Abstract
Background
ANGPTL4 (angiopoietin‐like protein 4) is a LPL (lipoprotein lipase) inhibitor and is present in high‐density lipoprotein (HDL). However, it is not defined whether ANGPTL4 in HDLs could affect HDL metabolism and function in type 2 diabetes mellitus (T2DM).
Methods and Results
ANGPTL4 levels in the circulation and HDLs were quantified in nondiabetic participants (n=201, 68.7% females) and T2DM patients (n=185, 66.5% females). HDLs were isolated from nondiabetic controls and T2DM patients to assess cholesterol efflux or subjected to endothelial lipase (EL)‐overexpressed HEK293 cells for EL hydrolysis in vitro. The association between ANGPTL4 in HDLs and HDL components and function was analyzed in nondiabetic participants or diabetic patients, respectively. Plasma or HDLs of ANGPTL4+/+ and ANGPTL4−/− mice was subjected for cholesterol efflux or EL hydrolysis, respectively. ANGPTL4 levels in the plasma and HDLs were 1.7‐ and 2.0‐fold higher in T2DM patients than nondiabetic controls, respectively (P<0.0001). Multivariable analysis demonstrated that per 1 doubling increase of ANGPTL4 levels in HDLs, the changes amounted to +0.27% cholesterol efflux (P=0.03), +0.06 μg/mL apolipoprotein A‐I (P=0.09) and −9.41 μg/L serum amyloid A (P=0.02) in nondiabetic controls. In T2DM patients, the corresponding estimates were −0.06% cholesterol efflux (P=0.10), −0.06 μg/mL apolipoprotein A‐I (P=0.38), and +3.64 μg/L serum amyloid A (P=0.72). HDLs isolated from ANGPTL4−/− mice showed accelerated hydrolysis by EL and reduced cholesterol efflux compared with ANGPTL4+/+ littermates.
Conclusions
Physically, ANGPTL4 in HDLs protected HDLs from hydrolysis. Resulting from increased circulating ANGPTL4 levels in T2DM, ANGPTL4 levels in HDLs were elevated but with compromised inhibitory effect on EL, leading to increased HDL hydrolysis and dysfunction.
Keywords: angiopoietin‐like protein 4, cholesterol efflux, diabetes mellitus, endothelial lipase, high‐density lipoproteins
Subject Categories: Clinical Studies, Lipids and Cholesterol, Metabolism
Clinical Perspective
What Is New?
Our study demonstrates that ANGPTL4 (angiopoietin‐like protein 4), as a component of HDL (high‐density lipoprotein), modulates HDL metabolism and function. As circulating angiopoietin‐like protein 4 levels increase in type 2 diabetes mellitus patients, HDL–associated angiopoietin‐like protein 4 increases but with skewed inhibition of endothelial lipase activity, leading to HDL hydrolysis and dysfunction.
What Are the Clinical Implications?
This study suggests that targeting angiopoietin‐like protein 4 for improved HDL function might confer reduction of cardiovascular risk and improve glycemic control in patients with type 2 diabetes mellitus.
Introduction
ANGPTL4 (angiopoietin‐like protein 4) belongs to the angiopoietin‐like protein family, which are secreted glycoproteins for angiogenic regulation.1, 2 So far, the origins of ANGPTL4 production include hepatocytes,3, 4 adipocytes,5, 6 skeletal muscle,7 glia cells7, and macrophages.8 Once secreted, the full‐length ANGPTL4 is processed by proprotein convertases at the linker region, releasing its N‐terminal region and the monomeric C‐terminal portion of ANGPTL4. The N‐terminal region is responsible for its assembly to form oligomerization, which mediates the inhibition of lipoprotein lipase LPL (lipoprotein lipase). In contrast, the C‐terminal region interacts with integrins and extracellular matrix proteins for cell adhesion and migration.2 Given that ANGPTL4 is produced by a variety of cells upon different stimuli and carries multiple functions through its N‐ and C‐terminal regions, it is not surprising that the biological function of ANGPTL4 could be complicated.
As a potent LPL inhibitor, ANGPTL4 disrupts LPL dimerization, converting this enzyme into inactive form.9, 10 Because of increased LPL activity, ANGPTL4‐null mice are featured as having lower triglycerides in the blood.11 The inverse correlation between ANGPTL4 and high‐density lipoprotein‐cholesterol HDL‐c (high‐density lipoprotein‐cholesterol) has been reported in European and American populations.12, 13 In line with these findings, ANGPTL4 variants have been identified in European subjects.14, 15, 16, 17 For instance, E40K carriers, who have loss‐of‐function mutation of ANGPTL4, display higher HDL‐c with reduced risk of coronary heart disease when compared with noncarriers.14, 15, 16, 17 Recently, plasma levels of ANGPTL4 have been found to be increased in patients with type‐2 diabetes mellitus (T2DM) and subjects with metabolic syndromes; both of them are featured as having reduced HDL‐c.8 These data suggest an intimate link between ANGPTL4 and HDLs.
It is well known that HDL function is critically determined by the components in HDLs such as apoA‐I (apolipoprotein A‐I).18, 19, 20 Pathological alteration of HDL metabolism imbalances HDL components and thus abrogates the protective effects of HDLs in cardiovascular events.18, 19, 20 Previously, we demonstrated that ANGPTL3, another family member of ANGPTL4, is positively associated with apolipoprotein A‐I and HDL function in nondiabetic individuals.21 Interestingly, ANGPTL4 is present in HDLs isolated from mice and human plasma.22 However, limited data have determined whether and how ANGPTL4, as a component of HDLs, has any direct impact on HDL metabolism and its function. Therefore, in the study, we tested the hypothesis that ANGPTL4 in HDLs could directly regulate HDL metabolism and function. Under the diabetic condition, the levels of ANGPTL4 in HDLs might alter following the elevated concentration of ANGPTL4 in the circulation, resulting in abnormal HDL metabolism and impaired HDL function.
Methods
Human Subjects, Sample Size, and Clinical Measurements
Because of limited data on ANGPTL4 expression in Asian subjects, we quantified circulating ANGPTL4 expression in a small number of nondiabetic participants and patients with T2DM. Based on the pilot experiment, we calculated the sample size by the standard formula: (http://powerandsamplesize.com/Calculators/Compare-2-Means/2-Sample-1-Sided), in which 1‐β is equal to 0.80 and α is equal to 0.01.
The formula used to compute sample size is
In which, σA and σB are SDs of ANGPTL4 levels in nondiabetic and T2DM subjects, respectively; μA and μB are means of ANGPTL4 levels in nondiabetic and T2DM subjects, respectively; κ=1; α=0.01; β=0.80.
After calculation, the minimum sample size was 91 for each group. From January 2016 until May 2016, 204 nondiabetic subjects and 186 T2DM patients were recruited to the Department of Endocrinology at Lu He Hospital in Beijing. All the participants were Han people from the same region. The criteria of T2DM diagnosis were adapted from that of the World Health Organization and the American Diabetes Association as follows: (1) fasting glucose level ≥7 mmol/L; or (2) 2‐hour oral glucose tolerance test ≥11.1 mmol/L; or (3) random glucose level ≥11.1 mmol/L and accompanied by typical DM symptoms such as polydipsia, polyuria, increased food intake, and loss of body weight. We excluded 3 nondiabetic subjects and 1 T2DM patient because either ANGPTL4 levels (n=2) or total cholesterol values (n=2) were deviated more than 3 SDs from the mean. Thus, the number of the analyzed subjects totaled 386.
After a 12‐hour overnight fast, venous blood samples were obtained to measure total cholesterol, triglyceride, HDL‐c, plasma glucose, insulin, and serum creatinine by the central laboratory in the hospital. LDL‐c (low‐density lipoprotein‐cholesterol) was computed from serum total cholesterol and HDL‐c and serum triglycerides by the Friedewald equation.23 Body mass index (BMI) was measured as described before.21 Pancreatic β‐cell function and insulin resistance were computed by Homeostasis Model of Assessment (Homa‐B and HOMA‐IR; http://www.dtu.ox.ac.uk/homacalculator/), using fasting insulin and glucose in the subjects. To assess kidney function, estimated glomerular filtration rate was calculated according to the formula.24
The study complied with the Helsinki Declaration for investigation of human subjects. The entire study obtained ethical approval from the competent Institutional Review Boards of Capital Medical University. All participants provided written informed consent.
Mice and Genotype
C57BL/6 mice at the age of 10 to 12 weeks old were used in the study. ANGPTL4−/− mice were kindly provided by Prof. Guo‐Qing Liu (Institute of Cardiovascular Sciences and Key Laboratory of Molecular Cardiovascular Sciences, Beijing University Health Science Center, China). To obtain stable expansion, ANGPTL4−/− were first crossed back with ANGPTL4+/+ mice to generate ANGPTL4+/− mice and then ANGPTL4−/− pups were screened out from ANGPTL+/− couples for further breeding. The phenotype of ANGPTL4−/− mice was determined by 2 pairs of primers (Table 1). Ethical approval for the entire study was obtained from the competent Institutional Review Boards of Capital Medical University.
Table 1.
Primer Sequences of ANGPTL4 Wild‐Type and ANGPTL4 Knockout Mice
| Primer Name | Sequence (5′ to 3′) | PCR Product |
|---|---|---|
| ANGPTL4‐wt‐F | CCAGCAGCAGAGATACCT | 240 bp |
| ANGPTL4‐wt‐R | GTCTTGTCTACTCCATTGTCT | |
| ANGPTL4‐ko‐F | GGTGAATAAGAGGAGGTTGC | 1000 bp |
| ANGPTL4‐ko‐R | CCGATTGTCTGTTGTGCC |
ANGPTL4 indicates angiopoietin‐like protein 4; PCR, polymerase chain reaction.
