Abstract
Background
Mean transaortic pressure gradient (MTPG) has never been validated as a predictor of mortality in patients with severe aortic stenosis. We sought to determine the value of MTPG to predict mortality in a large prospective cohort of severe aortic stenosis patients with preserved left ventricular ejection fraction and to investigate the cutoff of 60 mm Hg, proposed in American guidelines.
Methods and Results
A total of 1143 patients with severe aortic stenosis defined by aortic valve area ≤1 cm2 and MTPG ≥40 mm Hg were included. The population was divided into 3 groups according to MTPG: between 40 and 49 mm Hg, between 50 and 59 mm Hg, and ≥60 mm Hg. The end point was all‐cause mortality. MTPG was ≥60 mm Hg in 392 patients. Patients with MTPG ≥60 mm Hg had a significantly increase risk of mortality compared with patients with MTPG <60 mm Hg (hazard ratio [HR]=1.62 [1.27–2.05] P<0.001), even for the subgroup of asymptomatic or minimally symptomatic patients (HR=1.56 [1.04–2.34] P=0.032). After adjustment for established outcome predictors, patients with MTPG ≥60 mm Hg had a significantly higher risk of mortality than patients with MTPG <60 mm Hg (HR=1.71 [1.33–2.20] P<0.001), even after adjusting for surgery as a time‐dependent variable (HR=1.71 [1.43–2.11] P<0.001). Similar results were observed for the subgroup of asymptomatic or minimally symptomatic patients (HR=1.70 [1.10–2.32] P=0.018 and HR=1.68 [1.20–2.36] P=0.003, respectively).
Conclusions
This study shows the negative prognostic impact of high MTPG (≥60 mm Hg), on long‐term outcome of patients with severe aortic stenosis with preserved left ventricular ejection fraction, irrespective of symptoms.
Keywords: asymptomatic and minimally symptomatic patients, mean transaortic pressure gradient, mortality, prognosis, severe aortic stenosis
Subject Categories: Valvular Heart Disease, Cardiovascular Disease, Echocardiography, Mortality/Survival
Introduction
Severe aortic stenosis (SAS) constitutes a public health issue with a serious impact for healthcare providers. Elective surgery is recommended for severe symptomatic aortic stenosis (AS) and for some groups of asymptomatic individuals with SAS and preserved left ventricular (LV) ejection fraction (LVEF).1, 2 The cutoff values for grading the severity of aortic valve stenosis have been changed. For example, the current definition of severe aortic valve stenosis includes an aortic valve area (AVA) <1.0 cm2, mean transaortic pressure gradient (MTPG) >40 mm Hg, or aortic maximal velocity (Vmax) >4.0 m/s1 introducing somewhat lower MTPG cutoff values for severe stenosis compared with the previous values of AVA ≤1.0 cm2 and MTPG >50 mm Hg.3 The management of asymptomatic patients with SAS by either conservative treatment or aortic valve replacement remains controversial. The American College of Cardiology/American Heart Association guidelines define “very severe aortic stenosis” when Vmax is ≥5 m/s and/or when MTPG is ≥60 mm Hg.2 In comparison, European Society of Cardiology guidelines define very severe aortic stenosis as Vmax >5.5 m/s1 with no reference to MTPG. This cut‐off of 60 mm Hg, defined by American guidelines, is based on expert opinion, but is not supported by scientific evidence. There is an excellent correlation between MTPG measured by continuous‐wave Doppler and mean gradient measured by catheter,4 which make it one of the most powerful markers of the severity of AS provided cardiac output is normal and LVEF is preserved. However, MTPG has never been validated as a predictor of outcome in SAS.
The present study included consecutive patients diagnosed with SAS in the echocardiography laboratories of 2 French tertiary centers (Amiens and Lille). The aims of the study were 2‐fold: (1) to evaluate the prognostic impact of MTPG on all‐cause mortality in a large cohort of SAS patients with preserved LVEF, and in a subroup of asymptomatic or minimally symptomatic patients, and (2) to evaluate the cut‐off of 60 mm Hg defined by American guidelines to predict mortality in these 2 populations.
Methods
Study Population
Between 2000 and 2015, patients aged ≥18 years diagnosed with ≥ mild AS (aortic valve calcification with restricted systolic motion and AVA <2 cm2) and LVEF ≥50% were prospectively identified and included in an electronic database. The following patients were excluded: (1) patients with more than mild aortic and/or mitral regurgitation; (2) patients with prosthetic valves, congenital heart disease (with the exception of bicuspid aortic valves), supravalvular or subvalvular AS, or dynamic LV outflow tract obstruction; and (3) patients who refused to participate in the study. The present analysis focused on 1143 patients with severe AS defined by AVA ≤1 cm2 and/or AVA normalized to body surface area (BSA) ≤0.6 cm2/m2 and MTPG ≥40 mm Hg.
Patients were retrospectively divided into 3 groups according to their baseline MTPG: Group 1 included patients with MTPG between 40 and 49 mm Hg; group 2 included patients with MTPG between 50 and 59 mm Hg; and group 3 included patients with MTPG ≥60 mm Hg. We subsequently carried out subgroup analyses on the asymptomatic or minimally symptomatic SAS population (n=559). Symptoms were ascertained by each patient's personal cardiologist. Patients with atypical chest pain and elderly patients with minimal dyspnea not clearly related to AS were considered to be minimally symptomatic. A comorbidity index comprising the sum of the patient's individual comorbidities was calculated.5 Coronary artery disease was defined by the presence of a documented history of acute coronary syndrome, coronary artery disease previously confirmed by coronary angiography (reduction of normal diameter ≥50% in the left main coronary artery and ≥70% in the right coronary, left anterior descending, and circumflex arteries), or history of coronary revascularization. Institutional review board approval was obtained before conducting the study, which was conducted in accord with institutional policies, national legal requirements, and the revised Helsinki declaration.
