Abstract
Background
Despite a moderate correlation between angiographical stenosis and physiological significance, the mechanism of discordance has not been fully elucidated, particularly regarding the significance of microvascular function. This study sought to clarify whether microvascular function affects visual‐functional mismatch between quantitative coronary angiography (QCA) and fractional flow reserve (FFR).
Methods and Results
We assessed QCA, FFR, coronary flow reserve, and the index of microcirculatory resistance in 849 non‐left‐main coronary lesions with visually estimated intermediate stenoses from 532 patients. Clinical and lesion‐specific characteristics and physiological parameters associated with mismatch and reverse mismatch were studied. Coronary flow reserve and index of microcirculatory resistance showed a weak, but significant, correlation with FFR (R=0.306, P<0.001 and R=0.158, P<0.001, respectively). Four hundred twenty‐two lesions were visually nonsignificant (diameter stenosis assessed by QCA [QCA‐DS] ≤50%) and 427 lesions were visually significant (QCA‐DS >50%). Among visually nonsignificant lesions, FFR ≤0.80 (reverse mismatch) was observed in 129 lesions (30.6%). Among visually significant lesions, FFR >0.80 (mismatch) were observed in 179 lesions (41.9%). The significant predictors of reverse mismatch were male sex, nonculprit lesions of acute coronary syndrome, left anterior descending artery location, smaller QCA reference diameter, greater QCA‐DS, lower coronary flow reserve, and lower index of microcirculatory resistance. Mismatch was associated with right coronary artery location, greater QCA reference diameter, smaller QCA‐DS, lesion length, higher coronary flow reserve, and higher index of microcirculatory resistance.
Conclusions
There was a high prevalence of visual‐functional mismatches between QCA and FFR. The discrepancy was related to clinical characteristics, lesion‐specific factors, and microvascular resistance that was undistinguishable by coronary angiography, thus suggesting the importance of physiological lesion assessment.
Keywords: angiography, coronary artery disease, fractional flow reserve, microvascular dysfunction, percutaneous coronary intervention
Subject Categories: Coronary Circulation, Angiography, Catheter-Based Coronary and Valvular Interventions
Clinical Perspective
What is New?
High coronary flow reserve and high index of microcirculatory resistance were associated with visual‐functional mismatch in which angiographic diameter stenosis exceeds 50% and FFR was not indicative of ischemia (FRR >0.80).
Low CFR and low IMR were associated with visual‐functional reverse mismatch in which angiographic diameter stenosis was less than 50% and FFR suggested the presence of ischemia (FFR≤0.80).
What are the Clinical Implications?
Visual‐functional mismatch and reverse mismatch are not rare.
In addition to clinical characteristics and lesion location, microvascular function may play important roles for visual‐functional mismatch and reverse mismatch, which corroborates the importance of coronary flow physiological assessment.
Introduction
In addition to clinical characteristics and lesion location, microvascular function may play important roles for visual‐functional mismatch and reverse mismatch, which corroborates the importance of coronary flow physiological assessment.
Invasive coronary angiography is a well‐accepted method for identifying the presence of flow‐limiting epicardial coronary artery stenosis and for guiding revascularization. The seminal study by Gould et al reported that hyperemic flow begins to decline in the presence of stenosis with a reduction in diameter larger than 50%.1 This cut‐off value has been used for the threshold of inducible ischemia; therefore, it is accepted as the gold standard for guiding revascularization, validating noninvasive testing, and evaluating outcomes after revascularization strategies.2, 3, 4 However, cumulative evidence suggests that angiographically determined anatomical stenosis severity often underestimates or overestimates the functional significance of lesions.5, 6, 7 Fractional flow reserve (FFR) is currently the standard for decision‐making regarding revascularization in the catheter laboratory and has become part of the clinical guidelines for the assessment of the physiological significance of epicardial coronary stenosis based on sound concepts and randomized clinical trials.8, 9, 10, 11 However, FFR evaluation is still underutilized; instead, coronary angiography is widely used as a gatekeeper for decision‐making of revascularization even in large clinical trials.2, 12 Subanalysis of the FAME (Fractional Flow Reserve Versus Angiography for Multivessel Evaluation) trial has shown that angiography is inaccurate for assessing the functional significance of coronary stenosis when compared with FFR guidance, especially for intermediate stenosis.6 Although such “visual‐functional mismatch” or “reverse visual‐functional mismatch” are not uncommon, the mechanism of these phenomena has not been fully elucidated. Recent studies further specifically suggest the relevance of microvascular function to FFR.13, 14, 15 Visual‐functional mismatch has important implications in overcoming limitations related to angiography‐guided decision‐making to reduce unnecessary revascularization and avoiding missed appropriate revascularization. Therefore, we sought to identify lesion‐specific, patient‐related factors associated with visual‐functional mismatch using a large cohort of the institutional database that includes FFR, coronary flow reserve (CFR), and the index of microcirculatory resistance (IMR) information with a particular focus on microvascular function.
Methods
Study Population
A retrospective analysis of pooled data was performed in the institutional FFR registry listing pressure and ECG tracings between December 2010 and May 2016 that included consecutive 1645 FFR measurements of 815 patients referred for diagnostic or therapeutic catheterization. Institutional indication criteria for coronary physiological assessment including FFR, CFR, and IMR were intermediate coronary stenosis showing 30% to 80% diameter stenosis by visual estimation in patients with stable angina pectoris; in those with suspected asymptomatic ischemia and/or microvascular dysfunction; or in nonculprit lesions of acute coronary syndrome (ACS) more than 2 weeks after onset. We selected 1042 eligible lesions from 623 patients who met the following criteria (Figure 1): age older than 20 years; detection of an identifiable de novo lesion located at the proximal to middle portion of a native coronary artery; and patients with stable coronary disease or those with ACS in whom the culprit lesions were treated >2 weeks before the examination. Patients with a history of coronary artery bypass surgery, culprit lesions of ACS, left main disease, and in‐stent restenosis were excluded from the analysis. Although left main disease has been reported to be an important factor in reverse visual‐functional mismatch,16 the present study excluded those lesions because the relationship between left main disease and microvascular function is not able to be assessed by the IMR. In those 1042 eligible lesions, we further identified lesions with sufficient physiological data for the determination of FFR, CFR, and IMR. Insufficient acquisition of physiological data, such as pressure drift (PD) >3 mm Hg, insufficient waveform during the examination, and repeated FFR measurements irrespective of PD within 3 mm Hg, were excluded. The study was approved by the local ethics committee and conformed to the Declaration of Helsinki statement on research involving human subjects. Informed consent was provided by all participants after a complete explanation of the protocol and potential risks.
Figure 1.
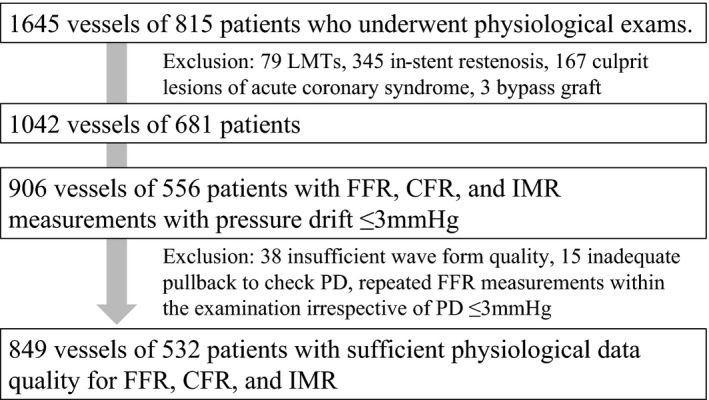
Patient population. CFR indicates coronary flow reserve; FFR, fractional flow reserve; IMR, index of microcirculatory resistance; LMT, left main trunk disease; PD, pressure drift.
