Abstract
Background
The assessment of myocardial perfusion (MP) and wall motion (WM) using contrast dipyridamole echocardiography (cSE‐WMP) improves the sensitivity to detect coronary artery disease and the stratification of cardiac events, but its long‐term value for fatal and nonfatal ischemic cardiac events, also with respect to patients undergoing revascularization or not, remains to be determined.
Methods and Results
One‐thousand three‐hundred and twenty‐nine patients with suspect or known CAD who underwent cSE‐WMP were followed for a median 5.5 years. The independent prognostic value of cSE‐WMP regarding cardiac death or nonfatal myocardial infarction was related to stress WM and MP, rest ejection fraction, clinical risk factors, and medications. Patients revascularized after cSE‐WMP were separately analyzed to determine whether the procedure influenced outcome and whether this depends on cSE‐WMP results. A total of 125 cardiac fatal and nonfatal ischemic events (9.4%) occurred during the follow‐up (61 deaths, 64 myocardial infarctions). The 5‐year event rate with normal MP and WM was 5.9%, 9.9% with isolated MP defects (normal WM), and 15.5% with both MP and WM abnormalities. In patients not undergoing revascularization (n=1111), reversible MP defects added discrimination value over WM response and clinical factors/medication data (P=0.001), while in the cohort undergoing revascularization (n=218), cSE‐WMP results did not influence outcome.
Conclusions
cSE‐WMP, with both contrast MP and WM assessments, provides independent, incremental prognostic information regarding ischemic cardiac events at 5 years in patients with known or suspected coronary artery disease. Revascularization reduces cardiac events after an abnormal cSE‐WMP, resulting in outcomes not different from those in patients with normal cSE‐WMP.
Keywords: cardiac events, contrast echocardiography, coronary artery disease, death, dipyridamole, ischemia, myocardial perfusion, prognosis, stress‐echocardiography, wall motion
Subject Categories: Echocardiography, Prognosis
Clinical Perspective
What Is New?
Cardiac ischemic fatal and nonfatal events are predicted in the long‐term by contrast pharmacologic vasodilator stress‐echocardiography, relying either on WM or on myocardial perfusion, incrementally to clinical and drug therapy data.
Such prognostic value is now demonstrated in a truly contemporary population, pharmacologically and interventionally treated according to most recent guidelines.
Such prognostic value of the index stress‐echocardiogram is apparently lost after patients with a positive test undergo revascularization, indirectly indicating a prognostic benefit of revascularization itself in such patients with significant reversible ischemia.
Such significant and beneficial effect of revascularization was not recorded in patients demonstrating only reversible myocardial perfusion defects, in the absence of WM, generally representing less extensive and less severe reversible ischemia.
What Are the Clinical Implications?
Our data add to few other retrospective studies suggesting that revascularization is prognostically beneficial only in the presence of a certain amount of demonstrable reversible ischemia at provocative testing, suggesting that revascularization probably should not be considered the primary therapy in patients with suspect or known CAD with only mild reversible ischemia.
Introduction
Pharmacologic stress echocardiography using wall motion (WM) assessment is an established technique for the detection and prognostication of coronary artery disease (CAD) using dobutamine1, 2, 3, 4, 5, 6 or vasodilators.7, 8, 9 Contrast stress echocardiography with additional myocardial perfusion assessment (cSE‐WMP) has demonstrated further increase in predictive accuracy for hard cardiac events in patients with suspected or known CAD, either during dobutamine10, 11 or vasodilator12, 13, 14 stress. As far as WM assessment is concerned, cSE‐WMP also has the advantage of maximizing test feasibility over standard stress echocardiography, with quality of acoustic windows not representing an issue when taking advantage of ultrasound contrast media.15 Still, the few studies assessing cSE‐WMP for prognosis do not address its value specifically in predicting true ischemic events (cardiac death and nonfatal acute myocardial infarction [MI]),10, 11, 12, 13, 14, 15, 16, 17, 18, 19, 20 and it is not known how the use of cSE‐WMP as a gatekeeper to coronary angiography and subsequent revascularization does relate to long‐term outcome in subsequently revascularized and nonrevascularized patients. Previous cSE‐WMP studies reported only on combined end points with prevalence of all‐cause deaths, half of them represented by cancer deaths, pneumonia, or other diseases that may not be directly influenced by coronary ischemia at stress testing.10, 11, 12, 13, 14 Cardiac ischemic events are instead clinically relevant end points, currently modifiable by timely aggressive medical or surgical therapy, if highest‐risk subjects can be identified.21 In this article, we report long‐term follow‐up in the largest published cSE‐WMP database, comprising 1329 contemporary patients, with an average 5.5 years follow‐up, more than double the follow‐up of previously published cSE‐WMP studies.
The hypotheses of the current study are that both the perfusion and WM analysis during cSE‐WMP are able to predict the incidence of cardiac ischemic events at 5 years in patients with known or suspected CAD. Secondly, we hypothesized that aggressive medical and revascularization therapy following an abnormal cSE‐WMP would significantly influence outcome.
Methods
Patients
We analyzed the outcome of 1329 consecutive patients with known or suspected CAD who were referred for cSE‐WMP using commercially available contrast agents in our laboratory from January 2008 to December 2011. Among them 1252 were already included in a prior prognostic analysis reporting on a significantly shorter follow‐up.12 Reasons for referral were evaluation of chest pain or dyspnea for suspect CAD in 941 (71%), preoperative risk assessment in 128 (10%), evaluation of multiple cardiac risk factors in 59 (4%), or functional assessment of known CAD in 201 (15%). The study was approved by the Institutional Review Board of the Parma Medical Center and all patients gave informed consent. Follow‐up was completed in September 2016. The population consisted of 794 men (60%) and mean age in the study population was 66±11 years. Risk factors for CAD (Table 1) were systemic hypertension in 940 (71%), hypercholesterolemia in 760 (57%), cigarette smoking in 314 (24%), and diabetes mellitus in 334 (25%). Two hundred eighty‐eight patients (22%) had a history of a previous MI and 372 overall (28%) had a history of at least 1 (primary or elective) percutaneous or surgical coronary revascularization procedure. Four hundred forty‐five patients had known CAD, in the form of previous revascularization or MI. Drug therapy was initially recorded for all at the time of testing, in patients who underwent early revascularization within 90 days it was updated to the therapy prescribed after their routine 6‐month postrevascularization visit, so that the therapy early revascularized patients were truly on for most of their follow‐up period was the only medications considered in statistical analysis. In this context, 798 patients (60%) were on β‐blockers, 823 (62%) angiotensin‐converting enzyme inhibitors or angiotensin receptor blockers, 830 (62%) were on aspirin, 246 (19%) on clopidogrel, and 749 (56%) were taking statins.
Table 1.
Clinical Characteristics and Echocardiographic Findings
| Overall (n=1329) | No PCI/CABG (n=1111) | PCI/CABG (n=218) | P Value | |
|---|---|---|---|---|
| Risk factors and patient history | ||||
| Mean age±SD | 66±11 | 66±11 | 68±9 | 0.021 |
| Male sex, n (%) | 794 (60) | 636 (57) | 158 (72) | <0.001 |
| Family history of CAD, n (%) | 389 (29) | 335 (30) | 54 (25) | 0.110 |
| Current cigarette smoking, n (%) | 314 (24) | 264 (24) | 50 (23) | 0.793 |
| Hypercholesterolemia, n (%)b | 760 (57) | 611 (55) | 149 (68) | <0.001 |
| Diabetes mellitus, n (%) | 334 (25) | 266 (24) | 68 (31) | 0.024 |
| Hypertension, n (%)a | 940 (71) | 774 (70) | 166 (76) | 0.055 |
| Obesity, n (%) | 175 (13) | 153 (14) | 22 (10) | 0.142 |
| Known CAD (prior MI or revascularization) | 445 (34) | 352 (32) | 102 (47) | <0.001 |
| Previous MI | 288 (22) | 217 (20) | 71 (33) | <0.001 |
| Previous revascularization | 372 (28) | 290 (26) | 82 (38) | 0.001 |
| Medications | ||||
| ASA, n (%) | 830 (62) | 647 (58) | 183 (84) | <0.001 |
| Plavix/Ticlop, n (%) | 246 (19) | 146 (13) | 100 (46) | <0.001 |
| β‐Blockers, n (%) | 798 (60) | 620 (56) | 178 (82) | <0.001 |
| ACE‐I/ARB, n (%) | 823 (62) | 659 (59) | 164 (75) | <0.001 |
| Statins, n (%) | 749 (56) | 578 (52) | 171 (78) | <0.001 |
| Echocardiography | ||||
| Reduced rest LVEF (<50%), n (%) | 323 (24) | 264 (24) | 59 (27) | 0.299 |
| Fixed WM abnormalities, n (%) | 382 (29) | 282 (25) | 100 (46) | <0.001 |
| Inducible WM abnormalities, n (%) | 248 (19) | 79 (7) | 169 (78) | <0.001 |
| Fixed MP abnormalities, n (%) | 330 (25) | 245 (22) | 85 (39) | <0.001 |
| Inducible MP abnormalities, n (%) | 411 (31) | 219 (20) | 192 (88) | <0.001 |
| No inducible MP abnormalities, n (%) | 918 (69) | 892 (80) | 26 (12) | <0.001 |
| Only inducible MP abnormalities, n (%) | 163 (12) | 140 (13) | 23 (11) | <0.001 |
Data presented are mean value±SD or n (%) of patients. ACE‐I indicates angiotensin‐converting enzyme inhibitors; ARBs, angiotensin receptor blockers; ASA, acetylsalicylic acid; CABG, coronary artery bypass graft; CAD, coronary artery disease; LVEF, left ventricular ejection fraction; MI, myocardial infarction; MP, myocardial perfusion; PCI, percutaneous coronary revascularization; WM, wall motion.
