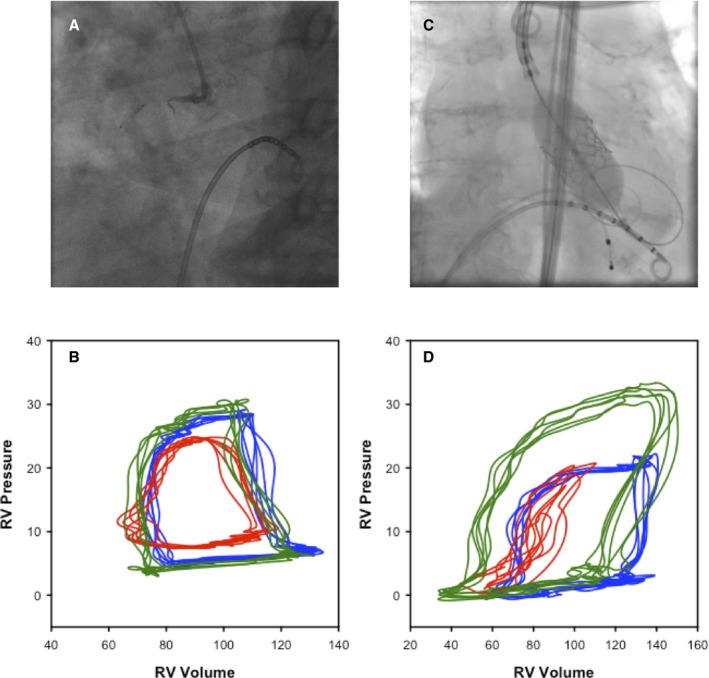
A, Fluoroscopic image of the conductance catheter located in the right ventricle (RV) during low‐pressure balloon occlusion of the right coronary artery. B, RV pressure volume (PV)–loops recorded at baseline (blue), at the end of the low‐pressure balloon occlusion (red), and at 15‐minute recovery (green). C, Fluoroscopic image of the conductance catheter located in the RV during SAPIEN 3‐valve deployment. D, RV PV‐loops recorded at baseline (blue), at the end of the rapid paced valve deployment (red), and at 15‐minute recovery (green).
Conductance Calibration
A 20‐kHz current was applied to the outermost electrodes to generate an intracavity electric field. The time varying conductance, G(t), was calculated by measuring the sum of the conductance between the 5 remaining sensing electrode pairs. The conductance catheter was calibrated using the technique first described in the LV by Baan et al19 and has subsequently been used for the RV.20, 21 The time varying volume, V(t), was calculated as follows: V(t)=1/α×L2/σ×[G(t)−G(p)]; α is the ratio of the conductance‐derived stroke volume to the true stroke volume (calculated from the indirect Fick measure of cardiac output), L is the interelectrode distance, σ is the blood conductivity (the reciprocal of the specific resistivity of the blood measured directly with the Millar cuvette), and G(p) is the parallel conductance (conductance of fluids and tissues surrounding the RV). G(p) was calculated using the hypertonic saline injection technique as previously described by Baan et al.19
Pressure Volume Loop Data Acquisition
The conductance technique was used to measure the pressure‐volume‐loop relationship during breath hold in midexpiration to provide a beat‐to‐beat assessment of RV function at steady state for at least 5 cardiac cycles (Figure 1). Pressure‐volume‐loop data were continuously recorded at baseline, during a 1‐minute low‐pressure (<4 atm) BO (BO group) or during a median of 38.5 (24.8–62.3) s at a mean rate of 200±18 beats per minute of cumulative RP (RP group). The rate, frequency, and duration of RP was at the operator's discretion guided by the surrogate of reduced LV ejection—a systolic blood pressure <40 mm Hg. RP was applied intermittently for pacing test capture, balloon aortic valvuloplasty, and TAVR (Edwards SAPIEN III Transcatheter Heart Valve (Edwards Lifesciences, Irvine, CA) positioning and deployment. Pressure‐volume‐loop data were recorded continuously postintervention over a 15‐minute period of recovery. Once data collection was completed, PCI was performed in the BO group at operator discretion. In the RP group, transient balloon occlusion of the inferior vena cava with a 35‐mm Amplatzer™ Sizing Balloon II (Abbott Vascular, Santa Clara, CA) was performed before RP and at 15‐minutes recovery (post‐TAVR) to also generate load‐independent indices of RV contractility.
Offline RV Hemodynamic Measurements
The conductance catheter data were analyzed offline using LabChart software (LabChart 7.0; ADInstruments). Five steady‐state pressure‐volume‐loops were recorded at baseline, during intervention (BO or RP), and at 1, 2, 3, 4, 5, 10, and 15‐minute intervals of recovery, to generate load‐dependent parameters of systolic and diastolic function. The systolic parameters of ventricular function were cardiac output, stroke volume, stroke work (SW), ejection fraction (EF), end‐systolic pressure, and the maximum rate of isovolumic contraction (dP/dtmax). Effective arterial elastance (Ea) to assess afterload was also assessed. The diastolic parameters of ventricular function were end‐diastolic pressure (EDP), the maximum rate of isovolumic relaxation (dP/dtmin), and the time constant of diastolic relaxation (Tau).22, 23, 24 Tau represents the exponential decay of the RV pressure during isovolumic relaxation. Tau was determined using the Weiss method.22 Tau was calculated as a parameter in an exponential fit using the following equation: P(t)=A exp (−t/Tau). The fit is calculated using nonlinear least‐squares regression. Although Tau is considered load dependent, it is predominantly only affected by heart rate. We also measured load‐independent measures of RV function in the RP group, recording preload‐recruitable stroke work and RV end‐systolic elastance and ventriculo‐arterial coupling.
