Abstract
Background
Ovarian cancer is the most common gynecological malignancies in women, with high mortality rates worldwide. Tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) is a member of the tumor necrosis factor (TNF) superfamily which preferentially induces apoptosis of cancer cells. However, acquired resistance to TRAIL hampers its therapeutic application. Identification of compounds that sensitize cancer cells to TRAIL is vital in combating resistance to TRAIL. The effect of kaempferol, a flavonoid enhancing TRAIL-induced apoptosis in ovarian cancer cells, was investigated in this study.
Material/Methods
The cytotoxic effects of TRAIL (25 ng/mL) and kaempferol (20–100 μM) on human ovarian cancer cells OVCAR-3 and SKOV-3 were assessed. Effect of kaempferol on the expression patterns of cell survival proteins (Bcl-xL, Bcl-2, survivin, XIAP, c-FLIP) and apoptotic proteins (caspase-3, caspase-8, caspase-9, Bax) were studied. The influence of kaempferol on expression of DR4 and DR5 death receptors on the cell surface and protein and mRNA levels was also analyzed. Apoptosis following silencing of DR5 and CHOP by small interfering RNA (siRNA), and activation of MAP kinases were analyzed as well.
Results
Kaempferol enhanced apoptosis and drastically up-regulated DR4, DR5, CHOP, JNK, ERK1/2, p38 and apoptotic protein expression with decline in the expression of anti-apoptotic proteins. Further transfection with siRNA specific to CHOP and DR5 indicated the involvement of CHOP in DR5 up-regulation and also the contribution of DR5 in kaempferol-enhanced TRAIL-induced apoptosis.
Conclusions
Kaempferol sensitized ovarian cancer cells to TRAIL-induced apoptosis via up-regulation of DR4 and DR5 through ERK/JNK/CHOP pathways.
MeSH Keywords: Apoptosis, MAP Kinase Kinase Kinase 3, Ovarian Neoplasms, Transcription Factor CHOP
Background
Ovarian cancer is most prevalent cancers among women and is the second most frequent gynecological cancer [1]. Ovarian cancer manifests very few symptoms in the initial stages and is usually diagnosed at an advanced stage [2] making treatment of the malignancy difficult [3]. Further, development of chemoresistance also presents a challenge to the clinical management of advanced epithelial ovarian cancer [4]. An effective strategy in cancer treatment is to induce apoptosis of cancer cells. Zhang et al. [5] reported that Pyrvinium, anti-helminthic drug inhibited cell proliferation and induced apoptosis of paclitaxel- and cisplatin-resistant ovarian cancer cells A2278/PTX and SK-OV-3 via down-regulating Wnt/β-catenin signaling pathway. Tumor necrosis factor (TNF)-related apoptosis-inducing ligand (TRAIL) is a member of the TNF family that specifically induces apoptosis in cancer cells and exhibiting negligible effects on normal cells [6].
TRAIL exerts its effects through its interaction with five receptors – the death receptors DR4/TRAIL-R1 [7], DR5/TRAIL R2 and the decoy receptors – DcR1/TRAIL-R3 [8] and DcR2/TRAIL-R4 [9] and osteoprotegerin, a cytoplasmic receptor [10]. Binding of TRAIL to DR4 and DR5, leads to the enlistment of Fas- associated protein with death domain (FADD) and pro-caspases-8/-10 [11]. This results in a multi-protein death-inducing signaling complex (DISC) [12] that eventually leads to initiation of the initiator caspases [13] resulting in transduction of apoptosis pathway.
Despite of favorable results, many studies have demonstrated that a several cancer cells are TRAIL-resistant, including malignant tumors such as ovarian cancer, pancreatic cancer, gliomas, malignant melanoma and neuroblastoma [14]. A number of molecular events have been associated with TRAIL-resistance as increased expression of anti-apoptotic proteins such as cellular (FLICE)-like inhibitory protein (c-FLIP), X-linked inhibitor of apoptosis protein (XIAP) and Bcl-2 [15], overexpression of decoy receptors (DcRs) and restricted expression of cell signaling DRs on the cell surface [16]. Furthermore, transcription factor NF-κB, PI3K/Akt and MAP kinase signaling pathways have all been implicated to be involved in resistance to TRAIL-induced apoptosis [14,15], thus making identification of TRAIL-sensitizers as crucial in cancer therapy. Several reports indicate that plant-derived compounds could effectively enhance and sensitize cancer cells to TRAIL-induced apoptosis [17]. 6-Shogaol, a potent bioactive compound in ginger, has been found to sensitize renal carcinoma cells to TRAL-induced apoptosis [18]. Henrich et al. [19] reported that Withanolide E, a steroidal lactone from Physalis peruviana, was effective in sensitizing cancer cells to TRAIL.