Blood pressure ≥140/90 mm Hg or treatment of hypertension.
Total cholesterol >200 mg/dL or treatment of hypercholesterolemia.
Diabetes mellitus was defined as a fasting plasma glucose level >125 mg/dL or the need for insulin or oral hypoglycemic agents. Hypercholesterolemia was defined as total cholesterol >200 mg/dL or treatment with lipid‐lowering medications. Hypertension was defined as blood pressure >140/90 mm Hg or use of antihypertensive medication.
Stress Protocol
Dipyridamole was infused at the total dose of 0.84 mg/kg in all patients. A total of 461 underwent a 10‐minute 0.84 mg/kg dipyridamole infusion+atropine administration (up to 1 mg), while the majority of patients (n=868) underwent a 6‐minute protocol, consisting of the 0.84 mg/kg dipyridamole infusion, which does not require additional atropine administration. Two‐dimensional echocardiography, 12‐lead ECGs, and blood pressure monitoring were performed in accordance with established standard protocols.1 Aminophylline was routinely used to reverse dipyridamole effect. When an obvious new WM abnormality (≥1 akinetic segment) was observed by the physician performing the test, dipyridamole infusion was stopped, and aminophylline was administered.
cSE‐WMP Imaging
The test was performed with a commercially available ultrasound scanner (ie33; Philips Medical Systems) equipped with low mechanical‐index real‐time pulse sequence schemes that deploy interpulse phase‐amplitude modulation.12 cSE‐WMP was performed using the phospholipid‐encapsulated microbubble SonoVue® (Bracco Imaging, Milan, Italy), either in repeated slow 0.5‐mL boluses (850 patients) or continuous infusion at 0.8 to 1.2 mL/min (479 patients) using a dedicated rotating pump.
Myocardial perfusion (MP) was studied at rest and after dipyridamole infusion by activation of low mechanical‐index power‐modulation imaging so that cineloops of flash‐replenishment sequences (both real‐time and end‐systolic triggered at every cardiac cycle) were digitally acquired in the apical 4‐, 2‐, and 3‐chamber views. The low‐mechanical index setting was activated just before administration of contrast and the time gain compensation and 2‐dimensional gain settings were adjusted to suppress any nonlinear signals from tissue at a mechanical index=0.08 to 0.12 and frame rate >30 Hz. In case of bolus contrast administration (SonoVue® 0.5 mL), the ideal timing to start acquiring the MP flash‐replenishment sequences was when attenuation from left ventricular cavity contrast had resolved, usually 15 s after peak video intensity was reached, during the initial washout of contrast. WM was assessed at rest and after dipyridamole infusion by activation of a specific preset for contrast WM analysis (left ventricular opacification, with harmonic imaging, a higher mechanical index=0.27 and frame rate=40 Hz) or with the same preset used for MP assessment depending on which allowed technically better WM imaging in the specific patient, while the standard 2‐dimensional preset was only resumed, after complete microbubble clearance, for continuous WM monitoring through the remainder of the stress test.
Image Analysis
The left ventricle was divided into 17 segments according to the recommendations of the American and European Societies of Echocardiography.1, 2
MP was visually assessed, using the following criteria: normal MP after dipyridamole was assigned if myocardium was fully replenished 1.5 to 2 s after the end of the flash impulse, and stress perfusion was defined as abnormal if myocardium was not fully replenished after this time in ≥1 contiguous segment. Normal myocardial replenishment at rest was defined as complete replenishment within 4 s after the flash impulse. A MP defect was scored as fixed or reversible based on its presence at rest. Left ventricle segments were excluded from MP reading if not clearly visualized, because of shadowing artifacts or low ultrasound penetration, especially in the basal segments. Segmental WM was graded as follows: normal=1; hypokinetic=2; akinetic=3; and dyskinetic=4. Reversible ischemia was defined as the occurrence of a stress‐induced new dyssynergy or worsening of rest hypokinesia in ≥1 segment.
Patients were also classified according to the extent of abnormality. They were considered to have single‐vessel abnormality when the MP defect or WM abnormality involved only 1 coronary artery territory and multivessel abnormality when the MP defect or WM abnormality involved >1 coronary artery territory. The left ventricular apex, anteroseptal, distal septum, and anterior walls were assigned to the left anterior descending coronary artery, the lateral wall to the left circumflex, and the inferior wall and basal septum to the right coronary artery. The results of both MP and WM analyses were made available to the referring physicians. The interobserver agreement data assessed in 2 previous studies in our lab are between 80% and 87.5% (k=0.60–0.75) for MP and between 90% and 95% (k=0.80–0.83) for WM assessments.12, 20
Follow‐Up
Follow‐up was obtained by review of the patient's hospital electronic records, regional health database, national cancer registry, and telephone interview when inconsistencies were found.
The study primary end point was only for ischemic cardiac events defined as death from an ischemic cardiac cause+nonfatal MI. If a patient first had a nonfatal MI and also died subsequently in the follow‐up, only the first event was recorded. In the current study, we selected cardiac death and not all‐cause death22 to exclude deaths related to cancer, infections, or other causes, which in cardiac studies generally account for half of total mortality, and cannot be attributed directly to CAD although they may have some association, possibly based on shared risk factors.23 Nonfatal MI was defined by means of a serial increase in cardiac‐specific enzymes and development of new ECG changes.
Statistical Analysis
Continuous variables are expressed as mean and SD, categorical variables as number of patients and proportions. Kaplan–Meier event‐free survival curves for the prespecified end points across categorical variables were estimated and compared using the Log‐rank test. For the analysis of cardiac mortality, patients dying of other causes were censored from follow‐up at the time of death.
Univariate and multivariable Cox proportional hazard models were used to estimate the risk of events.
Rest left ventricular ejection fraction (LVEF) was analyzed as a dichotomous variable (<50% or ≥50%); additional clinical variables considered in the analyses were defined according to the Framingham risk score assessment. They included diabetes mellitus, hypercholesterolemia, and hypertension. Clinical variables such as history of prior MI, known CAD, and prior revascularization were also considered. Echocardiographic parameters were rest LVEF, WM, and MP ischemic responses to dipyridamole. Acetylsalicylic acid, angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers, clopidogrel, and statin therapy at enrollment were considered, but in patients who underwent revascularization within 90 days it was updated to the therapy prescribed after their routine 6‐month postrevascularization visit. All clinical and cSE‐WMP variables with P<0.1 at univariate analysis were considered for multivariable models.
A first multivariable clinical model was derived, then cSE‐WMP variables were added sequentially to the model. The significance of additional variables to previous modeling steps was based on the likelihood ratio test. The usefulness of contrast cSE‐WMP variables—MP and WM— over clinical variables was then verified in terms of discrimination power using the Harrell's C index.
All analyses were also repeated after breaking the study population into those who did and did not undergo a revascularization procedure following cSE‐WMP.
In the overall population, risk reclassification was evaluated in terms of continuous net reclassification index (NRI) rather than categorical NRI, because of unclear cutoffs to be used for the definition of low, intermediate, and high‐risk groups in a selected population undergoing provocative testing.
For all Cox models, the proportional hazard assumption was assessed using the Schoenfeld test.
Results
There were 1329 patients enrolled, mean age was 66±11 years, 60% were males, 25% were diabetic, and 33% had previously known CAD, in the form of prior MI or prior revascularization. Baseline LVEF was 57±8%, with 323 (25%) having an LVEF at baseline <50% (range 25–49%). During the study period, 218 patients (16%) underwent revascularization procedures, either percutaneous (n=207) or surgical (n=16), while 7 of such patients underwent both types of revascularization. Most of these patients (n=179, 82%) were electively revascularized within 3 months of the cSE‐WMP. Of the 218 revascularized patients, 169 (78%) had a cSE‐WMP with reversible abnormality for both WM and MP, 23 (10%) only for MP, and 26 (12%) did not demonstrate any WM or MP abnormality. These 26 patients were referred for angiography and revascularization on the basis of persistent symptoms and in some cases (18 out of 26) according to a second provocative test discordant with the negative cSE‐WMP.