Statistical Analysis
Data are expressed as mean±SD unless otherwise stated. Analysis was performed using SigmaPlot 12.5 (Systat Software Inc, San Jose, CA) statistical analysis package. In view of the early nature of this work, we estimated group sizes from previous work.25 The group sizes were powered to detect a change of 10±8 ms in Tau between baseline and 15‐minute recovery in each group, with a significance level of 0.05 and a power of 0.8. We decided to recruit 10 patients per group. The study was not powered to detect changes between groups. RV hemodynamic data were converted to a percentage change from baseline values (on a patient basis) to facilitate data comparison between BO and RP groups. Comparison between baseline and recovery were assessed with paired Student t test. For comparisons between groups, non‐normally distributed data were compared using a Mann–Whitney U test, whereas for normally distributed data an unpaired Student t test was used. Categorical data are expressed as number (percentage) and were compared with the Fisher exact test. A probability level of P<0.05 was considered statistically significant.
Results
Patient demographic data are summarized in Table 1. Patients in the RP group were older, had more heart failure symptoms, and had higher pulmonary pressures. There was also a trend toward increased creatinine and reduced hemoglobin in the RP group, reflecting the frailty and comorbidity of an older population. Those in the BO group were more likely to have angina. Baseline hemodynamic data were broadly similar between groups, although the RP group had greater RV dP/dtmax.
Table 1.
Patient Demographic and Hemodynamic Data
| BO Group (n=10) | RP Group (n=10) | P Value | |
|---|---|---|---|
| Demographics | |||
| Age, y | 74 [67.5–75] | 80 [76–87] | 0.02 |
| Male sex, n (%) | 8 (80) | 7 (70) | 1.00 |
| BMI, kg/m2 | 28.9±4.1 | 25.8±4.9 | 0.13 |
| Active or ex‐smoker, n (%) | 5 (50) | 5 (50) | 1.00 |
| CCS (II and above), n (%) | 9 (90) | 2 (20) | 0.005 |
| NYHA Class (II and above), n (%) | 3 (30) | 10 (100) | 0.003 |
| Hypertension, n (%) | 3 (30) | 7 (70) | 0.179 |
| Diabetes mellitus, n (%) | 1 (10) | 3 (30) | 0.582 |
| Previous MI, n (%) | 2 (20) | 2 (20) | 1.00 |
| Hb | 13.7±1.9 | 12.0±2.1 | 0.09 |
| Cr, mg/dL | 1.1±0.3 | 1.4±0.5 | 0.09 |
| Baseline hemodynamics—right heart catheterization data | |||
| Systolic BP, mm Hg | 135 [121–142] | 146 [138–160] | 0.22 |
| Diastolic BP, mm Hg | 68 [64–83] | 66 [55–72] | 0.29 |
| Systemic MAP, mm Hg | 91 [80–96] | 86 [76–99] | 0.60 |
| MRAP, mm Hg | 5 [4–8] | 9.5 [6.5–10] | 0.10 |
| MPAP, mm Hg | 18 [17.25–21] | 22.5 [19–26] | 0.07 |
| PA Sats, % | 71 [69.8–72.4] | 72.1 [68.9–77.2] | 0.62 |
| Ao Sats, % | 94.7 [93.1–95.8] | 95.8 [94.9–96.9] | 0.14 |
| CO, L/min | 5.05 [4.4–5.3] | 5.2 [4.6–5.9] | 0.49 |
| CI, L/min per kg | 2.6 [2.3–3.0] | 2.8 [2.4–3.0] | 0.65 |
| Baseline hemodynamics—RV conductance catheter data | |||
| SW, mm Hg·mL | 1246 [897–1571] | 1775 [1511–2148] | 0.14 |
| SV, mL | 83 [71–86] | 81 [65–110] | 0.85 |
| ESP, mm Hg | 26.8 [25.1–32.4] | 28.4 [26.3–35.7] | 0.57 |
| EDP, mm Hg | 6.8 [5.2–11.4] | 6.5 [5.6–9.9] | 0.97 |
| ESV, mL | 102 [84–116] | 86 [65–98] | 0.19 |
| EDV, mL | 140 [127–180] | 140 [94–183] | 0.47 |
| EF, % | 56.5 [53.8–60.9] | 60.4 [56–65] | 0.21 |
| dP/dtmax, mm Hg/s | 349 [309–403] | 462 [379–578] | 0.02 |
| dP/dtmin, mm Hg/s | −272 [−292 to 259] | −264 [−305 to 227] | 0.67 |
| Tau, ms | 52 [47–61] | 53 [44–58] | 0.97 |
| Ea, mm Hg/mL | 0.35 [0.30–0.44] | 0.41 [0.29–0.45] | 0.73 |
Values are mean±SD, median [interquartile range], or n (%). Ao Sats indicates aortic saturations; BMI, body mass index; BO, balloon occlusion; BP, blood pressure; CCS, Canadian Cardiovascular Society Functional Classification of Angina; CI, cardiac index; CO, cardiac output; Cr, creatinine; dP/dtmax, maximum rate of isovolumic contraction; dP/dtmin, maximum rate of isovolumic relaxation; Ea, effective arterial elastance; EDP, end‐diastolic pressure; EDV, end‐diastolic volume; EF, ejection fraction; ESP, end‐systolic pressure; ESV, end‐systolic volume; Hb, hemoglobin; MAP, mean arterial pressure; MI, myocardial infarction; MPAP, mean pulmonary arterial pressure; MRAP, mean right atrial pressure; NYHA Class, New York Heart Association Classification; PA Sats, pulmonary artery saturations; RP, rapid pacing; SV, stroke volume; SW, stroke work; Tau, time constant of diastolic relaxation.
