Abstract
Background
Arrhythmogenic right ventricular dysplasia/cardiomyopathy is characterized by ventricular arrhythmias and sudden cardiac death. Once the diagnosis is established, risk stratification to determine whether implantable cardioverter‐defibrillator (ICD) placement is warranted is critical.
Methods and Results
The cohort included 312 patients (163 men, age at presentation 33.6±13.9 years) with definite arrhythmogenic right ventricular dysplasia/cardiomyopathy who received an ICD. Over 8.8±7.33 years, 186 participants (60%) had appropriate ICD therapy and 58 (19%) had an intervention for ventricular fibrillation/flutter. Ventricular tachycardia at presentation (hazard ratio [HR]: 1.86; 95% confidence interval [CI], 1.38–2.49; P<0.001), inducibility on electrophysiology study (HR: 3.14; 95% CI, 1.95–5.05; P<0.001), male sex (HR: 1.62; 95% CI, 1.20–2.19; P=0.001), inverted T waves in ≥3 precordial leads (HR: 1.66; 95% CI, 1.09–2.52; P=0.018), and premature ventricular contraction count ≥1000/24 hours (HR: 2.30; 95% CI, 1.32–4.00; P=0.003) were predictors of any appropriate ICD therapy. Inducibility at electrophysiology study (HR: 2.28; 95% CI, 1.10–4.70; P=0.025) remained as the only predictor after multivariable analysis. The predictors for ventricular fibrillation/flutter were premature ventricular contraction ≥1000/24 hours (HR: 4.39; 95% CI, 1.32–14.61; P=0.016), syncope (HR: 1.85; 95% CI, 1.10–3.11; P=0.021), aged ≤30 years at presentation (HR: 1.76; 95% CI, 1.04–3.00; P<0.036), and male sex (HR: 1.73; 95% CI, 1.01–2.97; P=0.046). Younger age at presentation (HR: 3.14; 95% CI, 1.32–7.48; P=0.010) and high premature ventricular contraction burden (HR: 4.43; 95% CI, 1.35–14.57; P<0.014) remained as independent predictors of ventricular fibrillation/flutter. Complications occurred in 66 participants (21%), and 64 (21%) had inappropriate ICD interventions. Overall mortality was low at 2%, and 4% underwent heart transplantation.
Conclusion
These findings represent an important step in identifying predictors of ICD therapy for potentially fatal ventricular fibrillation/flutter and should be considered when developing a risk stratification model for arrhythmogenic right ventricular dysplasia/cardiomyopathy.
Keywords: arrhythmogenic right ventricular cardiomyopathy/dysplasia, implantable cardioverter defibrillator, sudden cardiac death, tachyarrhythmias, ventricular fibrillation
Subject Categories: Arrhythmias, Catheter Ablation and Implantable Cardioverter-Defibrillator, Ventricular Fibrillation, Sudden Cardiac Death, Cardiomyopathy
Clinical Perspective
What Is New?
This study reports the outcomes of the largest series of patients with arrhythmogenic ARVD/C to undergo ICD implantation.
Among the 312 patients with ARVD/C, 186 (60%) received an appropriate ICD therapy and 58 (19%) received potentially lifesaving ICD therapy for ventricular fibrillation/flutter.
Variables identified as markers of high risk for ventricular fibrillation/flutter include a high burden of premature ventricular contractions, history of syncope, younger age at presentation, and male sex.
Variables identified as predictors of first appropriate ICD therapy were a history of ventricular tachycardia at presentation, inducibility at electrophysiology study, male sex, ≥3 T‐wave inversions in precordial leads, and a high burden of premature ventricular contractions.
What Are the Clinical Implications?
The high rate of appropriate ICD therapy for ventricular fibrillation/flutter is an important reminder of the lethal nature of ARVD/C and the importance of risk stratification for ICD placement.
The risk markers identified in this study should ultimately guide clinicians in their recommendation for ICD implantation in patients with arrhythmogenic ARVD/C.
Introduction
Arrhythmogenic right ventricular dysplasia/cardiomyopathy (ARVD/C) is an inherited cardiomyopathy characterized pathologically by myocyte loss with fibrofatty replacement and clinically by an increased risk of sustained ventricular arrhythmias and sudden cardiac death (SCD). Once the diagnosis of ARVD/C is established, a critical decision is whether a particular patient's SCD risk is sufficiently elevated to justify placement of an implantable cardioverter‐defibrillator (ICD).1 This decision is particularly important because these are often young patients who are expected to live for many years with a device that that is associated with both short‐ and long‐term complications.2
The purpose of this study was to expand the body of knowledge concerning the outcomes of ICD therapy in patients with ARVD/C. Although a number of prior studies have reported outcomes of ICD therapy in patients with ARVD/C, these studies were limited by small sample size, the use of the 1994 task force diagnostic criteria,3 the inclusion of patients who did not fully meet diagnostic criteria for ARVD/C, and the lack of genetic testing as a standard part of the diagnostic evaluation. Furthermore, the primary end point in many of these studies was the prediction of appropriate ICD therapy, including both sustained ventricular tachycardia (VT) and ventricular fibrillation/ventricular flutter (VF/VFL). In designing this study, we sought to overcome many of these limitations.
This study had 4 primary objectives. The first objective was to describe, in detail, the clinical characteristics and outcomes of a large series of ARVD/C patients who underwent ICD implantation. The second objective was to identify clinical and electrophysiological factors that best identified ARVD/C patients who experienced appropriate ICD therapy. We were particularly interested in better defining the risk factors for VF/VFL. The third objective was to report the complications associated with ICD implantation. And our fourth and final objective was to describe the long‐term outcomes of this cohort of ARVD/C patients, each of whom had undergone placement of an ICD.
Methods
Patient Population and Follow‐up
The Johns Hopkins ARVD/C program (ARVD.com) was established in 1999 to provide clinical care for patients with ARVD/C and to provide new knowledge concerning this disease. The Johns Hopkins ARVD/C Registry prospectively follows patients and at‐risk family members. All those in the registry who had a definite diagnosis of ARVD/C based on the 2010 task force criteria (TFC),4 who had implantation of an ICD to prevent sudden death, and who were followed for at least 30 days after ICD implantation were included in the study. All participants gave written informed consent to participate in this study, which was approved by the Johns Hopkins School of Medicine institutional review board.
Detailed clinical information regarding demographics, symptoms, 12‐lead ECG findings, and the results of 24‐hour Holter monitoring was reviewed. In addition, information regarding ICD therapies and arrhythmia occurrence was obtained for each patient. Magnetic resonance imaging reports were obtained, and images were reviewed for structural abnormalities to determine the severity and extent of right and left ventricular dysfunction. Programmed ventricular stimulation was performed on a case‐by‐case basis for clinical reasons, as determined by each patient's clinical electrophysiologist. An electrophysiological study (EPS) was considered positive if a sustained VT—VT or VF that lasted ≥30 seconds or that required termination due to hemodynamic compromise—was induced, independently of the use of isoproterenol. Device interrogations and stored electrograms were obtained from referring institutions and individual patients throughout the duration of follow‐up.