Lipoprotein Isolation
Plasma samples were separated by density gradient ultracentrifugation in a swing‐out rotor described by Chapman et al.25 Based on the density, very low‐density lipoprotein/intermediate‐density lipoprotein (g <1.019 g/mL), LDL (1.019 g/mL < g <1.063 g/mL), and HDLs (1.063 g/mL < g <1.21 g/mL) fractions were isolated. After being dialyzed in 1 mmol/L EDTA overnight, fractions were subjected to Western blot. Thereafter, HDL fractions were concentrated by centrifugation at 3220g for 20 minutes to assess cholesterol efflux or to evaluate the inhibition of ANGPTL4 on endothelial lipase (EL) activity in vitro.
HDL Fractionation and HDL Component Quantification
To quantify HDL components, plasma samples were precipitated by very low‐density lipoprotein/LDL precipitation buffer to obtain an HDL fraction, following the manual's instruction (Biovision, CA). The concentrations of apoA‐I, phospholipid, and serum amyloid A in HDL fraction were quantified following the instruction, respectively (MLBio, Shanghai, China). Triglyceride concentration in HDL fractions was determined (BioSino Biotechnology).
Total Cholesterol and Triglyceride Determination in Mice
After overnight fasting, mice were bled and plasma levels of cholesterol and triglyceride in ANPTL4+/+ and ANGPTL4−/− mice were measured (BioSino Biotechnology).
Enzyme‐Linked Immunosorbent Assay
The levels of ANGPTL4 in the plasma and HDL fraction were determined by Human ANGPTL4 assay kit according to the manufacturer's instruction (Catalogue number 27749, IBL International GMBH, Japan) as described before.26 After overnight fasting, plasma levels of ANGPTL4 in ANPTL4+/+ and ANGPTL4−/− mice were quantified by enzyme‐linked immunosorbent assay (SEB019Mu, Cloud‐Clone Corp).
Cholesterol Efflux Assay
Cholesterol efflux assay was performed following the manual's instruction (Catalog K582‐100, Biovision) as described before.21 Briefly, RAW264.7 macrophages were plated at the density of 1×105 cells/well in a 96‐well plate and maintained in DMEM plus 10% fetal bovine serum (Sigma‐Aldrich) for 2 hours. The adherent cells were first loaded with labeled cholesterol for 16 hours and then exposed to 2.5 μL plasma or 100 μg/mL HDLs for 4 hours.21, 27 When using plasma as cholesterol acceptor, they were treated with Serum Treatment Reagent prior to loading (Catalog K582‐100, Biovision). The supernatant was transferred to a 96‐well plate to measure the fluorescence (Ex/Em=482/515 nm). The adherent cells were solubilized by cell lysis buffer to measure the fluorescence (Ex/Em=482/515 nm). Cholesterol efflux %=fluorescence intensity of the media/(fluorescence intensity of the cell lysate+media)×100.28
Cell Culture, Transfection, and Fluorescence‐Activated Cell Sorting
HEK293 cells were maintained in DMEM supplemented with 10% fetal bovine serum and 1% penicillin/streptomycin. When cells reached 80% confluence, they were transfected with plasmid construction containing GFP‐flag using Lipofectamine 2000 reagent (Invitrogen) according to manufacturer's instructions. After transfection, cells were cultured for 48 hours. To obtain pure EL–expressing HEK 293 cells, cells were harvested and sorted by its GFP expression. After sorting, purified cells were expanded in vitro for EL hydrolysis assay.
EL Hydrolysis Assay
HDLs were concentrated by centrifugation, and HDL components (cholesterol, phospholipid, and triglyceride) were determined as aforementioned. EL‐expressing HEK293 cells were exposed to serum‐free medium containing HDLs (100 μg/mL) and incubated at 37°C. After 4 hours of incubation, the medium was collected to quantify the concentrations of cholesterol, phospholipid, and free fatty acid using commercially available kits (MLBio, Shanghai, China). The absolute amounts of phospholipid and cholesterol before and after EL assay were calculated to obtain the amounts of metabolized cholesterol and phospholipid. The hydrolyzed triglyceride was expressed by the ratio between the amount of free fatty acid generated after EL hydrolysis assay and the amount of triglyceride loaded before the assay.
Immunoprecipitation and Western Blot
HDL fractions (10 μL) were separated using 10% SDS‐PAGE. After transfer, nitrocellulose membranes were probed with anti‐ANGPTL4 antibody 1/1000 (Abcam, Cambridge, UK) overnight and then incubated with horseradish peroxidase–conjugated IgG secondary antibody 1/3000 (Amersham Biosciences, Piscataway, NJ) for 1 hour. Signals were detected by ECL chemiluminescence (Thermo Fisher Scientific, Rockford, IL).
To further confirm the presence of ANGPTL4 on HDL fractions, HDLs (400 μL) and lysis buffer (400 μL) were incubated with anti‐ANGPTL4 antibody (Santa Cruz, Dallas, TX) or IgG (Cell Signaling Technology, Beverly, MA) coupled with protein G plus/protein A‐agarose (Santa Cruz, Dallas, TX) overnight. After washing, the immunoprecipitated proteins were processed for Western blotting as described above.
HEK 293 cells transfected with plasmid containing EL cDNA (GFP‐EL‐flag) were lysed in 1 mL ice‐cold lysis buffer (10 mmol/L HEPES, 50 mmol/L NaCl, 5 mmol/L EDTA, 1 mmol/L benzamidine, and 0.5% Triton X‐100 [pH 7.4]). The lysates were analyzed by Western blotting with anti‐flag antibody (Medical and biological laboratories, Nagoya, Japan) and anti‐GAPDH antibody (Santa Cruz, Dallas, TX).
Statistical Analysis
The statistical analysis was performed using the SAS system, version 9.4 (SAS Institute Inc, Cary, NC). For untransformed and logarithmically transformed variables, we expressed the central tendency and spread of the distributions by the arithmetic mean and SD and the geometric mean and interquartile range, respectively. To achieve normal distribution, logarithmic transformation was performed in ANGPTL4 levels in plasma and HDL fractions, serum triglyceride, fasting insulin in blood, Homa‐B, Homa‐IR, and phospholipid and triglyceride levels in HDLs. We compared means by the large‐sample z‐test and proportions by Fisher exact test. Pearson's correlation was used for univariate association analysis. Multivariate analysis was performed by adjusting for age, sex, BMI, mean arterial pressure, and use of lipid‐lowering drugs. When analyzing the kinetics of EL hydrolysis, Dunnett's multiple comparison test was used for subjects with medium‐ or high‐ANGPTL4 levels in HDLs versus ones with low ANGPTL4 levels in HDLs. In the mice study, unpaired t‐test was used to compare the difference between ANGPTL4+/+ and NAGPTL4−/− mice. Significance was a 2‐tailed α‐levendothelial lipase of 0.05 or less.
Results
General Characterization of the Study Subjects
Table 2 lists the characteristics of nondiabetic participants and T2DM patients. In 201 nondiabetic participants, age averaged 53.1 years and the proportion of women was 68.7%. In 185 T2DM patients, age averaged 55.9 years and the proportion of women was 66.5%. All T2DM patients were received antidiabetic treatment; among them 58 (31.8%) used metformin and 19 (10.3%) took insulin injection. Among all T2DM patients, 78 subjects were just diagnosed diabetic during enrollment. Compared with nondiabetic controls, T2DM patients were featured as having a higher prevalence of hypertension, increased systolic blood pressure, plasma glucose and serum triglyceride, but reduced HDL‐c (Table 2). To further compare the HDL components between the 2 groups, HDLs were fractionated. In addition to decreased HDL‐c, the concentration of apoA‐I was 1.3‐fold higher in nondiabetic subjects than T2DM patients (P<0.0001). By contrast, the concentrations of serum amyloid A and phospholipid were 1.4‐ and 1.5‐fold increase in T2DM patients when compared with controls, respectively (P<0.0001 for both).
Table 2.
General Characteristics of Study Participants
| Nondiabetic | Type 2 Diabetic | P Value | |
|---|---|---|---|
| N | 201 | 185 | |
| Female (%) | 138 (68.7%) | 123 (66.5%) | 0.649 |
| Hypertension (%) | 63 (16.3%) | 73 (18.9%) | 0.030 |
| Coronary heart disease (%) | 6 (1.6%) | 11 (2.9%) | 0.157 |
| Medications (%) | |||
| Lipid‐lowering drugs (0, 1) | 3 (0.8%) | 17 (4.4%) | 0.001 |
| Antihypertensive (0, 1) | 25 (12.4%) | 33 (17.8%) | 0.224 |
| Insulin (0, 1) | NA | 19 (4.9%) | NA |
| Metformin (0, 1) | 2 | 58 (15.0%) | <0.0001 |
| Mean of characteristics | |||
| Age, y | 53.1 (9.4) | 55.9 (7.4) | 0.001 |
| Body mass index, kg/m2 | 26.2 (3.8) | 26.8 (4.0) | 0.094 |
| Systolic blood pressure, mm Hg | 128.6 (18.3) | 135.0 (18.9) | 0.001 |
| Diastolic blood pressure, mm Hg | 77.8 (11.0) | 79.4 (10.4) | 0.144 |
| Fasting blood glucose, mg/dL | 5.0 (0.5) | 9.8 (3.1) | <0.0001 |
| Insulin, pmol/L | 51 (34–71) | 52 (27–85) | 0.42 |
| Homa‐B, % | 93 (72–112) | 29 (13–47) | <0.0001 |
| Homa‐IR, % | 0.9 (0.7–1.3) | 1.1 (0.6–1.9) | 0.24 |
| eGFR, mL/min per 1.73 m2 | 124.3 (45.6) | 103.4 (45.5) | <0.0001 |
| Total cholesterol, mmol/L | 5.04 (1.03) | 5.21 (1.16) | 0.130 |
| Triglyceride, mg/dL | 114 (81–183) | 158 (92–246) | <0.0001 |
| LDL‐c, mmol/L | 3.04 (0.88) | 3.19 (0.88) | 0.102 |
| HDL‐c, mmol/L | 1.36 (0.34) | 1.27 (0.32) | 0.007 |
| HDL components | |||
| Apolipoprotein A‐I, μg/mL | 8.8 (2.3) | 7.0 (3.6) | <0.0001 |
| Serum amyloid A, μg/L | 657 (462–831) | 892 (537–1129) | <0.0001 |
| Phospholipid, mg/dL | 460 (291–744) | 678 (518–904) | <0.0001 |
| Triglyceride, mg/dL | 17 (10–22) | 17 (13–22) | 0.08 |
eGFR, estimated glomerular filtration rate calculated according to the CKD‐EPI formula. Hypertension was a blood pressure of ≥140 mm Hg systolic, or ≥90 mm Hg diastolic, or use of antihypertensive drugs. Pancreatic β‐cell function (Homa‐B) and insulin resistance (Homa‐IR) were computed by Homeostasis Model Assessment (Homa‐B and HOMA‐IR; http://www.dtu.ox.ac.uk/homacalculator/), using fasting insulin and glucose in the subjects. For untransformed and logarithmically transformed variables, we expressed the central tendency and spread of the distributions by the arithmetic mean and SD and the geometric mean and interquartile range, respectively. HDL‐c, high‐density lipoprotein cholesterol; LDL‐c, low‐density lipoprotein cholesterol; NA, not applicable.