Effect of BO on RV Function
Occlusion of the RCA was associated with deterioration in markers of systolic and diastolic function compared to baseline. Table 2 shows that at the end of BO, SW, ejection fraction, and dP/dtmax were significantly reduced; Tau and EDP increased. Systolic function reached suprabaseline levels at 1 minute after reperfusion with significant increases in dP/dtmax. SW, Ea, and ejection fraction were also elevated but not significantly so. Although diastolic parameters improved modestly 1 minute after reperfusion, they did not return to baseline levels. Figure 2 compares systolic and diastolic function at baseline and 15‐minute recovery. The RV was stunned at 15‐minute recovery; RV diastolic function remained impaired with elevated Tau and EDP compared with baseline. Systolic function (ejection fraction) was also impaired at 15 minutes. SW, dP/dtmax, and Ea also trended toward impairment.
Table 2.
RV Hemodynamic Data for BO Group
| Baseline | BO | 1‐Min Recovery | P Value | 15‐Min Recovery | P Value | |
|---|---|---|---|---|---|---|
| Heart rate, beats/min | 63±12 | 60±10.6 | 65±13 | 0.09 | 64±11 | 0.64 |
| SW, mm Hg·mL | 1369±660 | 678±354 | 1466±780 | 0.30 | 1162±446 | 0.07 |
| CO, L/min | 5.1±0.9 | 3.5±1.0 | 5.4±1.0 | 0.14 | 4.8±0.7 | 0.35 |
| SV, mL | 81.6±14.7 | 59.3±15.4 | 83.7±13.4 | 0.50 | 76.6±14.9 | 0.37 |
| ESP, mm Hg | 29.5±9.3 | 28.6±8.9 | 31.2±12.3 | 0.14 | 31.5±11.1 | 0.04 |
| EDP, mm Hg | 8.1±4.0 | 9.6±4.5 | 8.7±4.0 | 0.03 | 9.2±3.9 | <0.01 |
| ESV, mL | 111.8±44.2 | 122.7±31.0 | 106.8±37.3 | 0.43 | 128.6±44.4 | 0.19 |
| EDV, mL | 154.2±40.9 | 151.0±26.8 | 152.2±26.4 | 0.82 | 165.4±38.7 | 1.0 |
| EF, % | 55.7±9.0 | 41.2±11.9 | 57.8±11.0 | 0.22 | 47.8±6.3 | 0.005 |
| dP/dtmax, mm Hg/s | 359±84 | 294±98 | 434±141 | 0.02 | 342±99 | 0.40 |
| dP/dtmin, mm Hg/s | −278±96 | −191±76 | −264±119 | 0.19 | −278±114 | 0.99 |
| Tau, ms | 54.2±14.7 | 107.0±48.6 | 69.2±18.7 | <0.001 | 67.3±18.9 | <0.01 |
| Ea, mm Hg/mL | 0.37±0.09 | 0.52±0.20 | 0.38±0.12 | 0.50 | 0.44±0.18 | 0.08 |
Values are mean±SD. P values are displayed for comparison with baseline values. BO indicates balloon occlusion; CO, cardiac output; dP/dtmax, maximum rate of isovolumic contraction; dP/dtmin, maximum rate of isovolumic relaxation; Ea, effective arterial elastance; EDP, end‐diastolic pressure; EDV, end‐diastolic volume; EF, ejection fraction; ESP, end‐systolic pressure; ESV, end‐systolic volume; SV, stroke volume; SW, stroke work; Tau, time constant of diastolic relaxation.
Figure 2.