ICDs and Classification of Discharges
All patients received multifunctional third‐ or fourth‐generation transvenous ICDs, with the exception of 7 patients who had a subcutaneous ICD implanted. Decisions regarding ICD implantation and programming of these devices were made by the managing cardiologist and/or electrophysiologist. Stored intracardiac electrograms were analyzed to classify arrhythmias responsible for precipitating defibrillator discharges, according to following definitions.5 VF or VFL was defined as an irregular or regular tachycardia with a mean cycle length (CL) of ≤240 ms. VT was defined as a regular tachycardia arising from the ventricle with a CL >240 and <600 ms. First appropriate ICD intervention was defined as an ICD therapy for VT/VF. An inappropriate intervention was defined as a device discharge for supraventricular tachycardia (ie, sinus tachycardia, atrial fibrillation, atrial flutter) or oversensing due to electrical noise. Data on device interrogation were available for 120 of the 186 patients included in this series. Electrogram documentation of an appropriate therapy was available in 73 of these patients. When complete ICD interrogation information was not available, ICD interrogation interpretation by the outside referring electrophysiologist was used to classify arrhythmic events. The available electrograms were reviewed by 2 electrophysiologists who are part of the Johns Hopkins ARVD/C program and members of the study team who collaboratively determined the final adjudication. In case of disagreements between our adjudication and that of the local electrophysiologist, we used the read done by our study team.
Definitions
Syncope was defined as a transient loss of consciousness and postural tone with spontaneous recovery. A proband is an affected individual ascertained independently of family history of ARVD/C, whereas a family member is an affected person ascertained through family screening. Nonsustained VT was defined as 3 consecutive ventricular premature beats with a rate >100 beats/min, lasting ≤30 seconds, which was documented during exercise testing, loop monitoring, or 24‐hour Holter monitoring. An electrical or VT storm was defined as the occurrence of VT or VF that resulted in ≥3 ICD interventions (shock or antitachycardia pacing) in a 24‐hour period.6 Major structural abnormalities were defined based on the 2010 revised TFC.4
Survival Data and Statistical Analyses
Continuous variables are summarized as either mean±SD or median (interquartile range) and compared across groups using a Mann–Whitney U test or a t test. Categorical variables are reported as frequency (percentage) and compared between groups by a χ2 or Fisher exact test. The cumulative probability of survival free from first appropriate ICD intervention (VT/VF) and from intervention for VF/VFL was determined by the Kaplan–Meier method, and differences in survival between groups were evaluated with the log‐rank test. In patients without an ICD intervention, follow‐up was to the most recent evaluation, transplantation, or date of death, whichever came first. Univariate Cox regression analysis identified baseline variables that were significantly associated with appropriate ICD therapy. Significantly associated variables (P≤0.05) were integrated into multivariable analysis using the Cox proportional hazards model to identify independent predictors of appropriate ICD intervention for VT/VF and VF/VFL. All analyses were performed using STATA statistical software (version 13.1). A P≤0.05 was considered significant.
Results
Patient Population
The patient population studied consisted of 312 patients who met 2010 TFC4 for definite ARVD/C and received an ICD for prevention of SCD. Details of fulfillment of TFC are shown in Table S1. As shown in Table 1, the study population was mainly white (298, 96%), 163 (52%) were male, and 252 (81%) were probands. The overall mean age at presentation was 33.6±13.9 years, with 209 (67%) of patients aged ≤40 years at initial evaluation.
Table 1.
Clinical Features in Patients With and Without ICD Therapy for VT/VF and VF/VFL (Cycle Length ≤240 ms)
| Clinical Variables | Overall Population (n=312) | ICD Therapy for VT/VF (n=186) | No ICD Therapy (n=126) | ICD Therapy for VF/VFL (≤240 ms) (n=58) | No ICD Therapy for VF/VFL (n=254) | P Value | |
|---|---|---|---|---|---|---|---|
| VT/VF vs No ICD Therapy | VF/VFL vs No VF/VFL | ||||||
| Demographics | |||||||
| Male | 163 (52) | 115 (62) | 48 (38) | 38 (66) | 125 (49) | <0.001 | 0.025 |
| White | 298 (96) | 179 (96) | 119 (94) | 56 (97) | 242 (95) | 0.453 | 0.672 |
| Age at presentation, y | 33.6±13.9 | 32.9±14.0 | 34.7±13.8 | 28.6±12.9 | 34.7±13.9 | 0.327 | 0.003 |
| Follow‐up, y, median (IQR) | 7 (3–13) | 8.2 (3.68–13.95) | 4.7 (2.44–10.39) | 7 (3–12) | 7 (3–13) | <0.001 | 0.803 |
| Proband | 252 (81) | 169 (91) | 83 (66) | 53 (91) | 199 (78) | <0.001 | 0.023 |
| Mutation carrier | 184/307 (60) | 116/184 (63) | 68/123 (37) | 37/57 (64) | 147/250 (59) | 0.174 | 0.395 |
| Clinical characteristics | |||||||
| Syncope | 96 (31) | 59 (32) | 37 (29) | 25 (43) | 71 (28) | 0.658 | 0.024 |
| Inducibility at EPS | 165/217 (76) | 120/139 (86) | 45/78 (58) | 33/37 (89) | 132/180 (73) | <0.001 | 0.040 |
| Inverted T waves in ≥3 precordial leads | 209/268 (78) | 124/150 (83) | 85/118 (72) | 40/49 (82) | 169/219 (77) | 0.037 | 0.495 |
| PVCs ≥1000/24 h on Holter monitoring | 118/166 (71) | 64/80 (80) | 54/86 (63) | 27/30 (90) | 91/136 (67) | 0.015 | 0.012 |
| NSVT | 114 (37) | 66 (35) | 48 (38) | 24 (41) | 90 (35) | 0.638 | 0.396 |
| Major RV structural abnormality on CMR | 114/185 (62) | 64 (67) | 50 (56) | 21/32 (66) | 93/153 (61) | 0.143 | 0.609 |
| ICD characteristicsa | |||||||
| Age at ICD implantation, y | 36.5±13.5 | 35.6±13.6 | 37.9±13.4 | 30.8±12.7 | 37.8±13.4 | 0.147 | <0.001 |
| Primary prevention | 135 (43) | 61 (33) | 74 (59) | 27 (47) | 108 (43) | <0.001 | 0.576 |
| Secondary prevention | 177 (57) | 125 (67) | 52 (41) | 31 (53) | 146 (57) | ||
| Sustained VT | 158 (51) | 113 (61) | 45 (36) | 26 (45) | 132 (52) | <0.001 | 0.326 |
| VF at presentation | 19 (6) | 13 (7) | 7 (6) | 5 (9) | 14 (6) | 0.612 | 0.372 |
| Single‐chamber ICD | 184 (61) | 106/185 (57) | 78/117 (67) | 36 (62) | 148/244 (61) | 0.104 | 0.843 |
| Dual‐chamber ICD | 118 (39) | 79/185 (43) | 39/117 (33) | 22 (38) | 96/244 (39) | ||
| Subcutaneous ICD | 7 (2) | 1 (0.54) | 6 (5) | 0 (0) | 7 (3) | 0.010 | 0.355 |
Values are mean±SD, n (%), or n/N (%). CMR indicates cardiac magnetic resonance; EPS, electrophysiology study; ICD, implantable cardioverter‐defibrillator; IQR, interquartile range; NSVT, nonsustained ventricular tachycardia; PVC, premature ventricular contraction; RV, right ventricular; VF, ventricular fibrillation; VFL, ventricular flutter; VT, ventricular tachycardia.
The decision to implant an ICD for primary prevention was made by the patient's electrophysiologist.
