Abstract
In this issue of Blood, Nagel et al provide evidence supporting the presence of premalignant leukemic clones in nonlymphoid compartments of patients with BCR-ABL1–positive B-cell precursor acute lymphoblastic leukemia (ALL) as a cause of lineage switch after CD19 immunotherapy, extending the lineage switch mechanism beyond mixed-lineage leukemia (MLL)-rearranged ALL.1 This observation suggests that CD19-targeted therapies alone may not suffice in the eradication of underlying disease in BCR-ABL1 p210- and p190-positive patients, and that strategies to target the stem cell compartment may be needed for durable remissions in this high-risk population.
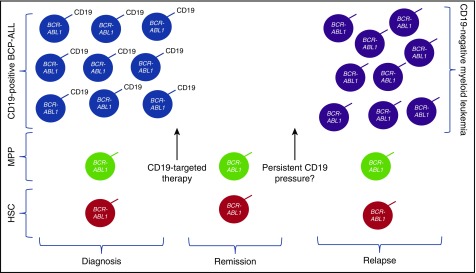
Potential mechanism of relapse after CD19-targeted immunotherapy in patients with Ph+ ALL and BCR-ABL fusion in the CD19+ leukemic compartment, as well as multipotent progenitor (MPP) cells and/or other hematopeietic stem cell (HSC) progenitor compartments. With anti-CD19 immune pressure and eradication of CD19+ leukemia, CD19– myeloid phenotype leukemia can emerge, likely redifferentiated from the progenitor populations. Whether persistence of anti-CD19 pressure will increase the likelihood of this mechanism of resistance remains a question.
With the advent of successful CD19-targeted immunotherapies, such as blinatumomab (CD19/CD3 bispecific T-cell engager) and CD19-directed chimeric antigen receptor (CAR) T cells, the emergence of leukemic resistance due to loss of CD19 is increasingly recognized as a limitation that impacts the durability of remissions in up to 30% to 40% of patients receiving anti-CD19 therapy. The primary mechanism of CD19 antigen loss seems to involve alternative splicing that results in loss of the targeted epitope.2 There is some evidence that this alternatively spliced CD19 exists prior to CD19 immunotherapy in some patients.3 An alternative cause of leukemic resistance is a lineage switch, identified predominantly in patients with MLL-rearranged ALL.4,5 Although lineage switch is a much rarer cause of resistance, aspects of leukemia biology may provide insights into possible strategies to improve the durability of remissions induced by immune-based therapies.
As pointed out by Nagel et al, the presence of leukemic clones in the stem cell compartments in subtypes of Philadelphia chromosome–positive (Ph+) pre-B cell ALL has been described previously, as has the emergence of myeloid phenotype relapses of Ph+ ALL. Nagel et al provide relevance to the field of immunotherapy by demonstrating that the existence of BCR-ABL1 fusion-positive hematopoietic stem cells and myeloid progenitors directly contributes to immunotherapeutic resistance. Indeed, the lineage-specificity of CD19-targeted immunotherapy and, possibly, the persistence of the immune pressure in the case of CAR T cells suggest that the lineage phenomenon will be relatively more common than observed in the context of standard cytotoxic agents. Walter et al6 demonstrated a similar pattern of evolution and clonal selection leading from myelodysplastic syndrome to acute myeloid leukemia, and the findings provocatively suggest that leukemogenesis in BCR-ABL1 ALL may predate the development of B-lineage leukemia. Given this paradigm of ancestral populations where BCR-ABL1 is not limited to the CD19-expressing lymphoid compartment alone, B-lineage–targeted therapies may provide only transient benefit, given the potential for the emergence of an antigen-negative population harboring the driver mutation.