Flavonoids are natural polyphenols in fruits and vegetables. They possess wide array of biological properties including anti-proliferative, antioxidant and anti-inflammatory activities [20]. Kaempferol and quercetin are flavonoids that belong to the subclass referred to as flavonols. They are commonly present in many foods including grapefruits, onions and tea [21]. Kaempferol has been reported to have numerous health benefits including antioxidant, cardioprotective and anti-carcinogenic effects [22]. Considering the previous studies, we explored kaempferol for possible enhancement of TRAIL-induced apoptosis of ovarian cancer cells.
Material and Methods
Cell lines
OVCAR-3 and SKOV-3 – human ovarian cancer cells were obtained from ATCC and cultured according to their instructions. Briefly, SKOV-3 cells were cultured in RPMI 1640 medium (Invitrogen, CA, USA) supplemented with fetal bovine serum (FBS) (10%). OVCAR-3 cells were also cultured in RPMI 1640, but were supplemented with 20% FBS and bovine insulin (0.01 mg/mL).
Chemicals and reagents
Kaempferol (Sigma-Aldrich, St. Louis, MO, USA) and human TRAIL from PeproTech (Rocky Hill, NJ, USA) were used in the study. Antibodies against Bcl-xL, Bcl-2, survivin, Bax, c-FLIP, CHOP (CEBP homologous protein), XIAP, caspase-3, caspase-8, caspase-9, DR4, DR5 were procured from Cell Signaling Technology (Danvers, MA, USA). β-actin, JNK, phospho-JNK, ERK1/2, phospho-ERK1/2, p38 and phospho-p38 were procured from Santa Cruz Biotechnology (Santa Cruz, CA, USA) and were used for expression analysis. All other analytical-grade chemicals and reagents were from Sigma-Aldrich unless noted otherwise.
Cell viability assay
OVCAR-3 and SKOV-3 – human ovarian cancer cells were seeded into 96-well plates (0.5×106 cells/well) and incubated for 24 h. The cells were then exposed to various concentrations of kaempferol (20–100 μM) for 24 h. Cells were then incubated with 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide (MTT). After 4 h, the formed formazan crystals were dissolved in DMSO and the absorbance was measured at 570 nm using a multiscan spectrum (Thermo Electron Co., Vantaa, Finland). The inhibition of cell proliferation was estimated using the formula – (A570 control cells – A570 treated cells)/A570 control cells ×100%.
Live/dead assay
To assess apoptosis following exposure to various concentrations of kaempferol (25, 50 and 100 μM), the Live/Dead kit assay (Invitrogen, CA, USA) was performed. The assay ascertains membrane integrity and intracellular esterase activity as a measure of apoptosis using non-fluorescent polyanionic dye, calcein-AM. The live cells retain the dye, thereby producing intense green fluorescence through conversion of esterase and the ethidium homodimer binds to nucleic acids inside the damaged/dead cells and gives a bright red fluorescence. Briefly, OVCAR-3 and SKOV-3 - human ovarian cancer cells that were treated with kaempferol and or TRAIL (25ng/mL) were stained with the Live/Dead reagent (5 μmol/L ethidium homodimer and 5 μmol/L calcein-AM) and incubated at 37°C for 30 min. The cells were examined for fluorescence under a fluorescence microscope (Labophot-2, Nikon).