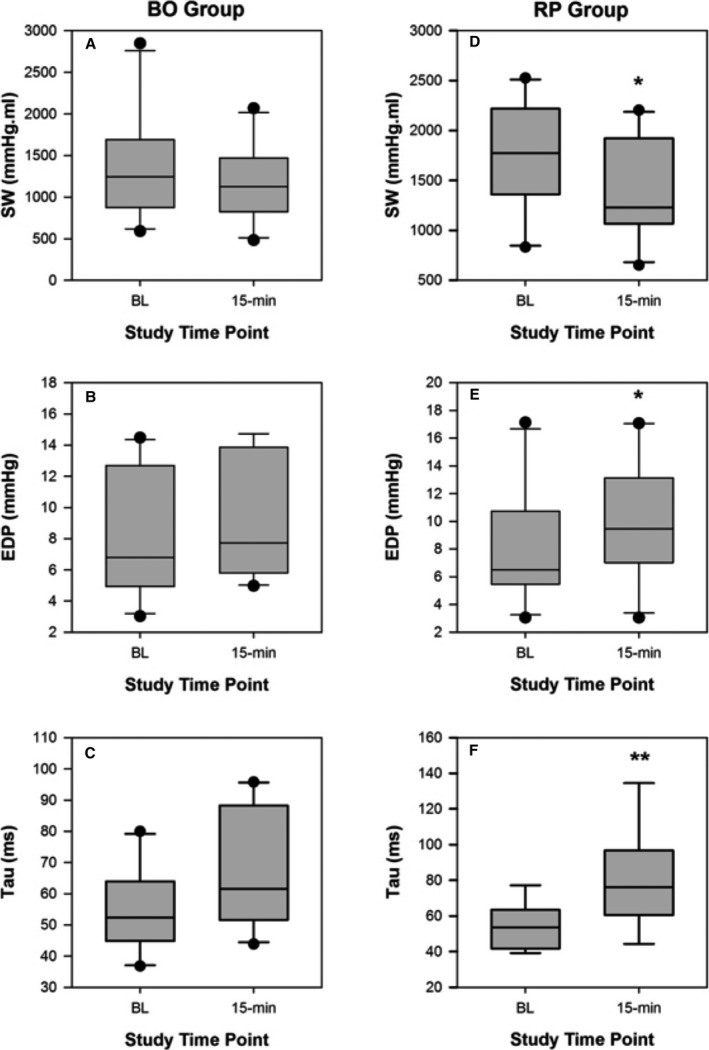
Comparisons of systolic function (A) stroke work (SW) and diastolic function (B) end‐diastolic pressure (EDP) and (C) Tau, at baseline (BL) and 15‐minute recovery following low‐pressure balloon occlusion (BO) of the right coronary artery (15 minutes). Comparisons of systolic function (D) SW and diastolic function (E) EDP and (F) Tau, at BL and 15 minutes after rapid pacing (RP) for valve deployment (15 minutes). *P<0.05, **P<0.01.
Effect of RP on RV Function
Table 3 also shows the changes in parameters of RV function throughout the study. There was a decline in all parameters of RV systolic function during RP. EDP and Tau both rose during RP. At 1‐minute recovery after RP, again there was improvement in parameters of RV systolic function to suprabaseline levels. As stroke volume recovered to almost baseline levels, but end‐systolic pressure remained elevated, the Ea was elevated at 1 minute. Improvement peaked at 2 minutes, shown in Figure 3. Diastolic function remained impaired, with EDP remaining elevated and Tau not returning to baseline levels at 1 minute into recovery. Ischemic dilatation of the RV was noted at 1‐minute recovery.
Table 3.
Hemodynamic Data for RP Group
| Baseline | RP | 1‐Min Recovery | P Value | 15‐Min Recovery | P Value | |
|---|---|---|---|---|---|---|
| Heart rate, beats/min | 65±14 | 194.3±33.4 | 72.1±19.0 | 0.11 | 64±14 | 0.80 |
| SW, mm Hg·mL | 1760±566 | 153±172 | 1871±759 | 0.68 | 1426±522 | 0.02 |
| CO, L/min | 5.5±1.7 | 7.1±4.1 | 5.7±1.8 | 0.76 | 5.4±1.3 | 0.79 |
| SV, mL | 88.3±31.6 | 36.7±18.7 | 85.8±43.2 | 0.81 | 87.2±24.6 | 0.82 |
| ESP, mm Hg | 30.8±7.6 | 28.2±5.9 | 43.0±16.0 | 0.01 | 30.6±6.4 | 0.94 |
| EDP, mm Hg | 8.1±4.2 | 11.9±3.7 | 12.1±4.1 | <0.001 | 9.9±4.4 | 0.03 |
| ESV, mL | 87.8±35.9 | 124.4±45.6 | 115.1±62.4 | 0.03 | 107.1±52.0 | 0.08 |
| EDV, mL | 139.1±52.5 | 118.5±35.0 | 152.6±58.8 | 0.14 | 143.3±57.3 | 0.67 |
| EF, % | 61.5±10.3 | 30.7±16.6 | 55.8±23.2 | 0.41 | 59.6±13.4 | 0.68 |
| dP/dtmax, mm Hg/s | 493±134 | 315±224 | 668±341 | 0.05 | 427±133 | 0.09 |
| dP/dtmin, mm Hg/s | −262±59 | −202±102 | −395±244 | 0.07 | −226±51 | 0.07 |
| Tau, ms | 54.2±12.7 | 139.7±49.4 | 79.6±41.9 | 0.07 | 81.0±27.5 | <0.01 |
| Ea, mm Hg/mL | 0.40±0.17 | 1.06±0.64 | 0.61±0.37 | 0.03 | 0.38±0.12 | 0.60 |
| Ees/Ea, mm Hg/mL | 1.25±0.31 | ··· | ··· | ··· | 0.85±0.43 | < 0.01 |
| PRSW, mm Hg/mL3 | 23.1±12.1 | ··· | ··· | ··· | 16.9±10.9 | 0.06 |
Values are mean±SD. P values are displayed for comparison with baseline values. CO indicates cardiac output; dP/dtmax, maximum rate of isovolumic contraction; dP/dtmin, maximum rate of isovolumic relaxation; Ea, effective arterial elastance; EDP, end‐diastolic pressure; EDV, end‐diastolic volume; Ees, end‐systolic elastance; EF, ejection fraction; ESP, end‐systolic pressure; ESV, end‐systolic volume; PRSW, preload recruitable stroke work; RP, rapid pacing; SV, stroke volume; SW, stroke work; Tau, time constant of diastolic relaxation.
Figure 3.