Echocardiography
All patients underwent a comprehensive Doppler‐echocardiographic study, using commercially available ultrasound systems. LV outflow tract was measured in parasternal long‐axis view with zoom on the aortic valve. LV outflow tract velocity time integral was recorded in apical 5 chambers view. Transaortic mean pressure gradient and peak aortic jet velocity were systematically measured in several acoustic windows (apical 5 chambers, right parasternal, suprasternal, and epigastric views), and the highest recorded value was taken into account. Pressure gradients were calculated using the simplified Bernoulli equation.4 AVA was calculated using the continuity equation AVA=(2πR2/4)×aortic velocity time integral/LV outflow tract velocity time integral.6 The alignment of both pulsed‐ and continuous‐wave Doppler was optimized to be parallel with the flow. When patients were in sinus rhythm, 3 cardiac cycles were averaged for all measures. For patients in atrial fibrillation, 5 cardiac cycles were averaged. LV dimensions were assessed from parasternal long‐axis views by 2‐dimensional–guided M‐mode using the leading‐edge methodology at end‐diastole and end‐systole. LVEF was calculated using Simpson's biplane method. LV mass was estimated by the formula on the basis of linear measurements and indexed for BSA.7 Stroke volume was calculated by multiplying the LV outflow tract area with the LV outflow tract velocity time integral. Left atrial volume was measured in LV end‐systole by Simpson's biplane method in apical 2‐ and 4‐chamber views and indexed to BSA.8, 9 LV filling pressures were estimated by E/e′ ratio.10 Systolic pulmonary artery pressure was recorded from the maximum peak tricuspid regurgitation velocity in any view using the simplified Bernoulli equation. Right atrial pressure was estimated from the inspiratory collapse of the inferior vena cava.11
Follow‐up
Median follow‐up was 38.0 [6–190] months. Patients were followed by clinical consultations and echocardiography in the outpatient clinics of the 2 tertiary centers. A few patients were followed in public hospitals or private practices by referring cardiologists working in collaboration with the tertiary centers. All surviving patients had a minimum follow‐up of at least 2 years. In surviving patients (n=840), follow‐up was completed until the end of the study (2015 or 2016) for 762 (91%). Events were ascertained by direct patient interview and clinical examination and/or by repeated follow‐up letters, questionnaires, and telephone calls to physicians, patients, and (if necessary) next of kin. The primary end point was all‐cause mortality. Clinical decisions regarding medical management and referral for surgery were taken by the heart team with the approval of the patients’ cardiologist in accord with current practice guidelines.1
Statistical Analysis
SPSS software (v 18.0; IBM Corp, Armonk, NY) was used for statistical analysis. Continuous variables are expressed as mean value ±1 SD or median and interquartile range, and categorical variables are expressed as percentages and counts. The relationship between baseline continuous variables and the various groups was explored using 1‐way ANOVA tests (for normally distributed variables) or Kruskal–Wallis tests (for non‐normally distributed variables). Pearson's chi‐square statistic or Fisher's exact test were used to examine the association between the various groups and baseline categorical variables. The significance between the referent group and the other groups was examined when a significant difference across categories was observed. Individual differences were compared with Mann–Whitney U tests (with Bonferroni correction for multiple comparisons) and Tukey tests for normally distributed data. Multiple linear regressions with backward elimination were used for multivariable analysis. MTPG was used as the dependent variable. P<0.05 was considered statistically significant. All P values are the results of 2‐tailed tests. Event rates ±1 SE of the 3 groups were estimated according to the Kaplan–Meier method and compared with 2‐sided log‐rank tests. Uni‐ and multivariate analyses of time to events were performed using Cox proportional hazards model with MTPG as independent variable in categorical format. Model‐building techniques were not used, and covariates considered to have a potential prognostic impact on an epidemiological basis were entered into the models. These covariates were: age, sex, BSA, hypertension, New York Heart Association class, coronary artery disease, history of atrial fibrillation, comorbidity index, LVEF, and aortic valve surgery, which was treated as a time‐dependent covariate. Hazard ratios (HRs) and 95% CIs were estimated for all‐cause mortality. The proportional hazards assumption was confirmed using statistics and graphs based on the Schoenfeld residuals. For continuous variables, the assumption of linearity was assessed by plotting residuals against independent variables. Penalized smoothing splines (P‐splines) were used to illustrate the association between MTPG as a continuous variable and the risk of mortality. Subgroup analyses (stratified by age, sex, coronary artery disease, hypertension, New York Heart Association class, atrial fibrillation, and BSA) were conducted to determine the homogeneity of the association between high MTPG (≥60 mm Hg) and the outcome variable. First, we estimated the effect of MTPG on the risk of overall mortality in each subgroup using a Cox univariate model and then we formally tested for first‐order interactions in Cox models by entering interaction terms, separately for each subgroup.
Results
Baseline Demographic and Clinical Characteristics
The study population consisted of 1143 patients (Table 1) with a mean age of 74 years (range, 62–86), comprising 52% of men; 25% of patients were in New York Heart Association class 3 or 4. Almost three quarters of patients had a history of hypertension, around one quarter of patients had a history of atrial fibrillation, and just over 50% had a history of coronary artery disease. Table 1 shows the baseline demographic clinical and echocardiographic characteristics of the study patients according to MTPG: 426 patients had an MTPG between 40 and 49 mm Hg (37%), 325 had an MTPG between 50 and 59 mm Hg (29%), and 392 had an MTPG ≥60 mm Hg (34%). No significant differences were observed between the study groups in terms of age, sex, BSA, hypertension, diabetes mellitus, coronary artery disease, history of atrial fibrillation, and Charlson comorbidity index. Compared with patients with MTPG <60 mm Hg, patients with high MTPG (≥60 mm Hg) less commonly had a diagnosis of hypertension (P=0.048) or diabetes mellitus (P=0.026; Table 2). Baseline clinical and echocardiographic characteristics of asymptomatic or minimally symptomatic SAS populations are presented in Tables S1 and S2. Nine‐hundred thirty‐three patients underwent aortic valve replacement (AVR), and 199 (24%) had at least 1 associated coronary artery bypass graft. Surgical AVR was performed in 834 patients (287 in the MTPG between 40 and 49 mm Hg group, 237 in the MTPG between 50 and 59 mm Hg group and 310 in the MTPG ≥60 mm Hg group), and transcatheter aortic valve replacement (TAVR) was performed in 99 patients (11%; 36 in the MTPG between 40 and 49 mm Hg group, 29 in the MTPG between 50 and 59 mm Hg group, and 34 in the MTPG ≥60 mm Hg group). Baseline clinical and echocardiographic characteristics of patients who underwent AVR are displayed in online Table 3. Compared with patients who underwent surgical AVR, patients who underwent TAVR were older (P<0.001), with smaller BSA (P<0.001), more often in New York Heart Association class III and IV (P=0.001), and, as expected, they had more comorbidities (P<0.001).
Table 1.
