Abstract
Background
Early life risk factors are associated with cardiometabolic disease, but have not been fully studied in atrial fibrillation (AF). There are discordant results from existing studies of birth weight and AF, and the impact of maternal body size, gestational age, placental size, and birth length is unknown.
Methods and Results
The Helsinki Birth Cohort Study includes 13 345 people born as singletons in Helsinki in the years 1934–1944. Follow‐up was through national registries, and ended on December 31, 2013, with 907 incident cases. Cox regression analyses stratified on year of birth were constructed for perinatal variables and incident AF, adjusting for offspring sex, gestational age, and socioeconomic status at birth. There was a significant U‐shaped association between birth weight and AF (P for quadratic term=0.01). The lowest risk of AF was found among those with a birth weight of 3.4 kg (3.8 kg for women [85th percentile] and 3.0 kg for men [17th percentile]). High maternal body mass index (≥30 kg/m2) predicted offspring AF; hazard ratio 1.36 (95% CI 1.07–1.74, P=0.01) compared with normal body mass index (<25 kg/m2). Maternal height was associated with early‐onset AF (<65.3 years), hazard ratio 1.47 (95% CI 1.24–1.74, P<0.0001), but not with later onset AF. Results were independent of incident coronary artery disease, hypertension, or diabetes mellitus.
Conclusions
High maternal body mass index during pregnancy and maternal height are previously undescribed predictors of offspring AF. Efforts to prevent maternal obesity might reduce later AF in offspring. Birth weight has a U‐shaped relation to incident AF independent of other perinatal variables.
Keywords: atrial fibrillation, hypertension, population science, population studies, risk prediction
Subject Categories: Arrhythmias, Epidemiology, Obesity, Risk Factors, Primary Prevention
Clinical Perspective
What Is New?
End gestational maternal body mass index predicts atrial fibrillation in offspring, independently of birth weight.
The association between birth weight and atrial fibrillation is U‐shaped.
What Are the Clinical Implications?
Efforts to prevent maternal obesity may prevent offspring atrial fibrillation.
Introduction
Low birth weight is a well‐established risk factor for cardiovascular disease,1 type 2 diabetes mellitus,2 and hypertension,3 as well as several other known atrial fibrillation (AF) risk factors, such as pulmonary function.4, 5 There are 3 previous studies that report on associations between self‐reported birth weight and incident AF, with divergent results. Conen et al report a positive association between self‐reported birth weight and incident AF among participants in the Women's Health Study, with the risk increase attributable to high birth weight mediated by adult height.6 The Atherosclerosis in Communities study, however, reports an inverse association,7 and finally data from a Swedish cohort showed both high and low birth weight to be associated with increased risk.8
The reported discrepancy in the findings between birth weight and AF suggests that there may be other factors that influence the association between birth weight and AF that have not been accounted for. Possible confounders include maternal body size, which is known to have consequences for offspring health, placental weight, and socioeconomic status at birth. Furthermore, birth weight shows a strong correlation with gestational age, which has not been adjusted for in previous publications. Another possible confounder is birth length, which has been shown to be a better predictor of adult height and weight than birth weight.9 Questions have also been raised concerning possible intermediate causes and diseases that may link birth weight and AF.
Therefore, the aim of the present article was to determine which early life factors, such as body size at birth, placental size, and socioeconomic status at birth, were related to incident AF in the Helsinki Birth Cohort Study. We further aimed to determine whether maternal height and body mass index (BMI) were associated with incident AF, and how maternal factors affect the association between body size at birth and AF. We also sought to describe the influence of intermediate incident coronary heart disease (CAD), diabetes mellitus, and hypertension during follow‐up on the association between perinatal factors and AF.
Methods
Data Collection and End Point Retrieval
In the Helsinki Birth Cohort Study, perinatal data were recorded in 13 345 singletons (6975 men and 6370 women) born in Helsinki between 1934 and 1944, who had visited child welfare clinics and were still alive and residing in Finland in 1971, at which time all Finnish citizens were given a unique personal identification number. Besides newborn weight and length, the cohort contains information on placental weight and diameters, marital status, parity, time of last menstrual period, maternal age, height and weight (at the end of gestation), as well as father's occupation, as a proxy for socioeconomic status. Details of the birth records have been described previously.10 Maternal BMI was calculated as kg/m2. Offspring ponderal index was calculated as kg/m3. Socioeconomic status at birth was defined by the father's occupation, which was recorded on the birth records. Classification of the father's occupational status was retrieved from the 1980 Classification of Occupation compiled by Statistics Finland, and has been described elsewhere.11 Missing data for father's occupation were assumed to be because of unmarried social status, and therefore these data were not excluded from analyses.