In the remaining 1111 patients who did not undergo revascularization at any time, the cSE‐WMP study was interpreted as normal for both WM and MP in 892 patients (80%), abnormal for MP only in 140 patients (13%), and ischemic for both MP and WM in 79 (7%). In the study cohort, no patient had abnormal WM with normal MP. The distribution of the results of WM and MP analysis in the overall cohort as well as stratified by subsequent revascularization is presented in Table 1, together with baseline characteristics, and drug therapy.
Patients who underwent subsequent revascularization differed from the group of patients who did not regarding more frequent abnormal cSE‐WMP results and higher medication use, as expected, while among risk factors they differed for being more frequently male, hypercholesterolemic, and more frequently with a known history of CAD. Association of cSE‐WMP results with the subsequent coronary revascularization procedure are graphically depicted in Figure 1.
Figure 1.
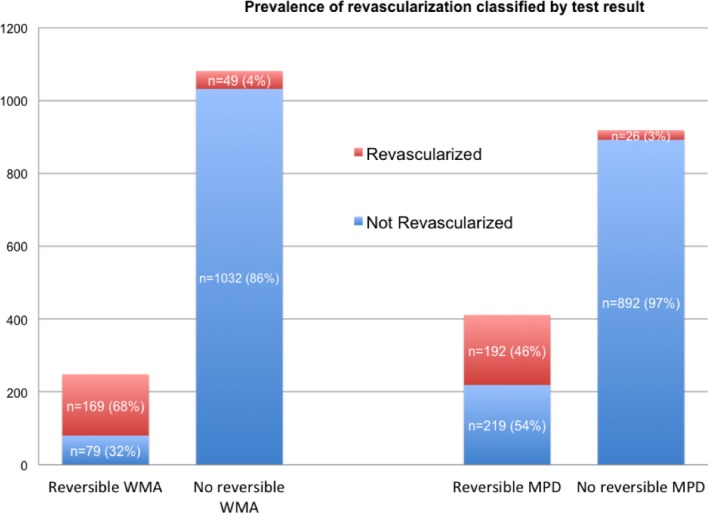
Bar graph demonstrating that, as expected, the percentage of subjects undergoing a revascularization procedure after contrast dipyridamole echocardiography was higher after abnormal tests, compared with the very limited number of subjects who underwent revascularization notwithstanding a normal test. MPD indicates myocardial perfusion defect; WMA, wall motion abnormality.
Outcome
The median follow‐up was 2013 days (lower, upper quartiles 1555, 2299 days). During the entire follow‐up, ischemic cardiac deaths were 61 (4.6%) and 64 patients had a nonfatal MI (4.8%). During the follow‐up period, patients who were not revascularized (n=1111) had 90 ischemic fatal or nonfatal cardiac events, while revascularized patients (n=218) had 35 ischemic fatal or nonfatal cardiac events.
Outcome According to cSE‐WMP Results
The outcome of patients, according to a normal test, presence of isolated reversible MP defect (normal WM), or the presence of both reversible MP and WM abnormalities, is graphically depicted as 5‐year event rates in Figure 2, and using Kaplan–Meier event‐free survival curves in Figure 3 for the primary end point of cardiac ischemic fatal and nonfatal events, and in Figure 4 separately showing event‐free Kaplan–Meier curves for either cardiac death (Figure 4A) or MI (Figure 4B). Depending on cSE‐WMP results, patients with reversible WM and perfusion abnormalities demonstrated the worst outcome, patients with isolated reversible MP abnormalities (in the absence of WM abnormality) had an intermediate outcome, while the best outcome was for patients with fully normal cSE‐WMP. Event‐free survival curves were significantly different for primary cardiac ischemic events (Log‐rank P<0.001) and also for cardiac death and nonfatal MI as single end points (Log‐rank <0.01).
Figure 2.
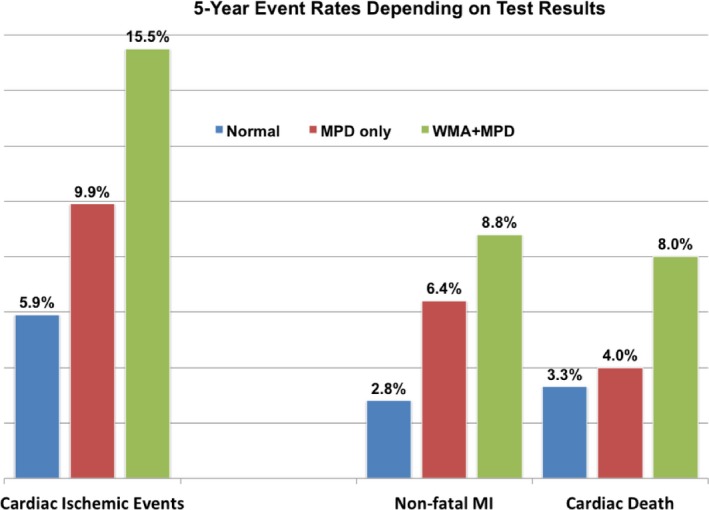
Five‐year event rate for primary cardiac ischemic events end point, and its breakdown into cardiac death and nonfatal myocardial infarction based on contrast dipyridamole echocardiography results. MI indicates myocardial infarction; MPD, myocardial perfusion defect; WMA, wall motion abnormality.
Figure 3.

Kaplan–Meier event‐free survival curves for cardiac ischemic events based on results of contrast dipyridamole echocardiography. The overall difference among the curves is highly statistically significant (Log‐rank <0.001). MPD indicates myocardial perfusion defect; WMA, wall motion abnormality.
Figure 4.
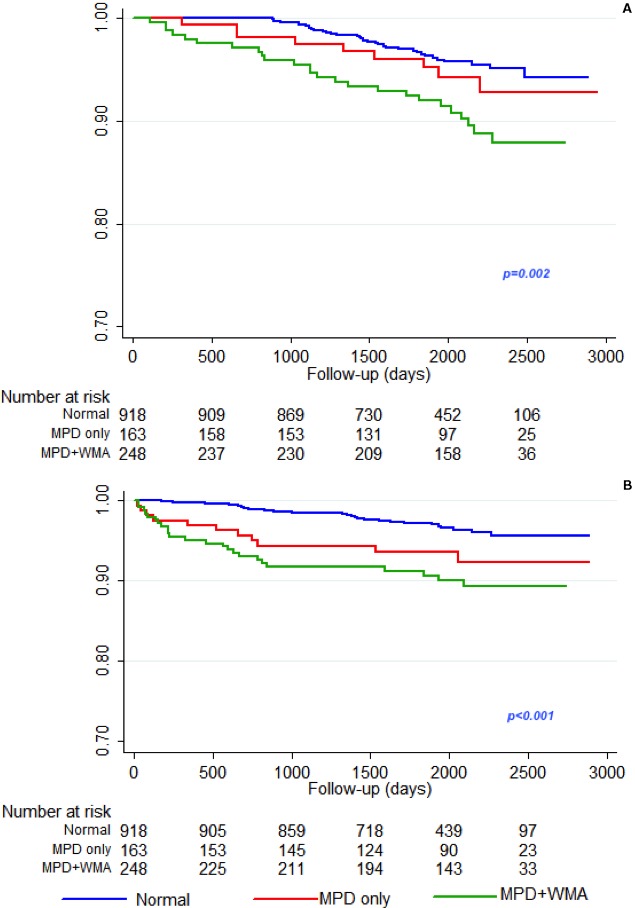
Event‐free survival curves for single components of the primary end point, cardiac death (A) and nonfatal MI (B), based on results of contrast dipyridamole echocardiography, The overall difference among the curves is highly statistically significant (Log‐rank=0.002 for cardiac death and <0.001 for nonfatal myocardial infarction). MI indicates myocardial infarction; MPD, myocardial perfusion defect; WMA, wall motion abnormality.
Predictors of Ischemic Cardiac Events
Table 2 shows univariable predictors of the primary end point. Age (HR 1.052, 95% CI 1.032–1.071), known CAD (2.510, 95% CI 1.766–3.569), diabetes mellitus (1.780, 95% CI 1.235–2.565), and undergoing revascularization after cSE‐WMP (HR=1.872, 95% CI 1.266–2.767) were the clinically significant univariable predictors. Among the drugs, therapy with acetylsalicylic acid (HR=2.355, 95% CI 1.538–3.606), clopidogrel (HR 2.461, 95% CI 1.705–3.554), angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers (HR 1.495, 95% CI 1.021–2.189), or statin (HR 1.895, 95% CI 1.291–2.782) were significant predictors. Rest and stress echocardiography demonstrated the following significant predictors: LVEF <50% (HR 1.701, 95% CI 1.180–2.453), reversible WM abnormality (HR 2.451, 95% CI 1.701–3.534), reversible MP abnormalities (HR 2.337, 95% CI 1.645–3.320), fixed MP abnormalities (HR=2.066, 95% CI 1.443–2.959), and reversible WM or MP abnormalities (HR 2.729, 95% CI 1.856–4.013).