Baseline Demographic, Clinical, and Echocardiographic Characteristics of the Study Patients With Severe Aortic Stenosis According to MTPG
| Variable | Overall Population (n=1143) | MTPG 40 to 49 mm Hg (n=426) | MTPG 50 to 59 mm Hg (n=325) | MTPG ≥60 mm Hg (n=392) | P Value |
|---|---|---|---|---|---|
| Demographics, baseline data, and symptoms | |||||
| Age, y | 74±12 | 74±11 | 75±12 | 74±12 | 0.096 |
| Male sex, n (%) | 597 (52.2) | 229 (53.8) | 163 (50.2) | 205 (52.3) | 0.619 |
| BSA, m2 | 1.87±0.2 | 1.87±0.2 | 1.87±0.2 | 1.86±0.2 | 0.822 |
| NYHA, n (%) | |||||
| 1 to 2 | 857 (75) | 324 (76.1) | 244 (72.9) | 296 (75.5) | 0.591 |
| 3 to 4 | 286 (25) | 102 (23.9) | 88 (27.1) | 96 (24.5) | |
| Medical history and risk factors | |||||
| Hypertension, n (%) | 829 (72.5) | 315 (73.9) | 244 (75.1) | 270 (68.9) | 0.128 |
| Diabetes mellitus, n (%,n) | 311 (27.2) | 127 (29.8) | 93 (28.6) | 91 (23.2) | 0.085 |
| Coronary artery disease, n (%) | 588 (51.4) | 216 (50.7) | 183 (56.3)a | 189 (48.2) | 0.090 |
| Past atrial fibrillation, n (%) | 305 (26.7) | 112 (26.3) | 96 (29.5) | 97 (24.7) | 0.343 |
| Charlson comorbidity index | 1.9±1.8 | 2±2.1 | 1.9±1.7 | 1.8±1.7 | 0.138 |
| Echocardiographic parameters | |||||
| Aortic valve | |||||
| Aortic valve area, cm2 | 0.70 (0.58–0.82) | 0.77 (0.64–0.90) | 0.70 (0.59–0.82)b | 0.62 (0.52–0.74)b | <0.001 |
| Aortic valve area indexed to BSA, cm2/m2 | 0.37 (0.32–0.44) | 0.42 (0.35–0.48) | 0.37 (0.32–0.43)b | 0.34 (0.29–0.39)b | <0.001 |
| Peak aortic jet velocity, m/s | 4.6 (4.27–5.0) | 4.2 (4.1–4.3) | 4.6 (4.4–4.7)b | 5.2 (5–5.6)b | <0.001 |
| Transaortic mean pressure gradient, mm Hg | 53 (46–65) | 44 (42–46) | 53 (51–56)b | 70 (64–79)b | <0.001 |
| Stroke volume, mL | 79 (66–92) | 76 (63–90) | 79 (66–91) | 82 (69–96)b | <0.001 |
| Indexed stroke volume, mL/m2 | 42 (36–49) | 41 (34.6–47.6) | 42 (35.5–49) | 44 (38–51)b | <0.001 |
| LV function | |||||
| LV end‐diastolic diameter, mm | 49 (45–54) | 49 (44–54) | 49 (45–54) | 50 (46–53) | 0.376 |
| LV end‐systolic diameter, mm | 30 (26–35) | 30 (26–35) | 30 (26–35) | 30 (27–34) | 0.994 |
| Ejection fraction, % | 64 (59–69) | 63 (58–68) | 64 (60–70) | 65 (60–70)a | 0.024 |
| Indexed LV mass, g/m2 | 132 (108–159) | 121 (101–147) | 131 (104–155)a | 142 (119–169)b | <0.001 |
| LV end‐diastolic volume, mL | 113 (88–153) | 111 (85–151) | 112 (85–149) | 115 (93–160) | 0.298 |
| LV end‐systolic volume, mL | 41 (28–58) | 40 (28–58) | 42 (28–58) | 41 (29–58) | 0.959 |
| E/E′ | 12.2 (8.7–16.9) | 12.2 (8.7–17.1) | 12 (8.5–17) | 12.4 (8.7–16.4) | 0.776 |
| Left atrial volume index, mL/m2 | 41 (31–52) | 41 (30–52) | 40 (31–52) | 41 (32–51) | 0.674 |
| sPAP, mm Hg | 33 (28–40) | 33 (28–40) | 33 (28–41) | 33 (28–40) | 0.953 |
Continuous normally distributed variables are expressed as mean ±1 SD, non‐normally distributed continuous variables are expressed as median (25th and 75th percentiles), and categorical variables as percentages and counts. BSA indicates body surface area; LV, left ventricular; MTPG, mean transaortic pressure gradient; NYHA, New York Heart Association class; sPAP systolic pulmonary artery pressure.
P<0.05 individual category vs MTPG 40 to 49 mm Hg.
P<0.001 individual category vs MTPG 40 to 49 mm Hg.
Table 2.
Baseline Demographic, Clinical, and Echocardiographic Characteristics of the Study Patients With Severe Aortic Stenosis According to MTPG < or ≥60 mm Hg
| Variable | Overall Population (n=1143) | MTPG <60 mm Hg (n=751) | MTPG ≥60 mm Hg (n=392) | P Value |
|---|---|---|---|---|
| Demographics, baseline data, and symptoms | ||||
| Age, y | 74±12 | 75±11 | 74±12 | 0.095 |
| Male sex, n (%) | 597 (52.2) | 392 (52.2) | 205 (52.3) | 0.975 |
| BSA, m2 | 1.87±0.2 | 1.87±0.2 | 1.86±0.2 | 0.550 |
| NYHA, n (%) | ||||
| 1 to 2 | 857 (75) | 561 (74.7) | 296 (75.5) | 0.764 |
| 3 to 4 | 286 (25) | 190 (25.3) | 96 (24.5) | |
| Medical history and risk factors | ||||
| Hypertension, n (%) | 829 (72.5) | 559 (74.7) | 270 (68.9) | 0.048 |
| Diabetes mellitus, n (%) | 311 (27.2) | 220 (29.5) | 91 (23.2) | 0.026 |
| Coronary artery disease, n (%) | 588 (51.4) | 399 (53.1) | 189 (48.2) | 0.115 |
| Past atrial fibrillation, n (%) | 305 (26.7) | 208 (27.7) | 97 (24.7) | 0.284 |
| Charlson comorbidity index | 1.9±1.8 | 2±1.9 | 1.8±1.7 | 0.074 |
| Echocardiographic parameters | ||||
| Aortic valve | ||||
| Aortic valve area, cm2 | 0.70 (0.58–0.82) | 0.74 (0.61–0.86) | 0.62 (0.52–0.74) | <0.001 |
| Aortic valve area indexed to BSA, cm2/m2 | 0.37 (0.32–0.44) | 0.39 (0.34–0.46) | 0.34 (0.29–0.39) | <0.001 |
| Peak aortic jet velocity, m/s | 4.6 (4.27–5.0) | 4.36 (4.18–4.6) | 5.2 (5–5.6) | <0.001 |
| Transaortic mean pressure gradient, mm Hg | 53 (46–65) | 48 (44–52) | 70 (64–79) | <0.001 |
| Stroke volume, mL | 79 (66–92) | 76 (65–90) | 82 (69–96) | <0.001 |
| Indexed stroke volume, mL/m2 | 42 (36–49) | 41 (35–48) | 44 (38–51) | <0.001 |
| LV function | ||||
| LV end‐diastolic diameter, mm | 49 (45–54) | 49 (44–54) | 50 (46–53) | 0.175 |
| LV end‐systolic diameter, mm | 30 (26–35) | 30 (26–35) | 30 (27–34) | 0.915 |
| Ejection fraction, % | 64 (59–69) | 64 (59–69) | 65 (60–70) | 0.011 |
| Indexed LV mass, g/m2 | 132 (108–159) | 125 (102–150) | 142 (119–169) | <0.001 |
| LV end‐diastolic volume, mL | 113 (88–153) | 111 (85–150) | 115 (93–160) | 0.120 |
| LV end‐systolic volume, mL | 41 (28–58) | 41 (28–58) | 41 (29–58) | 0.773 |
| E/E′ | 12.2 (8.7–16.9) | 12.1 (8.6–17) | 12.4 (8.7–16.4) | 0.638 |
| Left atrial volume index, mL/m2 | 41 (31–52) | 40 (30–52) | 41 (32–51) | 0.379 |
| sPAP, mm Hg | 33 (28–40) | 33 (28–40) | 33 (28–40) | 0.757 |
Continuous normally distributed variables are expressed as mean ±1 SD, non‐normally distributed continuous variables are expressed as median (25th and 75th percentiles), and categorical variables as percentages and counts. BSA indicates body surface area; LV, left ventricular; MTPG, mean transaortic pressure gradient; NYHA, New York Heart Association class; sPAP systolic pulmonary artery pressure.
Table 3.