Cardiac Catheterization
Each patient initially underwent standard selective coronary angiography through the radial artery using a 6‐Fr system. Quantitative analyses of angiograms were performed using offline analysis software (QAngio XA; Medis Medical Imaging Systems, Leiden, The Netherlands) to measure minimum lumen diameter, (QCA) reference lumen diameter (QCA‐RD), QCA percent diameter stenosis (QCA‐DS), and lesion length of the target lesion. All patients received a bolus injection of heparin (5000 IU) before the procedure and an additional bolus injection of 2000 IU every hour if the procedure required more than 1 hour. An intracoronary bolus injection of nitroglycerin (0.2 mg) was administered at the beginning of the procedure and repeated every 30 minutes. Intravenous infusion of ATP (150 μg/kg/min) was used to induce hyperemia. According to the manufacturer's specifications, which reported an acceptable pressure error range of 3%, we analyzed FFR measurements with PD ≤3 mm Hg in the present study. Our institutional standard protocol recommends repeated FFR measurements when PD ≥4 mm Hg; however, the final decision to repeat the measurements is at the discretion of the operator. Lesions with PD >3 mm Hg were excluded from the analysis, and FFR was calculated as a corrected value of distal coronary pressure (Pd)/proximal coronary pressure (Pa) after correction of Pd by PD.
Intracoronary Physiological Indices
FFR, CFR, and IMR were measured with a RadiAnalyzer Xpress instrument and a PressureWire Certus (pressure‐temperature sensor wire; St. Jude Medical, Uppsala, MN).5, 17, 18 The pressure wire was introduced, zeroed, and equalized to the catheter tip pressure before crossing the lesion. Afterward, the pressure sensor was positioned 8 to 10 cm distal to the ostium of the studied artery across the lesion. Baseline pressures were recorded for at least 20 seconds after the guiding catheter was flushed with saline. Thereafter, FFR measurements were performed at stable hyperemia. PD was determined when the pressure sensor was pulled back and reached the tip of the guiding catheter. All pressure and ECG tracings in the catheterization laboratory's monitoring system (RMC‐4000 Cardio Master with EP amplifier system JB400G; Nihon Koden, Tokyo, Japan) were submitted to the in‐hospital core cardiac physiology and morphological analysis laboratory. Insufficient waveform quality, including absence of pressure signal, catheter‐damped waveform, and inappropriate Pd waveform, were excluded. Analyzed FFR data were compared with the original readout values determined by the catheterization laboratory; a consensus reading was agreed on by 2 expert physicians if there were discordant values. In the present study, IMR was calculated as the product of the mean distal coronary pressure during stable hyperemia and mean hyperemic transit time (Tmn) and corrected by using the following formula proposed by Yong et al19: IMR=Pa×Tmn×([1.35×Pd/Pa]–0.32). In the absence of a validated cutoff to identify abnormally increased hyperemic microvascular resistance and the reported variability of IMR in patients with or without coronary heart disease, IMR values ≥75th percentile (28.0) of the present cohort were arbitrarily assumed as high IMR.20, 21 CFR was also measured simultaneously with FFR using the thermodilution method, as described elsewhere.18 In this study, low CFR was defined as a value <2.0, consistent with previous studies.22
Definition of Visual‐Functional Reverse Mismatch and Mismatch
The clinical cut‐off point of functionally significant ischemia was defined as FFR of 0.80. Visually significant stenosis was defined as QCA‐DS >50%. Lesions were categorized into 4 groups according to the FFR and QCA‐DS thresholds: concordantly nonsignificant group showing QCA‐DS ≤50% and FFR >0.80; visual‐functional reverse mismatch group; visual‐functional mismatch group; or concordantly significant group showing QCA‐DS >50% and FFR ≤0.80. Visual‐functional reverse mismatch was defined as QCA‐DS ≤50% and FFR ≤0.8. Visual‐functional mismatch was defined as QCA‐DS >50% and FFR >0.80. Determinants of reverse mismatch in visually nonsignificant stenosis and those of mismatch in visually significant stenosis were investigated in the present study.
Statistical Analysis
Statistical analysis was performed by using SPSS (version 23.0; SPSS Inc, Chicago, IL) and R software (version 3.2.3; R Foundation for Statistical Computing, Vienna, Austria). Patient demographics are presented as n (%), when appropriate. Categorical data are expressed as absolute frequencies and percentages and were compared using χ2 or Fisher's exact tests, as appropriate. Correlations between FFR and QCA data, CFR, and IMR were assessed by Pearson correlation analysis. Data were analyzed on a per‐patient and per‐lesion basis for the corresponding calculations. For per‐patient data, continuous variables are expressed as mean±SD for normally distributed variables or as median (25th–75th percentile) for non‐normally distributed variables and compared using Student t tests and Mann–Whitney U tests, respectively. For per‐lesion data, a logistic generalized estimated equation model with robust SEs that accounted for the clustering between lesions in the same subject was created. A multivariable logistic regression analysis was performed to identify the determinants for QCA‐FFR visual functional reverse mismatch and mismatch; results were presented as odds ratios (ORs) and 95% CI. The associated variables in univariate analysis (P≤0.20) and physiological parameters, including CFR and IMR, were entered into the final multivariable model. P<0.05 was considered statistically significant.
Results
Patient Characteristics and Angiographic, Procedural, and Hemodynamic Results
Of the 1042 eligible lesions with intermediate stenosis, 906 lesions from 556 patients in whom FFR, CFR, and IMR determinations were performed with a pullback PD ≤3 mm Hg were identified (Figure 1). Thereafter, 57 lesions were excluded from the final analysis because of insufficient waveform quality (n=38), inadequate pullback maneuver to check PD (n=15), or the performance of repeated FFR measurements irrespective of PD within 3 mm Hg (n=4), leaving 849 FFR measurements from 532 patients in the final analysis. Patient and lesion characteristics of the final cohort are summarized in Table 1. Average age was 66.9 years, 82.7% were male, 37.0% had a history of diabetes mellitus, and 71.1% had a history of hypertension. In QCA analysis, mean QCA‐DS was 48.3±14.9% and 427 lesions (50.3%) had QCA‐DS >50%. FFR ≤0.80 was observed in 377 (44.4%) lesions. Reverse mismatch was observed in 129 lesions, which accounted for 15.2% of the total cohort and 30.6% of visually nonsignificant stenosis, whereas mismatch was observed in 179 lesions, accounting for 21.1% of the total cohort and 41.9% of visually significant stenosis (Table 2). In the present cohort, median IMR was 18.0 (12.2–28.1). Lesion characteristics and physiological data of the groups stratified by QCA‐DS and FFR are summarized in Table 2. CFR and IMR were significantly lower in the reverse mismatch group than in the concordantly nonsignificant group, whereas the mismatch group showed higher CFR and IMR as compared with concordantly significant lesions. Correlations of FFR and other parameters, including QCA‐DS, CFR, and IMR, are depicted in Figure 2. QCA‐DS showed a moderate correlation with FFR (correlation coefficient, r=−0.468; P<0.001). CFR and IMR showed weak, but significant, correlations with FFR (r=0.306, P<0.001 and r=0.158, P<0.001, respectively).