Table 2.
Univariate Analysis of Main Clinical, Medications, and Imaging Parameters as Predictors of Cardiac Ischemic Events
| Ischemic Cardiac Events | ||
|---|---|---|
| Univariate Analysis | HR (95% CI) | P Value |
| Clinical | ||
| Age | 1.052 (1.032–1.071)a | 0.000a |
| Male sex | 1.282 (0.888–1.850) | 0.185 |
| Known CAD (MI/PCI/CABG) | 2.510 (1.766–3.569)a | 0.000a |
| Family history of CAD | 0.965 (0.651–1.431) | 0.860 |
| Smoke | 0.763 (0.489–1.191) | 0.233 |
| Hypercholesterolemia | 1.242 (0.865–1.784) | 0.240 |
| Diabetes mellitus | 1.780 (1.235–2.565)a | 0.002a |
| Hypertension | 1.242 (0.827–1.863) | 0.296 |
| Obesity | 0.835 (0.479–1.455) | 0.524 |
| Revascularization within 90 d after stress echocardiogram | 1.872 (1.266–2.767)a | 0.002a |
| Drugs | ||
| ASA | 2.355 (1.538–3.606)a | 0.000a |
| Clopidogrel | 2.461 (1.705–3.554)a | 0.000a |
| β‐Blockers | 1.364 (0.941–1.977) | 0.101 |
| ACE‐I/ARBs | 1.495 (1.021–2.189)a | 0.039a |
| Statin | 1.895 (1.291–2.782)a | 0.001a |
| Imaging | ||
| Rest LVEF reduction (<50%) | 1.701 (1.180–2.453)a | 0.004a |
| Reversible WM abnormality | 2.451 (1.701–3.534)a | 0.000a |
| Fixed WM abnormalities | 1.367 (0.949–1.970) | 0.094 |
| Reversible MP abnormalities | 2.337 (1.645–3.320)a | 0.000a |
| Fixed MP abnormalities | 2.066 (1.443–2.959)a | 0.000a |
| Reversible WM or MP abnormalities | 2.729 (1.856–4.013)a | 0.000a |
All patients with inducible WM abnormalities also had reversible MP defect. ACE‐I indicates angiotensin‐converting enzyme inhibitors; ASA, acetylsalicylic acid; ARBs, angiotensin receptor blockers; CAD, coronary artery disease; HR, hazard ratio; LVEF, left ventricular ejection fraction; MI, myocardial infarction; MP, myocardial perfusion; PCI/CABG, percutaneous/surgical coronary revascularization; WM, wall motion.
Statistically significant predictor.
Multivariable Predictors and Incremental Value of cSE‐WMP Over Clinical Characteristics
Table 3 shows sequential Cox regression models fit to test the incremental performance of prediction models when cSE‐WMP variables were added over clinical variables and drug therapy, reporting χ2 and Harrell's C index for discrimination for each of them.
Table 3.
Multivariable Models to Predict Ischemic Cardiac Events
| Multivariable Models | Ischemic Cardiac Events | |||
|---|---|---|---|---|
| HR (95% CI) | P Value | χ2 | Harrell's C (95% CI) | |
| Clinical model | ||||
| Age | 1.046 (1.03–1.07) | 0.000 | 67.5 | 0.701 (0.658–0.744) |
| Diabetes mellitus | 1.431 (0.987–2.074) | 0.059 | ||
| Known CAD | 1.733 (1.158–2.593) | 0.008 | ||
| ASA | 1.522 (0.970–2.389) | 0.068 | ||
| Clopidogrel | 1.483 (0.978–2.247) | 0.063 | ||
| Clinical+stress‐echocardiography WM | ||||
| Age | 1.045 (1.025–1.065) | 0.000 | 73.5a | 0.720b (0.678–0.763) |
| Diabetes mellitus | 1.383 (0.953–2.006) | 0.088 | ||
| Known CAD | 1.775 (1.195–2.637) | 0.004 | ||
| ASA | 1.391 (0.881–2.195) | 0.157 | ||
| Clopidogrel | 1.310 (0.862–1.989) | 0.206 | ||
| Reversible WM abnormality | 1.758 (1.196–2.585) | 0.004 | ||
| Clinical+stress‐echocardiography MP | ||||
| Age | 1.046 (1.026–1.066) | 0.000 | 76.3a | 0.723b (0.681–0.764) |
| Diabetes mellitus | 1.409 (0.972–2.041) | 0.070 | ||
| Known CAD | 1.810 (1.217–2.693) | 0.003 | ||
| ASA | 1.339 (0.847–2.118) | 0.212 | ||
| Clopidogrel | 1.299 (0.857–1.969) | 0.218 | ||
| Reversible MP abnormality | 1.839 (1.275–2.652) | 0.001 | ||
| Clinical+stress‐echocardiography WM and MP | ||||
| Age | 1.45 (1.025–1.066) | 0.000 | 76.7a, b | 0.725b (0.683–0.766) |
| Diabetes mellitus | 1.395 (0.961–2.024) | 0.080 | ||
| Known CAD | 1.806 (1.215–2.684) | 0.003 | ||
| ASA | 1.335 (0.843–2.111) | 0.218 | ||
| Clopidogrel | 1.281 (0.844–1.944) | 0.245 | ||
| Reversible MP abnormality | 1.633 (0.971–2.747) | 0.064 | ||
| Reversible WM abnormalities | 1.197 (0.695–2.063) | 0.517 | ||
All patients with inducible WM abnormalities also had reversible MP defect. The last model (WM and MP) with both WM and MP is not significantly more accurate than the previous models with only WM or MP, while they maintain higher Harrell's C and χ2 compared with the clinical model. ASA indicates acetylsalicylic acid; CAD, coronary artery disease; HR, hazard ratio; MP, myocardial perfusion; WM, wall motion.
Means P<0.01 all compared with clinical model.
Means P<0.05.
The model comprising abnormal stress WM demonstrated a significantly higher χ2 (P=0.005) and Harrell's C for discrimination (difference [95% CI]=0.019 [0.001–0.038]; P=0.037) compared with the initial reference clinical+drug therapy model and the same was true for the model adding reversible MP data to the baseline model with clinical and drug therapy data, with both higher χ2 (P=0.001) and Harrell's C for discrimination (difference [95% CI]=0.022 [0.001–0.042]; P=0.039). On the contrary, there was no additional independent value or increase of the model discrimination ability when adding MP and WM sequentially (last model in Table 3).
Similarly for risk reclassification, models in which either WM or MP was added to the model including only clinical+drug therapy data significantly improved risk reclassification (respectively continuous NRI [95% CI]=0.382 [0.209–0.556], P<0.001 and continuous NRI [95% CI]=0.448 [0.265–0.630], P<0.001). When both WM and MP were added to the initial reference clinical+drug therapy model, the ability to reclassify risk was even better as compared with the model with the sole WM (continuous NRI [95% CI]=0.306 [0.134–0.478], P<0.001) but not in comparison with the model including clinical+drug therapy and MP (continuous NRI [95% CI]=−0.105 [−0.279 to 0.069], P=0.238; categorical NRI [95% CI]=−0.004 [−0.009 to 0], P=0.095), showing superiority of MP to risk reclassify patients compared with WM.
Predictors of Ischemic Cardiac Events Stratifying for Revascularization
Table 4 shows univariable predictors of the primary end point after dividing patients into the 1111 who did not have subsequent coronary revascularization and the 218 who did.
Table 4.