Circulating ANGPTL4 in the Study Subjects
Consistent with previous reports,8, 12 plasma level of ANGPTL4 was 1.7‐fold higher in T2DM patients than controls (74.4 pg/mL versus 132.1 pg/mL, P<0.0001). The distribution of plasma ANGPTL4 levels in nondiabetic participants and T2DM patients is shown in Figure 1A and 1B, respectively. When comparing by sexes, plasma ANGPTL4 level in women was lower than in men in nondiabetic controls (87.9 pg/mL versus 66.2 pg/mL, P=0.0003). When compared with male and female controls, ANGPTL4 level was 1.5‐ and 1.9‐fold increased in male and female T2DM patients, respectively (Figure 1C).
Figure 1.
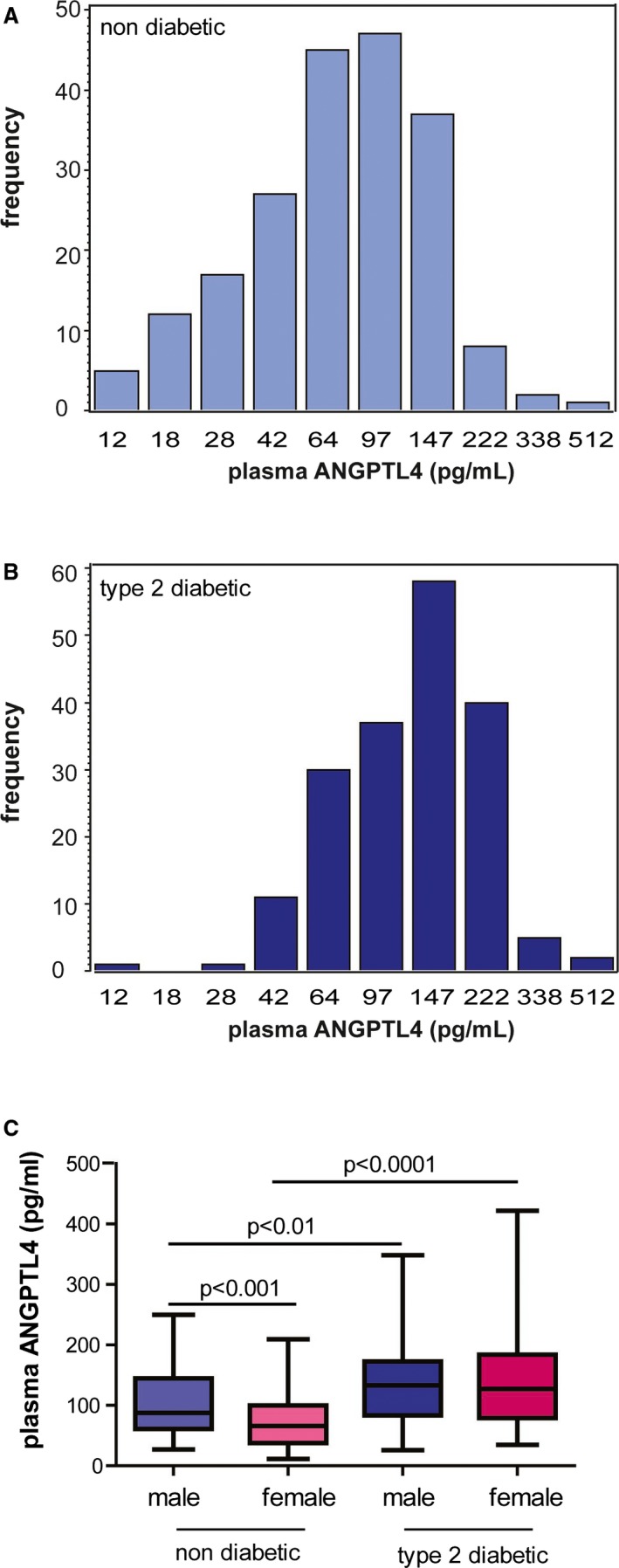
Circulating ANGPTL4 levels in the study subjects. The distribution of circulating ANGPTL4 in 201 nondiabetic subjects (A) and 185 diabetic patients (B) is shown. C, Plasma levels of ANGPTL4 in male and female nondiabetic controls and type 2 diabetic patients are shown. ANGPTL4 indicates angiopoietin‐like protein 4.
As ANGPTL4 is critically involved in metabolic syndrome,12 the association between circulating ANGPTL4 level and the individual metabolic parameter was examined in nondiabetic participants and T2DM patients (Table 3). Based on Pearson correlation, circulating ANGPTL4 levels were positively correlated with BMI (r=0.176, P=0.017), systolic blood pressure (r=0.188, P=0.010), serum triglyceride (r=0.153, P=0.171), and insulin resistance index (Homa‐IR) (r=0.181, P=0.014) but negatively correlated with HDL‐c (r=−0.176, P=0.017) in T2DM patients. Different from T2DM patients, circulating ANGPTL4 did not correlate with any of these parameters mentioned above in controls (P>0.17 for all) (Table 3).
Table 3.
Unadjusted Analysis of the Relationship Between ANGPTL4 and Metabolic Parameters in the Study Subjects
| Nondiabetic | Type‐2 Diabetic | |||
|---|---|---|---|---|
| R | P Value | r | P Value | |
| Number, n | 201 | 185 | ||
| Age, y | −0.001 | 0.99 | 0.071 | 0.34 |
| Body mass index, kg/m2 | 0.060 | 0.40 | 0.176 | 0.017 |
| Systolic blood pressure, mm Hg | 0.033 | 0.65 | 0.188 | 0.010 |
| Diastolic blood pressure, mm Hg | 0.071 | 0.32 | 0.054 | 0.47 |
| Plasma glucose, mmol/L | 0.084 | 0.24 | 0.108 | 0.15 |
| Homa‐B, % | −0.033 | 0.64 | 0.059 | 0.42 |
| Homa‐IR, % | −0.003 | 0.96 | 0.181 | 0.014 |
| Serum triglyceride, mg/dL | 0.024 | 0.74 | 0.153 | 0.037 |
| Total cholesterol, mmol/L | 0.094 | 0.19 | 0.101 | 0.17 |
| LDL‐c, mmol/L | 0.097 | 0.17 | 0.069 | 0.35 |
| HDL‐c, mmol/L | −0.07 | 0.34 | −0.176 | 0.017 |
Pancreatic β‐cell function and insulin resistance were computed by Homeostasis Model of Assessment (Homa‐B and HOMA‐IR; http://www.dtu.ox.ac.uk/homacalculator/), using fasting insulin and glucose in the subjects. ANGPTL4 indicates angiopoietin‐like protein 4; HDL‐c, high‐density lipoprotein cholesterol; LDL‐c, low‐density lipoprotein cholesterol.
ANGPTL4 in HDLs
As ANGPTL4 was identified as a component of HDLs in humans,22 hereby, we investigated whether the concentration of ANGPTL4 in HDLs was changed in T2DM patients. Firstly, by Western blot, ANGPTL4 was found present in HDLs isolated both from nondiabetic controls and T2DM patients, which was further confirmed by immunoprecipitation followed by Western blot (Figure 2A and 2B).
Figure 2.
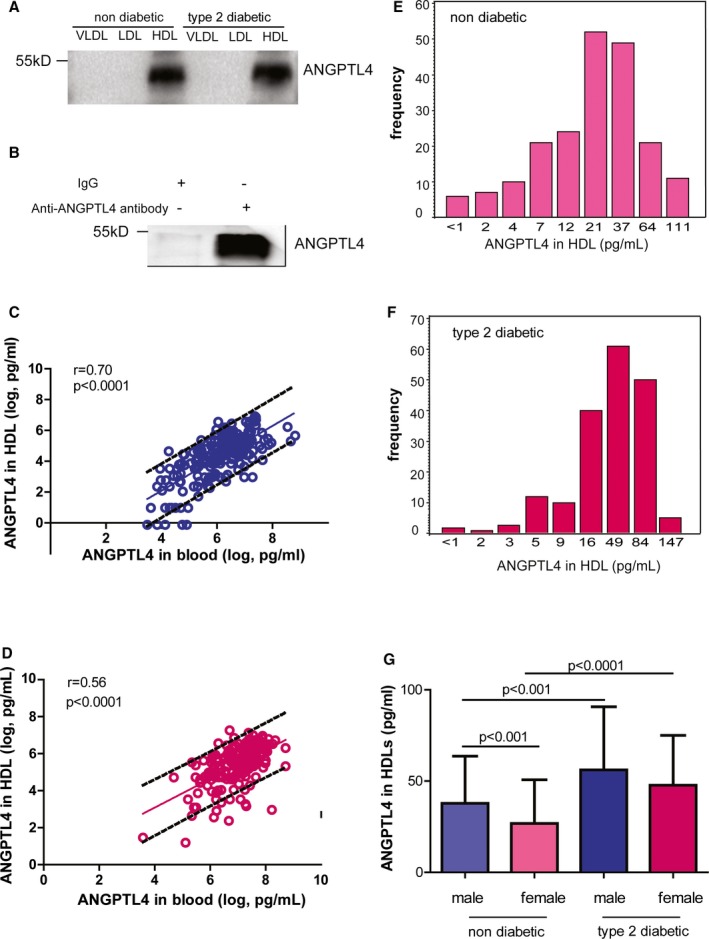
ANGPTL4 in human HDLs. The presence of ANGPTL4 in HDLs by Western blot (A) and immunoprecipitation followed by Western blot (B). n=5 to 6. The correlation is shown between circulating ANGPTL4 in the circulation and HDLs of nondiabetic controls (C) and diabetic patients (D). The distribution of ANGPTL4 in HDLs of nondiabetic controls (E) and diabetic patients is shown (F). ANGPTL4 levels in HDLs of nondiabetic controls and diabetic patients (G). ANGPTL4 indicates angiopoietin‐like protein 4; HDL, high‐density lipoprotein; IgG, immunoglobulin G; LDL, high‐density lipoprotein; VLDL, very low‐density lipoprotein.