Table 1.
Patient and Lesion Characteristics of Overall Cohort
| Patient Characteristics | |
|---|---|
| n | 532 |
| Age | 66.9±9.7 |
| Male sex | 440 (82.7) |
| Height, m | 1.62±8.9 |
| Weight, kg | 64.4±12.3 |
| BMI, kg/m2 | 24.4±3.5 |
| Hypertension | 378 (71.1) |
| Diabetes mellitus | 197 (37.0) |
| Dyslipidemia | 309 (58.1) |
| Current smoker | 137 (25.8) |
| Atrial fibrillation | 44 (8.3) |
| Chronic kidney disease | 47 (8.8) |
| Previous MI | 140 (26.3) |
| Previous PCI | 379 (71.2) |
| Acute coronary syndrome | 99 (18.6) |
| Laboratory data | |
| CRP, mg/dL | 0.42±0.98 |
| BUN, mg/dL | 18.0±8.1 |
| Creatinine, mg/dL | 0.82 (0.70–0.98) |
| eGFR, mL/min/1.73 m2 | 68.7±22.2 |
| HbA1c, % | 6.3±1.1 |
| LDL‐cholesterol, mg/dL | 101±31 |
| HDL‐cholesterol, mg/dL | 47±13 |
| Triglyceride | 148±106 |
| Medication | |
| Antiplatelet therapy | 385 (72.4) |
| Calcium blocker | 234 (44.0) |
| Statin | 308 (57.9) |
| Lesion characteristics | |
| n | 849 |
| Lesion location | |
| RCA | 175 (20.6) |
| LAD | 510 (60.1) |
| Cx | 164 (19.3) |
| Nonculprit lesion of ACS | 134 (15.8) |
| Previous MI‐related artery | 84 (9.9) |
| Quantitative coronary angiography | |
| Minimal lumen diameter | 1.46±0.63 |
| Reference diameter | 2.85±0.64 |
| Diameter stenosis | 48.3±14.9 |
| Lesion length | 12.4±7.6 |
| Physiological data | |
| Baseline HR, bpm | 68 (61–75) |
| Baseline Pa, mm Hg | 93 (84–103) |
| Baseline Pd, mm Hg | 86 (76–96) |
| Baseline Tmn, s | 0.87 (0.59–1.23) |
| Hyperemic Pa, mm Hg | 83 (75–92) |
| Hyperemic Pd, mm Hg | 67 (57–77) |
| Hyperemic Tmn, s | 0.29 (0.20–0.44) |
| FFR | 0.82 (0.75–0.89) |
| CFR | 2.81 (1.89–4.00) |
| IMR | 18.0 (12.2–28.1) |
ACS indicates acute coronary syndrome; BMI, body mass index; BUN, blood urea nitrogen; CFR, coronary flow reserve; CRP, C‐reactive protein; Cx, left circumflex artery; eGFR, estimated glomerular filtration rate; FFR, fractional flow reserve; HbA1c, glycated hemoglobin; HDL, high‐density lipoprotein; HR, heart rate; IMR, the index of microcirculatory resistance; LAD, left anterior descending artery; LDL, low‐density lipoprotein; MI, myocardial infarction; Pa, arterial pressure; PCI, percutaneous coronary intervention; Pd, distal pressure; RCA, right coronary artery; Tmn, mean transit time.
Table 2.
Lesion Characteristics Stratified by Diameter Stenosis and FFR (849 lesions)
| Visually Nonsignificant Lesions QCA‐DS ≤50% | Visually Significant Lesions QCA‐DS >50% | |||||
|---|---|---|---|---|---|---|
| FFR>0.80 Concordantly Non‐Significant | FFR≤0.80 “Reverese Mismatch” | P Value | FFR>0.80 “Mismatch” | FFR≤0.80 Concordantly Significant | P Value | |
| n | 293 | 129 | 179 | 248 | ||
| Lesion location | ||||||
| RCA | 66 (22.5) | 13 (10.1) | <0.001 | 59 (33.0) | 37 (14.9) | <0.001 |
| LAD | 165 (56.3) | 113 (87.6) | 70 (39.1) | 162 (65.3) | ||
| Cx | 62 (21.2) | 3 (2.3) | 50 (27.9) | 49 (19.8) | ||
| ACS nonculprit | 32 (10.9) | 30 (23.3) | 0.002 | 33 (18.4) | 39 (15.7) | 0.513 |
| Infarction‐related vessel | 35 (12.7) | 14 (11.5) | 0.869 | 15 (8.7) | 20 (8.3) | 1.000 |
| FFR | 0.89 (0.85–0.93) | 0.76 (0.70–0.79) | <0.001 | 0.86 (0.83–0.90) | 0.73 (0.64–0.77) | <0.001 |
| CFR | 3.07 (2.20–4.18) | 2.79 (1.83–4.00) | 0.032 | 3.09 (2.22–4.30) | 2.16 (1.43–3.03) | <0.001 |
| IMR | 19.3 (12.8–30.5) | 15.0 (10.9–21.5) | <0.001 | 19.2 (12.2–28.3) | 17.5 (11.9–28.1) | 0.277 |
ACS indicates acute coronary syndrome; CFR, coronary flow reserve; Cx, left circumflex artery; FFR, fractional flow reserve; IMR, the index of microcirculatory resistance; LAD, left anterior descending artery; RCA, right coronary artery.
Figure 2.
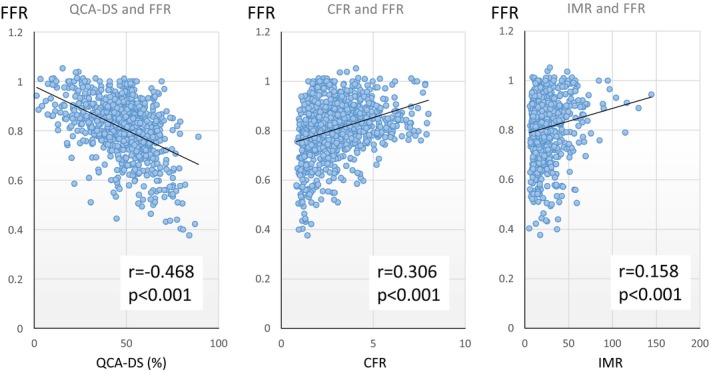
Correlation between FFR and angiographical and physiological indices. CFR indicates coronary flow reserve; FFR, fractional flow reserve; IMR, index of microcirculatory resistance; QCA‐DS, percent diameter stenosis determined by quantitative coronary angiography. QCA‐DS showed moderate correlations with FFR and CFR (left panel). CFR and IMR showed weak, but significant, correlations with FFR (middle and right panels).