Analysis of apoptosis by Annexin V assay
Human ovarian carcinoma cells, OVCAR-3 and SKOV-3 were incubated with kaempferol (50 or 100 μM) and/or TRAIL (25 ng/mL) for 24 h. Percentage of apoptosis was detected using Annexin V-FITC detection kit II (BD Biosciences Pharmingen, San Diego, CA, USA). Following treatments, the cancer cells were incubated for about 30 min at room temperature with 5 μL annexin V-FITC reagent and were examined using a flow cytometer (FACS Calibur, BD Biosciences).
Analysis of DR4 and DR5 expression
The cell-surface expression of death receptors DR4 and DR5 in the ovarian carcinoma cells treated with kaempferol (50 and 100 μM) for 24 h were assessed using mouse anti-human DR4 or DR5 monoclonal antibodies conjugated with phycoerythrin (R&D Systems). The cells were treated with the antibodies and incubated for 45 min at 4°C and were examined by flow cytometry [23].
Analysis of gene expression by real-time PCR
Using TRIzol reagent (Invitrogen, CA, USA) total RNA was isolated from tumor cells according to the manufacturer’s protocol. Quantitative real-time PCR for DR4, DR5 and GAPDH genes was carried out as previously described by Wei et al. [24]. The primer sequences DR4 5′-TTGTGTCCACCAGGATCTCA-3′ and 5′ GTCACTCC AGGGCGTACAAT-3′ [25], DR5 5′-ACT CCTGGAATGACTACCTG-3′ and 5′-ATCCCAAGTGAA CTTGA GCC-3′ [25], GAPDH, 5′-GTCATCCATGACAACTTTGG-3′ and 5′-GA GCTTGACAAAGTGGTCGT-3′ [26] were used. The relative expression levels of DR4 and DR5 genes were standardized with the expression of internal control (GAPDH).
Transfection with siRNA
Expression of DR5 and CHOP were analyzed following silencing with 25-nucleotide siRNA (Invitrogen, CA, USA) to assess the influence of kaempferol on DR5 and CHOP expression. The carcinoma cells OVCAR-3 and SKOV-3 were transfected with siRNA oligonucleotides (30 nmol/L) using lipofectamine 2000 (Invitrogen, CA, USA) as per the manufacturer’s protocols. Following transfection, cells were treated with kaempferol (100 μM) for 12 h and then incubated with TRAIL (25 ng/mL) for 24 h [27]. The cells were collected and analyzed for expression and for apoptosis. The percentage of apoptosis was assessed by Live/Dead assay as described earlier.
Western blot analysis
Proteins extracted from the cells treated with kaempferol (50 and 100 μM) and/or TRAIL (25 ng/mL) for 24 h were subjected to western blot analysis. Western blot analysis was performed as described previously by Sung et al. [28]. Following treatments, the cells were incubated in 0.5 mL of ice-cold whole-cell lysate buffer (5 M NaCl, 10% Nonidet P-40, 0.2 M sodium orthovanadate, 1 M HEPES, 0.1 M EGTA, 0.5 M EDTA, 0.1 M phenylmethylsulfonyl fluoride, 1 M sodium fluoride, 2 μg/mL aprotinin and 2 μg/mL leupeptin) on ice for 30 min. The isolated proteins from the cell lysates were separated by SDS-PAGE and electrotransferred to nitrocellulose membranes. Respective antibodies were used for hybridization and the immune-reactive bands were analyzed by enhanced chemiluminescence (GE Healthcare). The band densities were normalized to those of control – β-actin using anti-β-actin (Cell Signaling Technology Company, USA).
Statistical analysis
The results are reported as means ±SDs, from three or six different experiments. Data were evaluated using one-way analysis of variance (ANOVA) followed by Duncan’s multiple range test (DMRT) using the SPSS software (ver. 16.0). P values <0.05 were considered to indicate statistical significance.
Results
Kaempferol inhibited cancer cell proliferation
Kaempferol at 20–100 μM concentrations efficiently inhibited the proliferation of ovarian cancer cells OVCAR-3 and SKOV-3. Upon incubation with kaempferol, the cell viability significantly (p<0.05) decreased (Table 1). While 20 and 40 μM kaempferol reduced the viability, doses above 40 μM brought more reduction in cell viability in both the cell lines.
Table 1.