Relative Risk of Events (All‐Cause Mortality) of the Study Population With Severe AS and With Asymptomatic or Minimally Symptomatic Severe AS During Follow‐up Associated With MTPG
| MTPG | HR [CI] | P Value | HR [CI] | P Value |
|---|---|---|---|---|
| Severe AS (n=1143) | Asymptomatic or Minimally Symptomatic Severe AS (n=559) | |||
| Univariate analysis | P<0.001a | P=0.211a | ||
| MTPG 40 to 49 mm Hg | Referent group | Referent group | ||
| MTPG 50 to 59 mm Hg | 1.16 [0.87–1.55] | 0.311 | 0.88 [0.55–1.41] | 0.597 |
| MTPG ≥60 mm Hg | 1.30 [1.14–1.49] | <0.001 | 1.17 [0.94–1.47] | 0.164 |
| Multivariate analysis | ||||
| Model 1b | P<0.001a | P=0.042a | ||
| MTPG 40 to 49 mm Hg | Referent group | Referent group | ||
| MTPG 50 to 59 mm Hg | 1.04 [0.76–1.42] | 0.796 | 1.02 [0.61–1.71] | 0.930 |
| MTPG ≥60 mm Hg | 1.32 [1.15–1.52] | <0.001 | 1.29 [1–1.65] | 0.042 |
| Model 2c | P<0.001a | P=0.009a | ||
| MTPG 40 to 49 mm Hg | Referent group | Referent group | ||
| MTPG 50 to 59 mm Hg | 0.99 [0.78–1.27] | 0.958 | 0.99 [0.65–1.49] | 0.949 |
| MTPG ≥60 mm Hg | 1.32 [1.18–1.47] | <0.001 | 1.28 [1.05–1.55] | 0.012 |
AS indicates aortic stenosis; MTPG, mean transaortic pressure gradient.
P for global comparison.
Model 1: adjustment for age, sex, body surface area, hypertension, coronary artery disease, history of atrial fibrillation, comorbidity index, and ejection fraction.
Model 2: adjustment for age, sex, body surface area, hypertension, coronary artery disease, history of atrial fibrillation, comorbidity index, ejection fraction, and aortic valve surgery as a time‐dependent covariate.
Echocardiographic Characteristics
Echocardiographic variables (Table 1) showed that, as MTPG increased, AVA (P<0.001) and indexed AVA (P<0.001) decreased. As expected, peak aortic jet velocity (P<0.001) increased with increasing MTPG. Stroke volume (P<0.001), indexed stroke volume (P<0.001), and ejection fraction (P=0.024) were higher in the high‐MTPG group (MTPG ≥60 mm Hg) than in the referent group (MTPG between 40 and 49 mm Hg). Indexed LV mass (P<0.001) was higher in the group with MTPG ≥60 mm Hg than in the referent group. No significant difference was observed between groups in terms of left atrial volume index and LV filling pressures. Compared with patients with MTPG <60 mm Hg, patients with high MTPG (≥60 mm Hg) had more‐severe AS with smaller AVA (P<0.001) and indexed AVA (P<0.001), higher stroke volumes (P<0.001), LVEF (P=0.011), and indexed LV mass (P<0.001; Table 2). They were no difference in aortic stenosis severity between the TAVR group and the surgical AVR group, but the LV repercussion of AS was more important in the TAVR group, with greater LV systolic and diastolic diameters (respectively P=0.005 and <0.001), higher LV mass (P=0.002), lower LVEF (P=0.005), and more‐elevated LA volume (P=0.034) and pulmonary pressures (P=0.007; Table S3).
Prognostic Impact of High MTPG
Three hundred three patients died during follow‐up, 84 in the MTPG between 40 and 49 mm Hg group, 66 in the MTPG between 50 and 59 mm Hg group, and 153 in the MTPG ≥60 mm Hg group. Among patients who underwent surgical AVR, 180 died (36 in the MTPG between 40 and 49 mm Hg group, 33 in the MTPG between 50 and 59 mm Hg group, and 111 in the MTPG ≥60 mm Hg group). In the TAVR population, we recorded 17 deaths (4 in the MTPG between 40 and 49 mm Hg group, none in the MTPG between 50 and 59 mm Hg group, and 13 in the MTPG ≥60 mm Hg group).
Prognosis value of MTPG in overall population
Four‐year survival was 86±4% for patients with MTPG between 40 and 49 mm Hg, 85±3% for patients with MTPG between 50 and 59 mm Hg, and 72±4% for patients with MTPG ≥60 mm Hg (log rank, P<0.001; Figure 1A). On univariate Cox analysis, overall mortality was globally different between groups (HR=1.31 [1.15–1.50]; P<0.001; Table 3). No significant difference was observed between the MTPG 40 to 49 mm Hg group and the MTPG 50 to 59 mm Hg group (P=0.311). On multivariate analysis, the MTPG ≥60 mm Hg group exhibited a significant excess mortality after covariate adjustment (P<0.001; Figure 2A; Table 3, model 1) and after further adjustment for surgery (P<0.001; Figure S1A; Table 3, model 2) compared with the MTPG 40 to 49 mm Hg group. No significant difference was observed between the MTPG 40 to 49 mm Hg group and the MTPG 50 to 59 mm Hg group after covariate adjustment (P=0.796; Table 3, model 1) and after further adjustment for surgery (P=0.958; Table 3, model 2). Comparison of the high‐MTPG group (MTPG ≥60 mm Hg) and the intermediate group (MTPG 50–59 mm Hg) showed that this excess risk persisted after covariate adjustment and after further adjustment for surgery (P=0.002 and <0.001, respectively). No significant difference was observed between patients with MTPG between 60 and 69 mm Hg and patients with MTPG ≥70 mm Hg, (P=0.260) even after covariate adjustment. By dividing the whole population into 2 groups with a 60 mm Hg MTPG cutoff, 4‐year survival was 86±4% for patients with MTPG <60 mm Hg and 72±4% for patients with MTPG ≥60 mm Hg (log rank, P<0.001; Figure 1B). On univariate Cox analysis, overall mortality was significantly higher in the MTPG ≥60 mm Hg group (HR=1.62 [1.27–2.05]; P<0.001; Table 4), and this excess risk persisted after covariate adjustment (HR=1.71 [1.33–2.2]; P<0.001; Figure 2B; Table 4) and after further adjustment for surgery (HR=1.71 [1.43–2.11]; P<0.001; Figure S1B; Table 4). After excluding patients who underwent TAVR, overall mortality remained significantly higher in the MTPG ≥60 mm Hg group compared with patients with MTPG <60 mm Hg (HR=1.53 [1.21–1.93]; P<0.001), and this excess risk persisted after covariate adjustment (HR=1.68 [1.32–2.15]; P<0.001) and after further adjustment for surgery (HR=1.69 [1.45–2.01]; P<0.001). The nature of the relationship between MTPG as a continuous variable and the risk of mortality during follow‐up was estimated using spline functions for MTPG (Figure 3). The association between MTPG ≥60 mm Hg and risk of death was consistent in subgroups of patients with SAS, and no significant interactions were observed between MTPG ≥60 mm Hg and any of the subgroups (Figure 4).
Figure 1.