Using the unique personal identification number, the cohort was linked to the Finnish National Care Register for Health Care (formerly the FNHDR), which has been maintained from 1969, and the Finnish National Mortality Register, which include all hospitalizations and deaths in Finland. Subjects were followed until the end point of hospitalization for incident AF (International Classification of Disease [ICD] codes 427.92 in the eighth revision, 427D in the ninth revision, and I48 in the tenth revision) or censoring because of death, migration from Finland, or end of follow‐up at December 31, 2013. The Finnish National Care Register for Health Care is very similar to the Swedish National Hospital Discharge Register, in which the AF diagnosis has been validated recently and found to be of good quality.12 We have not differentiated between paroxysmal and permanent AF, nor have we differentiated between AF and atrial flutter, because of the progressive nature of AF and the similarities between these diagnoses.13, 14 Intervening diagnoses (CAD, type 2 diabetes mellitus, and hypertension) were retrieved from the same registers using ICD diagnoses codes for CAD (ICD‐8 and 9: 410–414, ICD‐10: I21–I25), for hypertension (ICD‐8: 400–404, ICD‐9 401–405, ICD‐10: I10–I15), and for diabetes mellitus: (ICD‐8: 250, ICD‐9: 250, ICD 10: E10–E14).
The study has been approved by the ethics committee at the National Public Health Institute in Helsinki, Finland. Data were linked by permission from National Institute for Health and Welfare.
Statistical Methods
All data were analyzed using Stata for Macintosh version 12.1 (Stata Corporation, College Station, TX). Perinatal factors were analyzed in relation to incident AF using multivariable adjusted Cox regression stratified on year of birth and using the Breslow method for ties. The proportional hazards assumption was assessed using −log‐log plots and using Schoenfeld residuals. There was evidence of nonproportionality for maternal height (P for test of nonzero slope 0.008) and these results are reported separately for the group above and below median occurrence of AF in the study population. Co‐linearity was assessed using the variance inflation factor. Birth weight was analyzed as a continuous variable, with and without a quadratic term, and is also presented by strata (<2500, 2500–2999, 3000–3999, and >4000 g), with the 3000 to 3999 strata as reference category. Socioeconomic status was analyzed as a categorical variable with manual work as baseline. Two models were used; Model 1 adjusted for offspring sex, socioeconomic status at birth, and gestational age, and Model 2 adjusted for Model 1 covariates+maternal BMI. The association between maternal BMI and AF was also analyzed with birth weight added to Model 1. The limit for statistical significance was set at P<0.05. There was no evidence of colinearity (1/variance inflation factor >0.80). Models with interaction parameters between birth weight (as a linear or quadratic variable) and offspring sex, gestational age, maternal BMI, maternal height, and socioeconomic status were compared with models without interaction parameters using the likelihood ratio test. The same procedure was applied to offspring sex and maternal BMI, maternal height, and placental weight.
In order to assess whether any associations between perinatal factors and incident AF were mediated by incident diabetes mellitus, CAD, and hypertension, we also performed subanalyses with censoring at the time of any such incident diagnosis preceding a diagnosis of AF. Competing risks regression analyses, as described by Fine and Gray,15 were performed to account for the competing risk of mortality on results.
Results
Median follow‐up time (interquartile range) was 70.5 (9.4) years, during which time there were 907 cases of AF (cumulative incidence 9.1% among men and 4.3% among women). Over the entire duration of follow‐up, the incidence of AF was 1.04 cases per 1000 person‐years (95% CI 0.97–1.11). Median age (interquartile range) was 65.3 (10.6) years at AF diagnosis and 70.7 (8.1) years for censoring because of end of follow‐up or death. The mean birth weight (SD) was 3406 (479) g. Selected baseline characteristics are reported in Table 1.
Table 1.
Baseline Characteristics, by Birth Weight Categories
| All | Birth Weight Categories | ||||
|---|---|---|---|---|---|
| <2500 g | 2500 to 2999 g | 3000 to 3999 g | ≥4000 g | ||
| Number | 13 345 | 410 | 1926 | 9593 | 1416 |
| Maternal characteristics | |||||
| Height, cm | 159.9 (5.7) | 158.5 (5.9) | 158.3 (5.5) | 160.0 (5.6) | 161.5 (5.4) |
| Weight, kg | 67.0 (8.3) | 62.9 (7.7) | 63.3 (7.6) | 67.2 (7.9) | 72.0 (8.9) |
| BMI, kg/m2 | 26.2 (2.9) | 25.0 (2.7) | 25.3 (2.6) | 26.2 (2.8) | 27.6 (3.3) |
| Placenta | |||||
| Weight, g | 645 (120) | 504 (100) | 557 (92) | 650 (105) | 774 (118) |
| Newborn characteristics | |||||
| Male sex, % | 52.3 | 48.5 | 42.5 | 52.3 | 52.3 |
| Birth length, cm | 50.2 (1.9) | 45.8 (1.7) | 48.3 (1.2) | 50.5 (1.3) | 52.7 (1.5) |
| Ponderal index, kg/m3 | 26.7 (2.3) | 23.6 (2.2) | 24.9 (1.7) | 26.9 (1.9) | 29.0 (2.2) |
| Gestational age, days | 280 (13) | 260 (21) | 274 (14) | 280 (12) | 285 (11) |
| Father's occupation at birth | |||||
| Upper middle class, % | 11.1 | 11.5 | 9.0 | 11.2 | 12.8 |
| Lower middle class, % | 20.8 | 20.2 | 19.2 | 21.2 | 20.6 |
| Manual worker, % | 61.8 | 60.0 | 63.1 | 61.5 | 62.9 |
| Unknown, % | 6.3 | 8.3 | 8.7 | 6.1 | 3.7 |
All values are mean (SD), unless stated otherwise. BMI indicates body mass index.