Clinical Characteristics at ICD Implant
Table 1 shows that genetic testing was performed in 307 patients (98%). A pathogenic mutation was observed in 184 (60%) of these patients (Table S2). Two thirds of patients who had an EPS performed prior to implant were inducible for sustained VT (144/217, 66%), with a mean CL of 273±53.8 ms (range 170–450 ms). VF was induced in 5% (10/217), and both arrhythmias were induced in 5% (11/217). In addition, 64 patients (21%) had catheter ablation performed before ICD implantation. Holter monitoring was performed in 166 patients (53%). The median premature ventricular contraction (PVC) count was 2572 (interquartile range: 760–6010). Of these patients, 118 (71%) had ≥1000 PVCs per 24 hours. Nonsustained VT on Holter monitoring, exercise stress testing, or loop recording monitors was observed in 114 patients (37%). A total of 268 patients had ECGs available for review from before ICD implantation, of whom 209 (78%) had T‐wave inversions in ≥3 precordial leads. Cardiac magnetic resonance imaging was available for review in 185 (60%), with 114 patients (62%) patients meeting major TFC.4
ICD Implantation
Primary prevention devices were implanted in 135 patients (43%), whereas 177 (57%) received a device for secondary prevention of SCD (Table 1). Among the patients who underwent implantation of an ICD for primary prevention, 21 had a family history of SCD, 47 had a family history of ARVD/C, 48 had experienced syncope, 70 had a history of nonsustained VT, and 52 had inducible sustained ventricular arrhythmia during EPS. Among the 177 patients who underwent ICD implantation for secondary prevention, 158 had previously experienced sustained VT and 19 had presented with VF. The mean age at ICD implantation was 36.5±13.5 years (range: 13–73 years). Seven patients (2%) had subcutaneous ICDs, whereas 184 (61%) had single‐chamber devices and 118 (39%) had dual‐chamber ICDs. Documentation containing ICD programming information was available for review in 148 of 186 patients (80%) who received an appropriate ICD intervention. The slowest ICD therapy detection zone was programmed at heart rates of ≥200 bpm in 41 of the 148 patients, >180 and <200 bpm in 48 patients, ≥160 and <180 bpm in 44 patients, and <160 bpm in 15 patients. Documentation containing ICD programming information was available for review in 148 of 186 patients (80%) who received an appropriate ICD intervention. The slowest ICD therapy detection zone was programmed at heart rates of ≥200 bpm in 41 of the 148 patients, >180 and <200 bpm in 48 patients, ≥160 and <180 bpm in 44 patients, and <160 bpm in 15 patients.
First Appropriate ICD Therapy (VT/VF)
Figure 1A shows the Kaplan–Meier analysis of cumulative survival from first appropriate ICD therapy (VT/VF). Overall, the cumulative survival free from appropriate ICD interventions was 60%, 51%, 37% and 24% at 1, 2, 5 and 10 years, respectively. Over a median follow‐up of 7 years (interquartile range: 3–13 years), 186 patients (60%) received appropriate ICD therapy. Of those, 115 (62%) were male and 169 (91%) were probands. The mean age at presentation for those patients who received appropriate ICD therapy was 32.9±14.0 years, with almost half (n=92) presenting before 30 years of age. Other characteristics of this group included a positive EPS in 120 of 139 that underwent EPS (86%), T‐wave inversion in >3 precordial leads (124/150, 83%), and ≥1000 PVCs per 24 hours (64/80, 80%; Table 1). The mean CL of the arrhythmia at the first appropriate event was 278±59.8 ms (range: 178–545 ms). The median time from ICD implantation to first appropriate therapy was 5.9 months (range: 0.03–252 months). The majority (119, 64%) of initial appropriate ICD therapies occurred in the first year, with 63 in the first 3 months after implant. A total of 84 of 124 patients (68%) with available records were engaged in exercise at the time of first appropriate ICD intervention. At the time of first ICD intervention, 120 of 173 patients (70%) with available records were taking the following medications: β‐blockers, 73 (61%); class I or III antiarrhythmic agents, 38 (32%); and a combination of both, 9 (8%). In addition, we observed that among those who had an appropriate ICD therapy, VT storms occurred in 82 patients (44%). The median duration from ICD implant to the first VT storm was 27 months (range: 0.03–275 months).
Figure 1.
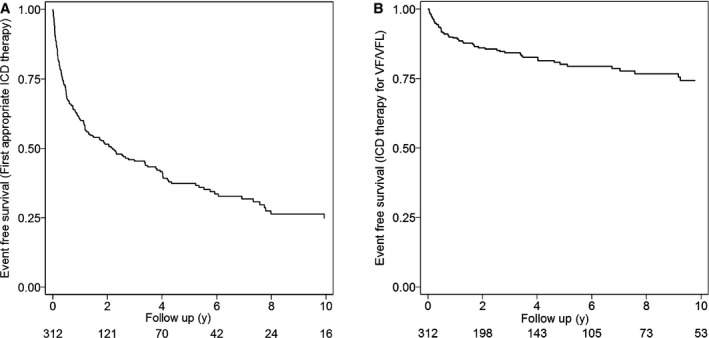
Appropriate ICD therapy in arrhythmogenic right ventricular dysplasia/cardiomyopathy patients. Kaplan–Meier analysis of cumulative survival free from any appropriate ICD interventions (A) and from ICD intervention for VF/VFL (B). ICD indicates implantable cardioverter‐defibrillator; VF/VFL, ventricular fibrillation/flutter.
Predictors of First Appropriate ICD Therapy
Table 2 and Table S3 show the variables analyzed as potential predictors of first appropriate ICD intervention (VT/VF). Univariate predictors of appropriate ICD intervention were history of a sustained VT at presentation (hazard ratio [HR]: 1.86; 95% confidence interval [CI], 1.38–2.49; P<0.001), inducibility on EPS (HR: 3.14; 95% CI, 1.95–5.05; P<0.001), male sex (HR: 1.62; 95% CI, 1.20–2.19; P=0.001), inverted T waves in ≥3 precordial leads (HR: 1.66; 95% CI, 1.09–2.52; P=0.018), and PVC count ≥1000/24 hours (HR: 2.30; 95% CI, 1.32–4.00; P=0.003). Only inducibility on EPS (HR: 2.28; 95% CI, 1.10–4.70; P=0.025) was found to be an independent predictor of any appropriate ICD therapy on multivariable analysis. Figure 2A through 2F shows Kaplan–Meier analysis of freedom from any ICD intervention stratified by inducibility on EPS, sex, number of T‐wave inversions on ECG, PVC count, history of VT at presentation, and primary versus secondary prevention (VT/VF).
Table 2.