Univariate Analysis of Main Clinical Variables, Medications, and Imaging Parameters as Predictors of Cardiac Ischemic Events, Once Patients Are Divided Based on Whether They Underwent Revascularization or Not After Their Stress Echocardiogram
| Ischemic Cardiac Events | ||||
|---|---|---|---|---|
| Univariate Analysis | Non Revascularized (n=1111) | Revascularized (n=218) | ||
| HR (95% CI) | P Value | HR (95% CI) | P Value | |
| Clinical | ||||
| Age | 1.056 (1.034–1.079)a | 0.000a | 1.034 (0.995–1.074)a | 0.087a |
| Male sex | 1.436 (0.933–2.212) | 0.100 | 0.685 (0.341–1.377) | 0.289 |
| Known CAD (MI/PCI/CABG) | 3.219 (2.123–4.881)a | 0.000a | 1.049 (0.541–2.036) | 0.887 |
| Family history of CAD | 1.118 (0.715–1.747) | 0.626 | 0.673 (0.279–1.623) | 0.378 |
| Smoke | 0.632 (0.363–1.100) | 0.104 | 1.204 (0.564–2.571) | 0.631 |
| Hypercholesterolemia | 1.169 (0.768–1.779) | 0.467 | 1.195 (0.574–2.488) | 0.635 |
| Diabetes mellitus | 1.895 (1.231–2.919)a | 0.004a | 1.295 (0.652–2.570) | 0.460 |
| Hypertension | 1.409 (0.864–2.296) | 0.169 | 0.801 (0.385–1.667) | 0.553 |
| Obesity | 0.877 (0.467–1.648) | 0.683 | 0.798 (0.244–2.607) | 0.709 |
| Drugs | ||||
| ASA | 2.984 (1.798–4.952)a | 0.000a | 0.601 (0.273–1.323) | 0.206 |
| Clopidogrel | 2.517 (1.577–4.018)a | 0.000a | 1.633 (0.836–3.191) | 0.151 |
| β‐Blockers | 1.529 (0.993–2.354)a | 0.054a | 0.509 (0.245–1.061)a | 0.071a |
| ACE‐I/ARBs | 1.567 (1.006–2.440)a | 0.047a | 0.878 (0.411–1.878) | 0.738 |
| Statin | 1.930 (1.245–2.991)a | 0.003a | 1.066 (0.466–2.441) | 0.879 |
| Imaging | ||||
| Rest LVEF reduction (<50%) | 1.905 (1.243–2.920)a | 0.003a | 1.213 (0.594–2.477) | 0.597 |
| Reversible WM abnormality | 3.585 (2.137–6.014)a | 0.000a | 1.018 (0.462–2.240) | 0.965 |
| Fixed WM abnormality | 1.291 (0.826–2.017) | 0.262 | 1.187 (0.611–2.306) | 0.612 |
| Reversible MP abnormality | 2.567 (1.676–3.933)a | 0.000a | 0.859 (0.333–2.216) | 0.754 |
| Fixed MP abnormality | 1.842 (1.188–2.856)a | 0.006a | 2.181 (1.119–4.247)a | 0.022a |
| Reversible WM/MP abnormality | 3.990 (2.344–6.793)a | 0.000a | 0.880 (0.339–2.287) | 0.794 |
All patients with inducible WM abnormalities also had reversible MP defect. ACE‐I indicates angiotensin‐converting enzyme inhibitor; ASA, acetylsalicylic acid; ARBs, angiotensin receptor blockers; CAD, coronary artery disease; HR, hazard ratio; LVEF, left ventricular ejection fraction; MI, myocardial infarction; MP, myocardial perfusion; PCI/CABG, percutaneous/surgical coronary revascularization; WM, wall motion.
Statistically significant predictor.
In patients who did not undergo revascularization, age (HR 1.056, 95% CI 1.034–1.079), known CAD (HR 3.219, 95% CI 2.123–4.881), and diabetes mellitus (HR 1.895, 95% CI 1.231–2.919) were the significant clinical predictors at univariable analysis. Among the drugs, therapy with acetylsalicylic acid (HR 2.984, 95% CI 1.798–4.952), clopidogrel (HR=2.517, 95% CI 1.577–4.018), β‐blockers (HR=1.529, 95% CI 0.993–2.354, borderline P=0.054), angiotensin‐converting enzyme inhibitors/angiotensin receptor blockers (HR=1.567, 95% CI 1.006–2.440), or statin (HR=1.930, 95% CI 1.245–2.991) were significant predictors. Rest and stress echocardiography demonstrated the following predictors: LVEF <50% (HR 1.905, 95% CI 1.243–2.920), reversible WM abnormality (HR 3.585, 95% CI 2.137–6.014), reversible MP abnormalities (HR=2.567, 95% CI 1.676–3.933), fixed MP abnormalities (HR=1.842, 95% CI 1.188–2.856), and reversible WM or MP abnormalities (HR=3.990, 95% CI 2.344–6.793).
In patients who did undergo revascularization, apart from age (HR 1.034, 95% CI 0.995–1.074) and β‐blockers (HR 0.509, 95% CI=0.245–1.061) resulting only in borderline significance, only a fixed MP abnormality was clearly significant (HR 2.181, 95% CI 1.119–4.247) at univariable analysis.
Table 5 shows sequential Cox regression multivariable models fit to test the incremental performance of prediction models when cSE‐WMP variables were added over clinical variables and drug therapy, reporting χ2 and Harrell's C index for discrimination for each of them. Compared with the results of the multivariable models in the entire population (Table 3), when dividing patients who did not or did undergo revascularization, diverse independent predictors were identified. In patients undergoing revascularization, not only do the models comprising either WM or MP on top of clinical and drug therapy both demonstrate a significant increase in χ2 and Harrell's C for discrimination compared with clinical and drug therapy variables, but the last model with both reversible WM and MP stress variables demonstrates a significant increase also compared with the model with only WM (χ2 from 74.82 to 78.42, borderline P=0.058, Harrell's C from 0.720 to 0.724, difference [95% CI]=0.004 [0.001–0.023]; P=0.044). It demonstrates that MP analysis in patients not undergoing revascularization was useful for better stratification of the primary end point, even when considering WM responses as well as clinical and drug therapy variables. In multivariable models for the 218 patients who underwent revascularization (Table 6), only the addition of fixed MP abnormalities significantly improved the clinical model including age and β‐blockers therapy (χ2 from 6.36 to 11.67, P=0.021, Harrell's C from 0.576 to 0.622, difference [95% CI]=0.046 [0.029–0.086]; P=0.009).
Table 5.
Multivariable Models to Predict Ischemic Cardiac Events in the 1111 Patients Who Did Not Undergo Revascularization After cSE‐WMP
| Multivariable Models | Ischemic Cardiac Events | |||
|---|---|---|---|---|
| HR (95% CI) | P Value | χ2 | Harrell's C (95% CI) | |
| Clinical model | ||||
| Age | 1.050 (1.027–1.074) | 0.000 | 63.72 | 0.692 (0.647–0.737) |
| Known CAD | 2.438 (1.578–3.766) | 0.000 | ||
| Diabetes mellitus | 1.587 (1.026–2.454) | 0.038 | ||
| ASA | 1.849 (1.084–3.153) | 0.024 | ||
| Clinical+echocardiography WM | ||||
| Age | 1.049 (1.025–1.073) | 0.000 | 74.82a | 0.720b (0.678–0.763) |
| Known CAD | 2.384 (1.543–3.684) | 0.000 | ||
| Diabetes mellitus | 1.545 (0.998–2.390) | 0.051 | ||
| ASA | 1.736 (1.015–2.969) | 0.044 | ||
| Reversible WM abnormalities | 2.662 (1.580–4.485) | 0.000 | ||
| Clinical+echocardiography MP | ||||
| Age | 1.051 (1.028–1.076) | 0.000 | 76.23a | 0.719b (0.677–0.760) |
| Known CAD | 2.456 (1.588–3.798) | 0.000 | ||
| Diabetes mellitus | 1.563 (1.010–2.418) | 0.045 | ||
| ASA | 1.673 (0.977–2.866) | 0.061 | ||
| Reversible MP abnormalities | 2.246 (1.461–3.454) | 0.000 | ||
| Clinical+echocardiography WM and MP | ||||
| Age | 1.050 (1.026–1.074) | 0.000 | 78.42c | 0.724c (0.683–0.765) |
| Known CAD | 2.415 (1.561–3.735) | 0.000 | ||
| Diabetes mellitus | 1.545 (0.998–2.391) | 0.051 | ||
| ASA | 1.668 (0.973–2.857) | 0.063 | ||
| Reversible WM abnormalities | 1.669 (0.848–3.284) | 0.138 | ||
| Reversible MP abnormalities | 1.771 (1.013–3.097) | 0.045 | ||
All patients with inducible WM abnormalities also had reversible MP defect. ASA indicates acetylsalicylic acid; CAD, coronary artery disease; cSE‐WMP, contrast stress echocardiography with additional myocardial perfusion assessment; HR, hazard ratio; MP, myocardial perfusion; WM, wall motion.
Means P<0.01 compared with clinical model.
Means P<0.05 compared with previous clinical model.
Means P≤0.05 compared with clinical+echocardiography WM model.
Table 6.