Results from analyses of body size at birth and incident AF are given in Table 2. There was no evidence of interaction between offspring sex and birth weight (as a linear or quadratic term P for interaction >0.30). Hazard ratios (HRs) were consistently positive above and below the reference group with birth weights 3000 to 3999 g in the full population as well as in both sexes. In the full population, there was a significant association between high birth weight (≥4000 g) and AF in Model 1 but not after adjustment for maternal BMI in Model 2. In sex‐stratified analyses, the only birth weight group that was significantly associated with increased risk of AF was medium‐low birth weight (2500–2999 g) in women. The P value for quadratic trend was 0.01 in the full population, and the likelihood ratio test testing analyses of birth weight as a continuous variable with and without quadratic term was 0.01. Figure depicts the modeled relationship between birth weight and AF after Model 2 adjustment. The lowest risk of AF was found in those born with a birth weight of 3.35 kg (3.80 kg among women and 3.03 kg among men). The separate estimates for men and women both fall within a low‐risk section of the curve. The addition of maternal BMI did not alter results substantially, nor did inclusion of maternal height, placental weight, or newborn length.
Table 2.
Cox Regression Analyses (Stratified on Year of Birth) for Body Size at Birth and Incident AF
| Model 1a | Model 2b | |||||||
|---|---|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | Individuals (Cases) | HR | 95% CI | P Value | Individuals (Cases) | |
| All | ||||||||
| Birth weightc | ||||||||
| <2500 g | 1.24 | 0.84 to 1.86 | 0.28 | 1.42 | 0.93 to 2.19 | 0.10 | ||
| 2500 to 2999 g | 1.19 | 0.99 to 1.45 | 0.07 | 1.22 | 1.00 to 1.50 | 0.06 | ||
| 3000 to 3999 g | 1 | 1 | ||||||
| ≥4000 g | 1.26 | 1.03 to 1.54 | 0.02 | 1.12 | 0.90 to 1.39 | 0.33 | ||
| Birth length, per cm increase | 1.03 | 0.99 to 1.07 | 0.17 | 12 769 (865) | 1.00 | 0.96 to 1.04 | 0.94 | 11 620 (788) |
| Men | ||||||||
| Birth weightd | 6707 (611) | 6044 (547) | ||||||
| <2500 g | 1.11 | 0.68 to 1.80 | 0.69 | 1.38 | 0.82 to 2.30 | 0.22 | ||
| 2500 to 2999 g | 1.07 | 0.84 to 1.38 | 0.59 | 1.06 | 0.81 to 1.38 | 0.70 | ||
| 3000 to 3999 g | 1 | 1 | ||||||
| ≥4000 g | 1.25 | 0.99 to 1.57 | 0.06 | 1.11 | 0.86 to 1.42 | 0.42 | ||
| Birth length, per cm increase | 1.06 | 1.01 to 1.11 | 0.01 | 6658 (603) | 1.04 | 0.99 to 1.09 | 0.17 | 6000 (541) |
| Women | ||||||||
| Birth weighte | 6169 (264) | 5576 (241) | ||||||
| <2500 g | 1.70 | 0.85 to 3.43 | 0.14 | 1.64 | 0.75 to 3.62 | 0.22 | ||
| 2500 to 2999 g | 1.44 | 1.06 to 1.96 | 0.02 | 1.54 | 1.12 to 2.12 | 0.008 | ||
| 3000 to 3999 g | 1 | 1 | ||||||
| ≥4000 g | 1.35 | 0.88 to 2.08 | 0.17 | 1.19 | 0.75 to 1.58 | 0.45 | ||
| Birth length, per cm increase | 0.95 | 0.88 to 1.02 | 0.18 | 6111 (262) | 0.92 | 0.86 to 1.00 | 0.05 | 5531 (239) |
AF indicates atrial fibrillation; BMI, body mass index; HR, hazard ratio.