Predictors Appropriate ICD Intervention for VT/VF and for ICD Therapy for VF/VFL (CL ≤240 ms)
| Variable | First Appropriate Therapy (VT/VF) | VF/VFL (CL ≤240 ms) | ||||||
|---|---|---|---|---|---|---|---|---|
| Univariate Analysis | Multivariable Analysis | Univariate Analysis | Multivariable Analysis | |||||
| HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | HR (95% CI) | P Value | |
| History of VT at presentation | 1.86 (1.38–2.49) | <0.001 | 1.18 (0.64–2.18) | 0.592 | 0.73 (0.43–1.23) | 0.244 | ··· | ··· |
| Inducibility at EPS | 3.14 (1.95–5.05) | <0.001 | 2.28 (1.10–4.70) | 0.025 | 2.65 (0.94–7.44) | 0.063 | ··· | ··· |
| Male sex | 1.62 (1.20–2.19) | 0.001 | 1.34 (0.74–2.40) | 0.325 | 1.73 (1.01–2.97) | 0.046 | 1.76 (0.81–3.84) | 0.155 |
| Inverted T waves in ≥3 precordial leads | 1.66 (1.09–2.52) | 0.018 | 1.49 (0.77–2.88) | 0.232 | 1.26 (0.61–2.62) | 0.531 | ··· | ··· |
| PVCs ≥1000/24 h on Holter monitoring | 2.30 (1.32–4.00) | 0.003 | 1.30 (0.68–2.49) | 0.414 | 4.39 (1.32–14.61) | 0.016 | 4.43 (1.35–14.57) | 0.014 |
| Major RV structural abnormality | 1.49 (0.98–2.28) | 0.065 | ··· | ··· | 1.26 (0.61–2.58) | 0.536 | ··· | ··· |
| Syncope | 1.19 (0.87–1.62) | 0.271 | ··· | ··· | 1.85 (1.10–3.11) | 0.021 | 2.05 (0.96–4.39) | 0.064 |
| Mutation carrier | 1.17 (0.87–1.58) | 0.298 | ··· | ··· | 1.14 (0.66–1.97) | 0.633 | ··· | ··· |
| NSVT | 1.09 (0.81–1.48) | 0.572 | ··· | ··· | 1.45 (0.86–2.44) | 0.163 | ··· | ··· |
| History of VF at presentation | 1.22 (0.64–2.29) | 0.534 | ··· | ··· | 1.63 (0.63–4.18) | 0.307 | ··· | ··· |
| Age at presentation ≤30 y | 1.03 (0.77–1.38) | 0.832 | ··· | ··· | 1.76 (1.04–3.00) | 0.036 | 3.14 (1.32–7.48) | 0.010 |
CI indicates confidence interval; CL, cycle length; EPS, electrophysiology study; HR, hazard ratio; ICD, implantable cardioverter‐defibrillator; NSVT, nonsustained ventricular tachycardia; PVC, premature ventricular contraction; RV, right ventricular; VF, ventricular fibrillation; VFL, ventricular flutter; VT, ventricular tachycardia.
Figure 2.
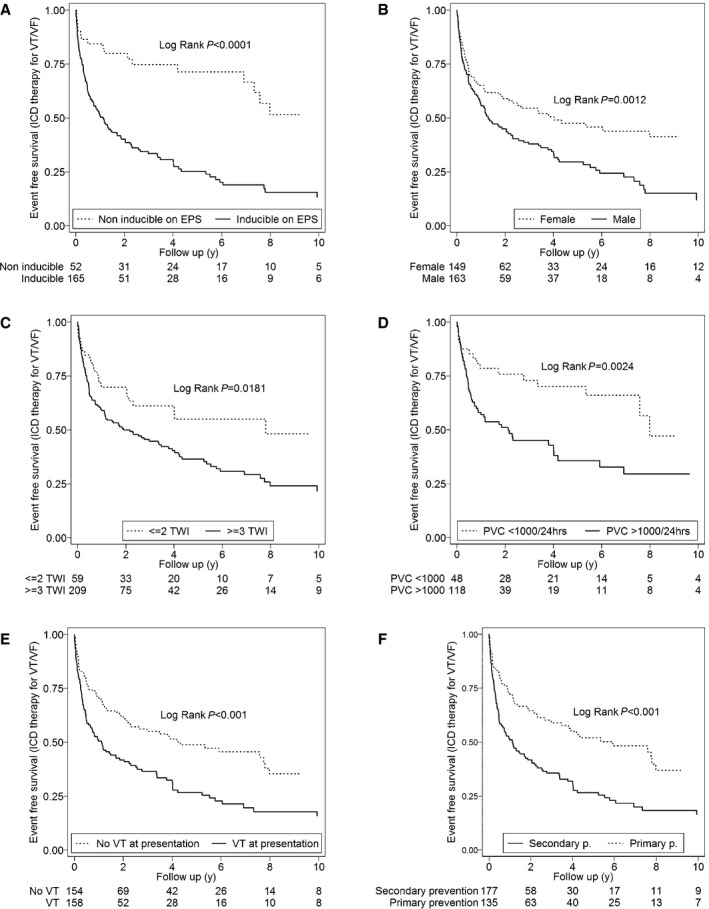
Cumulative survival from first appropriate ICD intervention (VT/VF) stratified by inducibility on EPS (A), sex (B), number of TWIs (C), PVCs on Holter monitor (D), history of VT at presentation (E), and primary vs secondary prevention (VT/VF) (F). EPS indicates electrophysiology study; ICD, implantable cardioverter‐defibrillator; PVC, premature ventricular contraction; TWI, T‐wave inversion; VT/VF, ventricular tachycardia/ventricular fibrillation.
ICD Therapy for VF/VFL (CL ≤240 ms)
Figure 1B shows the Kaplan–Meier analysis of cumulative survival from an ICD therapy for VF/VFL (CL ≤240 ms). The cumulative survival free from events was 89%, 85%, 79%, and 73% at 1, 2, 5, 10 years of follow‐up, respectively. An appropriate ICD intervention for VF/VFL was observed in 58 patients (19%). The majority of patients in this group were male (38, 66%) and probands (53, 91%). The mean age at presentation was 28±13.10 years, with approximately two thirds of patients who received an ICD therapy for VF/VFL presenting at ≤30 years of age (n=36; Figure S1). In addition, 27 of 30 (90%) had ≥1000 PVC/24 hours on Holter monitoring, and 33 of 37 (89%) were inducible for sustained ventricular arrhythmias on EPS (Table 1). The median time from ICD implantation to first ICD therapy for VF/VFL therapy was 9.1 months (range: 0.5–112.5 months). The CL for the first VF/VFL episode was 220±15.1 ms (range: 178–240 ms). The majority of patients (27/44, 61%) with available information for level of activity at first ICD therapy for VF/VFL were exercising at that time. In addition, 40 of 52 patients (77%) with available records were taking β‐blockers (24, 60%), class I or III antiarrhythmic drugs (12, 30%), or a combination of both medications (4, 10%) when the therapy occurred.
Predictors of ICD Therapy for VF/VFL (CL ≤240 ms)
The variables analyzed as potential predictors of appropriate ICD therapies for VF/VFL are shown in Table 2 and Table S3. Univariate predictors of appropriate ICD interventions were PVCs ≥1000/24 hours on Holter monitoring (HR: 4.39; 95% CI, 1.32–14.61; P=0.016), syncope (HR: 1.85; 95% CI, 1.10–3.11; P=0.021), age at presentation ≤30 years (HR: 1.76; 95% CI, 1.04–3.00; P=0.036), and male sex (HR: 1.73; 95% CI, 1.01–2.97; P=0.046). After multivariable analysis, age ≤30 years at presentation (HR: 3.14; 95% CI, 1.32–7.48; P=0.010) and PVC count ≥1000/24 hours (HR: 4.43; 95% CI, 1.35–14.57; P=0.014) remained as significant predictors of appropriate ICD interventions for VF/VFL. Figure 3A through 3F shows Kaplan–Meier analysis of freedom from ICD intervention stratified by sex, PVC count, syncope, age at presentation, history of VT at presentation, and primary versus secondary prevention (VT/VF).
Figure 3.