Determinants of FFR
In the overall population, a multivariable linear regression analysis showed that the significant factors affecting FFR as continuous variables were age (coefficient, ß=0.0016; P<0.001), QCA‐RD (coefficient ß=0.0358; P<0.001), QCA‐DS (coefficient ß=−0.0031; P<0.001), CFR (coefficient ß=−0.0223; P<0.001), and IMR (coefficient, ß=0.0013; P<0.001; Table 3). In addition, multivariable logistic regression analysis showed that the significant determinants for FFR ≤0.80 were male sex (OR, 2.60; 95% CI, 1.51–4.47; P=0.001), left anterior descending coronary artery (OR, 4.70; 95% CI, 3.10–7.12; P<0.001), smaller QCA‐RD (OR, 0.36; 95% CI, 0.24–0.56; P<0.001), greater QCA‐DS (OR, 1.07; 95% CI, 1.05–1.10; P<0.001), longer QCA lesion length (OR, 1.03; 95% CI, 1.00–1.06; P<0.001), lower CFR (OR, 0.66; 95% CI, 0.56–0.77; P<0.001), and lower IMR (OR, 0.98; 95% CI, 0.96–0.99; P<0.001; Table 4).
Table 3.
Determinants of FFR in Multivariate Linear Regression Analysis (849 Lesions)
| 95% CI | ||||
|---|---|---|---|---|
| Coefficient ß | Lower | Upper | P Value | |
| Age, y | 0.0016 | 0.0012 | 0.0020 | 0.001 |
| QCA‐DS, % | −0.0031 | −0.0033 | −0.0029 | <0.001 |
| QCA‐RD, mm | 0.0359 | 0.0309 | 0.0409 | <0.001 |
| CFR | 0.0223 | 0.0200 | 0.0246 | <0.001 |
| IMR | 0.0013 | 0.0011 | 0.0015 | <0.001 |
CFR indicates coronary flow reserve; FFR, fractional flow reserve; IMR, the index of microcirculatory resistance; QCA‐DS, diameter stenosis assessed by QCA; QCA‐RD, reference diameter assessed by quantitative coronary angiography (QCA).
Table 4.
Univariate and Multivariate Logistic Regression Analysis for Determinants of FFR ≤0.80 (849 Lesions)
| OR | 95% CI | P Value | OR | 95% CIl | P Value | |||
|---|---|---|---|---|---|---|---|---|
| Lower | Upper | Lower | Upper | |||||
| Male | 1.75 | 1.14 | 2.69 | 0.011 | 2.59 | 1.55 | 4.35 | <0.001 |
| Diabetes mellitus | 1.35 | 0.96 | 1.89 | 0.081 | 1.20 | 0.82 | 1.76 | 0.353 |
| Current smoking | 0.70 | 0.48 | 1.01 | 0.059 | 0.81 | 0.50 | 1.31 | 0.391 |
| ACS nonculprit lesion | 1.47 | 0.97 | 2.22 | 0.068 | 1.52 | 0.91 | 2.54 | 0.111 |
| eGFR, mL/min | 0.99 | 0.99 | 1.00 | 0.147 | 1.00 | 0.99 | 1.01 | 0.926 |
| LDL‐C, mg/dL | 1.00 | 0.99 | 1.00 | 0.079 | 0.99 | 0.99 | 1.00 | 0.057 |
| LAD | 2.86 | 2.07 | 3.95 | <0.001 | 4.30 | 2.88 | 6.44 | <0.001 |
| QCA‐RD, mm | 0.40 | 0.31 | 0.51 | <0.001 | 0.38 | 0.28 | 0.51 | <0.001 |
| QCA‐DS, % | 1.06 | 1.05 | 1.07 | <0.001 | 1.07 | 1.06 | 1.09 | <0.001 |
| QCA‐LL, mm | 1.06 | 1.03 | 1.09 | <0.001 | 1.03 | 1.00 | 1.06 | 0.021 |
| CFR | 0.69 | 0.61 | 0.78 | <0.001 | 0.69 | 0.60 | 0.80 | <0.001 |
| IMR | 0.99 | 0.98 | 0.99 | 0.003 | 0.98 | 0.96 | 0.99 | <0.001 |
ACS indicates acute coronary syndrome; CFR, coronary flow reserve; eGFR, estimated glomerular filtration rate; FFR, fractional flow reserve; IMR, the index of microcirculatory resistance; LAD, left anterior descending artery; LDL, low‐density lipoprotein; QCA‐DS, diameter stenosis assessed by QCA; QCA‐LL, lesion length assessed by QCA; QCA‐RD, reference diameter assessed by quantitative coronary angiography (QCA).
Determinants of Reverse Mismatch and Mismatch
Univariate and multivariable analyses of factors predicting reverse mismatch in visually nonsignificant lesions are shown in Table 5. Multivariable analysis identified male sex, nonculprit lesion of acute coronary syndrome, lower low‐density lipoprotein cholesterol level, left anterior descending coronary artery lesions, smaller QCA‐RD, larger QCA‐DS, lower CFR, and lower IMR as the significant predictors of reverse mismatch in visually nonsignificant lesions. In visually significant lesions, significant predictors of mismatch were right coronary artery, greater QCA‐RD, smaller QCA‐DS, shorter QCA lesion length, higher CFR, and higher IMR (Table 6). Figure 3 shows the frequency of reverse mismatch in visually nonsignificant lesions and that of mismatch in visually significant lesions stratified by IMR (>28.0) and CFR (≤2.0). In visually nonsignificant lesions, frequency of reverse mismatch was significantly different among the 4 groups categorized by IMR and CFR and highest in lesions with low IMR and low CFR values. In contrast, in visually significant lesions, frequency of mismatch was highest in the group with high IMR and high CFR (Figure 3).
Table 5.
Predictors of Reverse Mismatch in Visually Nonsignificant Lesions (422 lesions)
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P Value | OR | 95% CI | P Value | |
| Age, y | 0.98 | 0.96 to 1.00 | 0.087 | 0.98 | 0.95 to 1.01 | 0.194 |
| Male | 2.47 | 1.30 to 4.68 | 0.006 | 2.99 | 1.44 to 6.23 | 0.003 |
| ACS nonculprit lesion | 2.57 | 1.48 to 4.45 | 0.001 | 2.68 | 1.40 to 5.12 | 0.003 |
| LDL‐C, mg/dL | 0.99 | 0.99 to 1.00 | 0.133 | 0.99 | 0.98 to 1.00 | 0.017 |
| LAD | 6.03 | 3.15 to 11.57 | <0.001 | 6.43 | 3.07 to 13.47 | <0.001 |
| QCA‐RD, mm | 0.31 | 0.20 to 0.46 | <0.001 | 0.34 | 0.22 to 0.52 | <0.001 |
| QCA‐DS, % | 1.06 | 1.03 to 1.08 | <0.001 | 1.06 | 1.03 to 1.09 | <0.001 |
| QCA‐LL, mm | 1.04 | 1.01 to 1.08 | 0.019 | 1.03 | 0.99 to 1.06 | 0.121 |
| CFR | 0.86 | 0.73 to 1.01 | 0.071 | 0.77 | 0.63 to 0.95 | 0.017 |
| IMR | 0.97 | 0.95 to 0.99 | 0.011 | 0.97 | 0.94 to 0.99 | 0.012 |
ACS indicates acute coronary syndrome; CFR, coronary flow reserve; IMR, the index of microcirculatory resistance; LAD, left anterior descending artery; LDL, low‐density lipoprotein; MI, myocardial infarction; QCA‐DS, diameter stenosis assessed by QCA; QCA‐LL, lesion length assessed by QCA; QCA‐RD, reference diameter assessed by quantitative coronary angiography (QCA).
Table 6.