Kaplan–Meier curves of survival in the severe aortic stenosis population (n=1143) according to mean transaortic pressure gradient (MTPG). A, Dividing the population into 3 groups (MTPG 40–50 mm Hg, MTPG 50–59 mm Hg, and MTPG ≥60 mm Hg). B, Dividing the population into 2 groups (MTPG <60 mm Hg and MTPG ≥60 mm Hg).
Figure 2.
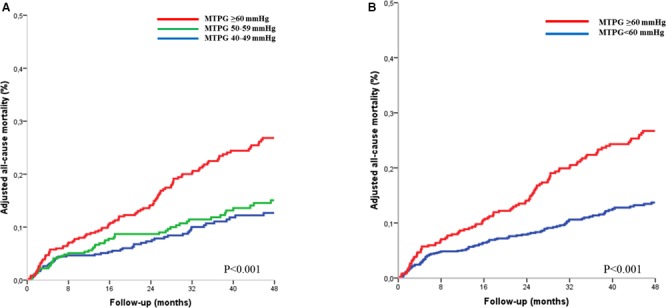
Adjusted all‐cause mortality curves in the severe aortic stenosis population (n=1143) according to mean transaortic pressure gradient (MTPG), divided into 3 groups (MTPG 40–50 mm Hg, MTPG 50–59 mm Hg, and MTPG ≥60 mm Hg) (A) and into 2 groups (MTPG <60 mm Hg and MTPG ≥60 mm Hg) (B). Curves are adjusted for age, sex, body surface area, hypertension, New York Heart Association class, coronary artery disease, history of atrial fibrillation, comorbidity index, and left ventricular ejection fraction.
Table 4.
Relative Risk of Events (All‐Cause Mortality) of the Study Population With Severe AS and With Asymptomatic or Minimally Symptomatic Severe AS During Follow‐up According to MTPG < or ≥60 mm Hg
| MTPG | HR [CI] | P Value | HR [CI] | P Value |
|---|---|---|---|---|
| Severe AS (n=1143) | Asymptomatic or Minimally Symptomatic Severe AS (n=559) | |||
| Univariate analysis | ||||
| MTPG <60 mm Hg | Referent group | Referent group | ||
| MTPG ≥60 mm Hg | 1.62 [1.27–2.05] | <0.001 | 1.56 [1.04–2.34] | 0.032 |
| Multivariate analysis | ||||
| Model 1a | ||||
| MTPG <60 mm Hg | Referent group | Referent group | ||
| MTPG ≥60 mm Hg | 1.71 [1.33–2.2] | <0.001 | 1.70 [1.10–2.32] | 0.018 |
| Model 2b | ||||
| MTPG <60 mm Hg | Referent group | Referent group | ||
| MTPG ≥60 mm Hg | 1.71 [1.43–2.11] | <0.001 | 1.68 [1.20–2.36] | 0.003 |
AS indicates aortic stenosis; HR, hazard ratio; MTPG, mean transaortic pressure gradient.
Model 1: adjustment for age, sex, body surface area, hypertension, coronary artery disease, history of atrial fibrillation, comorbidity index, and left ventricular ejection fraction.
Model 2: adjustment for age, sex, body surface area, hypertension, coronary artery disease, history of atrial fibrillation, comorbidity index, left ventricular ejection fraction, and aortic valve surgery as a time‐dependent covariate.
Figure 3.
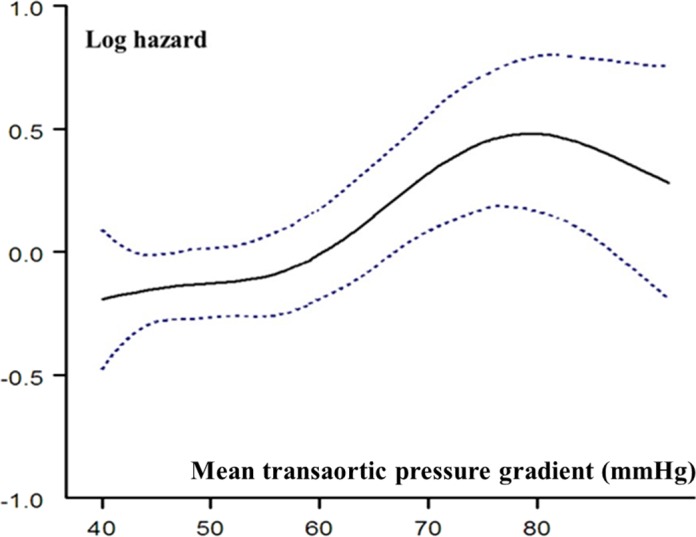
Relationship between MTPG and risk of mortality during follow‐up. Hazard ratio (solid line) and 95% CIs are estimated in a Cox model with MTPG represented as a spline function and adjusted for age, sex, body surface area, hypertension, New York Heart Association class, coronary artery disease, history of atrial fibrillation, comorbidity index, and left ventricular ejection fraction.
Figure 4.
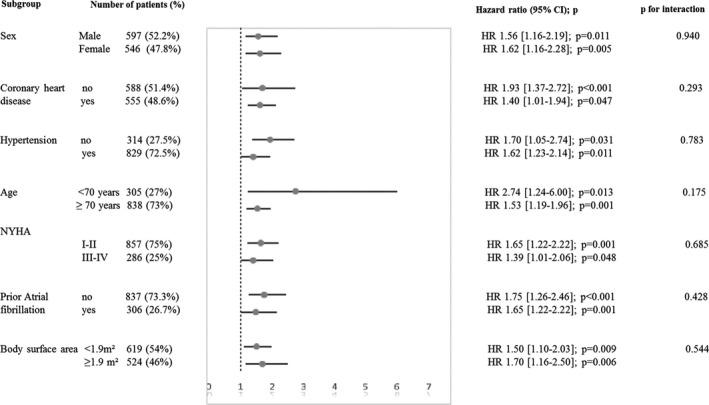
Hazard ratio (HR) and 95% confidence interval for risk of all‐cause mortality associated with mean transaortic pressure gradient ≥60 mm Hg in subgroups of patients with severe aortic stenosis. NYHA indicates New York Heart Association.
Prognostic value of MTPG in asymptomatic or minimally symptomatic SAS
Four‐year survival was 89±6% for patients with MTPG between 40 and 49 mm Hg, 87±5% for patients with MTPG between 50 and 59 mm Hg, and 81±4% for patients with MTPG ≥60 mm Hg (log rank, P=0.100; Figure 5A). On univariate Cox analysis, no significant difference was observed between groups (P=0.211; Table 3). On multivariate analysis, the MTPG ≥60 mm Hg group exhibited a significant excess mortality after covariate adjustment (P=0.042; Figure 6A; Table 3, model 1) and after further adjustment for surgery (P=0.012; Figure S2A; Table 3, model 2) compared with the MTPG 40 to 49 mm Hg group. No significant difference was observed between the MTPG 40 to 49 mm Hg group and the MTPG 50 to 59 mm Hg group after covariate adjustment (P=0.930; Table 3, model 1) and after further adjustment for surgery (P=0.949; Table 3, model 2). No significant difference was observed between the high‐MTPG group (MTPG ≥60 mm Hg) and the intermediate group (MTPG 50–59 mm Hg), after covariate adjustment (P=0.205), but the high‐MTPG group exhibited an excess risk of mortality after further adjustment for surgery (P=0.031). No significant difference was observed between patients with MTPG between 60 and 69 mm Hg and patients with MTPG ≥70 mm Hg patients (P=0.749), even after covariate adjustment (P=0.459). Dividing the whole population into 2 groups with a 60 mm Hg MTPG cutoff, 4‐year survival was 89±4% for patients with MTPG <60 mm Hg and 81±4% for patients with MTPG ≥60 mm Hg (log rank, P=0.032; Figure 5B). On Cox univariate analysis, overall mortality was higher in the MTPG ≥60 mm Hg group (HR=1.56 [1.04–2.34]; P=0.032; Table 4). This excess risk persisted after covariate adjustment (HR=1.70 [1.10–2.62]; P=0.018; Figure 6B; Table 4) and after further adjustment for surgery (HR=1.68 [1.20–2.36]; P=0.003; Figure S2B; Table 4).