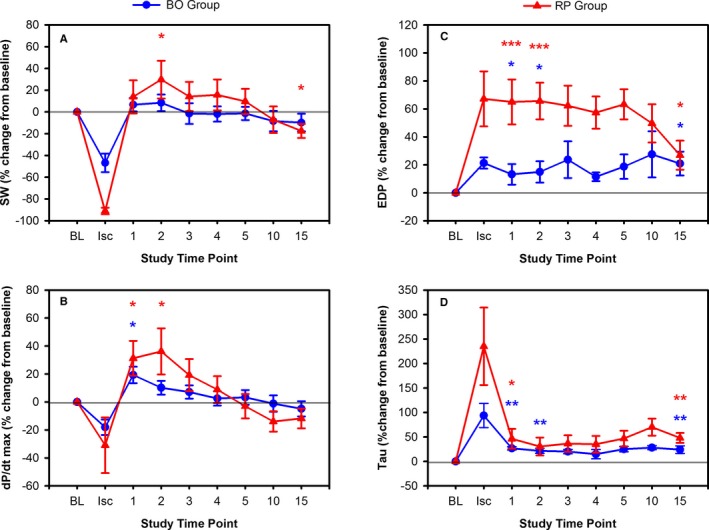
Comparison of change in systolic function for balloon occlusion (BO) (blue) and rapid pacing (RP) (red) groups using (A) stroke work (SW), (B) dP/dt max, and diastolic function (C) end diastolic pressure (EDP), (D) Tau. Values are normalized to baseline (BL), and shown at indicated time points including peak ischemia at the end of BO or during valve deployment (Isc), and throughout recovery. *P < 0.05, **P < 0.01, ***P < 0.001.
Figure 2 shows a comparison of baseline and 15‐minute recovery in the RP group. Over the 15‐minute recovery period, SW declined to below baseline levels, EDP reduced compared with its peak during RP but had not returned to baseline levels at 15 minutes, and Tau also remained elevated into recovery. End‐systolic pressure returned to baseline, with a subsequent normalization of Ea. There was a persistence of the increases in ESV and EDV at 15‐minute recovery. Load‐independent parameters also showed that RV systolic dysfunction persisted at 15 minutes into recovery: preload‐recruitable stroke work: baseline 23.1±12.1 versus recovery 16.9±10.9 mm Hg/mL3, P=0.06 and end‐systolic elastance and ventriculo‐arterial coupling: baseline 1.25±0.31 versus recovery 0.85±0.43 mm Hg/mL, P<0.01.
Comparison of Response to RP Versus BO
The RP group experienced more profound RV dysfunction than seen after RCA BO occlusion but because RP mechanically impairs the RV, as well as producing ischemia, these are not directly comparable. Table 4 compares conductance catheter data for load‐dependent parameters during recovery. At 1‐minute recovery, the RP group had a significantly higher EDP compared with the BO group and Tau also trended toward being worse in the RP group. However, dP/dtmin after RP experienced a greater early improvement at 1‐minute recovery compared with baseline. Ea was also significantly higher in the RP group at 1‐minute recovery driven by augmentation of end‐systolic pressure, rather than an increase in afterload. Both dP/dtmin and Ea findings are consistent with a larger hyperemic response, repaying a larger oxygen debt and transiently augmenting RV contractility during 1‐minute recovery. There was no significant difference in Ea at 15 minutes between RP and BO groups. Similarly, an intergroup difference in ESV was only observed at 1 minute. Diastolic dysfunction at 15‐minute recovery, measured by EDP and Tau, was noted in both groups but the groups were not significantly different. However, there was a trend toward more dysfunction as measured by dP/dtmin and Tau after RP.
Table 4.
Comparison of PCI Group and TAVR Group Following Injury and Recovery
| Δ vs Baseline, % ±SD | 1‐Min Recovery | 15‐Min Recovery | ||||
|---|---|---|---|---|---|---|
| BO | RP | P Value | BO | RP | P Value | |
| Heart rate | +2.49±4.1 | +11.7±21.8 | 0.20 | +1.57±7.39 | −0.61±12.7 | 0.64 |
| SW | +6.70±19.6 | +13.9±48.2 | 0.66 | −9.76±25.7 | −17.4±20.6 | 0.47 |
| CO | +5.70±10.9 | +6.93±29.7 | 0.90 | −3.77±18.5 | +1.95±22.8 | 0.55 |
| SV | +3.32±12.2 | −2.94±28.7 | 0.53 | −4.64±20.0 | +2.04±15.7 | 0.42 |
| ESP | +4.13±8.9 | +40.5±39.9 | 0.01 | +5.36±8.1 | +1.31±16.8 | 0.51 |
| EDP | +13.2±23.3 | +65.0±50.8 | <0.01 | +20.9±27.0 | +26.9±33.0 | 0.66 |
| ESV | −2.50±15.6 | +27.3±37.8 | 0.03 | +19.3±37.1 | 22.4±38.3 | 0.86 |
| EDV | +2.13±21.5 | +11.9±19.0 | 0.30 | +10.8±29.9 | 5.38±21.5 | 0.65 |
| EF | +3.58±8.5 | −9.69±31.3 | 0.21 | −12.9±13.1 | −1.36±25.6 | 0.22 |
| dP/dtmax | +19.3±18.5 | +31.2±39.4 | 0.40 | −4.88±17.2 | −11.8±22.1 | 0.45 |
| dP/dtmin | −8.14±13.2 | +44.6±66.4 | 0.02 | −0.42±26.1 | −12.2±21.6 | 0.29 |
| Tau | +26.7±10.6 | +46.3±60.5 | 0.32 | +24.5±24.5 | +48.1±30.5 | 0.07 |
| Ea | +1.37±11.7 | +55.3±57.8 | <0.01 | +16.0±29.5 | +2.16±26.5 | 0.28 |
Values are mean±SD. BO indicates balloon occlusion; CO, cardiac output; dP/dtmax, maximum rate of isovolumic contraction; dP/dtmin, maximum rate of isovolumic relaxation; Ea, effective arterial elastance; EDP, end‐diastolic pressure; EDV, end‐diastolic volume; EF, ejection fraction; ESP, end‐systolic pressure; ESV, end‐systolic volume; PCI, percutaneous coronary intervention; RP, rapid pacing; SV, stroke volume; SW, stroke work; TAVR, transcatheter aortic valve replacement; Tau, time constant of diastolic relaxation.