Adjusted for socioeconomic status at birth, gestational age, and sex (where applicable).
Adjusted for Model 1+maternal BMI.
P for quadratic trend=0.01 in Model 1 and 0.048 in Model 2.
P for quadratic trend=0.08 in Model 1 and 0.17 in Model 2.
P for quadratic trend=0.13 in Model 1 and 0.31 in Model 2.
Figure 1.
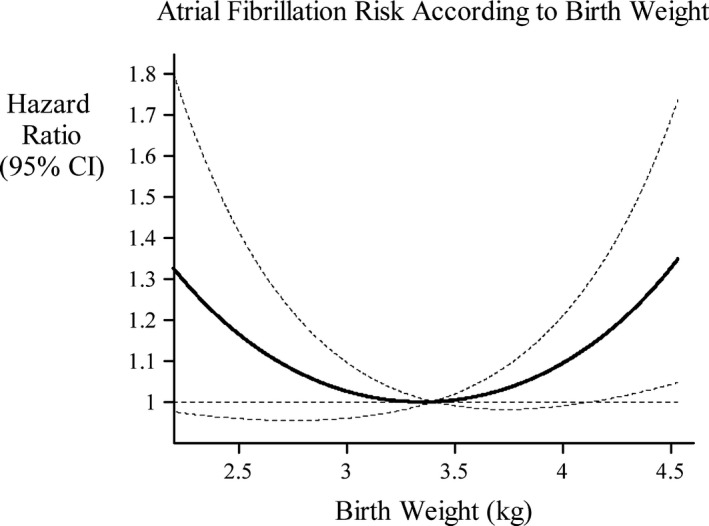
Hazard ratios and 95% confidence intervals implied by the quadratic model (adjusted for sex, gestational age, and socioeconomic status), using a birthweight of 3.4 kg as the baseline birthweight for comparison.
Results from analyses of maternal BMI and placental weight and incident AF are given in Table 3. There were positive associations between maternal BMI and incident AF in both sexes. The HR for incident AF associated with being born to a mother with a BMI ≥30 kg/m2 compared with a BMI <25 kg/m2 was 1.36 (95% CI 1.07–1.74, P=0.01), after Model 1 adjustment. There was no evidence of interaction between BMI and offspring sex (P for interaction >0.40). The association between maternal BMI and AF was not dependent on offspring birth weight; inclusion of birth weight (in categories or per kg with quadratic term) did not alter results substantially.
Table 3.
Cox Regression Analyses (Stratified on Year of Birth) for Maternal and Placental Factors and Incident AF in Offspring
| Model 1a | Model 2b | Individuals (Cases) | |||||
|---|---|---|---|---|---|---|---|
| HR | 95% CI | P Value | HR | 95% CI | P Value | ||
| All | |||||||
| Maternal BMI, per kg/m2 increase | 1.05 | 1.02 to 1.07 | <0.0001 | 1.05 | 1.02 to 1.07 | <0.0001 | 11 620 (788) |
| Placental weight, per 100 g increase | 0.97 | 0.92 to 1.03 | 0.27 | 0.94 | 0.87 to 1.00 | 0.07 | 12 850 (873) |
| Men | |||||||
| Maternal BMI, per kg/m2 increase | 1.04 | 1.01 to 1.07 | 0.005 | 1.04 | 1.01 to 1.07 | 0.01 | 6044 (547) |
| Placental weight, per 100 g increase | 1.00 | 0.94 to 1.07 | 0.99 | 0.95 | 0.87 to 1.02 | 0.19 | 6689 (610) |
| Women | |||||||
| Maternal BMI, per kg/m2 increase | 1.06 | 1.01 to 1.10 | 0.01 | 1.07 | 1.03 to 1.12 | 0.002 | 5576 (241) |
| Placental weight, per 100 g increase | 0.90 | 0.81 to 1.00 | 0.05 | 0.92 | 0.81 to 1.04 | 0.19 | 6161 (263) |
AF indicates atrial fibrillation; BMI, body mass index; HR, hazard ratio.
Adjusted for socioeconomic status at birth, gestational age, and offspring sex (where applicable).
Adjusted for Model 1+birth weight (in kg) as a linear and quadratic term.
The proportional hazards assumption was violated for the association between maternal height and incident AF in offspring. There was no evidence of interaction by offspring sex (P for interaction >0.60). There was a significant association between maternal height and AF in a subgroup with an AF diagnosis before the median age of onset (65.3 years), HR 1.47 (95% CI 1.24–1.76, P<0.0001) adjusted for Model 1, but not in a subgroup with later onset AF; HR 1.04 (95% CI 0.87–1.63, P=0.69).