Next, HDLs were fractionated from plasma and the concentration of ANGPTL4 in HDLs was measured by enzyme‐linked immunosorbent assay. ANGPTL4 was distributed in HDLs and peripheral blood in a linear mode in both groups (Figure 2C and 2D). Similar to its expression level in the blood, the concentration of ANGPTL4 in HDLs was 22.9 pg/mL (5th to 95th percentile interval: 2.0, 87.4 pg/mL) in controls but increased to 46.3 pg/mL (5th to 95th percentile interval: 8.5, 106.2 pg/mL) in T2DM patients (P<0.0001) (Figure 2E and 2F). When comparing ANGPTL4 level by sexes, the concentrations of ANGPTL4 in HDLs was 1.5‐ and 1.8‐fold higher in male and female T2DM patients compared with male and female controls, respectively (P<0.001 for both, Figure 2G).
Impact of ANGPTL4 on Other HDL Components
By unadjusted analysis, ANGPTL4 levels in HDLs were significantly correlated with apoA‐I but inversely associated with SAA in HDLs of nondiabetic controls (apoA‐I: r=0.156, P=0.027; SAA: r=−0.169, P=0.016) but not in T2DM patients (apoA‐I: r=−0.083, P=0.26; SAA: r=−0.039, P=0.59) (Table 4). Cholesterol level in HDLs was negatively associated with ANGPTL4 components in T2DM patients (r=−0.169, P=0.022) but displayed a trend of association in controls (r=−0.135, P=0.056).
Table 4.
Relationship Between ANGPTL4 in HDLs and Other Components in HDLs
| Nondiabetic Participants | T2DM Patients | |||
|---|---|---|---|---|
| Univariate | Multivariate | Univariate | Multivariate | |
| R | Estimate (95% CI) | r | Estimate (95% CI) | |
| Number | 201 | 201 | 185 | 185 |
| Cholesterol, mmol/L | −0.135 | −0.37 (−0.71, −0.03)* | −0.169* | −0.72 (−1.40, −0.04)* |
| apoA‐I, μg/mL | 0.156* | 0.06 (0.01, 0.12) | −0.083 | −0.06 (−0.19, 0.07) |
| Serum amyloid A, μg/L | −0.169* | −9.41 (−17.32, −1.50)* | −0.039 | 3.64 (−16.49, 23.77) |
| Phospholipid, mg/dL | −0.137 | −0.93 (−1.00, −0.87)* | 0.041 | 1.03 (0.96, 1.10) |
| Triglyceride, mg/dL | 0.130 | 1.04 (0.99, 1.10) | 0.113 | 1.06 (0.99, 1.12) |
All associations were adjusted for age, sex, body mass index, mean arterial pressure, and lipid‐lowering drugs. Estimates given with 95% CI express the difference in HDL components associated with 1 doubling increase of ANGPTL4 in HDLs. ANGPTL4 indicates angiopoietin‐like protein 4; apoA‐I, apolipoprotein A‐I; HDL, high‐density lipoprotein; T2DM, type 2 diabetes mellitus.
Significance: *P<0.05.
When adjusting for age, sex, BMI, mean arterial pressure, and lipid‐lowering drugs, an inverse correlation between cholesterol and ANGPTL4 in HDLs was seen in both nondiabetic controls and T2DM patients (controls: β=−0.32, P=0.044; T2DM: β=−0.72, P=0.041). The inverse associations between ANGPTL4 and phospholipid and SAA in HDLs remained in nondiabetic controls (phospholipid: β=−0.10, P=0.044; SAA: β=−31.4, P=0.021) (Table 4). A trend of positive association between ANGPTL4 and apoA‐I in HDLs was observed in controls (β=0.06, P=0.09). Multivariate‐adjusted analysis supported the above findings in T2DM patients (apoA‐I: β=−0.06, P=0.38; SAA: β=3.64, P=0.72; phospholipid: β=0.04, P=0.45) (Table 4).
Effect of ANGPTL4 on HDL Metabolism and Function in Mice
To directly assess the role of ANGPTL4 in HDL metabolism, ANGPTL4−/− mice were obtained (Figure 3A). As reported earlier, ANGPTL4−/− mice were featured as having low levels of cholesterol and triglyceride in the peripheral blood compared with their wild‐type littermates (Figure 3B). Plasma levels of ANGPTL4 were quantified by enzyme‐linked immunosorbent assay to further confirm the phenotype (Figure 3C). EL is a member of the LPL family and mediates cholesterol hydrolysis in HDLs.29 To further determine whether ANGPTL4 in HDLs acted on EL and thus led to the reduced HDL‐c in T2DM, HEK 293 cells were transfected with the plasmid encoding human EL cDNA and then purified by fluorescence‐activated cell sorting. The sorted cells were expanded in vitro and EL expression was checked by Western blot (Figure 3D). When HDLs were isolated from plasma and subjected to EL‐expressing 293 cells, both cholesterol and triglyceride content were metabolized faster in ANGPTL4−/− mice than ANGPTL4+/+ mice (Figure 3E and 3F). Consequently, cholesterol efflux was 15% reduced in ANGPTL4−/− mice compared with wild‐type mice (Figure 3G). Distinct from cholesterol and triglyceride, phospholipid hydrolysis was similar between the 2 groups (P=0.38). Altogether, these data indicate that ANGPTL4 in HDLs protect HDLs from EL‐mediated hydrolysis and maintain HDL function.
Figure 3.
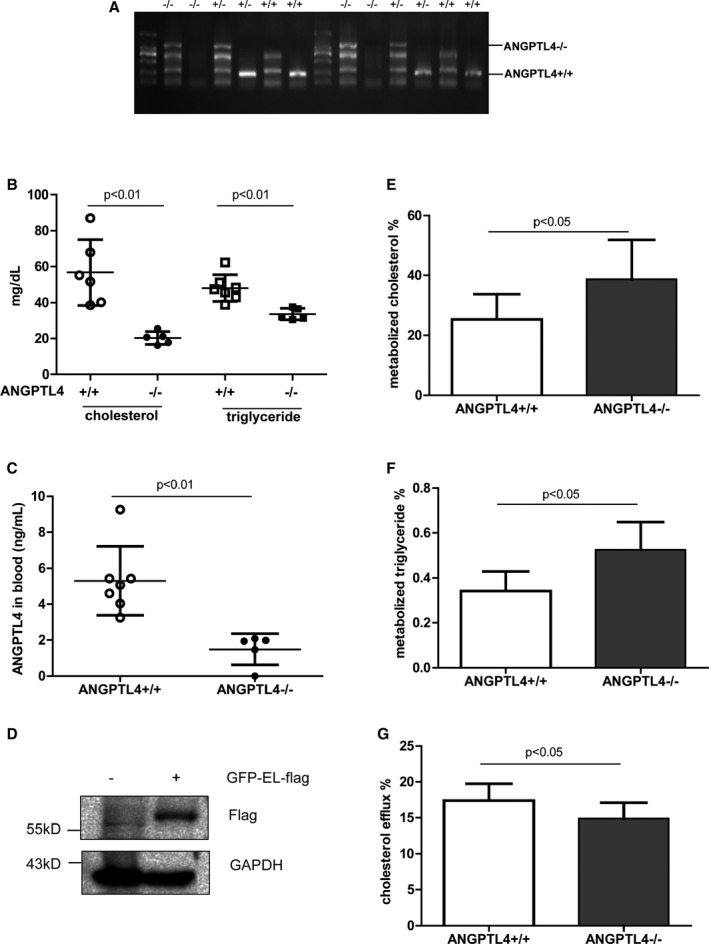
ANGPTL4 as a HDL component for HDL metabolism and function in mice. A, Phenotype determination by RT‐PCR. Two pairs of primers were used to screen ANGPTL4−/− mice. B, Total cholesterol and triglyceride levels in the blood of ANGPTL4+/+ and ANGPTL4−/− mice. n=5 to 7. C, Plasma levels of ANGPTL4. n=5 to 7. The filled circles and squares represent ANGPTL4+/+ mice and the open circles denote ANGPTL4−/− mice. HDLs were isolated from ANGPTL4+/+ and ANGPTL4−/− mice and subjected at a final concentration of 100 μg/mL onto endothelial lipase (EL)‐expressing 293 cells for hydrolysis. Human EL expression in purified HEK 293 cells transfected with the plasmid containing human EL cDNA (D). The percentage of metabolized cholesterol is shown in (E) and the ratio between the amount of free fatty acids produced by EL lysis vs total amount of triglyceride input before EL lysis is shown in (F). n=6 to 7. G, The percentage of cholesterol efflux against plasma of ANGPTL4+/+ and ANGPTL4−/− mice. n=8 to 18. ANGPTL4 indicates angiopoietin‐like protein 4; HDL, high‐density lipoprotein; RT‐PCR, reverse transcription polymerase chain reaction.