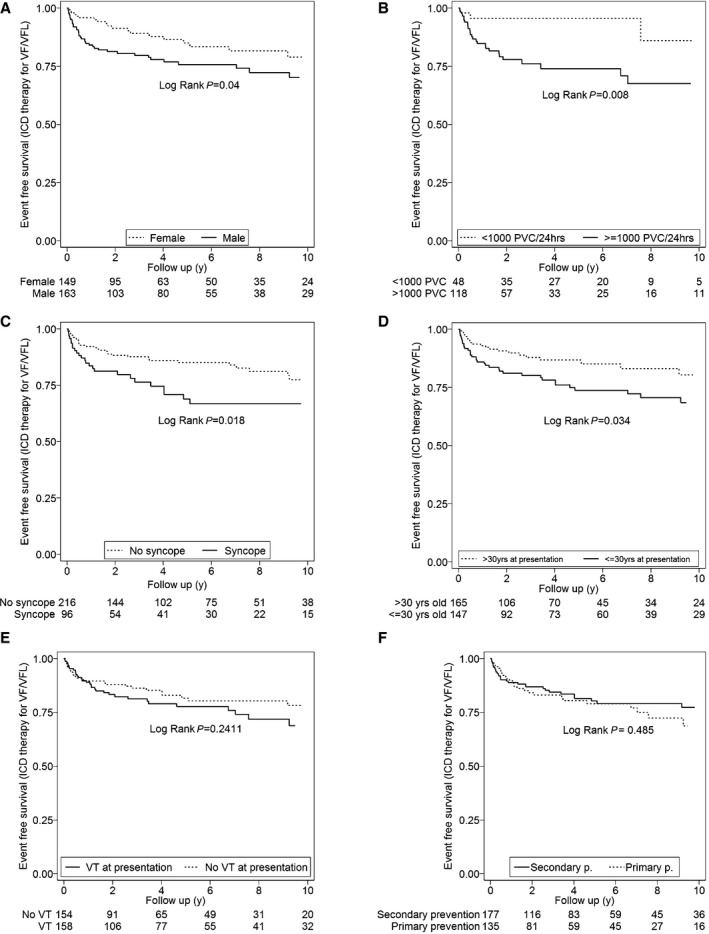
Cumulative survival from ICD intervention for VF/VFL (cycle length ≤240 ms) stratified by sex (A), PVCs on Holter monitor (B), syncope (C), age at presentation (D), history of VT at presentation (E), and primary vs secondary prevention (VT/VF) (F). ICD indicates implantable cardioverter defibrillator; PVC, premature ventricular contraction; VF/VFL, ventricular fibrillation/flutter; VT/VF, ventricular tachycardia/ventricular fibrillation.
In addition, Figure 3E and Figure S2 show that a history of sustained VT at presentation, although a predictor of all ICD therapies, was not a significant predictor of VF/VFL at follow‐up.
Inappropriate Interventions and Complications
In this study, we will separately report the rates of complications and inappropriate intervention. A total of 193 inappropriate ICD interventions were observed in 64 patients (21%). The majority (102, 53%) of inappropriate therapies occurred in patients with single‐chamber ICDs, with 85 (44%) in those with dual‐chamber devices, and 6 (3%) inappropriate shocks occurred in 1 patient with a subcutaneous ICD that eventually required replacement with a transvenous device. The median time from ICD implantation to first inappropriate intervention was 1.68 years (range: 0.01–19.8 years). The inappropriate discharges were due to sinus tachycardia (n=29), other type of supraventricular arrhythmia (n=28), inappropriate sensing (n=4), or lead malfunction (n=3).
Table 3 shows the ICD‐related complications in our patient population. There were 98 lead‐ or device‐related complications in 66 patients (21%). Among the 98 complications, 66 (67%) were lead related and the remainder were generator related (32, 33%). Six (6%) patients experienced decreased sensing on the right ventricular lead, requiring revision or replacement.
Table 3.
ICD–Related Complications
| Complication | Number (n=98) |
|---|---|
| Lead‐related complications (n=68) | |
| Lead fracture | 19 (19) |
| Decreased sensing on the RV | 6 (6) |
| Lead dislodgment | 8 (8) |
| Lead recall | 12 (12) |
| Subclavian/IJ vein thrombosis | 2 (2) |
| Lead malfunctiona | 19 (19) |
| Tamponade | 2 (2) |
| Generator‐related complications (n=30) | |
| Hematoma | 1 (1) |
| Generator recall | 7 (7) |
| Generator malposition | 4 (4) |
| Premature battery depletion | 2 (2) |
| Infection | 4 (4) |
| Generator malfunctionb | 11 (11) |
| Device explantation due to chronic pain | 1 (1) |
All complications listed required surgical intervention with the exception of venous thrombosis. ICD indicates implantable cardioverter‐defibrillator; IJ, internal jugular; RV, right ventricle.
RV lead showing high impedance (n=2), decreased sensing/high pacing threshold on right atrium lead (n=2), lead revision due to malposition (n=1), lead noise (n=8), concern for lead related proarrhythmia (n=2), lead revision/replacement for other reasons or unspecified malfunction issues (n=4).
Grommet torn from the header (n=1), generator noise (n=4), generator with prolonged charging times (n=2), failure to deliver a shock during a ventricular fibrillation episode (n=1), high impedance/inconsistent lead measurements (n=1), oversensing with multiple inappropriate shocks (n=1), high defibrillation threshold testing (n=1).
Long‐Term Outcomes and Survival
At last follow‐up, 307 patients (98%) were alive and 5 (2%) had died (1 due to complications after cardiac ablation, 2 related to heart failure, and 2 from noncardiac causes). A total of 12 patients (4%) underwent cardiac transplantation due to progressive heart failure and/or incessant arrhythmias. The age at cardiac transplantation averaged 45.84±8.83 years. The mean time from ICD implantation to cardiac transplant was 9.44±5.92 years.
Discussion
Main Findings
The present study represents the largest experience of ARVD/C patients with ICDs available to date. We present the outcome of a cohort of 312 patients with definite ARVD/C diagnosis per 2010 TFC who received an ICD for primary or secondary prevention of SCD. During a median follow‐up of 7 years (interquartile range: 3–13), 60% of ARVD/C patients received an appropriate ICD therapy. Among the 312 ARVD/C patients reported in this study, nearly 1 in 5 (19%) received potentially lifesaving ICD therapy for VF/VFL. The clinical variables that were identified as predictors of first appropriate ICD therapy were a history of VT at presentation, inducibility at EPS, male sex, inverted T waves in ≥3 precordial leads, and ≥1000 PVCs per 24 hours with inducibility at EPS being the only variable that remained significant after multivariable analysis. Predictors of ICD therapy for VF/VFL (CL ≤240 ms) were PVC count ≥1000/24 hours, syncope, ≤30 years of age at presentation, and male sex. Increased ventricular ectopy burden and younger age at presentation were found to independently increase the risk of ICD interventions for VF/VFL. Of particular note is our finding that a history of sustained VT at presentation, although predictive of an appropriate ICD therapy during follow‐up, was not a significant predictor of VF/VFL. Inappropriate ICD interventions were observed in 64 patients (21%) and ≥1 ICD‐related complication occurred in 66 patients (21%). Despite the high rate of appropriate ICD therapy, the overall mortality of this large cohort of patients was low, with 5 patients (2%) dying during a median follow‐up of 7 years, and 12 (4%) patients undergoing cardiac transplantation.
ICD Therapy in ARVD/C
ARVD/C is an inherited cardiomyopathy characterized by right ventricular dysfunction, ventricular arrhythmias, and SCD. It has been estimated that ARVD/C accounts for 13% to 16% of cases of SCD in children and young adults.1, 7, 8 Because of this markedly increased risk of SCD and sustained ventricular arrhythmias, ICD therapy represents one of the most important therapeutic modalities for patients diagnosed with ARVD/C. Since the first major description of ARVD/C in 1992 by Marcus et al,9 a number of articles have been published describing the clinical features and outcomes of ARVD/C patients who undergo placement of an ICD. Although these articles have provided important information, they also have important limitations including small sample sizes ranging from 26 to 132 patients,10, 11, 12, 13, 14, 15, 16, 17, 18, 19 the use of the 1994 task force diagnostic criteria,10, 11, 12, 13, 14, 16, 20, 21 inclusion of participants who did not fully meet diagnostic criteria for ARVD/C,13, 15 and lack of genetic testing.10, 11, 12, 13, 14, 16, 20, 21 Furthermore, the primary end point in many of these studies was the prediction of appropriate ICD therapy, including both sustained VT and VF/VFL.11, 12, 13, 15, 16, 17, 18, 19, 21 We undertook this study because of these limitations and because of the importance of risk stratification to prevent SCD in patients with ARVD/C.