In univariate analyses, as well as adjusted for birth weight, there was a significant interaction between gestational age and offspring sex (P for interaction 0.03). Among female offspring there was a positive association between gestational age and AF incidence, after adjustment for birth weight, HR 1.08 (95% CI 1.01–1.16, P=0.03, per 1‐week increase). Among male offspring there was a nonsignificant negative association, HR 0.96 (95% CI 0.92–1.00, P=0.07, per 1 week increase). There was no association between preterm birth (<260 days gestation) and incident AF in either sex.
Upper‐middle‐class social status at birth was associated with reduced incidence of AF, HR 0.77 (95% CI 0.61–0.98, P=0.03), compared with manual‐worker status, after Model 1 adjustment. No other socioeconomic status categories were significantly associated with incident AF (HR 1.05, 95% CI 1.01–1.38, P=0.68 for presumed unmarried status, HR 0.97, 95% CI 0.82–1.15, P=0.72 for lower‐middle‐class status). There was no evidence of interaction between social status and offspring sex (P for interaction >0.30).
Among individuals who went on to develop AF, this diagnosis of AF was preceded by a hospital diagnosis of diabetes mellitus in 81 cases (13.4%), by a diagnosis of hypertension in 200 cases (22.1%), and by a diagnosis of CAD in 110 cases (12.1%). There were 604 cases of AF that were not preceded by either of these diagnoses (66.6%). Subanalyses with censoring at the time of incident CAD, hypertension, or diabetes mellitus, if these preceded a diagnosis of AF, were not substantially altered.
We also tested whether results were independent of the competing risk of death, using competing‐risks regression. There was no effect on the association between maternal BMI and AF; subhazard ratio 1.05 (95% CI 1.02–1.07, P<0.0001) per kg/m2, in Model 2. The association between birth weight and AF was likewise mostly unchanged in the competing‐risks model.
Discussion
Maternal Factors and AF
The main finding of this study was previously unknown associations between maternal BMI and maternal height and incident AF in offspring.
No previous studies have reported on birth weight in the context of maternal body size and AF. Maternal height and maternal BMI can be assumed to reflect maternal lifetime nutrition as well as genetic factors. Offspring to obese mothers (BMI ≥30 kg/m2) were subject to a roughly 35% increased risk compared with offspring to mothers in the normal BMI category (BMI <25 kg/m2), after multivariable adjustment. To our best knowledge, this is the first study to have reported on maternal factors and incident AF in the offspring.
Maternal height was associated with incident AF among individuals with AF diagnosed before the median age of onset (65.3 years). The association could perhaps be explained by genetic factors associated with adult height in the offspring, a known AF risk factor,16, 17 but also reflects maternal lifelong nutrition. Genetic factors associated with height, as well as measured adult height, are both associated with ECG changes in young adults,18 and possibly this reflects a causal pathway linking maternal height to offspring AF.
Genetic factors seem less likely to explain the full effect of maternal BMI on offspring AF. Maternal BMI has been associated with less favorable body compositions in later adulthood among the offspring, as well as with CAD, type 2 diabetes mellitus, stroke, and cancer.19, 20 We did not find the association to be influenced by intermediate hypertension, CAD, or type 2 diabetes mellitus, but we have not been able to adjust for adult body composition. Besides genetic and epigenetic factors, BMI could also be inherited in families through cultural factors resulting in women with high BMI transmitting habits to their offspring that result in high adult BMI. This theory is in agreement with the high importance of adult BMI as an AF risk factor.21 The association between adult obesity and AF appears to be mediated through left atrial enlargement,22 and it is possible that left atrial enlargement is a pathway between maternal BMI to offspring AF.
However, since adult obesity is highly associated with CAD, type 2 diabetes mellitus, and hypertension, one would have expected the association between maternal BMI and AF to be attenuated somewhat by censoring of cases of AF at the time of these events. Therefore, there may be some effect of maternal BMI on the risk of offspring AF, which is not mediated by either adult body composition or increased incidence of the abovementioned diseases. The mechanisms by which maternal adiposity causes disease in the offspring are not known, but could perhaps include epigenetic changes. One hypothesis suggests that maternal and fetal overnutrition leads to permanent changes that induce vulnerability to obesity‐related health outcomes in the offspring, mediated by maternal hyperglycemia, hyperinsulinemia, and high levels of free fatty acids.20, 23 Maternal obesity has also been linked to inflammation.23 Since inflammation is a well‐known AF risk factor, one can speculate that some of the risk of AF associated with high maternal BMI could perhaps be mediated through upregulation of inflammatory pathways in the offspring.24 Prevalence of obesity among fertile women is rising, with implications for the health of future generations.25 Findings in the present study thus imply that incidence of AF may increase in conjunction with this trend. Another implication is that interventions to reduce maternal overweight, or indeed overweight among women of fertile age, may reduce the incidence of AF in future generations. Prepregnancy lifestyle interventions in obese women may have a positive effect on AF risk in offspring.