Discussion
This is the first study to assess the effect of coronary BO and RP on RV function and assess early recovery using the conductance technique. Although RP appears to elicit a more profound ischemic insult, both impaired RV systolic and diastolic function. Recovery of suprabaseline systolic function was observed initially followed by a steady decline to sub‐baseline levels at 15‐minute recovery in both groups. Diastolic parameters (RVEDP and Tau) also improved after reperfusion but did not recover to baseline values at 15‐minute recovery, indicating RV stunning.
Ischemic dysfunction following balloon occlusion of the LV has been demonstrated in a number of studies.25, 26, 27 These studies have shown that residual stunning affects both systolic and diastolic function, observed at up to 30 minutes after the initial ischemic insult. However, there is rapid improvement in systolic performance during reperfusion at 1 minute because of increased coronary flow. During reactive hyperemia, LV function can be augmented to suprabaseline levels, a phenomenon known as the Gregg effect.28 Increased volume of the microvasculature following reperfusion causes stretch‐activated calcium channels to open. The resultant influx of calcium increases myocyte contractility and briefly masks the effect of ischemic LV dysfunction. Detection of stunning is therefore confounded during this period of reactive hyperemia; the strict definition of stunning mandates that coronary flow must be normal. This occurs later at 15 to 30 minutes into recovery.
Previous investigation into the effect of coronary balloon occlusion on the RV did not report stunning, but this study only assessed RV function within the first minute of reperfusion.8 Others have reported that the RV is more resistant to ischemia than the LV.2 However, by assessing RV ischemic recovery for a longer time‐frame, we have confirmed that not only does balloon occlusion of the RCA and RP result in significant ischemic RV dysfunction, on a par with the LV, but this also persists (stunning) at 15 minutes. Diastolic indices are particularly affected, which possibly reflects their position in the ischemic cascade, although increased microcirculatory turgor during hyperemia (the scaffold effect) may also be responsible.9
RP has previously been shown to cause ischemia,11 and we observe it to cause a profound ischemic insult. It is likely that both supply and demand ischemia occur during RP, acting in concert to increase global metabolic demand of the myocardium, while coronary perfusion pressure gradient, and with it coronary flow, are also diminished.29 This is reflected in the larger hyperemic augmentation of RV function after RP we observe in RV systolic indices, repaying the larger oxygen debt.
Whereas BO causes both systolic and diastolic load‐dependent indices to remain impaired at 15 minutes into recovery, RP appears to predominantly affect diastolic load‐dependent indices. It is possible that ventricular interdependence and load dependency explain the lack of persistent systolic impairment observed after RP. Up to 50% of RV function is derived from the LV, so that improvement in LV function, as may occur after relief of aortic stenosis by TAVR, will augment load‐dependent RV systolic performance.30 When load‐independent indices of RV function are assessed, RV systolic dysfunction persists into recovery at 15 minutes after cessation of RP.
Clinical Relevance
The emergence of RV impairment that is persistent and may affect intraoperative hemodynamic stability during PCI and TAVR is relevant to clinical practice. In particular, although RP has been described as safe and reversible,10 there are case reports of hemodynamic collapse after RP for TAVR.31 Our study provides confirmatory evidence of the temporal pattern of the detrimental hemodynamic effects of RP that may be encountered during TAVR and provides a stimulus to explore new TAVR implantation techniques that do not require RP. Although speculative, the observation of RV dysfunction after BO may suggest that the duration of coronary BO required during PCI should be kept to a minimum to avoid potential hemodynamic compromise 10 to 15 minutes after the initial ischemic insult. Our findings may be particularly pertinent in patients with limited RV functional reserve.
Limitations
The 15‐minute recovery period was chosen for both ethical and practical reasons. Ideally a longer follow‐up to confirm that parameters eventually returned to baseline values would have fulfilled the reversible definition of stunning. Similarly, confirming normalization of coronary flow at 15‐minutes recovery would have been desirable, although for technical and ethical reasons this was not feasible. However, we and others have confirmed recovery of basal flow velocity within this time frame in the left coronary artery.9 It was not possible to directly compare the ischemic burden by RV functional indices as the action of RP mechanically impairs ventricular function. Measurement of lactate during the procedure would have allowed confirmation of the ischemic burden induced by each insult, but this was not assessed. Alternative methods for assessment of RV function such as echocardiography were not employed within this protocol, but may have provided data confirming the conductance catheter results, and could be considered for future studies. The study was not powered to test the hypothesis that there was a difference between the 2 types of RV ischemic insult, particularly with a number of confounding factors within the group demographics. Comparisons between BO and RP groups should therefore only be considered hypothesis‐generating.