Antigen loss after immunotherapy could result from the selection of a fully myeloid minor population or redifferentiation into a myeloid leukemia. The results presented by Nagel et al suggest that, at least for Ph+ ALL, the emergence of a myeloid leukemia occurs as a result of the “selection” of a CD19-negative progenitor cell that then differentiates into a myeloid phenotype (see figure). This mechanism has implications for the development of strategies to improve the durability of immunotherapy-induced remissions. One approach is through the use of combinatorial immunotherapeutic strategies, not dissimilar to the combinatorial chemotherapy approach that is well established in the treatment of acute leukemia. Multiantigen-targeted immunotherapeutic strategies to prevent antigen-negative relapse are in the earliest phases of development,7,8 but, at the present time, are still generally lineage specific. With the development of myeloid leukemia, as seen both in MLL-rearranged patients and now also with BCR-ABL1 populations, there may be a need to consider multilineage-specific targeting.
Another strategy involves a merger of immunotherapy and molecularly targeted therapy. As the prototypic model of a targetable kinase gene fusion, BCR-ABL1 targeting with imatinib, was once consider the “magic bullet” for CML. There has been increasing recognition of other potentially targetable kinases in ALL,9,10 with some proposed to be important to the leukemic stem cell. Treatment strategies to inhibit these kinase pathways are being developed, but activity in bulk disease may be challenging. Thus, the combination of kinase inhibition with immunotherapy, which has been shown to be quite effective at inducing remission even with large leukemic burdens, may be ideal.
So, what does the future hold? With the goal of developing novel, potentially curative therapies in a high percentage of patients, it is clear that single-antigen immunotherapeutic targeting may not be sufficient. The work presented by Nagel and colleagues elegantly highlights that this will best be achieved by returning to science to rationally design approaches to eradicate all malignant cells in a patient.
Footnotes
Conflict-of-interest disclosure: The authors declare no competing financial interests.
REFERENCES
- 1.Nagel I, Bartels M, Duell J, et al. . Hematopoietic stem cell involvement in BCR-ABL1–positive ALL as a potential mechanism of resistance to blinatumomab therapy. Blood. 2017;130(18):2027-2031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sotillo E, Barrett DM, Black KL, et al. . Convergence of acquired mutations and alternative splicing of CD19 enables resistance to CART-19 immunotherapy. Cancer Discov. 2015;5(12):1282-1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Fischer J, Paret C, El Malki K, et al. . CD19 isoforms enabling resistance to CART-19 immunotherapy are expressed in B-ALL patients at initial diagnosis. J Immunother. 2017;40(5):187-195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Jacoby E, Nguyen SM, Fountaine TJ, et al. . CD19 CAR immune pressure induces B-precursor acute lymphoblastic leukaemia lineage switch exposing inherent leukaemic plasticity. Nat Commun. 2016;7:12320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gardner R, Wu D, Cherian S, et al. . Acquisition of a CD19-negative myeloid phenotype allows immune escape of MLL-rearranged B-ALL from CD19 CAR-T-cell therapy. Blood. 2016;127(20):2406-2410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Walter MJ, Shen D, Ding L, et al. . Clonal architecture of secondary acute myeloid leukemia. N Engl J Med. 2012;366(12):1090-1098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Majzner RG, Heitzeneder S, Mackall CL. Harnessing the immunotherapy revolution for the treatment of childhood cancers. Cancer Cell. 2017;31(4):476-485. [DOI] [PubMed] [Google Scholar]
- 8.Ruella M, Barrett DM, Kenderian SS, et al. . Dual CD19 and CD123 targeting prevents antigen-loss relapses after CD19-directed immunotherapies. J Clin Invest. 2016;126(10):3814-3826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Reshmi SC, Harvey RC, Roberts KG, et al. . Targetable kinase gene fusions in high-risk B-ALL: a study from the Children’s Oncology Group. Blood. 2017;129(25):3352-3361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Roberts KG, Li Y, Payne-Turner D, et al. . Targetable kinase-activating lesions in Ph-like acute lymphoblastic leukemia. N Engl J Med. 2014;371(11):1005-1015. [DOI] [PMC free article] [PubMed] [Google Scholar]