Body Size at Birth and AF
To our best knowledge, this is the first study of body size at birth and AF to include adjustment for gestational age, birth length, and maternal BMI. There was some evidence of a U‐shaped relationship between birth weight and AF, but the association between birth weight and AF was not particularly strong. The present study uses recorded birth weights, which is an advantage compared with previous publications not only in that it is more precise, but also because it has allowed for the use of birth weight as a continuous variable, and the relationship between birth weight and AF to be modeled more precisely.6, 7, 8 It is biologically more plausible that both low and high birth weight should be associated with AF than that the association should be unidirectional, given that low birth weight is a known predictor of many adverse health outcomes and that tall stature is a known AF risk factor. The lowest risk of AF was found at a higher birth weight among women than men, and medium‐low birth weight was associated with increased incidence of AF among women. This could perhaps reflect that being generally taller, men are more exposed to the risk of AF, which is conferred by tall stature, and that higher birth weights among men are associated with adult height.
Strengths and Limitations
A major strength of the Helsinki Birth Cohort Study is that the birth data were recorded at the time of birth and are therefore precise and not subject to recall bias. Compared with previous studies of birth weight and incident AF, one can assume that the measurement error is smaller and that the perinatal data are more valid. Follow‐up has been through national registries. The Finnish National Care Register for Health Care and the Finnish National Mortality Register include all residents of Finland, based on individual social security numbers, and consequently there were no missing data at the time of linkage with registers, which is a strength. This approach has been tested in the neighboring country of Sweden that has a similar register, which has been validated and shown to be of high quality.12 However, there are some potential limitations to the study. The register became operational in 1969. Any cases occurring only before this will have been missed. Given the age of the population at this time, the incidence of AF would have been low before 1969, and considering the recurring and progressive nature of the disease,14 we consider it unlikely that any such cases would have influenced the results in a meaningful way. Subclinical cases of AF have also been missed, as well as cases treated only in an outpatient setting. Since the register is in continuous operation, and AF as a secondary diagnosis to other inpatient care has been registered, however, one can assume that most cases have eventually been detected, and this factor should not have influenced the results substantially either. Results may not be generalizable to AF diagnosed in an outpatient setting, however, and this is a limitation. The continuous operation of the registers has permitted censoring at the time of other incident conditions known to be associated with early‐life risk factors. We have not adjusted for subclinical diseases. Since AF risk factors within the normal range,26 and even midlife changes in risk factors within the normal range,27 have been associated with increased risk of AF, a risk of residual confounding remains. Again, however, if subclinical disease were to present a substantial source of residual confounding, one would have expected censoring at the time of clinical disease to have had substantial influence on results, which it did not. We therefore believe any residual confounding by subclinical disease to be inconsequential for the main results. We have not adjusted for adult body composition, or other physical examination data in midlife. This is a limitation, with the result that we can only speculate about mediating factors between perinatal factors and AF risk.
The cohort members were born in 1934–1944, during or before World War II, when Finland fought 2 wars against the Soviet Union (1939–1940 and 1941–1944). The duress associated with this may have influenced the pregnancies and the lives of the study subjects. The exposure variables are to some degree an effect of nutritional deprivation and other duress. In a homogeneous population, risk factors cannot be detected, and for this reason the timing of the study may be considered a strength. The results of the study are generalizable to the population of Europeans currently at the age where AF risk is highest, and therefore relevant. On the other hand, conditions can be assumed to differ from those experienced by later generations of Europeans, and this is a limitation. In a worldwide context, however, many children are born to mothers living in difficult conditions.
The database was assembled in 1974, at which time participants were aged 27 to 37 years. There is therefore some survivor bias. In comparison to most cohort studies with data collection later in life, the degree of survivor bias can be presumed to be lower, but it is present nonetheless.
There are no data concerning ethnicity. At the time, Helsinki was an ethnically homogeneous white society. Therefore, we cannot be sure that data are fully generalizable to a nonwhite population. There are no data for paternal characteristics, either. Paternal factors have been shown to be less important for childhood cardiometabolic outcomes, however, and for this reason we do not believe this to be a significant limitation.28
Conclusions
We confirm a significant association between birth weight and AF, and show it to be U‐shaped. This is the first study to show that maternal BMI and maternal height predict incident AF in the offspring. Should there be a causal component to these associations, the rising rates of obesity among fertile women could lead to rising incidence rates of AF in the future.