Conclusions
Ischemic RV dysfunction with residual stunning is observed after coronary BO and RP that may contribute to intraoperative hemodynamic instability when employing these interventional therapies, particularly when patients have limited RV functional reserve.
Sources of Funding
This study was funded by an NIHR CSO Healthcare Scientist Doctoral Fellowship Grant (NIHR‐HCS‐D12‐14) and supported by the Cambridge BRC. The views expressed are those of the authors and not necessarily those of the University of Cambridge, the NHS, the NIHR, or the Department of Health.
Disclosures
None.
Acknowledgments
The authors thank the staff in the cardiac catheter laboratory at Papworth Hospital for their assistance throughout the study and thank our patients for participating in this study.
(J Am Heart Assoc. 2017;6:e005820 DOI: 10.1161/JAHA.117.005820.)28588092
References
- 1. Haddad F, Hunt SA, Rosenthal DN, Murphy DJ. Right ventricular function in cardiovascular disease, part I: anatomy, physiology, aging, and functional assessment of the right ventricle. Circulation. 2008;117:1436–1448. [DOI] [PubMed] [Google Scholar]
- 2. O'Rourke RA, Dell'Italia LJ. Diagnosis and management of right ventricular myocardial infarction. Curr Probl Cardiol. 2004;29:6–47. [DOI] [PubMed] [Google Scholar]
- 3. de Groote P, Millaire A, Foucher‐Hossein C, Nugue O, Marchandise X, Ducloux G, Lablanche JM. Right ventricular ejection fraction is an independent predictor of survival in patients with moderate heart failure. J Am Coll Cardiol. 1998;32:948–954. [DOI] [PubMed] [Google Scholar]
- 4. Ito S, Pislaru SV, Soo WM, Huang R, Greason KL, Mathew V, Sandhu GS, Eleid MF, Suri RM, Oh JK, Nkomo VT. Impact of right ventricular size and function on survival following transcatheter aortic valve replacement. Int J Cardiol. 2016;221:269–274. [DOI] [PubMed] [Google Scholar]
- 5. Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, Tuzcu EM, Webb JG, Fontana GP, Makkar RR, Brown DL, Block PC, Guyton RA, Pichard AD, Bavaria JE, Herrmann HC, Douglas PS, Petersen JL, Akin JJ, Anderson WN, Wang D, Pocock S. Transcatheter aortic‐valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med. 2010;363:1597–1607. [DOI] [PubMed] [Google Scholar]
- 6. Farrer‐Brown G. Vascular pattern of myocardium of right ventricle of human heart. Br Heart J. 1968;30:679–686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Haupt HM, Hutchins GM, Moore GW. Right ventricular infarction: role of the moderator band artery in determining infarct size. Circulation. 1983;67:1268–1272. [DOI] [PubMed] [Google Scholar]
- 8. Bishop A, White P, Groves P, Chaturvedi R, Brookes C, Redington A, Oldershaw P. Right ventricular dysfunction during coronary artery occlusion: pressure‐volume analysis using conductance catheters during coronary angioplasty. Heart. 1997;78:480–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Hoole SP, Heck PM, White PA, Read PA, Khan SN, West NE, O'Sullivan M, Dutka DP. Stunning and cumulative left ventricular dysfunction occurs late after coronary balloon occlusion in humans insights from simultaneous coronary and left ventricular hemodynamic assessment. JACC Cardiovasc Interv. 2010;3:412–418. [DOI] [PubMed] [Google Scholar]
- 10. Webb JG, Pasupati S, Achtem L, Thompson CR. Rapid pacing to facilitate transcatheter prosthetic heart valve implantation. Catheter Cardiovasc Interv. 2006;68:199–204. [DOI] [PubMed] [Google Scholar]
- 11. Turer AT, Addo TA, Martin JL, Sabatine MS, Lewis GD, Gerszten RE, Keeley EC, Cigarroa JE, Lange RA, Hillis LD, de Lemos JA. Myocardial ischemia induced by rapid atrial pacing causes troponin T release detectable by a highly sensitive assay: insights from a coronary sinus sampling study. J Am Coll Cardiol. 2011;57:2398–2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hodgson JM, Mancini GB. Relation between graded, subcritical impairments of coronary flow reserve and regional myocardial dysfunction induced by atrial pacing in dogs. J Am Coll Cardiol. 1985;5:1116–1124. [DOI] [PubMed] [Google Scholar]
- 13. Forsberg LM, Tamas E, Vanky F, Nielsen NE, Engvall J, Nylander E. Left and right ventricular function in aortic stenosis patients 8 weeks post‐transcatheter aortic valve implantation or surgical aortic valve replacement. Eur J Echocardiogr. 2011;12:603–611. [DOI] [PubMed] [Google Scholar]
- 14. Okada DR, Rahmouni HW, Herrmann HC, Bavaria JE, Forfia PR, Han Y. Assessment of right ventricular function by transthoracic echocardiography following aortic valve replacement. Echocardiography. 2014;31:552–557. [DOI] [PubMed] [Google Scholar]