ANGPTL4‐Containing HDL on EL Activity in Human Subjects
We then determined how ANGPTL4, as a component of HDLs, influenced HDL metabolism in humans. HDLs isolated from nondiabetic participants and T2DM patients were subjected at the final concentration of 100 μg/mL onto EL‐overexpressed 293 cells. The hydrolysis of cholesterol, phospholipid, and triglyceride in the medium was assessed.
Following the increase of ANGPTL4 levels in HDLs, the percentage of hydrolyzed cholesterol did not differ in nondiabetic controls and T2DM patients with low‐, medium‐ and high‐level of ANGPTL4 in HDLs (P>0.11 for both) (Figure 4A). After EL hydrolysis, the percentage of metabolized phospholipid in HDLs was not different among nondiabetic subjects with low‐, medium‐, and high‐level of ANGPTL4 in HDLs (n=36, P=0.62). However, the percentage of metabolized phospholipid increased 2.6‐fold (P=0.0001) and 2.1‐fold (P=0.015) in T2DM patients with medium‐ and high‐level of ANGPTL4 in HDLs compared with those with low level of ANGPTL4 in HDLs, respectively (Figure 4B). Similar to the kinetics of phospholipid hydrolysis, the yield of free fatty acid was comparable among nondiabetic controls with the tertile levels of ANGPTL4 in HDLs (P=0.44) but was enhanced in the T2DM patients with medium‐ and high‐levels of ANGPTL4 when compared with ones with low ANGPTL4 levels in HDLs (P=0.037) (Figure 4C). Altogether, these data suggest that ANGPTL4 in HDLs of nondiabetic participants and T2DM patients had different inhibitory effect on EL and therefore influenced HDL metabolism differently.
Figure 4.
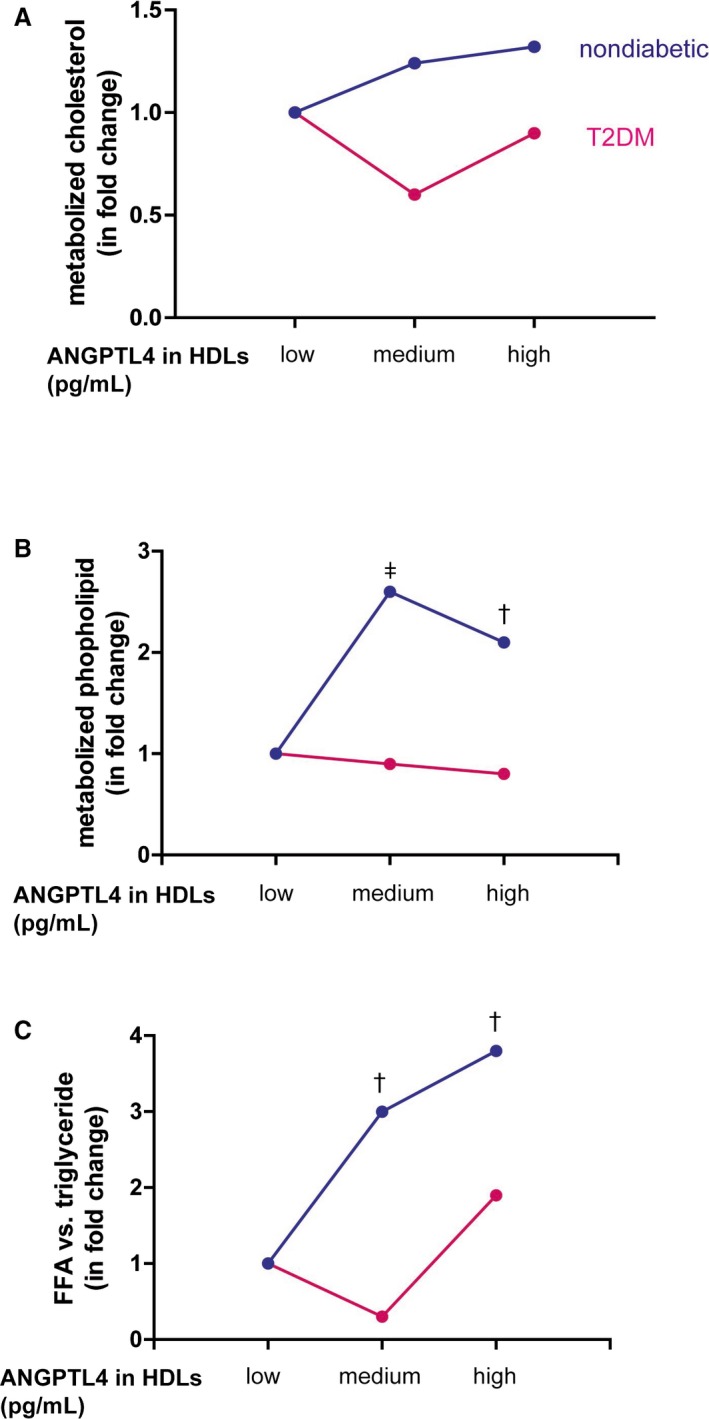
The influence of ANGPTL4 in HDLs on endothelial lipase hydrolysis in humans. Equal amount of HDLs were loaded into endothelial lipase (EL)‐expressing cells for hydrolysis for 4 hours. Before loading, cholesterol, phospholipid, and triglyceride in HDLs were quantified. After loading, medium was collected to measure cholesterol, phospholipid, and free fatty acid. The absolute amounts of metabolized cholesterol and phospholipid and triglyceride were calculated among the subjects with low‐, medium‐, and high‐levels of ANGPTL4 in HDLs in both groups. Metabolized cholesterol (A) and phospholipid (B) were expressed as the fold change when the subjects with medium‐ and high‐levels of ANGPTL4 compared with those having low ANGPTL4 levels in HDLs. n=11 to 12 per subgroup. C, To assess triglyceride metabolism, the ratio of the amount of free fatty acid after hydrolysis vs the amount of triglyceride loaded was calculated. Data are expressed as the fold change when comparing the subjects with the low ANGPTL4 levels in HDLs. n=11 to 12 per subgroup. Significance: ‡ P<0.0001; † P≤0.01. ANGPTL4 indicates angiopoietin‐like protein 4; FFA, free fatty acid; HDL, high‐density lipoprotein; T2DM, type 2 diabetes mellitus.
ANGPTL4 and HDL Function
Next, the relationship between ANGPTL4 and HDL function was studied. After isolation, HDLs were loaded to macrophages that were preloaded with fluorescently labeled cholesterol. HDL function was assessed as its capacity of cholesterol efflux. By Pearson correlation analysis, the percentage of cholesterol efflux was positively associated with ANGPTL4 levels in both peripheral blood and HDLs of nondiabetic participants (Figure 5A and 5B). The percentage of cholesterol efflux was positively associated with circulating ANGPTL4 levels but not with ANGPTL4 levels in HDLs in T2DM patients (Figure 5C and 5D).
Figure 5.
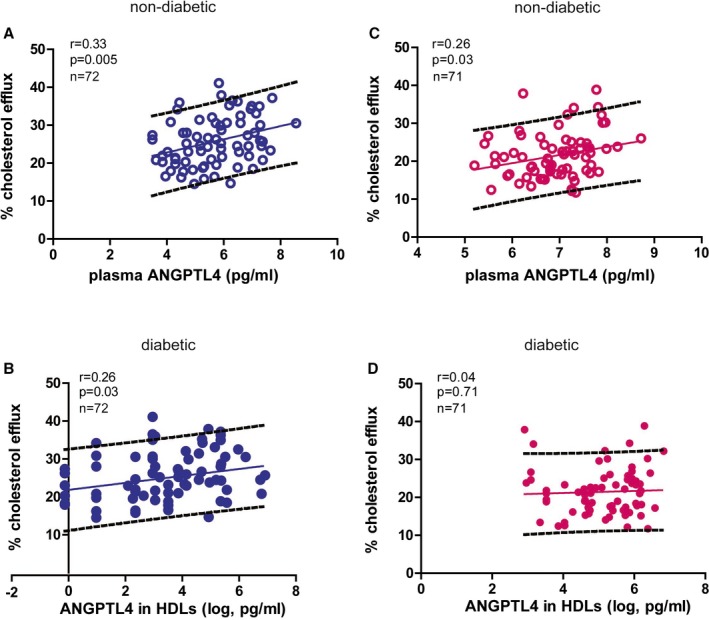
The relationship between ANGPTL4 and cholesterol efflux. Unadjusted association between circulating ANGPTL4 levels and the percentage of cholesterol efflux in nondiabetic controls (A) and diabetic patients is shown (B). Univariate association between ANGPTL4 in HDLs and the percentage of cholesterol efflux in nondiabetic controls (C) and diabetic patients is shown (D). ANGPTL4 indicates angiopoietin‐like protein 4; HDL, high‐density lipoprotein.
Multivariable analysis confirmed the positive association between ANGPTL4 levels and the percentage of cholesterol efflux in nondiabetic controls (circulating ANGPTL4: β=1.70, P=0.008; ANGPTL4 in HDLs: β=0.91, P=0.03). Distinct from nondiabetic controls, after adjusting for the covariables, the percentage of cholesterol efflux was neither associated with circulating ANGPTL4 levels nor with the concentrations of ANGPTL4 in HDLs in T2DM patients (circulating ANGPTL4: β=1.74, P=0.10; ANGPTL4 in HDLs: β=−0.19, P=0.81).
Taken together, per 1 doubling increase of ANGPTL4 level in HDLs, the changes amounted to +0.27% cholesterol efflux (95% CI: 0.03–0.51%; P=0.03), −0.37 mmol/L cholesterol (95% CI: −0.71 to −0.03 mmol/L; P=0.044), +0.06 μg/mL apoA‐I (95% CI: −0.01 to 0.12 μg/mL; P=0.09), and −9.41 μg/L SAA (95% CI: −17.3 to −1.5 μg/L; P=0.018) and −0.93 mg/dL phospholipid (95% CI: −0.99 to −0.87 mg/dL; P=0.044) in nondiabetic controls. In T2DM patients, the corresponding estimates were −0.06% cholesterol efflux (95% CI: −0.50% to 0.39%; P=0.10), −0.72 mmol/L cholesterol (95% CI: −1.40 to 0.04 mmol/L; P=0.041), −0.06 μg/mL apoA‐I (95% CI: −0.19 to 0.07 μg/mL; P=0.16), +3.64 μg/L SAA (95% CI: −16.5 to 23.8 μg/L; P=0.72), and +1.03 phospholipid (95% CI: 0.96–1.10; P=0.45) (Figure 6A through 6D).