Sources of Funding
Dr Johnson is supported by governmental funding within the Swedish National Health Services. The Helsinki Birth Cohort Study was supported by Signe and Ane Gyllenberg Foundation, Samfundet Folkhälsan, Finska Läkaresällskapet, Liv och Hälsa, and the Finnish Foundation for Cardiovascular Research. The Academy of Finland supported Dr Eriksson (grant no. 129369, 129907, 135072, 129255, and 126775). The research leading to these results has received funding from the European Commission within the 7th Framework Programme (DORIAN, grant agreement no. 278603) and EU H2020‐PHC‐2014‐DynaHealth (grant no. 633595). Dr Healey is supported by McMaster University, and a Heart and Stroke Foundation of Ontario Mid‐Career Award (MC7450). Dr Kajantie is supported by Academy of Finland grants (127437, 129306, 130326, 134791, 263924, 274794) as well as the Sigrid Juselius Foundation, the Finnish Foundation for Pediatric Research, the Finnish Foundation for Cardiovascular Research, the Novo Nordisk Foundation, and the Juho Vainio Foundation.
Disclosures
None.
(J Am Heart Assoc. 2017;6:e006036 DOI: 10.1161/JAHA.117.006036.)28649086
References
- 1. Lawlor DA, Ronalds G, Clark H, Smith GD, Leon DA. Birth weight is inversely associated with incident coronary heart disease and stroke among individuals born in the 1950s: findings from the Aberdeen Children of the 1950s prospective cohort study. Circulation. 2005;112:1414–1418. [DOI] [PubMed] [Google Scholar]
- 2. Whincup PH, Kaye SJ, Owen CG, Huxley R, Cook DG, Anazawa S, Barrett‐Connor E, Bhargava SK, Birgisdottir BE, Carlsson S, de Rooij SR, Dyck RF, Eriksson JG, Falkner B, Fall C, Forsen T, Grill V, Gudnason V, Hulman S, Hypponen E, Jeffreys M, Lawlor DA, Leon DA, Minami J, Mishra G, Osmond C, Power C, Rich‐Edwards JW, Roseboom TJ, Sachdev HS, Syddall H, Thorsdottir I, Vanhala M, Wadsworth M, Yarbrough DE. Birth weight and risk of type 2 diabetes: a systematic review. JAMA. 2008;300:2886–2897. [DOI] [PubMed] [Google Scholar]
- 3. Eriksson J, Forsén T, Tuomilehto J, Osmond C, Barker D. Fetal and childhood growth and hypertension in adult life. Hypertension. 2000;36:790–794. [DOI] [PubMed] [Google Scholar]
- 4. Johnson LS, Juhlin T, Engstrom G, Nilsson PM. Reduced forced expiratory volume is associated with increased incidence of atrial fibrillation: the Malmo Preventive Project. Europace. 2014;16:182–188. [DOI] [PubMed] [Google Scholar]
- 5. Baumann S, Godtfredsen NS, Lange P, Pisinger C. The impact of birth weight on the level of lung function and lung function decline in the general adult population. The Inter99 study. Respir Med. 2015;109:1293–1299. [DOI] [PubMed] [Google Scholar]
- 6. Conen D, Tedrow UB, Cook NR, Buring JE, Albert CM. Birth weight is a significant risk factor for incident atrial fibrillation. Circulation. 2010;122:764–770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lawani SO, Demerath EW, Lopez FL, Soliman EZ, Huxley RR, Rose KM, Alonso A. Birth weight and the risk of atrial fibrillation in whites and African Americans: the Atherosclerosis Risk in Communities (ARIC) study. BMC Cardiovasc Disord. 2014;14:69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Larsson SC, Drca N, Jensen‐Urstad M, Wolk A. Incidence of atrial fibrillation in relation to birth weight and preterm birth. Int J Cardiol. 2015;178:149–152. [DOI] [PubMed] [Google Scholar]
- 9. Sorensen HT, Sabroe S, Rothman KJ, Gillman M, Steffensen FH, Fischer P, Sorensen TI. Birth weight and length as predictors for adult height. Am J Epidemiol. 1999;149:726–729. [DOI] [PubMed] [Google Scholar]
- 10. Barker DJ, Osmond C, Forsen TJ, Kajantie E, Eriksson JG. Trajectories of growth among children who have coronary events as adults. N Engl J Med. 2005;353:1802–1809. [DOI] [PubMed] [Google Scholar]
- 11. Mikkonen HM, Salonen MK, Hakkinen A, Olkkola M, Pesonen AK, Raikkonen K, Osmond C, Eriksson JG, Kajantie E. The lifelong socioeconomic disadvantage of single‐mother background—the Helsinki Birth Cohort study 1934–1944. BMC Public Health. 2016;16:817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Smith JG, Platonov PG, Hedblad B, Engstrom G, Melander O. Atrial fibrillation in the Malmo Diet and Cancer study: a study of occurrence, risk factors and diagnostic validity. Eur J Epidemiol. 2010;25:95–102. [DOI] [PubMed] [Google Scholar]