Multivariable Models to Predict Ischemic Cardiac Events in the 218 Patients Who Did Undergo Revascularization After cSE‐WMP
| Multivariable Models | Ischemic Cardiac Events | |||
|---|---|---|---|---|
| HR (95% CI) | P Value | χ2 | Harrell's C (95% CI) | |
| Clinical model | ||||
| Age | 1.036 (0.997–1.076) | 0.071 | 6.36 | 0.576 (0.526–0.626) |
| β‐Blockers | 0.487 (0.234–1.015) | 0.055 | ||
| Clinical+echocardiography MP | ||||
| Age | 1.036 (0.997–1.076) | 0.072 | 11.67a | 0.622b (0.572–0.673) |
| β‐Blockers | 0.455 (0.216–0.955) | 0.037 | ||
| Fixed MP abnormalities | 2.200 (1.126–4.299) | 0.021 | ||
cSE‐WMP indicates contrast stress echocardiography with additional myocardial perfusion assessment; HR, hazard ratio; MP, myocardial perfusion.
Means P<0.01 compared with clinical model.
Means P<0.05 compared with previous clinical model.
Event Rates and Survival Free From Cardiac Events in Revascularized Versus Not‐Revascularized Patients
Figure 5 bar graph shows event rates at 2 different time points (3‐ and 6‐year follow‐up) for patients with normal or abnormal stress WM who did or did not undergo revascularization after the cSE‐WMP test.
Figure 5.
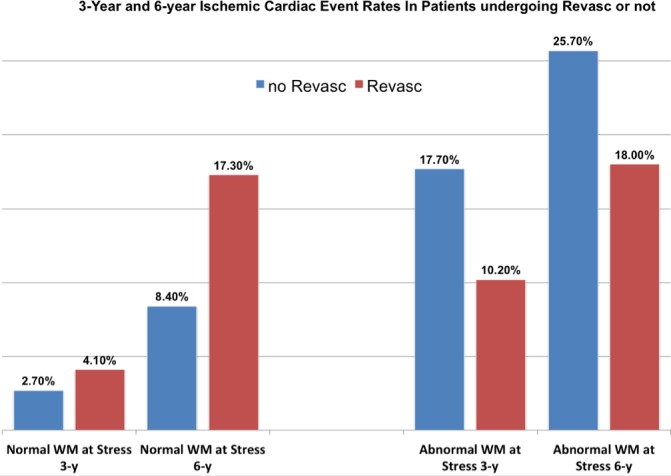
Histograms demonstrate how coronary revascularization was associated with both 3‐ and 6‐year better outcome in patients with a positive stress echocardiogram for wall motion assessment, while on the other hand it apparently negatively affected outcome in patients who underwent revascularization with a normal wall motion response at prior stress echocardiogram. WM indicates wall motion.
Such data suggest that revascularization improved both 3‐ and 6‐year outcome in patients with an abnormal stress‐echo for WM assessment, while on the other hand it apparently negatively affected outcome in patients who underwent revascularization, notwithstanding a normal WM response at prior stress‐echo.
Figure 6 shows survival free from cardiac ischemic events in patients who either did not undergo subsequent revascularization (Figure 6A) or who did undergo revascularization (Figure 6B). Survival curves for the 3 types of possible cSE‐WMP response (normal, isolated reversible MP abnormality, or both MP+WM abnormality) are shown in the upper panels while mid and lower panels dichotomize response based on WM or MP assessment. While test results appear to have a clear risk‐stratifying value in patients who subsequently did not undergo revascularization (Log‐rank P<0.001), such value is apparently completely lost in patients subsequently revascularized (Log‐rank not significant), indirectly indicating that revascularization acts as an outcome‐modifier after cSE‐WMP.
Figure 6.
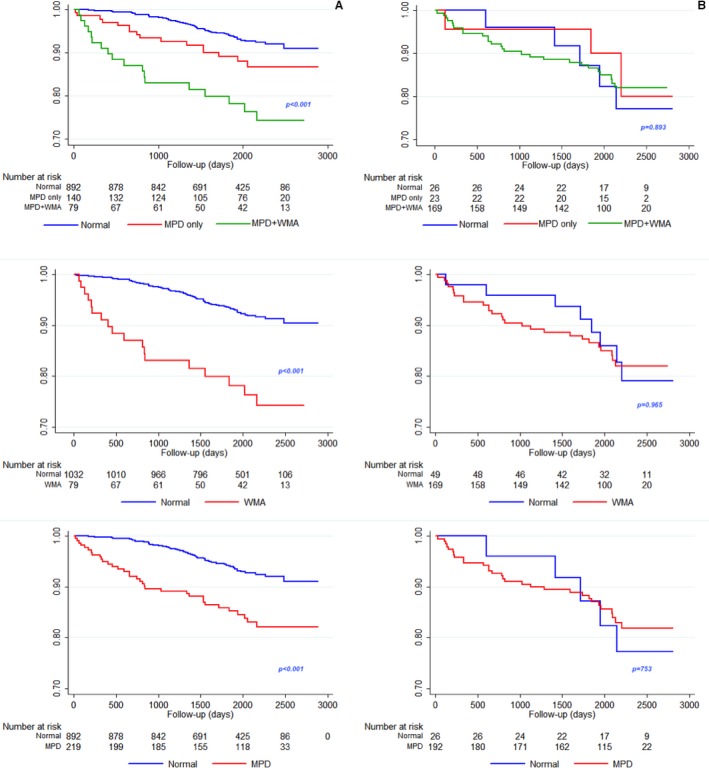
Shows survival free from cardiac ischemic events in patients who either did not undergo subsequent revascularization (A) or who did undergo revascularization (B). Survival curves for the 3 types of possible contrast dipyridamole echocardiography response (normal, isolated reversible myocardial perfusion defect [MPD], or both MPD+wall motion abnormality [WMA]) are shown in the upper panels while mid and lower panels dichotomize response based on wall motion or myocardial perfusion assessment. While test results have a clear prognostic value in patients who subsequently did not undergo revascularization, such value is lost in patients subsequently revascularized, indirectly indicating that revascularization acts as an outcome‐modifier.
Figure 7 shows the event‐free survival for patients testing abnormal or normal, respectively, at WM assessment (left) or myocardial perfusion assessment. It should be emphasized that only 49 patients were revascularized, notwithstanding normal stress WM and only 26 with normal myocardial perfusion at stress. Either considering WM (Log‐rank P=0.04) or myocardial perfusion assessment (Log‐rank P=0.027), the patients with a normal cSE‐WMP who were subsequently revascularized fared worse, while the opposite was not true for abnormal cSE‐WMP (upper panels).
Figure 7.
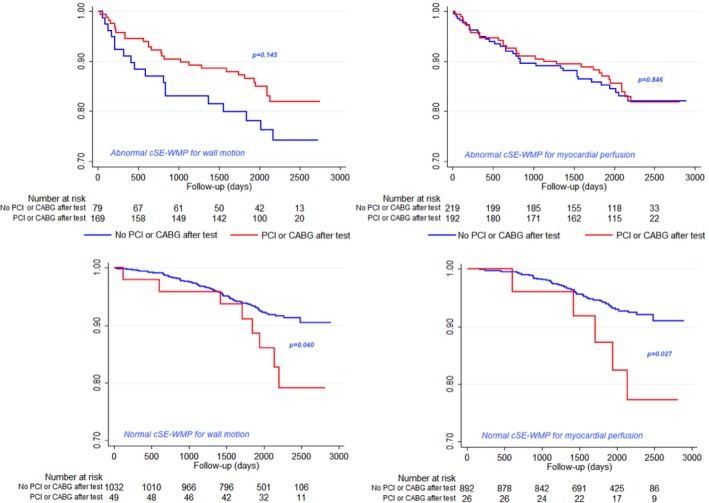
Event‐free survival curves for patients testing abnormal or normal, respectively, at wall motion assessment (left) or myocardial perfusion assessment. It should be emphasized that only 49 patients were revascularized notwithstanding normal stress wall motion and only 26 with normal myocardial perfusion at stress. Either for wall motion (Log‐rank P=0.04) or myocardial perfusion assessment (Log‐rank P=0.027), patients with a normal contrast dipyridamole echocardiography who are subsequently revascularized anyway fared worse, while the opposite was not true for abnormal contrast dipyridamole echocardiography (upper panels) not showing statistically significant differences, although a trend toward better outcome if a revascularized patient had abnormal stress wall motion was shown. CABG indicates coronary artery bypass graft; cSE‐WMP, contrast stress echocardiography with additional myocardial perfusion assessment; PCI, percutaneous coronary revascularization.
Discussion
In this study, we evaluated the ability of vasodilator cSE‐WMP, based on MP and WM assessments, to predict long‐term risk of incident fatal and nonfatal cardiac ischemic events, namely, nonfatal MI or cardiac death, in a contemporary group of patients with known or suspected CAD undergoing contrast stress‐echocardiography for clinical indications.
