Abstract
Background
Several markers detected on the routine 12‐lead ECG are associated with future heart failure events. We examined whether these markers are able to separate the risk of heart failure with reduced ejection fraction (HFrEF) from heart failure with preserved ejection fraction (HFpEF).
Methods and Results
We analyzed data of 6664 participants (53% female; mean age 62±10 years) from MESA (Multi‐Ethnic Study of Atherosclerosis) who were free of cardiovascular disease at baseline (2000–2002). A competing risks analysis was used to compare the association of several baseline ECG predictors with HFrEF and HFpEF detected during a median follow‐up of 12.1 years. A total of 127 HFrEF and 117 HFpEF events were detected during follow‐up. In a multivariable adjusted model, prolonged QRS duration, delayed intrinsicoid deflection, left‐axis deviation, right‐axis deviation, prolonged QT interval, abnormal QRS‐T axis, left ventricular hypertrophy, ST/T‐wave abnormalities, and left bundle‐branch block were associated with HFrEF. In contrast, higher resting heart rate, abnormal P‐wave axis, and abnormal QRS‐T axis were associated with HFpEF. The risk of HFrEF versus HFpEF was significantly differently for delayed intrinsicoid deflection (hazard ratio: 4.90 [95% confidence interval (CI), 2.77–8.68] versus 0.94 [95% CI, 0.29–2.97]; comparison P=0.013), prolonged QT interval (hazard ratio: 2.39 [95% CI, 1.55–3.68] versus 0.52 [95% CI, 0.23–1.19]; comparison P<0.001), and ST/T‐wave abnormalities (hazard ratio: 2.47 [95% CI, 1.69–3.62] versus 1.13 [95% CI, 0.72–1.77]; comparison P=0.0093).
Conclusions
Markers of ventricular repolarization and delayed ventricular activation are able to distinguish between the future risk of HFrEF and HFpEF. These findings suggest a role for ECG markers in the personalized risk assessment of heart failure subtypes.
Keywords: electrocardiography, epidemiology, heart failure
Subject Categories: Heart Failure, Electrocardiology (ECG)
Clinical perspective
What Is New?
Several markers detected on the routine 12‐lead ECG are predictive of future HF events.
Whether these ECG markers are able to distinguish between the risk of HF with reduced versus preserved ejection fraction is currently unknown.
This analysis from MESA (Multi‐Ethnic Study of Atherosclerosis) shows that markers of ventricular repolarization and delayed ventricular activation are able to distinguish between the future risk of HF with reduced versus preserved ejection fraction.
What Are the Clinical Implications?
Identifying specific ECG markers that separate the risk of heart failure with reduced versus preserved ejection fraction highlights the unique pathophysiological differences between these conditions and suggests a potential use of these markers in personalized risk assessment of specific types of HF.
Introduction
Heart failure (HF) is a major public health problem. Despite advances in treatment and improved survival in recent decades, the annual mortality for HF remains high, reaching proportions of all adult deaths of 40.5% in men and 59.5% in women.1 The diagnosis of HF is frequently made late, only when patients develop acute symptoms,2 making noninvasive, accurate, and cost‐effective means of detection a priority.
Approximately 50% of patients hospitalized for HF have preserved ejection fraction (HFpEF).2, 3 The management of HFpEF differs from the management of HF with reduced ejection fraction (HFrEF). Recent clinical trials have demonstrated that neurohormonal antagonists, such as β‐blockers, angiotensin‐converting enzyme inhibitors, and angiotensin receptor blockers, are effective in HFrEF.4, 5 However, the benefit of these therapies in HFpEF is unclear,6 suggesting that fundamental differences exist in the pathophysiology of both conditions.7
A number of studies have demonstrated that several markers detected on the routine ECG are associated with future HF events8, 9, 10, 11, 12, 13, 14, 15, 16, 17; however, it is currently unknown if a differential risk profile exists for these ECG markers in the prediction of HFrEF versus HFpEF. The ability to identify specific predictors for HFrEF and HFpEF is an important step to target appropriate preventive strategies for each HF subtype. Consequently, we conducted a competing risks analysis to identify specific ECG predictors that separate the risk of HFrEF from HFpEF in MESA.
Methods
Study Population
Details of MESA have been reported previously.18 Briefly, between July 2000 and September 2002, a total of 6814 persons were recruited at 6 field centers (Baltimore, Maryland; Chicago, Illinois; Forsyth County, North Carolina; Los Angeles, California; New York, New York; and St. Paul, Minnesota). Participants between 45 and 84 years of age with no clinical cardiovascular disease were recruited. All participants provided informed consent, and the study protocol was approved by the institutional review board at each participating institution. For the purpose of this analysis, participants were excluded if they were missing baseline ECG data, baseline characteristics, or HF follow‐up data.