Figure 6.
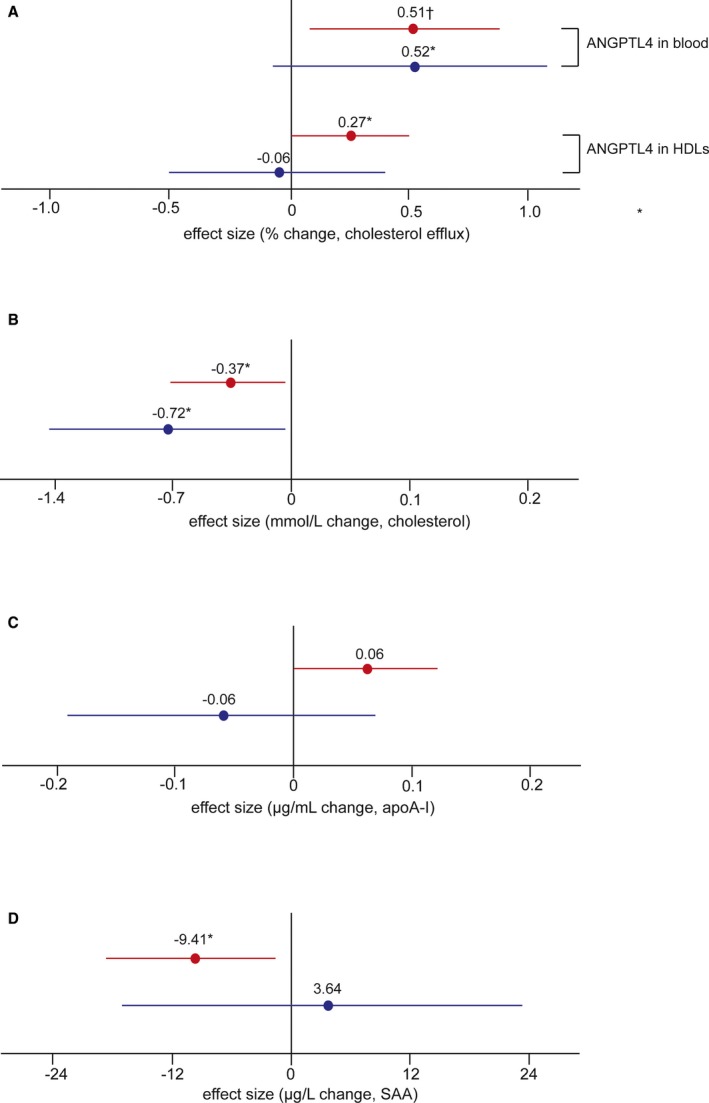
Multivariable analysis of the association between ANGPTL4 and HDL components and function. When adjusting for age, sex, body mass index, mean arterial pressure, and lipid‐lowering drugs, the association between the percentage of cholesterol efflux and circulating ANGPTL4 and ANGPTL4 levels in HDLs in both groups (A) is shown. The correlation between ANGPTL4 and cholesterol (B), apoA‐I (C), and SAA (D) in HDLs is shown. Red and blue lines present nondiabetic controls and T2DM patients, respectively. Data are expressed as the change per 1 doubling increase of ANGPTL4 in the circulation (A) or HDLs (A through D). Nondiabetic: n=201; T2DM: n=185. Significance: † P<0.01; *P<0.05. ANGPTL4 indicates angiopoietin‐like protein 4; apoA‐I, apolipoprotein A‐I; HDL, high‐density lipoprotein; SAA, serum amyloid A; T2DM, type 2 diabetes mellitus.
Discussion
To our best knowledge, our current study is the first to assess and compare whether and how ANGPTL4, as a component of HDLs, participates in HDL metabolism and function in nondiabetic participants and T2DM patients. The key findings can be summarized as follows: (1) the levels of ANGPTL4 in the circulation and HDLs were increased in T2DM patients compared with nondiabetic controls; (2) after adjusting for age, sex, BMI, and use of lipid‐lowering drugs, 1 doubling increase of ANGPTL4 in HDLs was associated with +0.06 μg/mL increase of apoA‐I and 0.27% increase of cholesterol efflux but 0.37 mmol/L decrease of cholesterol, 0.93 pg/mL decrease of phospholipid, and 9.41 μg/L reduction of SAA in HDLs in nondiabetic controls; (3) by contrast, multivariable analysis only illustrated the inverse correlation between cholesterol and ANGPTL4 in HDLs in T2DM patients; (4) in mice, the absence of ANGPTL4 in HDLs accelerated HDL hydrolysis and reduced HDL function; and (5) compared with mice, the role of ANGPTL4 in HDL metabolism was more complicated in human subjects, as evidenced by minor effect on HDL metabolism in nondiabetic controls and dramatic phospholipid and triglyceride hydrolysis in HDLs in T2DM patients when the increased concentrations of ANGPTL4 in HDLs were exposed to EL in vitro.
The incidence of cardiovascular diseases is high in diabetic patients.30 Population studies elucidated HDLs as an independent inverse predictor of cardiovascular disease.30, 31 These epidemiologic findings were confirmed in vitro and in animal models, as supported by promotion of cholesterol efflux from cells or tissues,32 inhibition of vascular inflammation33, and improved endothelial cell function.34 Whereas most studies reported the relationship between circulating ANGPTL4 levels and HDL‐c, they mainly recruited Europeans,8, 14 American,12 or Hispanic subjects.35 Few studies addressed the question of whether the concentration of ANGPTL4 in HDLs was associated with HDL‐c in Chinese subjects, nor did anyone evaluate whether and how ANGPTL4 directly affected HDL metabolism and/or function in T2DM patients.
Apart from these reports, it has been revealed that HDLs become glycated under hyperglycemia and thus render its atheroprotective properties.36 ANGPTL4 plays an important role in lipid metabolism via its inhibition of LPL and is present in HDLs in mice and humans. Thus, it becomes very crucial and relevant to investigate whether ANGPTL4, as a HDL component, modifies HDL metabolism and function in Chinese nondiabetic participants and T2DM patients.
In the literature, the relationship between circulating ANGPTL4 and cholesterol seems controversial. On the one hand, reports showed that plasma ANGPTL4 levels were inversely correlated with HDL‐c but not associated with LDL‐c or triglyceride in the British population.13 Circulating ANGPTL4 levels were negatively associated with HDL‐c and positively associated with triglyceride in Americans.12 On the other hand, no correlation was seen between circulating ANGPTL4 levels with any of these aforementioned parameters in Finnish subjects.37 In the study, we did not observe any significant association between circulating ANGPTL4 levels and HDL‐c, LDL‐c, or triglyceride in nondiabetic participants. Nevertheless, multivariate‐adjusted analysis delineated an inverse correlation between ANGPTL4 and cholesterol in HDLs in both controls and T2DM patients, refining a more specific relationship between ANGPTL4 and cholesterol in HDLs.
We then explored the impact of ANGPTL4 on HDL hydrolysis. As a member of the LPL family, EL catabolizes cholesterol and phospholipid in HDLs.29 Because EL shares a high degree of homology with LPL and hepatic lipase, it tends to be more specific at hydrolyzing phospholipids than LPL and hepatic lipase.38 Furthermore, serum EL activity was increased in T2DM patients.39 In the study, we explored whether ANGPTL4 could have any inhibitory impact on EL as it does for LPL. We first investigated the impact of ANGPTL4 on HDL metabolism and function in a simplified model (ie, ANGPTL4+/+ and ANGPTL4−/− mice). The absolute absence of ANGPTL4 led to prompt cholesterol and triglyceride hydrolysis when exposed to EL, which contributed to decreased HDL function. These data hint at the importance of ANGPTL4 for protecting HDLs from EL‐mediated hydrolysis and maintaining HDL function.
As always, human data are more complex than those of mice. Our data demonstrated that physical levels of ANGPTL4 in HDLs have a minor effect on cholesterol, phospholipid, and triglyceride metabolism in HDLs when exposed to EL. Different from controls, phospholipid and triglyceride were metabolized faster in T2DM patients with high levels of ANGPTL4 in HDLs when compared with ones with low ANGPTL4 levels in HDLs. These data imply that as circulating ANGPTL4 levels were increased in T2DM patients, the distribution of ANGPTL4 in HDLs was elevated but accompanied by reduced inhibition on EL, leading to the accelerated hydrolysis of phospholipid and triglyceride in HDLs and enhanced free fatty acid production. Despite dramatic hydrolysis of triglyceride and phospholipid in HDLs, cholesterol metabolism was similar among T2DM patients with different levels of ANGPTL4 in HDLs. Further studies are needed to determine how elevated ANGPTL4 levels in HDLs have reduced inhibitory property of EL or other lipases in the setting of diabetes mellitus.
Although the phospholipid levels in HDLs were observed to be higher in T2DM patients than that of controls (Table 2), phospholipid in HDLs of T2DM was hydrolyzed more than controls in EL‐expressing cells in vitro (Figure 4B). The contradictory observations might be explained as the following: (1) EL expression and activity are different between nondiabetic controls and T2DM patients,39 which could not be exactly examined in vitro; (2) we could not exclude the regulation of ANGPTL4 on other LPL members for HDL metabolism; and (3) we speculate that other HDL components might regulate ANGPTL4 activity. However, our in vivo and in vitro data verified that ANGPTL4 was critically involved in the diverse HDL metabolism patterns between nondiabetic controls and T2DM patients, all of which substantially translate to different HDL function.