Demographic Features of ARVD/C Patients Undergoing ICD Implantation
The results of this study provide important data concerning the demographic features of ARVD/C patients undergoing ICD implantation in the United States. Among the 312 ARVD/C patients in this series, more than half were male and the majority were probands. ICDs were implanted for secondary prevention in 57% of these patients. The median age at ICD implantation was 36.5 years. The demographic features of patients undergoing ICD implantation in this study are generally similar to those in prior reports.10, 11, 12, 13, 15, 16, 17, 18, 21 Nevertheless, this study is unique in that only patients who met diagnostic criteria were included, and a greater proportion of our ARVD/C population underwent ICD implantation for primary prevention than has been reported in prior series.10, 11, 12, 13, 16, 17, 18, 21 In addition, this cohort includes a lower proportion of male patients than most series of arrhythmogenic right ventricular cardiomyopathy patients with ICDs,10, 11, 14, 17, 20 and the median age at ICD implantation in our series was somewhat higher than in prior studies.17, 21
Incidence and Predictors of Sustained Ventricular Arrhythmias
The results of this study revealed that patients with ARVD/C who undergo ICD implantation for primary or secondary prevention have a remarkably high incidence of appropriate ICD therapies both for any sustained ventricular arrhythmia (22% annually) and for VF/VFL (3.6% annually). The clinical variables that were identified as predictors of any appropriate ICD therapy were a history of sustained VT at presentation, inducibility at EPS, male sex, ≥3 T‐wave inversions in ECG, and a PVC count ≥1000/24 hours. After multivariable analysis, inducibility at EPS was the only variable that remained as an independent risk factor for any ICD therapy.
These results confirm and extend the results of prior studies of ICD implantation in patients with ARVD/C. Although many of our findings are similar to those of prior reports, several are either new or differ from prior reports. In accordance with previous reports,10, 11, 12, 13, 16, 17, 18, 19, 20, 21 in which appropriate ICD interventions range from 34% to 78%, the results of our study revealed that 60% of patients had an appropriate ICD therapy on follow‐up, with the first ICD intervention occurring within a year of implantation in more than two thirds. The proportion of patients with a primary‐prevention ICD who experienced a sustained ventricular arrhythmia was 45% in our series, which is somewhat higher than has been described previously (24%).14
Several studies12, 13, 15 have reported that inducibility of sustained ventricular arrhythmias on EPS is a predictor of ICD therapy. Bhonsale et al15 showed that it is an independent predictor of ICD therapy in patients with ICDs implanted for primary prevention. Our findings confirm and extend these results, showing that EPS inducibility can provide considerable prognostic information for risk‐stratification purposes when evaluating patients with ARVD/C.
In addition, our results are consistent with previous studies13, 19, 20 that have reported that a history of sustained VT and/or VF are risk factors for having appropriate ICD therapy at follow‐up. Our results are also consistent with the results of prior studies that have reported that men are at higher arrhythmia risk than women.17, 19, 22, 23 Finally, our results are also consistent with prior studies that have reported that T‐wave inversions on precordial leads of the ECG13, 15, 20 and a PVC count of ≥1000 on 24‐hour Holter15 are predictors of appropriate ICD therapy for VT/VF. The design and findings of the present study allowed us to extend a prior observation15 that PVC frequency predicts appropriate ICD therapy in primary‐prevention ARVD/C patients compared with those undergoing ICD implantation for secondary prevention.
Incidence and Predictors of VF/VFL (CL ≤240 ms)
A main objective of this study was to define the incidence of VF/VFL (CL ≤240 ms) and identify factors that predict these malignant arrhythmias in ARVD/C patients. The results of this study revealed that a high PVC burden, history of syncope, proband status, ≤30 years old at presentation, and male sex were significant predictors of VF/VFL therapy, with PVC burden and younger age at presentation emerging as independent risk factors on multivariable analysis.
A review of the literature reveals that 3 prior studies have previously reported the incidence and predictors of VF/VFL. The first and largest of these studies consisted of 132 patients and was published in 2003 by Corrado et al.10 Similar to our study, the authors reported an annual incidence of VF/VFL of 3.3% per year, which is remarkably similar to the rate of 3.6% per year reported in our study. In addition, similar to the results of our study, they found that younger age and syncope were predictors of VF/VFL. A prior history of sustained VT or VF was another predictor of VF/VFL. A unique feature of our study was that we wanted to determine whether presentation with sustained VT (not VF/VFL) was predictive of a higher risk of VF/VFL during follow‐up. We found that sustained VT was not a predictor of VF/VFL. To the best of our knowledge, this study is the first to examine this relationship.
In the second study looking at predictors of VF/VFL,14 syncope was the only independent predictor of VF/VFL therapy in a primary‐prevention cohort. A recently published study19 looking at a cohort of ARVD/C patients that had ICDs predominantly for primary prevention of SCD (68/81, 84%) also found that syncope independently increased the risk of life‐threatening arrhythmic events, defined as SCD, aborted cardiac arrest, syncopal VT or electrical storm, or cardiovascular mortality. In the case of the present cohort, syncope was found to be a predictor of therapy for VF/VFL on univariate analysis but did not emerge as an independent risk factor on multivariable analysis.
In addition, Link et al20 showed that younger age at the time of ICD implantation is an independent factor for ICD therapy for VF/VFL; this is consistent with our findings. Furthermore, Mazzanti et al19 reported that patients aged between 21 and 40 years had higher risk of experiencing life‐threatening arrhythmic events.
Finally, our study demonstrates, for the first time, that a Holter‐monitor PVC count ≥1000/24 hours is an independent predictor of VF/VFL (CL ≤240 ms). This suggests that a high PVC burden might be a marker of electrical instability. This finding supports the role of Holter monitoring as a widely available and inexpensive, noninvasive way to obtain valuable information for the risk stratification of patients with ARVD/C.
Complications and Inappropriate ICD Therapies
One or more complications of ICD therapy occurred in 66 patients (21%) in this study. This high incidence of ICD‐related complications confirms the findings of prior studies.10, 12, 13, 14, 15, 19 Furthermore, a recently published meta‐analysis2 looking at ICD‐related complications in patients with inherited arrhythmia syndromes reported that 125 of 535 patients (24%) with ARVD/C have an ICD‐related complication.
Inappropriate ICD interventions were observed in 64 patients (21%). This finding is also similar to the rate of inappropriate ICD therapy reported in prior studies that have reported rates of inappropriate ICD therapy, ranging from 16% to 24%.2, 10, 11, 12, 13, 14, 15 This shows that the accurate identification of patients at high risk of SCD is critical not only to save lives but to protect low‐risk persons from potential complications and unnecessary ICD therapies.