Baseline Characteristics
Participant characteristics were collected during the initial MESA visit. Age, sex, race/ethnicity, income, and education were self‐reported. Annual income was categorized as <$20 000 or ≥$20 000, and education was categorized as high school or less or some college or more. Smoking was defined as ever (current or former) versus never smoker. Blood samples were obtained after a 12‐hour fast, and measurements of total cholesterol, high‐density lipoprotein cholesterol, and plasma glucose were used. Diabetes mellitus was defined as fasting glucose values ≥126 mg/dL or a history of diabetes medication use. Blood pressure was measured for each participant after 5 minutes in the seated position, and systolic measurements were recorded 3 separate times, and the mean of the last 2 values was used. The use of aspirin, statins, and antihypertensive medications was self‐reported. Body mass index was computed as the weight in kilograms divided by the square of the height in meters. Resting heart rate was obtained from baseline ECGs.
ECG Measurements
In MESA, 12‐lead digital ECGs were obtained by trained technicians using GE MAC 1200 electrocardiographs with standardized procedures. ECGs were transmitted electronically to the MESA ECG Reading Center located at the Epidemiological Cardiology Research Center (Wake Forest School of Medicine, Winston‐Salem, NC). According to MESA protocol, all filters in the ECG machines were disabled to provide unfiltered measurements. All ECGs were automatically processed, after visual inspection for technical errors and inadequate quality, using the 2001 version of the GE Marquette 12‐SL program. As part of routine quality control measures regarding ECG data processing, trained staff performed visual inspection of main ECG waveforms and confirmed computer‐detected ECG abnormalities.
Abnormal P‐wave duration, PR interval, and QRS duration were defined as values >120, >200, and >100 ms, respectively. Prolonged QT interval was defined as ≥460 ms for women and ≥450 ms for men using the Framingham formula: QTF=QT+0.154×[1−(60/heart rate)].19 Abnormal P‐wave axis was defined as values outside the range of 0° and 75°.20, 21 Left‐axis deviation was defined as QRS axis less between −90° and −30°, and right‐axis deviation was defined as QRS axis between −90 and +90°. Abnormal QRS‐T angle was defined as values greater than the sex‐specific 95th percentile values (men: >88°; women: >77°). Abnormal P‐wave terminal force in lead V1 (PTFV1) was defined as values >4000 μV×ms.22 Time to peak R wave (instrinsicoid deflection [ID]) was automatically measured from V5 and V6 (the left ventricular chest leads), and the maximum of both values was used in the main analysis. Time to ID values >50 ms were considered abnormal.23 Left ventricular hypertrophy was defined by the Cornell criteria (R wave amplitude AVL plus S wave amplitude V3 ≥2.8 mV in men and ≥2.0 mV in women).24 Low QRS voltage, ST/T‐wave abnormalities, right bundle‐branch block, and left bundle‐branch block were defined using Minnesota Code Criteria.25
Heart Failure
The ascertainment of incident HF events in MESA has been described previously.26 Participants were followed for incident cardiovascular events from baseline through December 31, 2013. At intervals of 9 to 12 months, a telephone interviewer contacted each participant to inquire about all interim hospital admissions, cardiovascular outpatient diagnoses, procedures, and deaths. In addition, MESA occasionally identified medical encounters through cohort clinic visits, participant call‐ins, medical record abstractions, or obituaries. Next‐of‐kin interviews for out‐of‐hospital cardiovascular deaths also were used.
The outcome of interest for this analysis was the composite of probable and definite HF events. Definite or probable HF required symptoms, such as shortness of breath or edema, because asymptomatic disease is not a MESA end point. In addition to symptoms, probable HF required a previous physician diagnosis and the patient to be receiving medical treatment for HF. Definite HF required ≥1 other criteria, such as pulmonary edema or congestion by chest x‐ray, dilated ventricle or poor left ventricular function by echocardiography or ventriculography, or evidence of left ventricular diastolic dysfunction. HF events were stratified by type as HFrEF or HFpEF. HFpEF events were defined as cases with ejection fraction ≥50%.
Statistics
Baseline characteristics were compared by HF status. Categorical variables were reported as frequency and percentage, whereas continuous variables were recorded as mean±SD. Statistical significance for categorical variables was tested using the χ2 method and the ANOVA procedure for continuous variables.
Follow‐up time was defined as the time between the baseline ECG measurement until a diagnosis of HF, death, loss to follow‐up, or end of follow‐up (December 31, 2013). Cox regression was used to compute hazard ratios (HRs) and 95% confidence intervals for the association between each ECG measurement and HF. P values for the HRs were computed using the likelihood ratio method. Separate analyses were conducted for HFrEF and HFpEF. Multivariable models were constructed as follows: model 1 adjusted for age, sex, race/ethnicity, income, and education; model 2 adjusted for model 1 covariates plus systolic blood pressure, heart rate, smoking, diabetes mellitus, body mass index, total cholesterol, high‐density lipoprotein cholesterol, aspirin, statins, and antihypertensive medications. A competing risks analysis was used to compare the association of several ECG predictors with HFrEF and HFpEF. Specifically, we used the Lunn–McNeil method to test whether ECG predictors that were significantly associated with each HF type were associated with a differential risk for HFrEF versus HFpEF.27 The proportional hazards assumption was not violated in our analyses. Statistical significance was defined as P<0.05. SAS version 9.4 was used for all analyses.