ANGPTL4 is expressed in multiple tissues, and cleavage of its N‐terminal and C‐terminal of this protein could not only inhibit LPL activity but also affect vasculature.2 Thus, ANGPTL4 comprises multifunctional secreted proteins with different targets. ANGPTL4 induced in adipocytes promotes α‐cell proliferation.40 The sialylation form of ANGPTL4 produced in podocytes interacts with αvβ5 intergrin in glomerular endothelial cells to govern glomerulus structure and stability.41 Blockade of this interaction could postpone the recovery from peak proteinuria in mice models of kidney diseases.41, 42 Furthermore, on the one hand, ANGPTL4 promotes endothelial cell integrity and improves angiogenesis for cerebral protection in ischemic stroke; on the other hand, upregulation of ANGPTL4 in hypoxic Müller cells accelerates abnormal angiogenesis and retinopathy.43 Collectively, ANGPTL4 is a double‐edged sword in different diseases.
The present study must be interpreted within the context of some other potential limitations. First, we did not compare LPL and EL activity in the peripheral blood of nondiabetic controls and T2DM patients, nor did we evaluate the effect of ANGPTL4 on LPL‐mediated HDL hydrolysis because HDLs were our main focus in the study. Second, we did not assess the angiogenic function of ANGPTL4 in the study subjects because it was not our focus. Third, we were not able to explore how ANGPTL4 levels were differently regulated in nondiabetic subjects and T2DM patients.
Metformin is the initial treatment for diabetes mellitus. Nevertheless, about 39.7% of T2DM patients could achieve HbA1c less than 7.0% in clinical practice.44 Consistent with the current status, fasting glucose levels of T2DM were much higher than controls in our study. Therefore, a stepwise intensified strategy for pharmaceutical interventions from metformin monotherapy to dual or triple therapies is recommended and being optimized at present.45
In conclusion, our data illustrated that ANGPTL4 in HDLs physically protects HDLs from hydrolysis and sustains HDL function. As circulating ANGPTL4 levels were increased in T2DM patients, the concentration of ANGPTL4 was elevated in HDLs in diabetic patients but with skewed inhibition of EL activity, which contributes substantially to HDL hydrolysis and dysfunction.
Sources of Funding
The study received support from the National Science Funding in China (#81470566 and #81670765), the Beijing Municipal Science & Technology Commission (#Z131100006813018), the Health and Research Bureau of Tongzhou District (#R5; KJ2016CX030; KJ2016CX025; KJ2016CX037‐08), Capital Health Development Research Project (2014‐3‐7081), and Lu He Hospital Funding (LH201504).
Disclosures
None.
Acknowledgments
We express our sincere appreciation to Prof Guo‐Qing Liu for providing ANGPTL4−/− mice for the study and Prof Jan A. Staessen for his guidance on statistical analysis using SAS software.
(J Am Heart Assoc. 2017;6:e005973 DOI: 10.1161/JAHA.117.005973.)28645936
Contributor Information
Dong Zhao, Email: zdoc66@126.com.
Ying‐Mei Feng, Email: yingmeif13@ccmu.edu.cn, Email: yingmeif13@sina.com.
References
- 1. Santulli G. Angiopoietin‐like proteins: a comprehensive look. Front Endocrinol (Lausanne). 2014;5:4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Zhu P, Goh YY, Chin HF, Kersten S, Tan NS. Angiopoietin‐like 4: a decade of research. Biosci Rep. 2012;32:211–219. [DOI] [PubMed] [Google Scholar]
- 3. Janssen AW, Betzel B, Stoopen G, Berends FJ, Janssen IM, Peijnenburg AA, Kersten S. The impact of PPARalpha activation on whole genome gene expression in human precision cut liver slices. BMC Genomics. 2015;16:760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Xu A, Lam MC, Chan KW, Wang Y, Zhang J, Hoo RL, Xu JY, Chen B, Chow WS, Tso AW, Lam KS. Angiopoietin‐like protein 4 decreases blood glucose and improves glucose tolerance but induces hyperlipidemia and hepatic steatosis in mice. Proc Natl Acad Sci USA. 2005;102:6086–6091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Drager LF, Yao Q, Hernandez KL, Shin MK, Bevans‐Fonti S, Gay J, Sussan TE, Jun JC, Myers AC, Olivecrona G, Schwartz AR, Halberg N, Scherer PE, Semenza GL, Powell DR, Polotsky VY. Chronic intermittent hypoxia induces atherosclerosis via activation of adipose angiopoietin‐like 4. Am J Respir Crit Care Med. 2013;188:240–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Gealekman O, Burkart A, Chouinard M, Nicoloro SM, Straubhaar J, Corvera S. Enhanced angiogenesis in obesity and in response to PPAR gamma activators through adipocyte VEGF and ANGPTL4 production. Am J Physiol Endocrinol Metab. 2008;295:E1056–E1064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Vienberg SG, Kleinridders A, Suzuki R, Kahn CR. Differential effects of angiopoietin‐like 4 in brain and muscle on regulation of lipoprotein lipase activity. Mol Metab. 2015;4:144–150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Tjeerdema N, Georgiadi A, Jonker JT, van Glabbeek M, Alizadeh Dehnavi R, Tamsma JT, Smit JW, Kersten S, Rensen PC. Inflammation increases plasma angiopoietin‐like protein 4 in patients with the metabolic syndrome and type 2 diabetes. BMJ Open Diabetes Res Care. 2014;2:e000034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Lafferty MJ, Bradford KC, Erie DA, Neher SB. Angiopoietin‐like protein 4 inhibition of lipoprotein lipase: evidence for reversible complex formation. J Biol Chem. 2013;288:28524–28534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Sukonina V, Lookene A, Olivecrona T, Olivecrona G. Angiopoietin‐like protein 4 converts lipoprotein lipase to inactive monomers and modulates lipase activity in adipose tissue. Proc Natl Acad Sci USA. 2006;103:17450–17455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Koster A, Chao YB, Mosior M, Ford A, Gonzalez‐DeWhitt PA, Hale JE, Li D, Qiu Y, Fraser CC, Yang DD, Heuer JG, Jaskunas SR, Eacho P. Transgenic angiopoietin‐like (ANGPTL)4 overexpression and targeted disruption of ANGPTL4 and ANGPTL3: regulation of triglyceride metabolism. Endocrinology. 2005;146:4943–4950. [DOI] [PubMed] [Google Scholar]
- 12. Mehta N, Qamar A, Qu L, Qasim AN, Mehta NN, Reilly MP, Rader DJ. Differential association of plasma angiopoietin‐like proteins 3 and 4 with lipid and metabolic traits. Arterioscler Thromb Vasc Biol. 2014;34:1057–1063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Smart‐Halajko MC, Robciuc MR, Cooper JA, Jauhiainen M, Kumari M, Kivimaki M, Khaw KT, Boekholdt SM, Wareham NJ, Gaunt TR, Day IN, Braund PS, Nelson CP, Hall AS, Samani NJ, Humphries SE, Ehnholm C, Talmud PJ. The relationship between plasma angiopoietin‐like protein 4 levels, angiopoietin‐like protein 4 genotype, and coronary heart disease risk. Arterioscler Thromb Vasc Biol. 2010;30:2277–2282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Dewey FE, Gusarova V, O'Dushlaine C, Gottesman O, Trejos J, Hunt C, Van Hout CV, Habegger L, Buckler D, Lai KM, Leader JB, Murray MF, Ritchie MD, Kirchner HL, Ledbetter DH, Penn J, Lopez A, Borecki IB, Overton JD, Reid JG, Carey DJ, Murphy AJ, Yancopoulos GD, Baras A, Gromada J, Shuldiner AR. Inactivating variants in ANGPTL4 and risk of coronary artery disease. N Engl J Med. 2016;374:1123–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Folsom AR, Peacock JM, Demerath E, Boerwinkle E. Variation in ANGPTL4 and risk of coronary heart disease: the Atherosclerosis Risk in Communities study. Metabolism. 2008;57:1591–1596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Myocardial Infarction G, Investigators CAEC , Stitziel NO, Stirrups KE, Masca NG, Erdmann J, Ferrario PG, Konig IR, Weeke PE, Webb TR, Auer PL, Schick UM, Lu Y, Zhang H, Dube MP, Goel A, Farrall M, Peloso GM, Won HH, Do R, van Iperen E, Kanoni S, Kruppa J, Mahajan A, Scott RA, Willenberg C, Braund PS, van Capelleveen JC, Doney AS, Donnelly LA, Asselta R, Merlini PA, Duga S, Marziliano N, Denny JC, Shaffer CM, El‐Mokhtari NE, Franke A, Gottesman O, Heilmann S, Hengstenberg C, Hoffman P, Holmen OL, Hveem K, Jansson JH, Jockel KH, Kessler T, Kriebel J, Laugwitz KL, Marouli E, Martinelli N, McCarthy MI, Van Zuydam NR, Meisinger C, Esko T, Mihailov E, Escher SA, Alvar M, Moebus S, Morris AD, Muller‐Nurasyid M, Nikpay M, Olivieri O, Lemieux Perreault LP, AlQarawi A, Robertson NR, Akinsanya KO, Reilly DF, Vogt TF, Yin W, Asselbergs FW, Kooperberg C, Jackson RD, Stahl E, Strauch K, Varga TV, Waldenberger M, Zeng L, Kraja AT, Liu C, Ehret GB, Newton‐Cheh C, Chasman DI, Chowdhury R, Ferrario M, Ford I, Jukema JW, Kee F, Kuulasmaa K, Nordestgaard BG, Perola M, Saleheen D, Sattar N, Surendran P, Tregouet D, Young R, Howson JM, Butterworth AS, Danesh J, Ardissino D, Bottinger EP, Erbel R, Franks PW, Girelli D, Hall AS, Hovingh GK, Kastrati A, Lieb W, Meitinger T, Kraus WE, Shah SH, McPherson R, Orho‐Melander M, Melander O, Metspalu A, Palmer CN, Peters A, Rader D, Reilly MP, Loos RJ, Reiner AP, Roden DM, Tardif JC, Thompson JR, Wareham NJ, Watkins H, Willer CJ, Kathiresan S, Deloukas P, Samani NJ, Schunkert H. Coding variation in ANGPTL4, LPL, and SVEP1 and the risk of coronary disease. N Engl J Med. 2016;374:1134–1144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Talmud PJ, Smart M, Presswood E, Cooper JA, Nicaud V, Drenos F, Palmen J, Marmot MG, Boekholdt SM, Wareham NJ, Khaw KT, Kumari M, Humphries SE; Consortium E, Consortium H . ANGPTL4 E40K and T266M: effects on plasma triglyceride and HDL levels, postprandial responses, and CHD risk. Arterioscler Thromb Vasc Biol. 2008;28:2319–2325. [DOI] [PubMed] [Google Scholar]