Survival and Transplantation
The overall mortality of this large cohort of patients was low, with 5 patients (2%) dying during a median follow‐up of 7 years. A total of 12 patients (4%) underwent cardiac transplantation. The age at first symptom averaged 25±11.25 years, and the age at cardiac transplantation averaged 45.84±8.83 years. These findings are consistent with prior reports.9, 11, 13, 15, 16
Study Limitations
The present study has several limitations that need to be considered when interpreting its results. First, the ARVD/C patients in this study were not an unselected cohort of ARVD/C patients but rather patients who were clinically assessed by their clinical cardiologist or electrophysiologist to have a sufficiently high arrhythmic risk to warrant ICD implantation. Consequently, the reported rate of arrhythmic events does not necessarily correspond to the true arrhythmic risk of an unselected general cohort of ARVD/C patients. This limitation also affects our ability to determine the sensitivity and specificity of all diagnostic tests, including EPS, in predicting arrhythmia risk because the treating cardiologists may have elected not to implant an ICD in some patients with a “positive” or “negative” test. Second, programming of ICDs was performed at the discretion of the implanting physician, often before publication of the Multicenter Automatic Defibrillator Implantation Trial ‐ Reduce Inappropriate Therapy (MADIT‐RIT) study. This approach to ICD programming likely resulted in an overestimation of the frequency of appropriate ICD therapies that would be anticipated today as a result of the revised ICD programming parameters that resulted from the results of the MADIT‐RIT study. Third, the survival benefit of ICD therapy was assessed assuming that VF/VFL would have been fatal in all cases without shock therapy. We recognize that this may overestimate the true benefit of ICD therapy. Fourth, we did not have complete information on the CL of treated arrhythmias in all patients. When intracardiac tracings were unavailable for review, we relied on the interpretation of the treating electrophysiologist or cardiologist with regard to the appropriateness of the ICD intervention. Last, not all patients had complete diagnostic testing performed. As noted in the results section of the study, ECGs, Holters, magnetic resonance images, and EPS at the time of ICD implant were available for 78%, 71%, 62%, and 76% of patients, respectively. This is a limitation of our study and affects the power of our study to identify the relative role of these tests in risk‐stratifying patients with ARVD/C. Although this is a limitation of our study, it is important to recognize that this is a limitation of all prior studies of ICD therapy in arrhythmogenic right ventricular cardiomyopathy therapy. This could potentially affect the precision of the HR estimates with resultant wide CIs due to a reduced number during multivariable modeling. In addition, we did not have complete details on the stimulation protocol and/or isoproterenol dose used during the EPS.
Conclusion
This study reports the outcomes of the largest series of ARVD/C patients to undergo ICD implantation. The results of this study provide important data concerning the outcomes of ICD therapy in patients with ARVD/C that should ultimately help guide clinicians in their recommendation for ICD implantation in patients with ARVD/C. The high rate of appropriate ICD therapy for VF/VFL is an important reminder of the lethal nature of ARVD/C. Variables that clinicians should consider to be markers of high risks for a malignant ventricular arrhythmia (VF/VFL) include high PVC burden, history of syncope, younger age at presentation, and male sex.
Sources of Funding
The authors wish to acknowledge funding from the Dr Francis P. Chiaramonte Private Foundation, the St Jude Medical Foundation, and Boston Scientific Corp. The Johns Hopkins ARVD/C Program is supported by the Leyla Erkan Family Fund for ARVD/C Research, the Dr. Satish, Rupal, and Robin Shah ARVD/C Fund at Johns Hopkins, the Bogle Foundation, the Healing Hearts Foundation, the Campanella Family, the Patrick J. Harrison Family, the Peter French Memorial Foundation, and the Wilmerding Endowments.
Disclosures
Dr Calkins is a consultant for Medtronic Inc. Dr Calkins receives research support from the St Jude Medical Foundation and Boston Scientific Corp. Tichnell and James receive salary support from these grants. Dr Riele receives research support from the Dutch Heart foundation. The remaining authors have no disclosures to report.
Supporting information
Table S1. The 2010 Revised Task Force Criteria in 312 Patients With Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy
Table S2. Details of Individual Mutations Identified in the Study Population
Table S3. Predictors Appropriate Implantable Cardioverter‐Defibrillator Intervention for VT/VF and for VF/VFL (Cycle Length ≤240 ms)*
Figure S1. A and B, Proportion of patients with implantable cardioverter‐defibrillators for primary and secondary prevention (ventricular tachycardia/ventricular fibrillation [VT/VF]) that develop VF/VFL (cycle length [CL] ≥240 ms) at follow up (A). Proportion of patients with and without history of ventricular tachycardia at presentation (VT) that develop ventricular fibrillation/flutter (CL ≥240 ms) at follow up (B).
Figure S2. Age at the time of first implantable cardioverter‐defibrillator intervention for ventricular fibrillation/flutter (cycle length ≥240 ms).
Acknowledgments
We are grateful to the ARVD/C patients and families who have made this work possible.
(J Am Heart Assoc. 2017;6:e006242 DOI: 10.1161/JAHA.117.006242.)28588093
References
- 1. Corrado D, Link MS, Calkins H. Arrhythmogenic right ventricular cardiomyopathy. N Engl J Med. 2017;376:61–72. [DOI] [PubMed] [Google Scholar]
- 2. Olde Nordkamp LR, Postema PG, Knops RE, van Dijk N, Limpens J, Wilde AA, de Groot JR. Implantable cardioverter‐defibrillator harm in young patients with inherited arrhythmia syndromes: a systematic review and meta‐analysis of inappropriate shocks and complications. Heart Rhythm. 2016;13:443–454. [DOI] [PubMed] [Google Scholar]
- 3. McKenna WJ, Thiene G, Nava A, Fontaliran F, Blomstrom‐Lundqvist C, Fontaine G, Camerini F. Diagnosis of arrhythmogenic right ventricular dysplasia/cardiomyopathy. Br Heart J. 1994;71:215–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Marcus FI, McKenna WJ, Sherrill D, Basso C, Bauce B, Bluemke DA, Calkins H, Corrado D, Cox MG, Daubert JP, Fontaine G, Gear K, Hauer R, Nava A, Picard MH, Protonotarios N, Saffitz JE, Sanborn DM, Steinberg JS, Tandri H, Thiene G, Towbin JA, Tsatsopoulou A, Wichter T, Zareba W. Diagnosis of arrhythmogenic right ventricular cardiomyopathy/dysplasia: proposed modification of the Task Force Criteria. Eur Heart J. 2010;31:806–814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Maron BJ, Shen WK, Link MS, Epstein AE, Almquist AK, Daubert JP, Bardy GH, Favale S, Rea RF, Boriani G, Estes NA III, Spirito P. Efficacy of implantable cardioverter‐defibrillators for the prevention of sudden death in patients with hypertrophic cardiomyopathy. N Engl J Med. 2000;342:365–373. [DOI] [PubMed] [Google Scholar]