Predictors of Mismatch in Visually Significant Lesions (427 Lesions)
| Univariate Analysis | Multivariate Analysis | |||||
|---|---|---|---|---|---|---|
| OR | 95% CI | P Value | OR | 95% CI | P Value | |
| Diabetes mellitus | 0.71 | 0.45 to 1.10 | 0.127 | 0.96 | 0.58 to 1.60 | 0.877 |
| Current smoking | 1.96 | 1.22 to 3.15 | 0.005 | 1.35 | 0.75 to 2.43 | 0.315 |
| RCA | 2.90 | 1.77 to 4.73 | <0.001 | 2.07 | 1.17 to 3.63 | 0.012 |
| QCA‐RD, mm | 2.37 | 1.70 to 3.30 | <0.001 | 2.28 | 1.54 to 3.39 | <0.001 |
| QCA‐DS, % | 0.93 | 0.90 to 0.96 | <0.001 | 0.92 | 0.88 to 0.96 | <0.001 |
| QCA‐LL, mm | 0.96 | 0.92 to 0.99 | 0.005 | 0.94 | 0.90 to 0.99 | 0.009 |
| CFR | 1.66 | 1.40 to 1.97 | <0.001 | 1.62 | 1.33 to 1.97 | <0.001 |
| IMR | 1.01 | 1.00 to 1.02 | 0.185 | 1.02 | 1.00 to 1.04 | 0.015 |
CFR indicates coronary flow reserve; IMR, the index of microcirculatory resistance; QCA‐DS, diameter stenosis assessed by QCA; QCA‐LL, lesion length assessed by QCA; QCA‐RD, reference diameter assessed by quantitative coronary angiography (QCA); RCA, right coronary artery.
Figure 3.
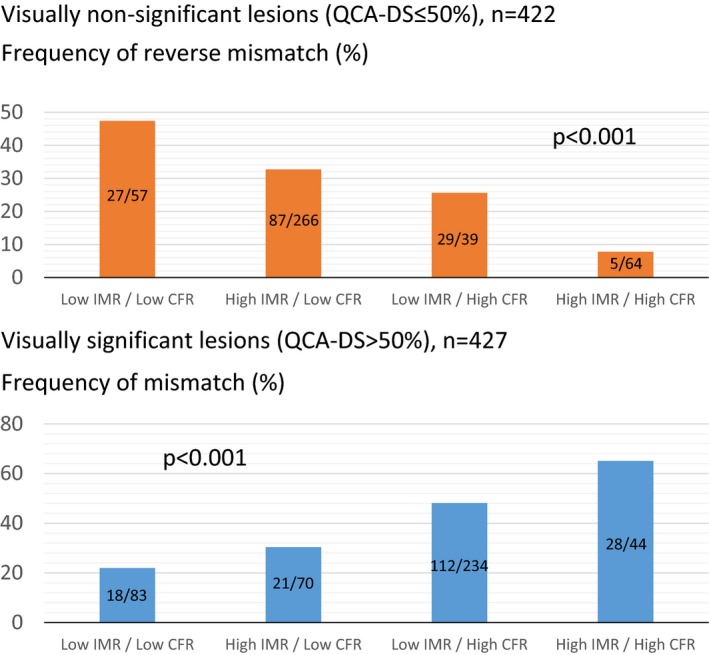
Frequency of visual—functional reverse mismatch and mismatch stratified by IMR and CFR values (lesion‐based analysis). CFR indicates coronary flow reserve; IMR, index of microcirculatory resistance; QCA‐DS, percent diameter stenosis determined by quantitative coronary angiography. Upper panel shows the frequencies of reverse mismatch in visually nonsignificant stenosis in the groups stratified by IMR and CFR thresholds. Reverse mismatch was most frequently observed in the group with low IMR (IMR ≤28.0) and low CFR (CFR <2.0). Lower panel shows the mismatch frequencies in visually significant stenosis in the groups stratified by IMR and CFR thresholds. Mismatch was most frequent in the group with high IMR (IMR >28.0) and high CFR (CFR ≥2.0).
Discussion
The main findings of the present study were as follows: (1) QCA‐derived visual and FFR‐defined functional discordance were not rare, with frequencies of mismatch and reverse mismatch being 21.1% and 15.2%, respectively; (2) both mismatch and reverse mismatch were related to the clinical characteristics, lesion‐specific factors, and microvascular resistance; (3) high hyperemic microvascular resistance and high CFR were significant predictors of mismatch; and (4) low hyperemic microvascular resistance and low CFR were significant predictors of reverse mismatch. To the best of our knowledge, this is the first study showing that microvascular function affects both visual‐functional mismatch and reverse mismatch by using IMR as a specific marker of microvascular resistance. Invasive coronary angiography remains a gatekeeper for decision‐making in revascularization because the utilization of FFR is still low, despite the recommendations in the guidelines.23 The relationship between angiographic stenosis severity and functional significance assessed by FFR and the mismatch between these 2 measures have been extensively investigated to optimize percutaneous coronary intervention (PCI).16, 24, 25, 26 Clinical factors, including age, sex, angiographical lesion‐related factors, and physiological factors, have been reported to contribute to visual‐functional mismatch and reverse mismatch.15, 16, 26, 27, 28 Our results were in line with these previous studies and further suggested the importance of microvascular function in both mismatch and reverse mismatch. These findings might be related to the fact that FFR as an index is affected by coronary flow volume through the stenosis. Decreased hyperemic microvascular resistance implies that coronary flow might increase for a fixed stenosis and a given driving pressure, and it may dictate a low FFR value despite relatively low resistance to coronary flow induced by the epicardial stenosis and microvascular resistance. In contrast, with increased microvascular resistance, coronary flow through the given fixed stenosis will decrease and FFR will increase without alteration in epicardial resistance to flow resulting from the epicardial stenosis.