Results
A total of 6664 participants (mean age 62±10 years, 53% women, 38% white, 12% Chinese American, 28% black, 22% Hispanic) were included in the final analysis. Baseline characteristics stratified by the development of HF are shown in Table 1. As shown, participants who did not develop HF were more likely to be young, to be female, to have higher educational attainment and income, and to have fewer cardiovascular risk factors compared with those who developed HFrEF or HFpEF. Compared with HFrEF, participants with HFpEF were more likely to be older, to be female, to report smoking, and to have higher systolic blood pressure and cholesterol values. Almost none of those with HFpEF had left bundle‐branch block, and those with HFrEF tended to have a higher prevalence of prolonged QRS duration, abnormal time to ID, prolonged QT duration, and ST/T‐wave abnormalities.
Table 1.
Baseline Characteristics by HF Subtype
| Characteristic | No HF (n=6420) | HFrEF (n=127) | HFpEF (n=117) | P Valuea |
|---|---|---|---|---|
| Age, mean±SD, y | 62±10 | 67±8.9 | 70±8.5 | <0.001 |
| Men, % | 2996 (47) | 91 (72) | 58 (50) | <0.001 |
| Race/ethnicity | 0.023 | |||
| White, % | 2446 (38) | 50 (40) | 50 (43) | |
| Chinese American, % | 782 (12) | 3 (2) | 12 (10) | |
| Black, % | 1769 (28) | 46 (36) | 32 (27) | |
| Hispanic, % | 1423 (22) | 28 (22) | 23 (20) | |
| Education, high school or less, % | 2328 (36) | 51 (40) | 49 (42) | 0.31 |
| Income <$20 000, % | 1703 (27) | 47 (37) | 40 (34) | 0.0060 |
| Ever smoker, % | 3156 (49) | 73 (57) | 69 (59) | 0.021 |
| Diabetes mellitus, % | 866 (13) | 39 (31) | 36 (31) | <0.001 |
| Body mass index, kg/m2, mean±SD | 28±5.4 | 29±5.5 | 30±6.4 | <0.001 |
| Systolic blood pressure, mm Hg, mean±SD | 126±21 | 137±22 | 139±23 | <0.001 |
| Total cholesterol, mg/dL, mean±SD | 194±36 | 187±36 | 189±33 | 0.017 |
| HDL cholesterol, mg/dL, mean±SD | 51±15 | 47±13 | 50±14 | 0.0083 |
| Antihypertensive medications, % | 2329 (36) | 76 (60) | 65 (56) | <0.001 |
| Aspirin, % | 1496 (23) | 46 (36) | 37 (32) | <0.001 |
| Statin, % | 938 (15) | 25 (20) | 17 (15) | 0.28 |
| Heart rate, mean±SD, bpm | 63±9.6 | 64±11 | 66±10 | 0.0014 |
| P‐wave duration, >120 ms, % | 699 (11) | 27 (21) | 21 (18) | <0.001 |
| PR interval, >200 ms, % | 492 (7.6) | 19 (15) | 15 (13) | 0.0014 |
| PTFV1, >4000 ms, % | 940 (15) | 30 (24) | 30 (26) | <0.001 |
| Abnormal P‐wave axis, % | 548 (8.5) | 11 (8.7) | 18 (15) | 0.033 |
| QRS duration, >100 ms, % | 1239 (19) | 56 (44) | 34 (29) | <0.001 |
| Time to ID, >50 ms, % | 113 (1.8) | 14 (11) | 3 (2.6) | <0.001b |
| Left‐axis deviation, % | 367 (5.7) | 21 (17) | 14 (12) | <0.001 |
| Right‐axis deviation, % | 23 (<1) | 2 (1.6) | 0 (0) | 0.15b |
| Prolonged QT interval, % | 481 (7.5) | 28 (22) | 6 (5.1) | <0.001 |
| Abnormal QRS‐T axis, % | 293 (4.6) | 22 (17) | 19 (16) | <0.001 |
| Left ventricular hypertrophy, % | 236 (3.7) | 12 (9.5) | 8 (6.8) | 0.0017b |
| Low voltage, % | 124 (1.9) | 1 (<1) | 3 (2.6) | 0.55b |
| ST/T‐wave abnormalities, % | 852 (13) | 44 (35) | 25 (21) | <0.001 |
| Right bundle‐branch block, % | 145 (2.3) | 6 (4.7) | 7 (5.9) | <0.001b |
| Left bundle‐branch block, % | 16 (<1) | 5 (3.9) | 1 (<1) | <0.001b |
bpm indicates beats per minute; HDL, high‐density lipoprotein; HF, heart failure; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; PTFV1, P‐wave terminal force in V1.
Statistical significance for continuous data was tested using the ANOVA procedure, and categorical data were tested using the χ2 test.
Statistical significance tested using the Fisher exact test because of low cell frequencies.
Over a median follow‐up of 12.1 years (25th–75th percentiles: 11.6–12.7 years), a total of 244 HF cases (incidence rate per 1000 person‐years: 3.33; 95% confidence interval, 2.94–3.77) were identified. Of these, 127 (52%) were HFrEF and 117 (48%) were HFpEF. Among the ECG markers examined, higher resting heart rate, prolonged QRS duration, abnormal time to ID, left‐axis deviation, abnormal QRS‐T angle, left ventricular hypertrophy, ST/T‐wave abnormalities, and left bundle‐branch block were significantly associated with all HF events (Figure).