- 6. Credner SC, Klingenheben T, Mauss O, Sticherling C, Hohnloser SH. Electrical storm in patients with transvenous implantable cardioverter‐defibrillators: incidence, management and prognostic implications. J Am Coll Cardiol. 1998;32:1909–1915. [DOI] [PubMed] [Google Scholar]
- 7. Bagnall RD, Weintraub RG, Ingles J, Duflou J, Yeates L, Lam L, Davis AM, Thompson T, Connell V, Wallace J, Naylor C, Crawford J, Love DR, Hallam L, White J, Lawrence C, Lynch M, Morgan N, James P, du Sart D, Puranik R, Langlois N, Vohra J, Winship I, Atherton J, McGaughran J, Skinner JR, Semsarian C. A prospective study of sudden cardiac death among children and young adults. N Engl J Med. 2016;374:2441–2452. [DOI] [PubMed] [Google Scholar]
- 8. Finocchiaro G, Papadakis M, Robertus JL, Dhutia H, Steriotis AK, Tome M, Mellor G, Merghani A, Malhotra A, Behr E, Sharma S, Sheppard MN. Etiology of sudden death in sports: insights from a United Kingdom regional registry. J Am Coll Cardiol. 2016;67:2108–2115. [DOI] [PubMed] [Google Scholar]
- 9. Marcus FI, Fontaine GH, Guiraudon G, Frank R, Laurenceau JL, Malergue C, Grosgogeat Y. Right ventricular dysplasia: a report of 24 adult cases. Circulation. 1982;65:384–398. [DOI] [PubMed] [Google Scholar]
- 10. Corrado D, Leoni L, Link MS, Della Bella P, Gaita F, Curnis A, Salerno JU, Igidbashian D, Raviele A, Disertori M, Zanotto G, Verlato R, Vergara G, Delise P, Turrini P, Basso C, Naccarella F, Maddalena F, Estes NA III, Buja G, Thiene G. Implantable cardioverter‐defibrillator therapy for prevention of sudden death in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia. Circulation. 2003;108:3084–3091. [DOI] [PubMed] [Google Scholar]
- 11. Wichter T, Paul M, Wollmann C, Acil T, Gerdes P, Ashraf O, Tjan TD, Soeparwata R, Block M, Borggrefe M, Scheld HH, Breithardt G, Böcker D. Implantable cardioverter/defibrillator therapy in arrhythmogenic right ventricular cardiomyopathy: single‐center experience of long‐term follow‐up and complications in 60 patients. Circulation. 2004;109:1503–1508. [DOI] [PubMed] [Google Scholar]
- 12. Roguin A, Bomma CS, Nasir K, Tandri H, Tichnell C, James C, Rutberg J, Crosson J, Spevak PJ, Berger RD, Halperin HR, Calkins H. Implantable cardioverter‐defibrillators in patients with arrhythmogenic right ventricular dysplasia/cardiomyopathy. J Am Coll Cardiol. 2004;43:1843–1852. [DOI] [PubMed] [Google Scholar]
- 13. Piccini JP, Dalal D, Roguin A, Bomma C, Cheng A, Prakasa K, Dong J, Tichnell C, James C, Russell S, Crosson J, Berger RD, Marine JE, Tomaselli G, Calkins H. Predictors of appropriate implantable defibrillator therapies in patients with arrhythmogenic right ventricular dysplasia. Heart Rhythm. 2005;2:1188–1194. [DOI] [PubMed] [Google Scholar]
- 14. Corrado D, Calkins H, Link MS, Leoni L, Favale S, Bevilacqua M, Basso C, Ward D, Boriani G, Ricci R, Piccini JP, Dalal D, Santini M, Buja G, Iliceto S, Estes NA III, Wichter T, McKenna WJ, Thiene G, Marcus FI. Prophylactic implantable defibrillator in patients with arrhythmogenic right ventricular cardiomyopathy/dysplasia and no prior ventricular fibrillation or sustained ventricular tachycardia. Circulation. 2010;122:1144–1152. [DOI] [PubMed] [Google Scholar]
- 15. Bhonsale A, James CA, Tichnell C, Murray B, Gagarin D, Philips B, Dalal D, Tedford R, Russell SD, Abraham T, Tandri H, Judge DP, Calkins H. Incidence and predictors of implantable cardioverter‐defibrillator therapy in patients with arrhythmogenic right ventricular dysplasia/cardiomyopathy undergoing implantable cardioverter‐defibrillator implantation for primary prevention. J Am Coll Cardiol. 2011;58:1485–1496. [DOI] [PubMed] [Google Scholar]
- 16. Dalal D, Nasir K, Bomma C, Prakasa K, Tandri H, Piccini J, Roguin A, Tichnell C, James C, Russell SD, Judge DP, Abraham T, Spevak PJ, Bluemke DA, Calkins H. Arrhythmogenic right ventricular dysplasia: a United States experience. Circulation. 2005;112:3823–3832. [DOI] [PubMed] [Google Scholar]
- 17. Martin A, Crawford J, Skinner JR, Smith W. High arrhythmic burden but low mortality during long‐term follow‐up in arrhythmogenic right ventricular cardiomyopathy. Heart Lung Circ. 2016;25:275–281. [DOI] [PubMed] [Google Scholar]
- 18. Schuler PK, Haegeli LM, Saguner AM, Wolber T, Tanner FC, Jenni R, Corti N, Lüscher TF, Brunckhorst C, Duru F. Predictors of appropriate ICD therapy in patients with arrhythmogenic right ventricular cardiomyopathy: long term experience of a tertiary care center. PLoS One. 2012;7:e39584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Mazzanti A, Ng K, Faragli A, Maragna R, Chiodaroli E, Orphanou N, Monteforte N, Memmi M, Gambelli P, Novelli V, Bloise R, Catalano O, Moro G, Tibollo V, Morini M, Bellazzi R, Napolitano C, Bagnardi V, Priori SG. Arrhythmogenic right ventricular cardiomyopathy: clinical course and predictors of arrhythmic risk. J Am Coll Cardiol. 2016;68:2540–2550. [DOI] [PubMed] [Google Scholar]
- 20. Link MS, Laidlaw D, Polonsky B, Zareba W, McNitt S, Gear K, Marcus F, Estes NA III. Ventricular arrhythmias in the North American multidisciplinary study of ARVC: predictors, characteristics, and treatment. J Am Coll Cardiol. 2014;64:119–125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Marcus GM, Glidden DV, Polonsky B, Zareba W, Smith LM, Cannom DS, Estes NA III, Marcus F, Scheinman MM; Multidisciplinary Study of Right Ventricular Dysplasia Investigators . Efficacy of antiarrhythmic drugs in arrhythmogenic right ventricular cardiomyopathy: a report from the North American ARVC Registry. J Am Coll Cardiol. 2009;54:609–615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bhonsale A, James CA, Tichnell C, Murray B, Madhavan S, Philips B, Russell SD, Abraham T, Tandri H, Judge DP, Calkins H. Risk stratification in arrhythmogenic right ventricular dysplasia/cardiomyopathy‐associated desmosomal mutation carriers. Circ Arrhythm Electrophysiol. 2013;6:569–578. [DOI] [PubMed] [Google Scholar]
- 23. Rigato I, Bauce B, Rampazzo A, Zorzi A, Pilichou K, Mazzotti E, Migliore F, Marra MP, Lorenzon A, De Bortoli M, Calore M, Nava A, Daliento L, Gregori D, Iliceto S, Thiene G, Basso C, Corrado D. Compound and digenic heterozygosity predicts lifetime arrhythmic outcome and sudden cardiac death in desmosomal gene‐related arrhythmogenic right ventricular cardiomyopathy. Circ Cardiovasc Genet. 2013;6:533–542. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1. The 2010 Revised Task Force Criteria in 312 Patients With Arrhythmogenic Right Ventricular Dysplasia/Cardiomyopathy
Table S2. Details of Individual Mutations Identified in the Study Population
Table S3. Predictors Appropriate Implantable Cardioverter‐Defibrillator Intervention for VT/VF and for VF/VFL (Cycle Length ≤240 ms)*
Figure S1. A and B, Proportion of patients with implantable cardioverter‐defibrillators for primary and secondary prevention (ventricular tachycardia/ventricular fibrillation [VT/VF]) that develop VF/VFL (cycle length [CL] ≥240 ms) at follow up (A). Proportion of patients with and without history of ventricular tachycardia at presentation (VT) that develop ventricular fibrillation/flutter (CL ≥240 ms) at follow up (B).
Figure S2. Age at the time of first implantable cardioverter‐defibrillator intervention for ventricular fibrillation/flutter (cycle length ≥240 ms).