- 18. Calkin AC, Drew BG, Ono A, Duffy SJ, Gordon MV, Schoenwaelder SM, Sviridov D, Cooper ME, Kingwell BA, Jackson SP. Reconstituted high‐density lipoprotein attenuates platelet function in individuals with type 2 diabetes mellitus by promoting cholesterol efflux. Circulation. 2009;120:2095–2104. [DOI] [PubMed] [Google Scholar]
- 19. Prufer N, Kleuser B, van der Giet M. The role of serum amyloid A and sphingosine‐1‐phosphate on high‐density lipoprotein functionality. Biol Chem. 2015;396:573–583. [DOI] [PubMed] [Google Scholar]
- 20. Siddiqi HK, Kiss D, Rader D. HDL‐cholesterol and cardiovascular disease: rethinking our approach. Curr Opin Cardiol. 2015;30:536–542. [DOI] [PubMed] [Google Scholar]
- 21. Zhao D, Yang LY, Wang XH, Yuan SS, Yu CG, Wang ZW, Lang JN, Feng YM. Different relationship between ANGPTL3 and HDL components in female non‐diabetic subjects and type‐2 diabetic patients. Cardiovasc Diabetol. 2016;15:132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mandard S, Zandbergen F, van Straten E, Wahli W, Kuipers F, Muller M, Kersten S. The fasting‐induced adipose factor/angiopoietin‐like protein 4 is physically associated with lipoproteins and governs plasma lipid levels and adiposity. J Biol Chem. 2006;281:934–944. [DOI] [PubMed] [Google Scholar]
- 23. Johnson R, McNutt P, MacMahon S, Robson R. Use of the Friedewald formula to estimate LDL‐cholesterol in patients with chronic renal failure on dialysis. Clin Chem. 1997;43:2183–2184. [PubMed] [Google Scholar]
- 24. Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF III, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J; Ckd EPI . A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Chapman MJ, Goldstein S, Lagrange D, Laplaud PM. A density gradient ultracentrifugal procedure for the isolation of the major lipoprotein classes from human serum. J Lipid Res. 1981;22:339–358. [PubMed] [Google Scholar]
- 26. Ito Y, Oike Y, Yasunaga K, Hamada K, Miyata K, Matsumoto S, Sugano S, Tanihara H, Masuho Y, Suda T. Inhibition of angiogenesis and vascular leakiness by angiopoietin‐related protein 4. Can Res. 2003;63:6651–6657. [PubMed] [Google Scholar]
- 27. Wang X, Collins HL, Ranalletta M, Fuki IV, Billheimer JT, Rothblat GH, Tall AR, Rader DJ. Macrophage ABCA1 and ABCG1, but not SR‐BI, promote macrophage reverse cholesterol transport in vivo. J Clin Invest. 2007;117:2216–2224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Agarwala AP, Rodrigues A, Risman M, McCoy M, Trindade K, Qu L, Cuchel M, Billheimer J, Rader DJ. High‐density lipoprotein (HDL) phospholipid content and cholesterol efflux capacity are reduced in patients with very high HDL cholesterol and coronary disease. Arterioscler Thromb Vasc Biol. 2015;35:1515–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Maugeais C, Tietge UJ, Broedl UC, Marchadier D, Cain W, McCoy MG, Lund‐Katz S, Glick JM, Rader DJ. Dose‐dependent acceleration of high‐density lipoprotein catabolism by endothelial lipase. Circulation. 2003;108:2121–2126. [DOI] [PubMed] [Google Scholar]
- 30. Shah AD, Langenberg C, Rapsomaniki E, Denaxas S, Pujades‐Rodriguez M, Gale CP, Deanfield J, Smeeth L, Timmis A, Hemingway H. Type 2 diabetes and incidence of cardiovascular diseases: a cohort study in 1.9 million people. Lancet Diabetes Endocrinol. 2015;3:105–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Saleheen D, Scott R, Javad S, Zhao W, Rodrigues A, Picataggi A, Lukmanova D, Mucksavage ML, Luben R, Billheimer J, Kastelein JJ, Boekholdt SM, Khaw KT, Wareham N, Rader DJ. Association of HDL cholesterol efflux capacity with incident coronary heart disease events: a prospective case‐control study. Lancet Diabetes Endocrinol. 2015;3:507–513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Didichenko SA, Navdaev AV, Cukier AM, Gille A, Schuetz P, Spycher MO, Therond P, Chapman MJ, Kontush A, Wright SD. Enhanced HDL functionality in small HDL species produced upon remodeling of HDL by reconstituted HDL, CSL112: effects on cholesterol efflux, anti‐inflammatory and antioxidative activity. Circ Res. 2016;119:751–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Patel S, Drew BG, Nakhla S, Duffy SJ, Murphy AJ, Barter PJ, Rye KA, Chin‐Dusting J, Hoang A, Sviridov D, Celermajer DS, Kingwell BA. Reconstituted high‐density lipoprotein increases plasma high‐density lipoprotein anti‐inflammatory properties and cholesterol efflux capacity in patients with type 2 diabetes. J Am Coll Cardiol. 2009;53:962–971. [DOI] [PubMed] [Google Scholar]
- 34. Tabet F, Vickers KC, Cuesta Torres LF, Wiese CB, Shoucri BM, Lambert G, Catherinet C, Prado‐Lourenco L, Levin MG, Thacker S, Sethupathy P, Barter PJ, Remaley AT, Rye KA. HDL‐transferred microRNA‐223 regulates ICAM‐1 expression in endothelial cells. Nat Commun. 2014;5:3292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Smart‐Halajko MC, Kelley‐Hedgepeth A, Montefusco MC, Cooper JA, Kopin A, McCaffrey JM, Balasubramanyam A, Pownall HJ, Nathan DM, Peter I, Talmud PJ, Huggins GS, Look AS. ANGPTL4 variants E40K and T266M are associated with lower fasting triglyceride levels in Non‐Hispanic White Americans from the Look AHEAD Clinical Trial. BMC Med Genet. 2011;12:89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Tong X, Lv P, Mathew AV, Liu D, Niu C, Wang Y, Ji L, Li J, Fu Z, Pan B, Pennathur S, Zheng L, Huang Y. The compensatory enrichment of sphingosine ‐1‐ phosphate harbored on glycated high‐density lipoprotein restores endothelial protective function in type 2 diabetes mellitus. Cardiovasc Diabetol. 2014;13:82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Robciuc MR, Tahvanainen E, Jauhiainen M, Ehnholm C. Quantitation of serum angiopoietin‐like proteins 3 and 4 in a Finnish population sample. J Lipid Res. 2010;51:824–831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Paradis ME, Lamarche B. Endothelial lipase: its role in cardiovascular disease. Can J Cardiol. 2006;22(suppl B):31B–34B. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Shiu SW, Zhou H, Wong Y, Tan KC. Endothelial lipase and reverse cholesterol transport in type 2 diabetes mellitus. J Diabetes Investig. 2010;1:111–116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Ben‐Zvi D, Barrandon O, Hadley S, Blum B, Peterson QP, Melton DA. ANGPTL4 links alpha‐cell proliferation following glucagon receptor inhibition with adipose tissue triglyceride metabolism. Proc Natl Acad Sci USA. 2015;112:15498–15503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Clement LC, Mace C, Avila‐Casado C, Joles JA, Kersten S, Chugh SS. Circulating angiopoietin‐like 4 links proteinuria with hypertriglyceridemia in nephrotic syndrome. Nat Med. 2014;20:37–46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Chugh SS, Mace C, Clement LC, Del Nogal Avila M, Marshall CB. Angiopoietin‐like 4 based therapeutics for proteinuria and kidney disease. Front Pharmacol. 2014;5:23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Babapoor‐Farrokhran S, Jee K, Puchner B, Hassan SJ, Xin X, Rodrigues M, Kashiwabuchi F, Ma T, Hu K, Deshpande M, Daoud Y, Solomon S, Wenick A, Lutty GA, Semenza GL, Montaner S, Sodhi A. Angiopoietin‐like 4 is a potent angiogenic factor and a novel therapeutic target for patients with proliferative diabetic retinopathy. Proc Natl Acad Sci USA. 2015;112:E3030–E3039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Xu Y, Wang L, He J, Bi Y, Li M, Wang T, Wang L, Jiang Y, Dai M, Lu J, Xu M, Li Y, Hu N, Li J, Mi S, Chen CS, Li G, Mu Y, Zhao J, Kong L, Chen J, Lai S, Wang W, Zhao W, Ning G; China Noncommunicable Disease Surveillance G . Prevalence and control of diabetes in Chinese adults. JAMA. 2013;310:948–959. [DOI] [PubMed] [Google Scholar]
- 45. Xu W, Mu Y, Zhao J, Zhu D, Ji Q, Zhou Z, Yao B, Mao A, Engel SS, Zhao B, Bi Y, Zeng L, Ran X, Lu J, Ji L, Yang W, Jia W, Weng J. Efficacy and safety of metformin and sitagliptin based triple antihyperglycemic therapy (STRATEGY): a multicenter, randomized, controlled, non‐inferiority clinical trial. Sci China Life Sci. 2017;60:225–238. [DOI] [PubMed] [Google Scholar]