The current study is unique in that it studies the largest cohort ever studied with contrast myocardial perfusion and WM assessment stress‐echocardiography, reporting on a truly contemporary (enrolled 2008–2012) and hence presumably high‐intensity medical therapy cohort, with almost double follow‐up compared to existing similar studies. Large sample and long follow‐up contributed with achieve sufficient statistical power to aim for the first time at purely cardiac ischemic fatal and nonfatal events and draw robust conclusions regarding the incremental performance of prediction models when cSE‐WMP variables were added over clinical and drug therapy data.
Role of MP and WM Imaging in Predicting Events
Both reversible MP abnormalities or reversible WM abnormalities during cSE‐WMP were independently predictive of 5‐year ischemic events, with an isolated reversible MP defect portending an intermediate prognosis between a normal test and a fully ischemic test with both reversible MP and WM abnormality. This is in line with prior studies and pathophysiology, although to date no cSE‐WMP study addressed such a 5‐year event‐rate, but only shorter follow‐up periods.
Revascularization Impact
With all limitations of retrospective nonrandomized studies, by keeping patients who were subsequently revascularized, and not censoring them at the time of revascularization, we were allowed to infer that the long‐term impact of revascularization may be dependent on the results of the cSE‐WMP study. This specific patient population (ie, those revascularized following a normal or abnormal stress echocardiogram) has not been evaluated in terms of long‐term impact in the current era of background intensive medical therapy. Importantly, risk stratification operated by MP and WM variables during cSE‐WMP was much more robustly demonstrated in multivariable models in patients who did not undergo revascularization after their stress test. cSE‐WMP stratification value in fact did not hold in patients undergoing subsequent coronary revascularization; in this context, the result of their previous cSE‐WMP was apparently not significant for subsequent risk restratification. In other words, our study suggests that a revascularization procedure exerted an effect on outcome, which tended to finally equalize subsequent prognosis in all patients. The current study suggests that patients with a positive cSE‐WMP, specifically with reversible WM abnormalities, do benefit significantly from revascularization, while the ones with a normal cSE‐WMP probably should not undergo revascularization, at least as far as a beneficial effect on cardiac ischemic events is the aim of such procedure. However, the current study was retrospective and not randomized, and the decision to indicate revascularization was based on clinical grounds, heavily influenced by test positivity. Similar findings were found in at least 1 scintigraphic24 and 1 recent dobutamine echo25 which were also retrospective and observational studies; they enrolled patients in the 1990s, and their applicability to the current era may be limited; furthermore, they apparently did not correct for significant outcome‐modifying therapy, such as statins and antiplatelets, very tightly interacting with revascularization procedures, always upscaled after a revascularization procedure and currently much more intensive than was used 2 decades ago. While in such studies the amount of reversible ischemia needed to make revascularization prognostically useful is moderate to severe, in our study such differentiation appeared to be between milder forms of ischemia being related to isolated MP abnormalities and more severe forms being related to any amount of WM abnormality accompanying MP abnormalities. This may relate to the use of vasodilator stress versus dobutamine or exercise “demand” stressors.
Only prospective multicenter studies, such as the ISCHEMIA (International Study of Comparative Health Effectiveness with Medical and Invasive Approaches) trial, for example, will hopefully definitely confirm whether the presence of reversible ischemia, and which amount, is truly a key prerequirement to indicate revascularization in stable CAD on prognostic grounds.
Comparison With Fractional Flow Reserve
cSE‐WMP results might be useful for the decision to revascularize or not, similar to what fractional flow reserve (FFR) studies have recently proposed.26, 27, 28 Our findings point in the same direction to what has been observed with invasive FFR; such multicenter studies prospectively demonstrated that FFR is prognostically useful to proceed to revascularization, but in patients already selected to undergo diagnostic coronary angiography, although they fell short of demonstrating a benefit regarding hard events, death and MI, since in the FAME 2 study (Fractional Flow Reserve Versus Angiography for Multivessel Evaluation) the FFR‐guided percutaneous coronary revascularization arm was favored on comparison of the primary composite end point of death, MI, and target vessel revascularization, but such finding was driven by urgent target vessel revascularization.28, 29 cSE‐WMP is a gatekeeper that may be useful at a higher decisional level compared with FFR, when, in the work‐up of suspect/known CAD, chest pain needs to be categorized as ischemic or not, and cSE‐WMP may in some ways be more useful in identifying those likely to benefit from revascularization for truly severe ischemic events, in particular for the possibility to assess myocardial blood flow at tissue level using contrast MP.30
Study Limitations
Keeping revascularized patients has pros and cons, and is less typical than censoring patients at the time of revascularization, which is moreover also questionable because of the introduction of the bias of excluding most patients testing as abnormal at cSE‐WMP, who are the ones generally undergoing revascularization, also precluding assessing the potential effect of revascularization.
In our study, as in all studies considering drug therapy (in particular if per‐protocol updated after revascularization as in the current study), some drugs, such as antiplatelets and statins, were very tightly linked to the revascularization procedure and such drugs and revascularization to abnormal cSE‐WMP. Thus, it was not statistically fair to try to analyze revascularization in a multivariable analysis, because of interaction with the abovementioned parameters. We rather chose to stratify for revascularization, and also present the data separately for patients who did or did not undergo revascularization. However, we acknowledge that only 218 patients were finally revascularized and only 35 fatal and nonfatal events recorded during the follow‐up in this subgroup of patients, so that the sample size seems too small to make any meaningful inferences in this subset. Among the 1329 patients included in the study, 1252 were already included in a prior prognostic study with significantly shorter follow‐up.12
Electrocardiographic and hemodynamic changes during stress were not analyzed in our study, but these parameters have never been clearly associated with prognosis during dipyridamole stress echocardiography.7, 8, 9
Perfusion imaging during echocardiography requires significant specific expertise, not very differently from other advanced ultrasound, nuclear, or other imaging techniques.
Both bolus and continuous‐infusion contrast techniques were employed for this study. Although continuous infusion would have been the ideal technique for analyzing contrast replenishment following a high mechanical index impulse, we found that the bolus technique resulted in much lower contrast utilization and produced equivalent results for visual analysis.
Conclusions
cSE‐WMP provides independent and incremental information for the prediction of 5‐year ischemic cardiac events in addition to clinical and rest imaging variables in current‐era patients with known or suspected CAD, after adjustment for clinical and medications data. As expected and previously reported, an isolated reversible MP defect without WM abnormality is in general a more benign finding compared with a reversible MP defect associated with a reversible WMA. There is also a suggestion that revascularization reduces ischemic cardiac events only after a positive cSE‐WMP, more clearly if WM response is considered, while performing revascularization despite the fact that a negative cSE‐WMP for MP or WM might be detrimental. This was per se a collateral interesting result of our study, that long‐term ischemic cardiac event rate in patients undergoing stress echocardiography may be heavily influenced by subsequent revascularization and by cSE‐WMP results before revascularization, which is not a consolidated finding in the literature.
Disclosures
Dr Gaibazzi has received grant support from Bracco, and GE imaging. Dr Porter has received grant support from Philips Healthcare, Lantheus Medical, and Astellas Pharma.