Figure 1.
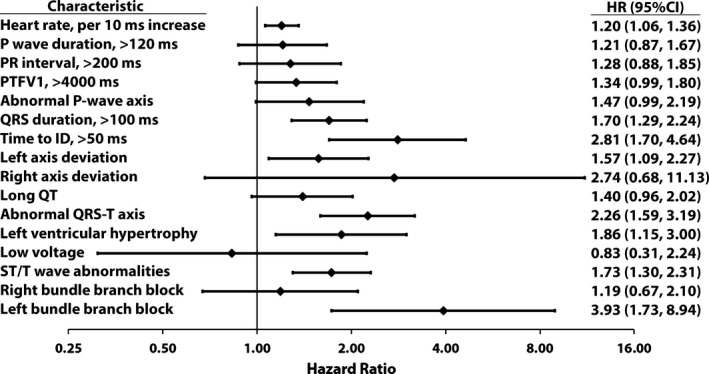
Association between electrocardiographic abnormalities and heart failure. Model adjusted for age, sex, race/ethnicity, education, income, systolic blood pressure, smoking, diabetes mellitus, body mass index, cholesterol, high‐density lipoprotein cholesterol, aspirin, statins, and antihypertensive medications. CI indicates confidence interval; HR, hazard ratio; ID, intrinsicoid deflection; PTFV1, P‐wave terminal force in V1.
Table 2 shows the multivariable HRs for the development of HFrEF and HFpEF associated with each ECG measurement separately. As shown, prolonged QRS duration, delayed time to ID, left‐axis deviation, right‐axis deviation, prolonged QT interval, abnormal QRS‐T axis, left ventricular hypertrophy, ST/T‐wave abnormalities, and left bundle‐branch block were associated with HFrEF. In contrast, higher resting heart rate, abnormal P‐wave axis, and abnormal QRS‐T axis were associated with HFpEF. The risk of HFrEF versus HFpEF was different for abnormal time to ID (comparison P=0.013), prolonged QT interval (comparison P<0.001), and ST/T‐wave abnormalities (comparison P=0.0093).
Table 2.
Electrocardiographic Predictors of HFrEF Versus HFpEF
| ECG Predictora | HFrEF (n=127) | HFpEF (n=117) | P Comparisond | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Model 1b HR (95% CI) | P Value | Model 2c HR (95% CI) | P Value | Model 1b HR (95% CI) | P Value | Model 2c HR (95% CI) | P Value | ||
| Heart rate, per 10‐ms increase | 1.15 (0.97–1.37) | 0.11 | 1.09 (0.91–1.30) | 0.34 | 1.43 (1.20–1.71) | <0.001 | 1.34 (1.12–1.60) | 0.0014 | 0.11 |
| P wave duration, >120 ms | 1.52 (0.98–2.35) | 0.059 | 1.32 (0.85–2.05) | 0.21 | 1.30 (0.80–2.10) | 0.29 | 1.09 (0.67–1.78) | 0.72 | ··· |
| PR interval, >200 ms | 1.50 (0.91–2.46) | 0.11 | 1.36 (0.83–2.23) | 0.23 | 1.33 (0.77–2.31) | 0.31 | 1.19 (0.69–2.08) | 0.53 | ··· |
| PTFV1, >4000 ms | 1.57 (1.04–2.37) | 0.033 | 1.32 (0.87–1.99) | 0.20 | 1.65 (1.09–2.51) | 0.019 | 1.35 (0.88–2.07) | 0.17 | ··· |
| Abnormal P‐wave axis | 0.83 (0.44–1.55) | 0.56 | 1.00 (0.53–1.89) | 0.99 | 1.64 (0.99–2.72) | 0.056 | 2.04 (1.22–3.42) | 0.0066 | 0.088 |
| QRS duration, >100 ms | 2.45 (1.70–3.53) | <0.001 | 2.14 (1.48–3.09) | <0.001 | 1.53 (1.01–2.32) | 0.048 | 1.28 (0.84–1.95) | 0.24 | 0.071 |
| Time to ID, >50 ms | 5.67 (3.25–9.90) | <0.001 | 4.90 (2.77–8.68) | <0.001 | 1.22 (0.39–3.83) | 0.74 | 0.94 (0.29–2.97) | 0.91 | 0.013 |
| Left‐axis deviation | 2.08 (1.29–3.36) | 0.0028 | 1.86 (1.15–3.02) | 0.012 | 1.47 (0.83–2.60) | 0.19 | 1.29 (0.73–2.28) | 0.38 | 0.34 |
| Right‐axis deviation | 4.94 (1.22–19.95) | 0.025 | 4.98 (1.22–20.40) | 0.025 | N/Ae | N/Ae | N/Ae | N/Ae | ··· |
| Prolonged QT interval | 2.55 (1.66–3.90) | <0.001 | 2.39 (1.55–3.68) | <0.001 | 0.52 (0.23–1.19) | 0.12 | 0.48 (0.21–1.11) | 0.086 | <0.001 |
| Abnormal QRS‐T axis | 3.30 (2.06–5.28) | <0.001 | 2.52 (1.56–4.05) | <0.001 | 2.66 (1.61–4.39) | <0.001 | 2.01 (1.21–3.33) | 0.0068 | 0.52 |
| Left ventricular hypertrophy | 3.36 (1.82–6.22) | <0.001 | 2.56 (1.36–4.82) | 0.0036 | 1.70 (0.82–3.54) | 0.15 | 1.31 (0.62–2.76) | 0.48 | 0.18 |
| Low voltage | 0.45 (0.06–3.19) | 0.42 | 0.43 (0.06–3.09) | 0.40 | 1.28 (0.40–4.04) | 0.68 | 1.20 (0.38–3.81) | 0.76 | ··· |
| ST/T‐wave abnormalities | 3.04 (2.09–4.43) | <0.001 | 2.47 (1.69–3.62) | <0.001 | 1.33 (0.85–2.08) | 0.22 | 1.13 (0.72–1.77) | 0.61 | 0.0093 |
| Right bundle‐branch block | 1.16 (0.51–2.67) | 0.72 | 1.03 (0.45–2.36) | 0.95 | 1.63 (0.75–3.55) | 0.22 | 1.38 (0.63–3.02) | 0.42 | ··· |
| Left bundle‐branch block | 9.29 (3.78–22.83) | <0.001 | 6.75 (2.70–16.86) | <0.001 | 1.95 (0.27–14.02) | 0.51 | 1.28 (0.17–9.30) | 0.81 | 0.14 |
CI indicates confidence interval; HFpEF, heart failure with preserved ejection fraction; HFrEF, heart failure with reduced ejection fraction; HR, hazard ratio; ID, intrinsicoid deflection; PTFV1, P‐wave terminal force in V1.
Selected from the list of ECG predictors that showed significant associations with total heart failure events in a model similar to model 2.