Microvascular Resistance and Functional Stenosis Significance
Although the unequivocal benefit of FFR‐guided revascularization over angiographic guidance has been established,9, 10, 11 it should be noted that FFR is determined under the assumption of minimal and constant microvascular resistance. Our results suggest that microvascular resistance independently and significantly influences visual‐functional mismatch and reverse mismatch in a large study population with intermediate epicardial coronary stenosis. Because FFR relies on a translesional hyperemic pressure gradient in a restricted model of coronary physiology, it is inevitably influenced by pharmacologically induced hyperemic coronary flow or by the individual's responsiveness to vasodilator drugs.15 The most important factor related to outcome in patients with coronary heart disease is the presence or absence of inducible myocardial ischemia and its extent. It has been proposed that the presence of microvascular dysfunction is not an obstacle for making decisions based on FFR, provided that minimal and constant microvascular resistance with induced hyperemia is deemed to be nonreversible or consistent before and after revascularization.28, 29 However, several studies reported the evidence of serial changes in adenosine‐induced microvascular resistance before, after, and during follow‐up post‐PCI.13, 30, 31 van de Hoef et al also reported that identification of epicardial disease severity by FFR is partly obscured by microvascular resistance and that FFR increased with increasing hyperemic microvascular resistance to epicardial disease of equivalent severity.15 Our results shed light on the relationship between FFR and microvascular function and indicate that low hyperemic microvascular resistance most likely influences reverse visual‐unctional mismatch for intermediate epicardial stenoses, and that increased microvascular resistance affects visual‐functional mismatch. These findings suggest that the mismatch group might include, at least in part, lesions with low pressure gradients attributed to the decreased coronary flow as a consequence of impaired microvascular resistance.32 In other words, the present study suggests that FFR >0.80 and QCA‐DS >50% not only identify vessels without the need for revascularization, but also include vessels with impaired and nonimpaired coronary flow. This potential abnormality in coronary physiology beyond the FFR assumption might provide reasons for why patients with revascularization deferral in randomized trials were not free from long‐term cardiac events, such as in DEFER and FAME follow‐up data, why 12% of patients with FFR >0.80 required revascularization within 2 years of deferral9, 10, 11 and, conversely, why some patients with FFR ≤0.80 but with preserved CFR have a low rate of major adverse cardiac events at follow‐up.33
CFR and Functional Stenosis Significance
In the present study, in addition to IMR, CFR was associated with the FFR value. CFR is the ratio of maximum stress flow to rest flow for the artery of interest; this fundamentally represents coronary flow capacity modified by the integration of epicardial stenosis, diffuse arterial narrowing, and microvascular dysfunction.32 Our results demonstrated that CFR positively correlated with the FFR value in the linear regression analysis. Furthermore, a high CFR value was a significant predictor of visual‐functional mismatch in visually significant lesions, and low CFR value was a significant predictor of reverse mismatch in visually nonsignificant lesions. Because CFR is likely to be composed of 3 elements (epicardial stenosis, diffuse narrowing, and microvascular function) and FFR represents the functional significance of epicardial stenosis, our results suggest that epicardial stenosis might influence a significant portion of CFR, rather than diffuse narrowing or microvascular function, in the setting of visual‐functional mismatch evaluation because CFR and FFR showed an independent, parallel correlation.
Microvascular Resistance, Coronary Flow, and Functional Stenosis Significance
In the present study, a non‐negligible proportion for whom the FFR value was >0.80 showed abnormal coronary microvascular resistance (IMR >28.0; 135 of 472; 28.6%) and disturbed coronary hemodynamics (CFR <2.0; 96 of 472; 20.3%). In those lesions, hyperemic coronary flow might increase if the increased resistance is reduced after revascularization, as suggested by our recent studies.13, 30 The exact mechanism and pathophysiology of increased or decreased hyperemic microvascular resistance were not known and the change in microvascular resistance and its effect on absolute coronary flow after revascularization remain elusive. Further studies are needed to elucidate the relationship between microvascular resistance and functional stenosis significance by considering the effect of revascularization and the change in microvascular resistance on absolute coronary flow and myocardial ischemia.
Clinical Implication of Mismatch and Reverse Mismatch
Although frequency and determinants of visual‐functional mismatch have been investigated in the present and previous studies,15, 16 clinical implication of those lesions has not been elucidated. Specifically, clinical outcomes of the lesions with mismatch or reverse mismatch have not been sufficiently investigated, and, moreover, best therapeutic strategies for those lesions have not been well discussed. If we follow the data from the FAME and FAME2 trials,10, 11 the mismatch group should be treated by optimal medical therapy alone and the reverse‐mismatch group should be treated by PCI and optimal medical therapy. However, given the substantial population of patients showing FFR within the gray zone ranging from 0.75 to 0.80 in the reverse mismatch group, superiority of PCI for those lesions may deserve further consideration.34 Previous studies have suggested the impact of plaque characteristics on FFR values for a given anatomical stenosis in an epicardial coronary artery.35 The present study reports the association of CFR and IMR with FFR. Although these factors might be represented by a low FFR value as a single variable, nevertheless, integrated information on patient characteristics, coronary flow, and plaque morphology may provide additive predictive values on FFR, which might enable tailored strategies for lesions, such as gray zone lesions, in which the prognostic values of FFR might be suboptimal.
Study Limitations
The results of the present study should be interpreted with the consideration of some limitations. First, this was a retrospective study performed at a single center without a dedicated core laboratory for angiographic analysis. Exclusion of patients with significant left main disease, renal impairment, heart failure, or acute coronary syndrome may have resulted in selection bias. This study enrolled patients with stable angina pectoris based on symptoms and noninvasive test results and those who were referred to the catheter laboratory for treatment or diagnosed by diagnostic catheterization at our institution, which might inevitably involve referral bias. There was no clinically validated or normal range of IMR. Therefore, the quartiles of IMR in the study population were used to define high (highest quartile) and low (lowest quartile) IMR. Although this approach provides a reasonable estimation of an abnormal IMR range for patients with coronary heart disease, our results should be tested in an independent study population. Coronary wedge pressure was not measured because this study cohort included lesions that were not treated with PCI, which might have led to overestimation of IMR in tight stenoses with significant collateral flow.36