(J Am Heart Assoc. 2017;6:e006202 DOI: 10.1161/JAHA.117.006202.)28566297
References
- 1. Sicari R, Nihoyannopoulos P, Evangelista A, Kasprzak J, Lancellotti P, Poldermans D, Voigt JU, Zamorano JL; European Association of Echocardiography . Stress echocardiography expert consensus statement: European Association of Echocardiography (EAE) (a registered branch of the ESC). Eur J Echocardiogr. 2008;9:415–437. [DOI] [PubMed] [Google Scholar]
- 2. Pellikka PA, Nagueh SF, Elhendy AA, Kuehl CA, Sawada SG; American Society of Echocardiography . American Society of Echocardiography recommendations for performance, interpretation, and application of stress echocardiography. J Am Soc Echocardiogr. 2007;20:1021–1041. [DOI] [PubMed] [Google Scholar]
- 3. Poldermans D, Fioretti PM, Boersma E, Bax JJ, Thomson IR, Roelandt JR, Simoons ML. Long‐term prognostic value of dobutamine‐atropine stress echocardiography in 1737 patients with known or suspected coronary artery disease: a single‐center experience. Circulation. 1999;99:757–762. [DOI] [PubMed] [Google Scholar]
- 4. Krivokapich J, Child JS, Walter DO, Garfinkel A. Prognostic value of dobutamine stress echocardiography in predicting cardiac events in patients with known or suspected coronary artery disease. J Am Coll Cardiol. 1999;33:708–716. [DOI] [PubMed] [Google Scholar]
- 5. Marwick TH, Case C, Sawada S, Rimmerman C, Brenneman P, Kovacs R, Short L, Lauer M. Prediction of mortality using dobutamine echocardiography. J Am Coll Cardiol. 2001;37:754–760. [DOI] [PubMed] [Google Scholar]
- 6. Bjork Ingul C, Rozis E, Slordahl SA, Marwick TH. Incremental value of strain rate imaging to wall motion analysis for prediction of outcome in patients undergoing dobutamine stress echocardiography. Circulation. 2007;115:1252–1259. [DOI] [PubMed] [Google Scholar]
- 7. Sicari R, Pasanisi E, Venneri L, Landi P, Cortigiani L, Picano E; Echo Persantine International Cooperative (EPIC) Study Group; Echo Dobutamine International Cooperative (EDIC) Study Group . Stress echo results predict mortality: a large‐scale multicenter prospective international study. J Am Coll Cardiol. 2003;41:589–595. [DOI] [PubMed] [Google Scholar]
- 8. Cortigiani L, Bigi R, Landi P, Bovenzi F, Picano E, Sicari R. Prognostic implication of stress echocardiography in 6214 hypertensive and 5328 normotensive patients. Eur Heart J. 2011;32:1509–1518. [DOI] [PubMed] [Google Scholar]
- 9. Cortigiani L, Borelli L, Raciti M, Bovenzi F, Picano E, Molinaro S, Sicari R. Prediction of mortality by stress echocardiography in 2835 diabetic and 11305 nondiabetic patients. Circ Cardiovasc Imaging. 2015;8:e002757 DOI: 10.1161/CIRCIMAGING.114.002757. [DOI] [PubMed] [Google Scholar]
- 10. Tsutsui JM, Elhendy A, Anderson JR, Xie F, McGrain AC, Porter TR. Prognostic value of dobutamine stress myocardial contrast perfusion echocardiography. Circulation. 2005;112:1444–1450. [DOI] [PubMed] [Google Scholar]
- 11. Tsutsui JM, Xie F, Cloutier D, Kalvaitis S, Elhendy A, Porter TR. Real‐time dobutamine stress myocardial perfusion echocardiography predicts outcome in the elderly. Eur Heart J. 2008;29:377–385. [DOI] [PubMed] [Google Scholar]
- 12. Gaibazzi N, Reverberi C, Lorenzoni V, Molinaro S, Porter TR. Prognostic value of high‐dose dipyridamole stress myocardial contrast perfusion echocardiography. Circulation. 2012;126:1217–1224. [DOI] [PubMed] [Google Scholar]
- 13. Gaibazzi N, Lorenzoni V, Reverberi C, Wu J, Xie F, Porter TR. Effect of pharmacologic stress test results on outcomes in obese versus nonobese subjects referred for stress perfusion echocardiography. J Am Soc Echocardiogr. 2016;29:899–906. [DOI] [PubMed] [Google Scholar]
- 14. Dawson D, Kaul S, Peters D, Rinkevich D, Schnell G, Belcik JT, Wei K. Prognostic value of dipyridamole stress myocardial contrast echocardiography: comparison with single photon emission computed tomography. J Am Soc Echocardiogr. 2009;22:954–960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Shah BN, Zacharias K, Pabla JS, Karogiannis N, Calicchio F, Balaji G, Alhajiri A, Ramzy IS, Elghamaz A, Gurunathan S, Khattar RS, Senior R. The clinical impact of contemporary stress echocardiography in morbid obesity for the assessment of coronary artery disease. Heart. 2016;102:370–375. [DOI] [PubMed] [Google Scholar]
- 16. Gaibazzi N, Squeri A, Reverberi C, Molinaro S, Lorenzoni V, Sartorio D, Senior R. Contrast stress‐echocardiography predicts cardiac events in patients with suspected acute coronary syndrome but nondiagnostic electrocardiogram and normal 12‐hour troponin. J Am Soc Echocardiogr. 2011;24:1333–1341. [DOI] [PubMed] [Google Scholar]
- 17. Wejner‐Mik P, Lipiec P, Kasprzak JD. Long‐term prognostic value of dipyridamole stress myocardial contrast echocardiography. Eur J Echocardiogr. 2011;12:762–766. [DOI] [PubMed] [Google Scholar]
- 18. Shah BN, Gonzalez‐Gonzalez AM, Drakopoulou M, Chahal NS, Bhattacharyya S, Li W, Khattar RS, Senior R. The incremental prognostic value of the incorporation of myocardial perfusion assessment into clinical testing with stress echocardiography study. J Am Soc Echocardiogr. 2015;28:1358–1365. [DOI] [PubMed] [Google Scholar]
- 19. Porter TR, Smith LM, Wu J, Thomas D, Haas JT, Mathers DH, Williams E, Olson J, Nalty K, Hess R, Therrien S, Xie F. Patient outcome following 2 different stress imaging approaches: a prospective randomized comparison. J Am Coll Cardiol. 2013;61:2446–2455. [DOI] [PubMed] [Google Scholar]
- 20. Gaibazzi N, Rigo F, Lorenzoni V, Molinaro S, Bartolomucci F, Reverberi C, Marwick TH. Comparative prediction of cardiac events by wall motion, wall motion plus coronary flow reserve, or myocardial perfusion analysis: a multicenter study of contrast stress echocardiography. JACC Cardiovasc Imaging. 2013;6:1–12. [DOI] [PubMed] [Google Scholar]
- 21. Ford I, Shah AS, Zhang R, McAllister DA, Strachan FE, Caslake M, Newby DE, Packard CJ, Mills NL. High sensitivity cardiac troponin, statin therapy, and risk of coronary heart disease. J Am Coll Cardiol. 2016;68:2719–2728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Lauer MS, Blackstone EH, Young JB, Topol EJ. Cause of death in clinical research: time for a reassessment? J Am Coll Cardiol. 1999;34:618–620. [DOI] [PubMed] [Google Scholar]
- 23. Wang EY, Dixson J, Schiller NB, Whooley MA. Causes and predictors of death in patients with coronary heart disease (from the Heart and Soul Study). Am J Cardiol. 2017;119:27–34. [DOI] [PubMed] [Google Scholar]
- 24. Hachamovitch R, Hayes SW, Friedman JD, Cohen I, Berman DS. Comparison of the short‐term survival benefit associated with revascularization compared with medical therapy in patients with no prior coronary artery disease undergoing stress myocardial perfusion single photon emission computed tomography. Circulation. 2003;107:2900–2907. [DOI] [PubMed] [Google Scholar]
- 25. Boiten HJ, Ekmen H, Zijlstra F, van Domburg RT, Schinkel AF. Impact of early coronary revascularization on long‐term outcomes in patients with myocardial ischemia on dobutamine stress echocardiography. Am J Cardiol. 2016;118:635–640. [DOI] [PubMed] [Google Scholar]
- 26. Pijls NH, van Schaardenburgh P, Manoharan G, Boersma E, Bech JW, van't Veer M, Bär F, Hoorntje J, Koolen J, Wijns W, de Bruyne B. Percutaneous coronary intervention of functionally nonsignificant stenosis: 5‐year follow‐up of the DEFER Study. J Am Coll Cardiol. 2007;49:2105–2111. [DOI] [PubMed] [Google Scholar]
- 27. Pijls NH, Fearon WF, Tonino PA, Siebert U, Ikeno F, Bornschein B, van't Veer M, Klauss V, Manoharan G, Engstrøm T, Oldroyd KG, Ver Lee PN, MacCarthy PA, De Bruyne B; FAME Study Investigators . Fractional flow reserve versus angiography for guiding percutaneous coronary intervention in patients with multivessel coronary artery disease: 2‐year follow‐up of the FAME (Fractional Flow Reserve Versus Angiography for Multivessel Evaluation) study. J Am Coll Cardiol. 2010;56:177–184. [DOI] [PubMed] [Google Scholar]
- 28. De Bruyne B, Fearon WF, Pijls NH, Barbato E, Tonino P, Piroth Z, Jagic N, Mobius‐Winckler S, Rioufol G, Witt N, Kala P, MacCarthy P, Engström T, Oldroyd K, Mavromatis K, Manoharan G, Verlee P, Frobert O, Curzen N, Johnson JB, Limacher A, Nüesch E, Jüni P; FAME 2 Trial Investigators . Fractional flow reserve‐guided PCI for stable coronary artery disease. N Engl J Med. 2014;371:1208–1217. [DOI] [PubMed] [Google Scholar]
- 29. Gibbons RJ, Miller TD. Should extensive myocardial ischaemia prompt revascularization to improve outcomes in chronic coronary artery disease? Eur Heart J. 2015;36:2281–2287. [DOI] [PubMed] [Google Scholar]
- 30. Wu J, Barton D, Xie F, O'Leary E, Steuter J, Pavlides G, Porter TR. Comparison of fractional flow reserve assessment with demand stress myocardial contrast echocardiography in angiographically intermediate coronary stenoses. Circ Cardiovasc Imaging. 2016;9:e004129 DOI: 10.1161/CIRCIMAGING.116.004129. [DOI] [PubMed] [Google Scholar]