Adjusted for age, sex, race/ethnicity, education, and income.
Adjusted for model 1 covariates plus systolic blood pressure, smoking, diabetes mellitus, body mass index, cholesterol, high‐density lipoprotein cholesterol, aspirin, statins, and antihypertensive medications.
P value comparison computed using effect estimates from model 2 for the variables that showed a significant P value in at least 1 of the heart failure subtypes.
Hazard ratio not computed due to 0% prevalence of the abnormality in HFpEF.
Discussion
In this analysis from MESA, we demonstrated that several ECG markers are associated with both HFrEF and HFpEF. In addition, markers of ventricular repolarization and delayed ventricular activation were able to distinguish between HFrEF and HFpEF events. These findings suggest that distinct ECG profiles exist in the prediction of HFrEF and HFpEF.
Although several reports have shown that findings on the routine ECG are associated with future HF events,8, 9, 10, 11, 12, 13, 14, 15, 16, 17 few have explored whether ECG predictors vary in their ability to distinguish between HFrEF and HFpEF. A recent examination from the Framingham Heart Study has shown that left ventricular hypertrophy and left bundle‐branch block are associated with HFrEF and that atrial fibrillation is associated with HFpEF.28 In that study, which was limited to white participants, the aforementioned markers were the only ECG abnormalities examined.
Similar to findings from the Framingham Heart Study, our data confirm that left ventricular hypertrophy is associated with HFrEF.28 The reason for this finding possibly is related to the fact that left ventricular hypertrophy detects abnormal left ventricular mass,29 which is a well‐known risk factor for HFrEF. In addition, men dominate the HFrEF population and, on average, have significantly higher left ventricular mass than women.30 Consequently, it is possible that sex differences in left ventricular mass contribute to the predilection of ECG left ventricular hypertrophy for HFrEF events. Furthermore, similar to findings from the Framingham Heart Study, left bundle‐branch block was associated with HFrEF and not HFpEF.
The current analysis represents the most comprehensive study to examine the differential predictive abilities of ECG abnormalities to distinguish between HFrEF and HFpEF risk. In our study, markers of abnormal ventricular depolarization (QRS duration, delayed time to ID), axis deviation (left and right), abnormal ventricular repolarization (ST/T‐wave abnormalities), and conduction disease (left bundle‐branch block) were associated with HFrEF. In contrast, higher resting heart rate and abnormal P‐wave axis were associated with HFpEF. Abnormal QRS‐T axis was associated with both subtypes. However, only abnormalities of ventricular depolarization (delayed time to ID) and repolarization (prolonged QT interval and ST/T‐wave abnormalities) were statistically different in terms of associations with HF subtypes. Overall, the unique findings presented support a role for the 12‐lead ECG to separate HF risk by subtype (eg, HFrEF versus HFpEF).
Delayed time to ID is thought to represent conduction delay secondary to increases in left ventricular cavity size and increases in left ventricular end‐diastolic volume.31, 32 Similarly, abnormalities of left ventricular repolarization possibly detect structural abnormalities that predispose to HFrEF rather than HFpEF.29, 33, 34 This is supported by data that have shown that ST/T‐wave abnormalities are not associated with diastolic dysfunction that would be expected in the development of HFpEF35. Therefore, abnormal ECG measures of ventricular repolarization would be expected to differentially predict HFrEF compared with HFpEF. Overall, these data suggest that delayed time to ID, prolonged QT interval, and ST/T‐wave abnormalities detect subclinical anatomical abnormalities that predispose to HFrEF instead of events with normal ejection fraction.