Figure 5.
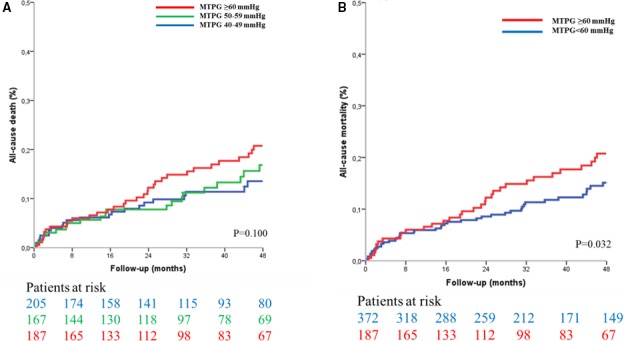
Kaplan–Meier curves of survival in asymptomatic and minimally symptomatic patients with severe aortic stenosis (n=559) according to mean transaortic pressure gradient (MTPG). A, Dividing the population into 3 groups (MTPG 40–50 mm Hg, MTPG 50–59 mm Hg, and MTPG ≥60 mm Hg). B, Dividing the population into 2 groups (MTPG <60 mm Hg and MTPG ≥60 mm Hg).
Figure 6.
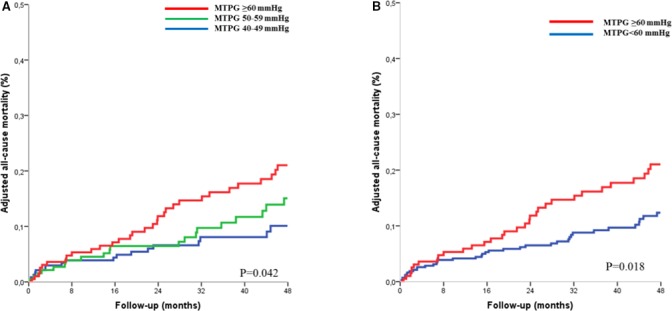
Adjusted all‐cause mortality curves in asymptomatic and minimally symptomatic patients with severe aortic stenosis (n=559) according to mean transaortic pressure gradient (MTPG), divided into 3 groups (MTPG 40–50 mm Hg, MTPG 50–59 mm Hg, and MTPG ≥60 mm Hg) (A) and into 2 groups (MTPG < 60 mm Hg and MTPG ≥60 mm Hg). (B). Curves are adjusted for age, sex, body surface area, hypertension, New York Heart Association class, coronary artery disease, history of atrial fibrillation, comorbidity index, and left ventricular ejection fraction.
Discussion
This study shows that MTPG is a powerful prognostic factor in patients with severe AS. Echocardiographic measurement of MTPG is considered more robust than AVA to evaluate the severity of AS in the presence of normal transvalvular flow.1 MTPG assessed by Doppler echocardiography is reliable and is well correlated with values obtained by cardiac catheterization.4, 12, 13, 14 Previous studies have focused exclusively on Vmax as a marker of severity and a predictor of outcome. American College of Cardiology/American Heart Association guidelines define “very severe aortic stenosis” in the presence of Vmax ≥5 m/s and/or MTPG ≥60 mm Hg.2 European Society of Cardiology guidelines use a different Vmax cutoff of 5.5 m/s for “very severe aortic stenosis”1 with no reference to MTPG.1 The present study is the first to investigate the prognostic impact of MTPG on mortality in patients with severe AS and preserved LVEF. Our results show that MTPG greater than the 60 mm Hg cutoff strongly impacts on outcome in patients with severe AS, and that the predictive power of MTPG ≥60 mm Hg remains unaltered after adjustment for factors known to be major prognostic determinants, such as age, sex, hypertension, symptoms, coronary artery disease, atrial fibrillation, comorbidity, LVEF, and aortic valve surgery. High MTPG at baseline is associated with a more than 70% increase in the risk of all‐cause mortality during follow‐up, even in the group of patients with no or minimal symptoms at diagnosis. Therefore, in clinical practice, MTPG should be systematically measured and taken into consideration for AS severity quantification and for treatment decisions.
The prognostic value of MTPG in patients with asymptomatic severe AS has been investigated only by a few exercise‐stress echocardiography studies. In a series of 69 patients with AS, an increase in MTPG ≥18 mm Hg at peak exercise was predictive of early cardiac events.15 Maréchaux et al16 highlighted that elevation of MTPG ≥20 mm Hg added an incremental prognostic value to resting echocardiography parameters in patients with AS and was associated with a 2‐fold increase in the relative risk of cardiovascular death or the need for AVR during follow‐up. However, solid outcome data on the impact of high MTPG detected at rest in patients with severe AS and preserved LVEF have not yet been reported.
AS management is difficult, particularly in asymptomatic patients, with a persistent controversy between conservative treatment and surgical management.17, 18, 19, 20 On the one hand, as reported,21, 22, 23 the 2‐year risk of cardiac events (onset of symptoms, AVR, or mortality) in asymptomatic patients with hemodynamically significant AS is high, whereas the risk of sudden death is low. Conversely, AVR should ideally be performed before the onset of irreversible left heart remodeling that reduces the long‐term benefit of surgery.24 Therefore, the decision to operate asymptomatic individuals with severe AS must be based on careful assessment of the benefit‐risk balance. Several studies have investigated the value of Vmax for risk stratification of patients with asymptomatic severe AS. In a study based on 163 asymptomatic patients with severe AS, Vmax >4.4 m/s was the best cutoff for the prediction of a composite end point, including onset of symptoms, AVR, and death.25 In this study,25 2‐year event‐free survival was 30% for patients with Vmax >4.4 m/s versus 60% for patients with Vmax <4.4 m/s. Among 622 asymptomatic patients with AS and Vmax ≥4 m/s, a Vmax ≥4.5 m/s was associated with an ≈50% increased risk of cardiac events (death or AVR)26 in a report on 116 asymptomatic patients with severe AS, a Vmax ≥5.5 m/s was associated with almost 90% increase in the risk of events (death or AVR).27 These studies undeniably validated Vmax as an outcome marker in AS, but the cutoff for identifying patients in whom the severity of the valvular obstacle is critical remains unclear, especially for patients with no or minimal symptoms, as reflected by the different criteria used to define “very severe AS” proposed by US and European guidelines.1, 2 Our study including a large number of patients has the advantage of identifying a unique MTPG cutoff value of 60 mm Hg as proposed by the American Heart Association/American College of Cardiology guidelines for risk stratification in severe AS with a clear impact on mortality risk irrespective of clinical presentation. We deliberately chose not to use a combined end point associating death and AVR because of the possible bias of AVR related to the personal physician's assessment of disease severity. Patients with severe AS and preserved LVEF in whom MTPG is ≥60 mm Hg at the time of diagnosis represent a high‐risk group with more than 70% increase in all‐cause mortality during follow‐up, even when they initially present no or minimal symptoms. Early elective surgery, at the asymptomatic stage, should be considered only for selected patients who present a low operative risk and high risk on medical management.2 From this point of view, MTPG represents a valuable marker of risk and should be integrated in the decision‐making process for surgery in asymptomatic AS.