- 13. Waldo AL, Feld GK. Inter‐relationships of atrial fibrillation and atrial flutter mechanisms and clinical implications. J Am Coll Cardiol. 2008;51:779–786. [DOI] [PubMed] [Google Scholar]
- 14. Kerr CR, Humphries KH, Talajic M, Klein GJ, Connolly SJ, Green M, Boone J, Sheldon R, Dorian P, Newman D. Progression to chronic atrial fibrillation after the initial diagnosis of paroxysmal atrial fibrillation: results from the Canadian Registry of Atrial Fibrillation. Am Heart J. 2005;149:489–496. [DOI] [PubMed] [Google Scholar]
- 15. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94:496–509. [Google Scholar]
- 16. Rosenberg MA, Patton KK, Sotoodehnia N, Karas MG, Kizer JR, Zimetbaum PJ, Chang JD, Siscovick D, Gottdiener JS, Kronmal RA, Heckbert SR, Mukamal KJ. The impact of height on the risk of atrial fibrillation: the Cardiovascular Health Study. Eur Heart J. 2012;33:2709–2717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Rosenberg MA, Kaplan RC, Siscovick DS, Psaty BM, Heckbert SR, Newton‐Cheh C, Mukamal KJ. Genetic variants related to height and risk of atrial fibrillation: the Cardiovascular Health Study. Am J Epidemiol. 2014;180:215–222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kofler T, Theriault S, Bossard M, Aeschbacher S, Bernet S, Krisai P, Blum S, Risch M, Risch L, Albert CM, Pare G, Conen D. Relationships of measured and genetically determined height with the cardiac conduction system in healthy adults. Circ Arrhythm Electrophysiol. 2017;10:pii:e004735. doi: 10.1161/CIRCEP.116.004735. [DOI] [PubMed] [Google Scholar]
- 19. Eriksson JG, Sandboge S, Salonen M, Kajantie E, Osmond C. Maternal weight in pregnancy and offspring body composition in late adulthood: findings from the Helsinki Birth Cohort Study (HBCS). Ann Med. 2015;47:94–99. [DOI] [PubMed] [Google Scholar]
- 20. Eriksson JG, Sandboge S, Salonen MK, Kajantie E, Osmond C. Long‐term consequences of maternal overweight in pregnancy on offspring later health: findings from the Helsinki Birth Cohort Study. Ann Med. 2014;46:434–438. [DOI] [PubMed] [Google Scholar]
- 21. Tedrow UB, Conen D, Ridker PM, Cook NR, Koplan BA, Manson JE, Buring JE, Albert CM. The long‐ and short‐term impact of elevated body mass index on the risk of new atrial fibrillation the WHS (Women's Health Study). J Am Coll Cardiol. 2010;55:2319–2327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Wang TJ, Parise H, Levy D, D'Agostino RB Sr, Wolf PA, Vasan RS, Benjamin EJ. Obesity and the risk of new‐onset atrial fibrillation. JAMA. 2004;292:2471–2477. [DOI] [PubMed] [Google Scholar]
- 23. Ramsay JE, Ferrell WR, Crawford L, Wallace AM, Greer IA, Sattar N. Maternal obesity is associated with dysregulation of metabolic, vascular, and inflammatory pathways. J Clin Endocrinol Metab. 2002;87:4231–4237. [DOI] [PubMed] [Google Scholar]
- 24. Adamsson Eryd S, Sjogren M, Smith JG, Nilsson PM, Melander O, Hedblad B, Engstrom G. Ceruloplasmin and atrial fibrillation: evidence of causality from a population‐based Mendelian randomization study. J Intern Med. 2014;275:164–171. [DOI] [PubMed] [Google Scholar]
- 25. Hanson M, Gluckman P, Bustreo F. Obesity and the health of future generations. Lancet Diabetes Endocrinol. 2016;4:966–967. [DOI] [PubMed] [Google Scholar]
- 26. Grundvold I, Skretteberg PT, Liestol K, Erikssen G, Kjeldsen SE, Arnesen H, Erikssen J, Bodegard J. Upper normal blood pressures predict incident atrial fibrillation in healthy middle‐aged men: a 35‐year follow‐up study. Hypertension. 2012;59:198–204. [DOI] [PubMed] [Google Scholar]
- 27. Johnson LS, Juhlin T, Engstrom G, Nilsson PM. Risk factor changes and incident atrial fibrillation among middle‐aged men in the Malmo Preventive Project cohort. Eur Heart J Cardiovasc Pharmacother. 2016;2:81–87. [DOI] [PubMed] [Google Scholar]
- 28. Gaillard R, Steegers EA, Duits L, Felix JF, Hofman A, Franco OH, Jaddoe VW. Childhood cardiometabolic outcomes of maternal obesity during pregnancy: the Generation R Study. Hypertension. 2014;63:683–691. [DOI] [PubMed] [Google Scholar]