By 2030, the prevalence of HF is projected to increase by 23%, with medical costs increasing to nearly $53.1 billion.36 Accordingly, the identification of at‐risk individuals by low‐cost, noninvasive cardiac assessment is of paramount importance due to the large burden that HF will place on the healthcare system. Our results suggest that simple markers detected on routine ECG are able to distinguish between persons who will develop HFrEF and HFpEF. In addition, the distinctive associations between certain ECG markers with different patterns of HF, which further emphasizes the unique differences between HFpEF and HFpEF,28 suggest that the ECG may have a role to guide targeted preventive strategies for each HF subtype. The ECG also could be a useful tool to select patients for clinical trials with aims to prevent specific HF subtypes. Further research, however, is needed to determine the cost‐effectiveness of using the ECG to characterize HF risk by subtype before recommendations regarding clinical practice or research applications are made.
The current study should be interpreted in the context of several limitations. Although rigorous methods were used to account for all HF cases, some events may have been missed. It is unlikely, however, that the resulting bias would have been differential in nature rather than merely reducing effect estimates toward the null. Because of the limited number of HF event subtypes, we were unable to explore whether racial or ethnic variation exists regarding the differential prediction of ECG abnormalities for HF subtype events. In addition, although numerous covariates were included in our multivariable models, we acknowledge that residual confounding remains a possibility.
In conclusion, our results indicate that HFrEF and HFpEF are preceded by distinct profiles on the routine 12‐lead ECG, suggesting a role for ECG recordings to better characterize the risk of HF by subtype. Further research is needed to confirm our findings and to determine whether these markers are able to identify individuals in whom targeted preventive therapies are warranted to reduce the current and future burden of HF.
Sources of Funding
This research was supported by contracts N01‐HC‐95159, N01‐HC‐95160, N01‐HC‐95161, N01‐HC‐95162, N01‐HC‐95163, N01‐HC‐95164, N01‐HC‐95165, N01‐HC‐95166, N01‐HC‐95167, N01‐HC‐95168 and N01‐HC‐95169 from the National Heart, Lung, and Blood Institute and by grants UL1‐TR‐000040 and UL1‐TR‐001079 from the National Center for Research Resources. Dr O'Neal is supported by the National Heart, Lung, and Blood Institute of the National Institutes of Health under award no. F32‐HL‐134290. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Disclosures
None.
Acknowledgments
The authors thank the other investigators, the staff, and the participants of MESA (Multi‐Ethnic Study of Atherosclerosis) for their valuable contributions. A full list of participating MESA investigators and institutions can be found at http://www.mesa-nhlbi.org.
(J Am Heart Assoc. 2017;6:e006023 DOI: 10.1161/JAHA.117.006023.)28546456
References
- 1. Benjamin EJ, Blaha MJ, Chiuve SE, Cushman M, Das SR, Deo R, de Ferranti SD, Floyd J, Fornage M, Gillespie C, Isasi CR, Jimenez MC, Jordan LC, Judd SE, Lackland D, Lichtman JH, Lisabeth L, Liu S, Longenecker CT, Mackey RH, Matsushita K, Mozaffarian D, Mussolino ME, Nasir K, Neumar RW, Palaniappan L, Pandey DK, Thiagarajan RR, Reeves MJ, Ritchey M, Rodriguez CJ, Roth GA, Rosamond WD, Sasson C, Towfighi A, Tsao CW, Turner MB, Virani SS, Voeks JH, Willey JZ, Wilkins JT, Wu JH, Alger HM, Wong SS, Muntner P; American Heart Association Statistics C and Stroke Statistics S . Heart disease and stroke statistics—2017 update: a report from the American Heart Association. Circulation. 2017;135:e146–e603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Yancy CW, Lopatin M, Stevenson LW, De Marco T, Fonarow GC; Committee ASA and Investigators . Clinical presentation, management, and in‐hospital outcomes of patients admitted with acute decompensated heart failure with preserved systolic function: a report from the Acute Decompensated Heart Failure National Registry (ADHERE) Database. J Am Coll Cardiol. 2006;47:76–84. [DOI] [PubMed] [Google Scholar]
- 3. Oktay AA, Rich JD, Shah SJ. The emerging epidemic of heart failure with preserved ejection fraction. Curr Heart Fail Rep. 2013;10:401–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Heidenreich PA, Lee TT, Massie BM. Effect of beta‐blockade on mortality in patients with heart failure: a meta‐analysis of randomized clinical trials. J Am Coll Cardiol. 1997;30:27–34. [DOI] [PubMed] [Google Scholar]