Conclusions
The present data, which are in line with those of previous studies, indicate that coronary angiography underestimates or overestimates physiological stenosis severity in comparison with FFR in non‐negligible proportion of visually intermediate lesions. In addition, our results indicate that these visual‐functional mismatch and reverse mismatch are related to coronary flow and microvascular function, which may emphasize the importance of coronary flow assessment and coronary pressure indices.
Disclosures
None.
(J Am Heart Assoc. 2017;6:e005916 DOI: 10.1161/JAHA.117.005916.)28566295
References
- 1. Gould KL, Lipscomb K. Effects of coronary stenoses on coronary flow reserve and resistance. Am J Cardiol. 1974;34:48–55. [DOI] [PubMed] [Google Scholar]
- 2. Boden WE, O'Rourke RA, Teo KK, Hartigan PM, Maron DJ, Kostuk WJ, Knudtson M, Dada M, Casperson P, Harris CL, Chaitman BR, Shaw L, Gosselin G, Nawaz S, Title LM, Gau G, Blaustein AS, Booth DC, Bates ER, Spertus JA, Berman DS, Mancini GB, Weintraub WS, Group CTR. Optimal medical therapy with or without pci for stable coronary disease. N Engl J Med 2007;356:1503–1516. [DOI] [PubMed] [Google Scholar]
- 3. Genders TS, Steyerberg EW, Alkadhi H, Leschka S, Desbiolles L, Nieman K, Galema TW, Meijboom WB, Mollet NR, de Feyter PJ, Cademartiri F, Maffei E, Dewey M, Zimmermann E, Laule M, Pugliese F, Barbagallo R, Sinitsyn V, Bogaert J, Goetschalckx K, Schoepf UJ, Rowe GW, Schuijf JD, Bax JJ, de Graaf FR, Knuuti J, Kajander S, van Mieghem CA, Meijs MF, Cramer MJ, Gopalan D, Feuchtner G, Friedrich G, Krestin GP, Hunink MG; CAD Consortium . A clinical prediction rule for the diagnosis of coronary artery disease: validation, updating, and extension. Eur Heart J 2011;32:1316–1330. [DOI] [PubMed] [Google Scholar]
- 4. Serruys PW, Farooq V, Vranckx P, Girasis C, Brugaletta S, Garcia‐Garcia HM, Holmes DR Jr, Kappetein AP, Mack MJ, Feldman T, Morice MC, Stahle E, James S, Colombo A, Pereda P, Huang J, Morel MA, Van Es GA, Dawkins KD, Mohr FW, Steyerberg EW. A global risk approach to identify patients with left main or 3‐vessel disease who could safely and efficaciously be treated with percutaneous coronary intervention: the SYNTAX trial at 3 years. JACC Cardiovasc Interv. 2012;5:606–617. [DOI] [PubMed] [Google Scholar]
- 5. Pijls NH, De Bruyne B, Peels K, Van Der Voort PH, Bonnier HJ, Bartunek JKJJ, Koolen JJ. Measurement of fractional flow reserve to assess the functional severity of coronary‐artery stenoses. N Engl J Med. 1996;334:1703–1708. [DOI] [PubMed] [Google Scholar]
- 6. Tonino PA, Fearon WF, De Bruyne B, Oldroyd KG, Leesar MA, Ver Lee PN, Maccarthy PA, Van't Veer M, Pijls NH. Angiographic versus functional severity of coronary artery stenoses in the fame study fractional flow reserve versus angiography in multivessel evaluation. J Am Coll Cardiol. 2010;55:2816–2821. [DOI] [PubMed] [Google Scholar]
- 7. Topol EJ, Nissen SE. Our preoccupation with coronary luminology. The dissociation between clinical and angiographic findings in ischemic heart disease. Circulation. 1995;92:2333–2342. [DOI] [PubMed] [Google Scholar]
- 8. Task Force on Myocardial Revascularization of the European Society of C, the European Association for Cardio‐Thoracic S, European Association for Percutaneous Cardiovascular I , Wijns W, Kolh P, Danchin N, Di Mario C, Falk V, Folliguet T, Garg S, Huber K, James S, Knuuti J, Lopez‐Sendon J, Marco J, Menicanti L, Ostojic M, Piepoli MF, Pirlet C, Pomar JL, Reifart N, Ribichini FL, Schalij MJ, Sergeant P, Serruys PW, Silber S, Sousa Uva M, Taggart D. Guidelines on myocardial revascularization. Eur Heart J. 2010; 31:2501–2555.20802248 [Google Scholar]
- 9. Pijls NH, van Schaardenburgh P, Manoharan G, Boersma E, Bech JW, van't Veer M, Bar F, Hoorntje J, Koolen J, Wijns W, de Bruyne B. Percutaneous coronary intervention of functionally nonsignificant stenosis: 5‐year follow‐up of the defer study. J Am Coll Cardiol 2007;49:2105–2111. [DOI] [PubMed] [Google Scholar]
- 10. Pijls NH, Fearon WF, Tonino PA, Siebert U, Ikeno F, Bornschein B, van't Veer M, Klauss V, Manoharan G, Engstrom T, Oldroyd KG, Ver Lee PN, MacCarthy PA, De Bruyne B; FAME Study Investigators . Fractional flow reserve versus angiography for guiding percutaneous coronary intervention in patients with multivessel coronary artery disease: 2‐year follow‐up of the FAME (fractional flow reserve versus angiography for multivessel evaluation) study. J Am Coll Cardiol 2010;56:177–184. [DOI] [PubMed] [Google Scholar]
- 11. De Bruyne B, Fearon WF, Pijls NH, Barbato E, Tonino P, Piroth Z, Jagic N, Mobius‐Winckler S, Rioufol G, Witt N, Kala P, MacCarthy P, Engstrom T, Oldroyd K, Mavromatis K, Manoharan G, Verlee P, Frobert O, Curzen N, Johnson JB, Limacher A, Nuesch E, Juni P; Fame2 Trial Investigators . Fractional flow reserve‐guided PCI for stable coronary artery disease. N Engl J Med. 2014;371:1208–1217. [DOI] [PubMed] [Google Scholar]
- 12. Serruys PW, Morice MC, Kappetein AP, Colombo A, Holmes DR, Mack MJ, Stahle E, Feldman TE, van den Brand M, Bass EJ, Van Dyck N, Leadley K, Dawkins KD, Mohr FW; Investigators S . Percutaneous coronary intervention versus coronary‐artery bypass grafting for severe coronary artery disease. N Engl J Med. 2009;360:961–972. [DOI] [PubMed] [Google Scholar]
- 13. Murai T, Kanaji Y, Yonetsu T, Lee T, Matsuda J, Usui E, Araki M, Niida T, Isobe M, Kakuta T. Preprocedural fractional flow reserve and microvascular resistance predict increased hyperaemic coronary flow after elective percutaneous coronary intervention. Catheter Cardiovasc Interv. 2017; 89:233–242. [DOI] [PubMed] [Google Scholar]
- 14. Murai T, Lee T, Yonetsu T, Isobe M, Kakuta T. Influence of microvascular resistance on fractional flow reserve after successful percutaneous coronary intervention. Catheter Cardiovasc Interv. 2015;85:585–592. [DOI] [PubMed] [Google Scholar]
- 15. van de Hoef TP, Nolte F, EchavarrIa‐Pinto M, van Lavieren MA, Damman P, Chamuleau SA, Voskuil M, Verberne HJ, Henriques JP, van Eck‐Smit BL, Koch KT, de Winter RJ, Spaan JA, Siebes M, Tijssen JG, Meuwissen M, Piek JJ. Impact of hyperaemic microvascular resistance on fractional flow reserve measurements in patients with stable coronary artery disease: insights from combined stenosis and microvascular resistance assessment. Heart. 2014;100:951–959. [DOI] [PubMed] [Google Scholar]
- 16. Park SJ, Kang SJ, Ahn JM, Shim EB, Kim YT, Yun SC, Song H, Lee JY, Kim WJ, Park DW, Lee SW, Kim YH, Lee CW, Mintz GS, Park SW. Visual‐functional mismatch between coronary angiography and fractional flow reserve. JACC Cardiovasc Interv. 2012;5:1029–1036. [DOI] [PubMed] [Google Scholar]