- 15. Lindman BR, Maniar HS, Jaber WA, Lerakis S, Mack MJ, Suri RM, Thourani VH, Babaliaros V, Kereiakes DJ, Whisenant B, Miller DC, Tuzcu EM, Svensson LG, Xu K, Doshi D, Leon MB, Zajarias A. Effect of tricuspid regurgitation and the right heart on survival after transcatheter aortic valve replacement: insights from the Placement of Aortic Transcatheter Valves II inoperable cohort. Circ Cardiovasc Interv. 2015;8:e002073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Axell RG, Hoole SP, Hampton‐Till J, White PA. RV diastolic dysfunction: time to re‐evaluate its importance in heart failure. Heart Fail Rev. 2015;20:363–373. [DOI] [PubMed] [Google Scholar]
- 17. Bishop A, White P, Chaturvedi R, Brookes C, Redington A, Oldershaw P. Resting right ventricular function in patients with coronary artery disease: pressure volume analysis using conductance catheters. Int J Cardiol. 1997;58:223–228. [DOI] [PubMed] [Google Scholar]
- 18. White PA, Bishop AJ, Conroy B, Oldershaw PJ, Redington AN. The determination of volume of right ventricular casts using a conductance catheter. Eur Heart J. 1995;16:1425–1429. [DOI] [PubMed] [Google Scholar]
- 19. Baan J, van der Velde ET, de Bruin HG, Smeenk GJ, Koops J, van Dijk AD, Temmerman D, Senden J, Buis B. Continuous measurement of left ventricular volume in animals and humans by conductance catheter. Circulation. 1984;70:812–823. [DOI] [PubMed] [Google Scholar]
- 20. McKay RG, Spears JR, Aroesty JM, Baim DS, Royal HD, Heller GV, Lincoln W, Salo RW, Braunwald E, Grossman W. Instantaneous measurement of left and right ventricular stroke volume and pressure‐volume relationships with an impedance catheter. Circulation. 1984;69:703–710. [DOI] [PubMed] [Google Scholar]
- 21. McCabe C, White PA, Hoole SP, Axell RG, Priest AN, Gopalan D, Taboada D, MacKenzie Ross R, Morrell NW, Shapiro LM, Pepke‐Zaba J. Right ventricular dysfunction in chronic thromboembolic obstruction of the pulmonary artery: a pressure‐volume study using the conductance catheter. J Appl Physiol (1985). 2014;116:355–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Weiss JL, Frederiksen JW, Weisfeldt ML. Hemodynamic determinants of the time‐course of fall in canine left ventricular pressure. J Clin Invest. 1976;58:751–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Raff GL, Glantz SA. Volume loading slows left ventricular isovolumic relaxation rate. Evidence of load‐dependent relaxation in the intact dog heart. Circ Res. 1981;48:813–824. [DOI] [PubMed] [Google Scholar]
- 24. Matsubara H, Takaki M, Yasuhara S, Araki J, Suga H. Logistic time constant of isovolumic relaxation pressure‐time curve in the canine left ventricle. Better alternative to exponential time constant. Circulation. 1995;92:2318–2326. [DOI] [PubMed] [Google Scholar]
- 25. Read PA, Hoole SP, White PA, Khan FZ, O'Sullivan M, West NE, Dutka DP. A pilot study to assess whether glucagon‐like peptide‐1 protects the heart from ischemic dysfunction and attenuates stunning after coronary balloon occlusion in humans. Circ Cardiovasc Interv. 2011;4:266–272. [DOI] [PubMed] [Google Scholar]
- 26. Giblett JP, Axell RG, White PA, Clarke SJ, McCormick L, Read PA, Reinhold J, Brown AJ, O'Sullivan M, West NE, Dutka DP, Hoole SP. Glucagon‐like peptide‐1 derived cardioprotection does not utilize a KATP‐channel dependent pathway: mechanistic insights from human supply and demand ischemia studies. Cardiovasc Diabetol. 2016;15:99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. McCormick LM, Hoole SP, White PA, Read PA, Axell RG, Clarke SJ, O'Sullivan M, West NE, Dutka DP. Pre‐treatment with glucagon‐like peptide‐1 protects against ischemic left ventricular dysfunction and stunning without a detected difference in myocardial substrate utilization. JACC Cardiovasc Interv. 2015;8:292–301. [DOI] [PubMed] [Google Scholar]
- 28. Gregg DE. Effect of coronary perfusion pressure or coronary flow on oxygen usage of the myocardium. Circ Res. 1963;13:497–500. [DOI] [PubMed] [Google Scholar]
- 29. Selle A, Figulla HR, Ferrari M, Rademacher W, Goebel B, Hamadanchi A, Franz M, Schlueter A, Lehmann T, Lauten A. Impact of rapid ventricular pacing during TAVI on microvascular tissue perfusion. Clin Res Cardiol. 2014;103:902–911. [DOI] [PubMed] [Google Scholar]
- 30. Santamore WP, Dell'Italia LJ. Ventricular interdependence: significant left ventricular contributions to right ventricular systolic function. Prog Cardiovasc Dis. 1998;40:289–308. [DOI] [PubMed] [Google Scholar]
- 31. Kim EH, Lee SM, Lee JH, Lee SH, Park PW, Gwon HC. Unexpected and fatal hemodynamic collapse during transapical transcatheter aortic valve implantation—a case report. Korean J Anesthesiol. 2013;64:360–362. [DOI] [PMC free article] [PubMed] [Google Scholar]