- 5. Cohn JN. ACE inhibitors in non‐ischaemic heart failure: results from the MEGA trials. Eur Heart J. 1995;16(suppl O):133–136. [DOI] [PubMed] [Google Scholar]
- 6. Pitt B, Pfeffer MA, Assmann SF, Boineau R, Anand IS, Claggett B, Clausell N, Desai AS, Diaz R, Fleg JL, Gordeev I, Harty B, Heitner JF, Kenwood CT, Lewis EF, O'Meara E, Probstfield JL, Shaburishvili T, Shah SJ, Solomon SD, Sweitzer NK, Yang S, McKinlay SM; Investigators T . Spironolactone for heart failure with preserved ejection fraction. N Engl J Med. 2014;370:1383–1392. [DOI] [PubMed] [Google Scholar]
- 7. Schwartzenberg S, Redfield MM, From AM, Sorajja P, Nishimura RA, Borlaug BA. Effects of vasodilation in heart failure with preserved or reduced ejection fraction implications of distinct pathophysiologies on response to therapy. J Am Coll Cardiol. 2012;59:442–451. [DOI] [PubMed] [Google Scholar]
- 8. Silva RM, Kazzaz NM, Torres RM, Moreira Mda C. P‐wave dispersion and left atrial volume index as predictors in heart failure. Arq Bras Cardiol. 2013;100:67–74. [DOI] [PubMed] [Google Scholar]
- 9. Zhang ZM, Rautaharju PM, Prineas RJ, Loehr L, Rosamond W, Soliman EZ. Ventricular conduction defects and the risk of incident heart failure in the Atherosclerosis Risk in Communities (ARIC) Study. J Card Fail. 2015;21:307–312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang ZM, Prineas RJ, Case D, Soliman EZ, Rautaharju PM; Group AR . Comparison of the prognostic significance of the electrocardiographic QRS/T angles in predicting incident coronary heart disease and total mortality (from the Atherosclerosis Risk in Communities Study). Am J Cardiol. 2007;100:844–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Dukes JW, Dewland TA, Vittinghoff E, Mandyam MC, Heckbert SR, Siscovick DS, Stein PK, Psaty BM, Sotoodehnia N, Gottdiener JS, Marcus GM. Ventricular ectopy as a predictor of heart failure and death. J Am Coll Cardiol. 2015;66:101–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Agarwal SK, Simpson RJ Jr, Rautaharju P, Alonso A, Shahar E, Massing M, Saba S, Heiss G. Relation of ventricular premature complexes to heart failure (from the Atherosclerosis Risk in Communities [ARIC] Study). Am J Cardiol. 2012;109:105–109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Zhang ZM, Rautaharju PM, Soliman EZ, Manson JE, Martin LW, Perez M, Vitolins M, Prineas RJ. Different patterns of bundle‐branch blocks and the risk of incident heart failure in the Women's Health Initiative (WHI) study. Circ Heart Fail. 2013;6:655–661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Rautaharju PM, Kooperberg C, Larson JC, LaCroix A. Electrocardiographic predictors of incident congestive heart failure and all‐cause mortality in postmenopausal women: the Women's Health Initiative. Circulation. 2006;113:481–489. [DOI] [PubMed] [Google Scholar]
- 15. Triola B, Olson MB, Reis SE, Rautaharju P, Merz CN, Kelsey SF, Shaw LJ, Sharaf BL, Sopko G, Saba S. Electrocardiographic predictors of cardiovascular outcome in women: the National Heart, Lung, and Blood Institute‐sponsored Women's Ischemia Syndrome Evaluation (WISE) study. J Am Coll Cardiol. 2005;46:51–56. [DOI] [PubMed] [Google Scholar]
- 16. Rautaharju PM, Prineas RJ, Wood J, Zhang ZM, Crow R, Heiss G. Electrocardiographic predictors of new‐onset heart failure in men and in women free of coronary heart disease (from the Atherosclerosis in Communities [ARIC] Study). Am J Cardiol. 2007;100:1437–1441. [DOI] [PubMed] [Google Scholar]
- 17. Rautaharju PM, Zhang ZM, Haisty WK Jr, Prineas RJ, Kucharska‐Newton AM, Rosamond WD, Soliman EZ. Electrocardiographic predictors of incident heart failure in men and women free from manifest cardiovascular disease (from the Atherosclerosis Risk in Communities [ARIC] Study). Am J Cardiol. 2013;112:843–849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Bild DE, Bluemke DA, Burke GL, Detrano R, Diez Roux AV, Folsom AR, Greenland P, Jacob DR Jr, Kronmal R, Liu K, Nelson JC, O'Leary D, Saad MF, Shea S, Szklo M, Tracy RP. Multi‐Ethnic Study of Atherosclerosis: objectives and design. Am J Epidemiol. 2002;156:871–881. [DOI] [PubMed] [Google Scholar]
- 19. Sagie A, Larson MG, Goldberg RJ, Bengtson JR, Levy D. An improved method for adjusting the QT interval for heart rate (the Framingham Heart Study). Am J Cardiol. 1992;70:797–801. [DOI] [PubMed] [Google Scholar]
- 20. Li Y, Shah AJ, Soliman EZ. Effect of electrocardiographic P‐wave axis on mortality. Am J Cardiol. 2014;113:372–376. [DOI] [PubMed] [Google Scholar]