Strengths and Limitations
Because follow‐up data were obtained retrospectively, our study therefore presents inherent limitations of this type of analysis. The specific indications for surgery during follow‐up were not recorded in our database. However, diagnosis and follow‐up were performed by cardiologists with expertise in valvular heart disease, and surgical decisions were taken by the heart team with the approval of the patient's physician in accord with current practice guidelines. We analyzed the subgroup of patients with no or minimal symptoms, because we consider that, in elderly patients with AS, it is often very difficult to differentiate asymptomatic individuals from patients with minimal symptoms. The mean pulmonary pressure observed in the study groups was lower than previously reported,28, 29 presumably because of exclusion of patients with LV dysfunction and with significant associated mitral regurgitation, which accounted for 27% to 53% in other studies.28, 29 Finally, this study exclusively concerned patients with severe AS and preserved LVEF and those without significant valve regurgitation. Further studies are needed to evaluate the role of MTPG in other subsets of patients with severe AS.
Conclusions
The present study shows that high MTPG is an independent predictor of mortality in patients with severe AS and preserved LVEF on medical and surgical treatment. Detection of MTPG ≥60 mm Hg at the time of AS diagnosis is associated with a greater than 70% increase in the risk of death during follow‐up, even for asymptomatic or minimally symptomatic patients. Our findings suggest that MTPG assessment should be part of the decision‐making process for surgery in asymptomatic or minimally symptomatic patients with severe AS.
Disclosures
None.
Supporting information
Table S1. Baseline Demographic, Clinical, and Echocardiographic Characteristics of the Study Patients With Asymptomatic or Minimally Symptomatic Severe Aortic Stenosis According to Mean Transaortic Pressure Gradient (MTPG)
Table S2. Baseline Demographic, Clinical, and Echocardiographic Characteristics of the Study Patients With Asymptomatic or Minimally Symptomatic Severe Aortic Stenosis According to Mean Transaortic Pressure Gradient (MTPG) < or ≥60 mm Hg
Table S3. Baseline Demographic, Clinical, and Echocardiographic Characteristics of Patients Who Underwent Aortic Valve Replacement (AVR)
Figure S1. Adjusted all‐cause mortality curves in the severe AS population (n=1143) according to mean transaortic pressure gradient (MTPG), divided into 3 groups (A) and into 2 groups (B).
Figure S2. Adjusted all‐cause mortality curves in asymptomatic and minimally symptomatic patients with severe aortic stenosis (n=559) according to mean transaortic pressure gradient (MTPG), divided into 3 groups (A) and into 2 groups (B).
(J Am Heart Assoc. 2017;6:e005850 DOI: 10.1161/JAHA.117.005850.)28572283
References
- 1. Joint Task Force on the Management of Valvular Heart Disease of the European Society of C, European Association for Cardio‐Thoracic S , Vahanian A, Alfieri O, Andreotti F, Antunes MJ, Baron‐Esquivias G, Baumgartner H, Borger MA, Carrel TP, De Bonis M, Evangelista A, Falk V, Iung B, Lancellotti P, Pierard L, Price S, Schafers HJ, Schuler G, Stepinska J, Swedberg K, Takkenberg J, Von Oppell UO, Windecker S, Zamorano JL, Zembala M. Guidelines on the management of valvular heart disease (version 2012). Eur Heart J. 2012;33:2451–2496. [DOI] [PubMed] [Google Scholar]
- 2. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP III, Guyton RA, O'Gara PT, Ruiz CE, Skubas NJ, Sorajja P, Sundt TM III, Thomas JD; Members AATF . 2014 AHA/ACC guideline for the management of patients with valvular heart disease: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. Circulation. 2014;129:2440–2492. [DOI] [PubMed] [Google Scholar]
- 3. Bonow RO, Carabello B, de Leon AC, Edmunds LH, Fedderly BJ, Freed MD, Gaasch WH, McKay CR, Nishimura RA, O'Gara PT, O'Rourke RA, Rahimtoola SH, Ritchie JL, Cheitlin MD, Eagle KA, Gardner TJ, Garson A, Gibbons RJ, Russell RO, Ryan TJ, Smith SC. ACC/AHA guidelines for the management of patients with valvular heart disease. Executive summary. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee on Management of Patients With Valvular Heart Disease). J Heart Valve Dis. 1998;7:672–707. [PubMed] [Google Scholar]
- 4. Currie PJ, Seward JB, Reeder GS, Vlietstra RE, Bresnahan DR, Bresnahan JF, Smith HC, Hagler DJ, Tajik AJ. Continuous‐wave Doppler echocardiographic assessment of severity of calcific aortic stenosis: a simultaneous Doppler‐catheter correlative study in 100 adult patients. Circulation. 1985;71:1162–1169. [DOI] [PubMed] [Google Scholar]
- 5. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. [DOI] [PubMed] [Google Scholar]
- 6. Skjaerpe T, Hegrenaes L, Hatle L. Noninvasive estimation of valve area in patients with aortic stenosis by Doppler ultrasound and two‐dimensional echocardiography. Circulation. 1985;72:810–818. [DOI] [PubMed] [Google Scholar]
- 7. Lang RM, Badano LP, Mor‐Avi V, Afilalo J, Armstrong A, Ernande L, Flachskampf FA, Foster E, Goldstein SA, Kuznetsova T, Lancellotti P, Muraru D, Picard MH, Rietzschel ER, Rudski L, Spencer KT, Tsang W, Voigt JU. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur Heart J Cardiovasc Imaging. 2015;16:233–270. [DOI] [PubMed] [Google Scholar]
- 8. Tsang TS, Barnes ME, Bailey KR, Leibson CL, Montgomery SC, Takemoto Y, Diamond PM, Marra MA, Gersh BJ, Wiebers DO, Petty GW, Seward JB. Left atrial volume: important risk marker of incident atrial fibrillation in 1655 older men and women. Mayo Clin Proc. 2001;76:467–475. [DOI] [PubMed] [Google Scholar]
- 9. Devereux RB, Alonso DR, Lutas EM, Gottlieb GJ, Campo E, Sachs I, Reichek N. Echocardiographic assessment of left ventricular hypertrophy: comparison to necropsy findings. Am J Cardiol. 1986;57:450–458. [DOI] [PubMed] [Google Scholar]
- 10. Yock PG, Popp RL. Noninvasive estimation of right ventricular systolic pressure by Doppler ultrasound in patients with tricuspid regurgitation. Circulation. 1984;70:657–662. [DOI] [PubMed] [Google Scholar]