- 17. Fearon WF, Balsam LB, Farouque HM, Caffarelli AD, Robbins RC, Fitzgerald PJ, Yock PG, Yeung AC. Novel index for invasively assessing the coronary microcirculation. Circulation. 2003;107:3129–3132. [DOI] [PubMed] [Google Scholar]
- 18. De Bruyne B, Pijls NH, Smith L, Wievegg M, Heyndrickx GR. Coronary thermodilution to assess flow reserve: experimental validation. Circulation. 2001;104:2003–2006. [DOI] [PubMed] [Google Scholar]
- 19. Yong AS, Layland J, Fearon WF, Ho M, Shah MG, Daniels D, Whitbourn R, Macisaac A, Kritharides L, Wilson A, Ng MK. Calculation of the index of microcirculatory resistance without coronary wedge pressure measurement in the presence of epicardial stenosis. JACC Cardiovasc Interv. 2013;6:53–58. [DOI] [PubMed] [Google Scholar]
- 20. Melikian N, Vercauteren S, Fearon WF, Cuisset T, MacCarthy PA, Davidavicius G, Aarnoudse W, Bartunek J, Vanderheyden M, Wyffels E, Wijns W, Heyndrickx GR, Pijls NH, de Bruyne B. Quantitative assessment of coronary microvascular function in patients with and without epicardial atherosclerosis. EuroIntervention. 2010;5:939–945. [PubMed] [Google Scholar]
- 21. Murai T, Lee T, Yonetsu T, Iwai T, Takagi T, Hishikari K, Masuda R, Iesaka Y, Isobe M, Kakuta T. Variability of microcirculatory resistance index and its relationship with fractional flow reserve in patients with intermediate coronary artery lesions. Circ J. 2013;77:1769–1776. [DOI] [PubMed] [Google Scholar]
- 22. Lee JM, Jung JH, Hwang D, Park J, Fan Y, Na SH, Doh JH, Nam CW, Shin ES, Koo BK. Coronary flow reserve and microcirculatory resistance in patients with intermediate coronary stenosis. J Am Coll Cardiol. 2016;67:1158–1169. [DOI] [PubMed] [Google Scholar]
- 23. Fihn SD, Gardin JM, Abrams J, Berra K, Blankenship JC, Dallas AP, Douglas PS, Foody JM, Gerber TC, Hinderliter AL, King SB III, Kligfield PD, Krumholz HM, Kwong RY, Lim MJ, Linderbaum JA, Mack MJ, Munger MA, Prager RL, Sabik JF, Shaw LJ, Sikkema JD, Smith CR Jr, Smith SC Jr, Spertus JA, Williams SV, Anderson JL; American College of Cardiology Foundation, American Heart Association Task F . ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2012;2012:e354–e471. [DOI] [PubMed] [Google Scholar]
- 24. Fischer JJ, Samady H, McPherson JA, Sarembock IJ, Powers ER, Gimple LW, Ragosta M. Comparison between visual assessment and quantitative angiography versus fractional flow reserve for native coronary narrowings of moderate severity. Am J Cardiol. 2002;90:210–215. [DOI] [PubMed] [Google Scholar]
- 25. Brosh D, Higano ST, Lennon RJ, Holmes DR Jr, Lerman A. Effect of lesion length on fractional flow reserve in intermediate coronary lesions. Am Heart J. 2005;150:338–343. [DOI] [PubMed] [Google Scholar]
- 26. Toth G, Hamilos M, Pyxaras S, Mangiacapra F, Nelis O, De Vroey F, Di Serafino L, Muller O, Van Mieghem C, Wyffels E, Heyndrickx GR, Bartunek J, Vanderheyden M, Barbato E, Wijns W, De Bruyne B. Evolving concepts of angiogram: fractional flow reserve discordances in 4000 coronary stenoses. Eur Heart J. 2014;35:2831–2838. [DOI] [PubMed] [Google Scholar]
- 27. Lee JM, Layland J, Jung JH, Lee HJ, Echavarria‐Pinto M, Watkins S, Yong AS, Doh JH, Nam CW, Shin ES, Koo BK, Ng MK, Escaned J, Fearon WF, Oldroyd KG. Integrated physiologic assessment of ischemic heart disease in real‐world practice using index of microcirculatory resistance and fractional flow reserve: insights from the international index of microcirculatory resistance registry. Circ Cardiovasc Interv. 2015;8:e002857. [DOI] [PubMed] [Google Scholar]
- 28. Echavarria‐Pinto M, Escaned J, Macias E, Medina M, Gonzalo N, Petraco R, Sen S, Jimenez‐Quevedo P, Hernandez R, Mila R, Ibanez B, Nunez‐Gil IJ, Fernandez C, Alfonso F, Banuelos C, Garcia E, Davies J, Fernandez‐Ortiz A, Macaya C. Disturbed coronary hemodynamics in vessels with intermediate stenoses evaluated with fractional flow reserve: a combined analysis of epicardial and microcirculatory involvement in ischemic heart disease. Circulation. 2013;128:2557–2566. [DOI] [PubMed] [Google Scholar]
- 29. Tamita K, Akasaka T, Takagi T, Yamamuro A, Yamabe K, Katayama M, Morioka S, Yoshida K. Effects of microvascular dysfunction on myocardial fractional flow reserve after percutaneous coronary intervention in patients with acute myocardial infarction. Catheter Cardiovasc Interv. 2002;57:452–459. [DOI] [PubMed] [Google Scholar]
- 30. Murai T, Lee T, Kanaji Y, Matsuda J, Usui E, Araki M, Niida T, Hishikari K, Ichijyo S, Hamaya R, Yonetsu T, Isobe M, Kakuta T. The influence of elective percutaneous coronary intervention on microvascular resistance: a serial assessment using the index of microcirculatory resistance. Am J Physiol Heart Circ Physiol. 2016;311:H520–H531. [DOI] [PubMed] [Google Scholar]
- 31. Verhoeff BJ, Siebes M, Meuwissen M, Atasever B, Voskuil M, de Winter RJ, Koch KT, Tijssen JG, Spaan JA, Piek JJ. Influence of percutaneous coronary intervention on coronary microvascular resistance index. Circulation. 2005;111:76–82. [DOI] [PubMed] [Google Scholar]
- 32. Gould KL, Johnson NP, Bateman TM, Beanlands RS, Bengel FM, Bober R, Camici PG, Cerqueira MD, Chow BJ, Di Carli MF, Dorbala S, Gewirtz H, Gropler RJ, Kaufmann PA, Knaapen P, Knuuti J, Merhige ME, Rentrop KP, Ruddy TD, Schelbert HR, Schindler TH, Schwaiger M, Sdringola S, Vitarello J, Williams KA Sr, Gordon D, Dilsizian V, Narula J. Anatomic versus physiologic assessment of coronary artery disease. Role of coronary flow reserve, fractional flow reserve, and positron emission tomography imaging in revascularization decision‐making. J Am Coll Cardiol. 2013;62:1639–1653. [DOI] [PubMed] [Google Scholar]
- 33. van de Hoef TP, van Lavieren MA, Damman P, Delewi R, Piek MA, Chamuleau SA, Voskuil M, Henriques JP, Koch KT, de Winter RJ, Spaan JA, Siebes M, Tijssen JG, Meuwissen M, Piek JJ. Physiological basis and long‐term clinical outcome of discordance between fractional flow reserve and coronary flow velocity reserve in coronary stenoses of intermediate severity. Circ Cardiovasc Interv. 2014;7:301–311. [DOI] [PubMed] [Google Scholar]
- 34. Johnson NP, Toth GG, Lai D, Zhu H, Acar G, Agostoni P, Appelman Y, Arslan F, Barbato E, Chen SL, Di Serafino L, Dominguez‐Franco AJ, Dupouy P, Esen AM, Esen OB, Hamilos M, Iwasaki K, Jensen LO, Jimenez‐Navarro MF, Katritsis DG, Kocaman SA, Koo BK, Lopez‐Palop R, Lorin JD, Miller LH, Muller O, Nam CW, Oud N, Puymirat E, Rieber J, Rioufol G, Rodes‐Cabau J, Sedlis SP, Takeishi Y, Tonino PA, Van Belle E, Verna E, Werner GS, Fearon WF, Pijls NH, De Bruyne B, Gould KL. Prognostic value of fractional flow reserve: linking physiologic severity to clinical outcomes. J Am Coll Cardiol. 2014;64:1641–1654. [DOI] [PubMed] [Google Scholar]
- 35. Ahmadi A, Stone GW, Leipsic J, Serruys PW, Shaw L, Hecht H, Wong G, Norgaard BL, O'Gara PT, Chandrashekhar Y, Narula J. Association of coronary stenosis and plaque morphology with fractional flow reserve and outcomes. JAMA Cardiol. 2016;1:350–357. [DOI] [PubMed] [Google Scholar]
- 36. Yong AS, Ho M, Shah MG, Ng MK, Fearon WF. Coronary microcirculatory resistance is independent of epicardial stenosis. Circ Cardiovasc Interv. 2012;5:S101–S102. [DOI] [PubMed] [Google Scholar]