- 21. Rangel MO, O'Neal WT, Soliman EZ. Usefulness of the electrocardiographic P‐wave axis as a predictor of atrial fibrillation. Am J Cardiol. 2016;117:100–104. [DOI] [PubMed] [Google Scholar]
- 22. Kamel H, O'Neal WT, Okin PM, Loehr LR, Alonso A, Soliman EZ. Electrocardiographic left atrial abnormality and stroke subtype in ARIC. Ann Neurol. 2015;78:670–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. O'Neal WT, Qureshi WT, Nazarian S, Kawel‐Boehm N, Bluemke DA, Lima JA, Soliman EZ. Electrocardiographic time to intrinsicoid deflection and heart failure: the Multi‐Ethnic Study of Atherosclerosis. Clin Cardiol. 2016;39:531–536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Devereux RB, Casale PN, Eisenberg RR, Miller DH, Kligfield P. Electrocardiographic detection of left ventricular hypertrophy using echocardiographic determination of left ventricular mass as the reference standard. Comparison of standard criteria, computer diagnosis and physician interpretation. J Am Coll Cardiol. 1984;3:82–87. [DOI] [PubMed] [Google Scholar]
- 25. Prineas RJ, Crow RS, Zhang ZM. The Minnesota Code Manual of Electrocardiographic Findings: Standards and Procedures for Measurement and Classification. 2nd ed London: Springer; 2010. [Google Scholar]
- 26. Bahrami H, Kronmal R, Bluemke DA, Olson J, Shea S, Liu K, Burke GL, Lima JA. Differences in the incidence of congestive heart failure by ethnicity: the Multi‐Ethnic Study of Atherosclerosis. Arch Intern Med. 2008;168:2138–2145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Lunn M, McNeil D. Applying Cox regression to competing risks. Biometrics. 1995;51:524–532. [PubMed] [Google Scholar]
- 28. Ho JE, Lyass A, Lee DS, Vasan RS, Kannel WB, Larson MG, Levy D. Predictors of new‐onset heart failure: differences in preserved versus reduced ejection fraction. Circ Heart Fail. 2013;6:279–286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Okin PM, Devereux RB, Nieminen MS, Jern S, Oikarinen L, Viitasalo M, Toivonen L, Kjeldsen SE, Julius S, Dahlof B. Relationship of the electrocardiographic strain pattern to left ventricular structure and function in hypertensive patients: the LIFE study. Losartan Intervention For End point. J Am Coll Cardiol. 2001;38:514–520. [DOI] [PubMed] [Google Scholar]
- 30. Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise J, Solomon S, Spencer KT, St John Sutton M, Stewart W; American Society of Echocardiography's N, Standards C, Task Force on Chamber Q, American College of Cardiology Echocardiography C, American Heart A and European Association of Echocardiography ESoC . Recommendations for chamber quantification. Eur J Echocardiogr. 2006;7:79–108. [DOI] [PubMed] [Google Scholar]
- 31. Recke S, Gansser R, Marienhagen J, Platsch G, Feistel H, Weniger J, von der Emde J. R peak time prolongation and R peak delay in leads I, V5, or V6. Diagnostic values as signs of myocardial dysfunction in chronic mitral incompetence. J Electrocardiol. 1994;27:129–136. [DOI] [PubMed] [Google Scholar]
- 32. Recke SH. Left ventricular function‐conduction impairment as reflected by the ECG in chronic aortic regurgitation. Wien Klin Wochenschr. 2011;123:502–507. [DOI] [PubMed] [Google Scholar]
- 33. Devereux RB, Reichek N. Repolarization abnormalities of left ventricular hypertrophy. Clinical, echocardiographic and hemodynamic correlates. J Electrocardiol. 1982;15:47–53. [DOI] [PubMed] [Google Scholar]
- 34. Okin PM, Devereux RB, Fabsitz RR, Lee ET, Galloway JM, Howard BV; Strong Heart S . Quantitative assessment of electrocardiographic strain predicts increased left ventricular mass: the Strong Heart Study. J Am Coll Cardiol. 2002;40:1395–1400. [DOI] [PubMed] [Google Scholar]
- 35. Palmieri V, Okin PM, Bella JN, Wachtell K, Oikarinen L, Gerdts E, Boman K, Nieminen MS, Dahlof B, Devereux RB. Electrocardiographic strain pattern and left ventricular diastolic function in hypertensive patients with left ventricular hypertrophy: the LIFE study. J Hypertens. 2006;24:2079–2084. [DOI] [PubMed] [Google Scholar]
- 36. Heidenreich PA, Albert NM, Allen LA, Bluemke DA, Butler J, Fonarow GC, Ikonomidis JS, Khavjou O, Konstam MA, Maddox TM, Nichol G, Pham M, Pina IL, Trogdon JG; American Heart Association Advocacy Coordinating C, Council on Arteriosclerosis T, Vascular B, Council on Cardiovascular R, Intervention, Council on Clinical C, Council on E, Prevention and Stroke C . Forecasting the impact of heart failure in the United States: a policy statement from the American Heart Association. Circ Heart Fail. 2013;6:606–619. [DOI] [PMC free article] [PubMed] [Google Scholar]