- 11. Reisner SA, Lysyansky P, Agmon Y, Mutlak D, Lessick J, Friedman Z. Global longitudinal strain: a novel index of left ventricular systolic function. J Am Soc Echocardiogr. 2004;17:630–633. [DOI] [PubMed] [Google Scholar]
- 12. Hatle L. Noninvasive assessment and differentiation of left ventricular outflow obstruction with Doppler ultrasound. Circulation. 1981;64:381–387. [DOI] [PubMed] [Google Scholar]
- 13. Hatle L. Noninvasive assessment of valve lesions with Doppler ultrasound. Herz. 1984;9:213–221. [PubMed] [Google Scholar]
- 14. Castel AL, Maréchaux S, Laaouaj J, Rusinaru D, Levy F, Tribouilloy C. Relationship between cutoff values of peak aortic valve velocity and those of other Doppler echocardiographic parameters of severity in patients with aortic stenosis and normal flow. Echocardiography. 2012;29:1150–1156. [DOI] [PubMed] [Google Scholar]
- 15. Lancellotti P, Lebois F, Simon M, Tombeux C, Chauvel C, Pierard LA. Prognostic importance of quantitative exercise Doppler echocardiography in asymptomatic valvular aortic stenosis. Circulation. 2005;112:I377–I382. [DOI] [PubMed] [Google Scholar]
- 16. Maréchaux S, Hachicha Z, Bellouin A, Dumesnil JG, Meimoun P, Pasquet A, Bergeron S, Arsenault M, Le Tourneau T, Ennezat PV, Pibarot P. Usefulness of exercise‐stress echocardiography for risk stratification of true asymptomatic patients with aortic valve stenosis. Eur Heart J. 2010;31:1390–1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Banovic M, Iung B, Bartunek J, Asanin M, Beleslin B, Biocina B, Casselman F, da Costa M, Deja M, Gasparovic H, Kala P, Labrousse L, Loncar Z, Marinkovic J, Nedeljkovic I, Nedeljkovic M, Nemec P, Nikolic SD, Pencina M, Penicka M, Ristic A, Sharif F, Van Camp G, Vanderheyden M, Wojakowski W, Putnik S. Rationale and design of the Aortic Valve replAcemenT versus conservative treatment in Asymptomatic seveRe aortic stenosis (AVATAR trial): a randomized multicenter controlled event‐driven trial. Am Heart J. 2016;174:147–153. [DOI] [PubMed] [Google Scholar]
- 18. Rosenhek R, Maurer G, Baumgartner H. Should early elective surgery be performed in patients with severe but asymptomatic aortic stenosis? Eur Heart J. 2002;23:1417–1421. [DOI] [PubMed] [Google Scholar]
- 19. Pai RG, Kapoor N, Bansal RC, Varadarajan P. Malignant natural history of asymptomatic severe aortic stenosis: benefit of aortic valve replacement. Ann Thorac Surg. 2006;82:2116–2122. [DOI] [PubMed] [Google Scholar]
- 20. Carabello BA. Should severe aortic stenosis be operated on before symptom onset? Aortic valve replacement should be operated on before symptom onset. Circulation. 2002;126:112–117. [DOI] [PubMed] [Google Scholar]
- 21. Pellikka PA, Nishimura RA, Bailey KR, Tajik AJ. The natural history of adults with asymptomatic, hemodynamically significant aortic stenosis. J Am Coll Cardiol. 1990;15:1012–1017. [DOI] [PubMed] [Google Scholar]
- 22. Lund O. Preoperative risk evaluation and stratification of long‐term survival after valve replacement for aortic stenosis. Reasons for earlier operative intervention. Circulation. 1990;82:124–139. [DOI] [PubMed] [Google Scholar]
- 23. Lund O, Larsen KE. Cardiac pathology after isolated valve replacement for aortic stenosis in relation to preoperative patient status. Early and late autopsy findings. Scand J Thorac Cardiovasc Surg. 1989;23:263–270. [DOI] [PubMed] [Google Scholar]
- 24. Taniguchi T, Morimoto T, Shiomi H, Ando K, Kanamori N, Murata K, Kitai T, Kawase Y, Izumi C, Miyake M, Mitsuoka H, Kato M, Hirano Y, Matsuda S, Nagao K, Inada T, Murakami T, Takeuchi Y, Yamane K, Toyofuku M, Ishii M, Minamino‐Muta E, Kato T, Inoko M, Ikeda T, Komasa A, Ishii K, Hotta K, Higashitani N, Kato Y, Inuzuka Y, Maeda C, Jinnai T, Morikami Y, Sakata R, Kimura T; CURRENT AS Registry Investigators . Initial surgical versus conservative strategies in patients with asymptomatic severe aortic stenosis. J Am Coll Cardiol. 2015;66:2827–2838. [DOI] [PubMed] [Google Scholar]
- 25. Lancellotti P, Donal E, Magne J, Moonen M, O'Connor K, Daubert J‐C, Pierard LA. Risk stratification in asymptomatic moderate to severe aortic stenosis: The importance of the valvular, arterial and ventricular interplay. Heart. 2010;96:1364–1371. [DOI] [PubMed] [Google Scholar]
- 26. Pellikka PA, Sarano ME, Nishimura RA, Malouf JF, Bailey KR, Scott CG, Barnes ME, Tajik AJ. Outcome of 622 adults with asymptomatic, hemodynamically significant aortic stenosis during prolonged follow‐up. Circulation. 2005;111:3290–3295. [DOI] [PubMed] [Google Scholar]
- 27. Rosenhek R, Zilberszac R, Schemper M, Czerny M, Mundigler G, Graf S, Bergler‐Klein J, Grimm M, Gabriel H, Maurer G. Natural history of very severe aortic stenosis. Circulation. 2010;121:151–156. [DOI] [PubMed] [Google Scholar]
- 28. Kapoor N, Varadarajan P, Pai RG. Echocardiographic predictors of pulmonary hypertension in patients with severe aortic stenosis. Eur J Echocardiogr. 2008;9:31–33. [DOI] [PubMed] [Google Scholar]
- 29. Pai RG, Varadarajan P, Kapoor N, Bansal RC. Aortic valve replacement improves survival in severe aortic stenosis associated with severe pulmonary hypertension. Ann Thorac Surg. 2007;84:80–85. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. Baseline Demographic, Clinical, and Echocardiographic Characteristics of the Study Patients With Asymptomatic or Minimally Symptomatic Severe Aortic Stenosis According to Mean Transaortic Pressure Gradient (MTPG)
Table S2. Baseline Demographic, Clinical, and Echocardiographic Characteristics of the Study Patients With Asymptomatic or Minimally Symptomatic Severe Aortic Stenosis According to Mean Transaortic Pressure Gradient (MTPG) < or ≥60 mm Hg
Table S3. Baseline Demographic, Clinical, and Echocardiographic Characteristics of Patients Who Underwent Aortic Valve Replacement (AVR)
Figure S1. Adjusted all‐cause mortality curves in the severe AS population (n=1143) according to mean transaortic pressure gradient (MTPG), divided into 3 groups (A) and into 2 groups (B).
Figure S2. Adjusted all‐cause mortality curves in asymptomatic and minimally symptomatic patients with severe aortic stenosis (n=559) according to mean transaortic pressure gradient (MTPG), divided into 3 groups (A) and into 2 groups (B).