Cytotoxicity of various concentrations of kaempferol as determined by MTT assay.
| Cell viability (%) | ||
|---|---|---|
| SKOV-3 | OVCAR-3 | |
| Control | 99.21±0.58f | 99.14±0.75f |
| 20 μM Kaempferol | 85.62±3.09ae | 86.36±3.62ae |
| 40 μM Kaempferol | 69.73±1.67ad | 74.09±1.54ad |
| 60 μM Kaempferol | 48.00±2.06ac | 49.90±1.78ac |
| 80 μM Kaempferol | 44.80±2.34ac | 45.98±3.06ac |
| 100 μM Kaempferol | 36.17±2.05ab | 40.26±1.28ab |
Values are represented as mean ±SD, n=6.
Denotes statistical significance at p<0.05 compared against control;
represents values of various treatment on the same cell line that differ from each other at p<0.05 as determined by one-way ANOVA followed by DMRT analysis.
Kaempferol enhanced TRAIL-induced apoptosis
Live/Dead assay for determining cell viability was performed after exposure to 25, 50 or 100 μM kaempferol. Exposure to 50 μM concentration resulted in 49.09% in OVCAR-3 cells and 48.93% in SKOV-3 cells. Striking increases (p<0.05) were observed in treatment with 100 μM kaempferol as compared with lower doses of 25 or 50 μM. However, exposure to TRAIL and kaempferol at 100 μM interestingly, bought a multi-fold increase in cytotoxicity levels to 73.19% in OVCAR-3 and 78.90% in SKOV-3 cells (Table 2). TRAIL alone caused viability of about 36.16% and 37.88% in SKOV-3 and OVCAR-3 cells respectively.
Table 2A.
Kaempferol enhanced TRAIL-induced apoptosis in ovarian cancer cells.
| Cytotoxicity (%) | ||
|---|---|---|
| SKOV-3 | OVCAR-3 | |
| Control | 2.1±0.66b | 1.7±0.59b |
| 25 μM Kaempferol | 29.25±1.98ac | 35.60±2.06ac |
| 50 μM Kaempferol | 48.93±2.73ad | 49.09±3.02ad |
| 100 μM Kaempferol | 66.12±4.36af | 60.05±3.97ae |
| 25 μM Kaempferol + TRAIL | 59.84±3.5ae | 62.96±3.86ae |
| 50 μM Kaempferol + TRAIL | 63.19±2.13ae | 68.90±2.50af |
Values are represented as mean ±SD, n=6.
Denotes statistical significance at p<0.05 compared against control;
represents values of various treatment on the same cell line that differ from each other at p<0.05 as determined by one-way ANOVA followed by DMRT analysis.
Translocation of membrane phosphatidylserine to the extracellular surface of the membrane from the cytoplasmic interface is a primary indicator of apoptosis. Annexin V/PI staining assay was performed to assess the integrity of the cell membrane. The results presented in Table 2A and 2B reveals that the combined exposure to TRAIL and kaempferol at 50 and 100 μM could induce more robust apoptosis in the cancer cells than the exposure to either TRAIL or kaempferol at both the doses. The results suggest that kaempferol was able to effectively enhance TRAIL-induced apoptosis.
Table 2B.
Kaempferol enhanced TRAIL-induced apoptosis in ovarian cancer cells as determined by Annexin V assay.
| Cytotoxicity (%) | ||
|---|---|---|
| SKOV-3 | OVCAR-3 | |
| Control | 1.59±0.05b | 1.98±0.10b |
| 50 μM Kaempferol | 30.39±1.56ac | 36.12±1.32ad |
| 100 μM Kaempferol | 57.90±2.86ad | 60.01±3.05ae |
| 50 μM Kaempferol + TRAIL | 56.12±1.98ad | 58.17±2.12ae |
| 100 μM Kaempferol + TRAIL | 68.54±3.71ae | 72.15±3.50af |
| TRAIL (25 ng/ml) | 28.70±1.75ac | 31.22±1.53ac |
Values are represented as mean ±SD, n=6.
Denotes statistical significance at p<0.05 compared against control;
represents values of various treatment on the same cell line that differ from each other at p<0.05 as determined by one-way ANOVA followed by DMRT analysis.
Kaempferol up-regulated the cell surface expression of DR4 and DR5
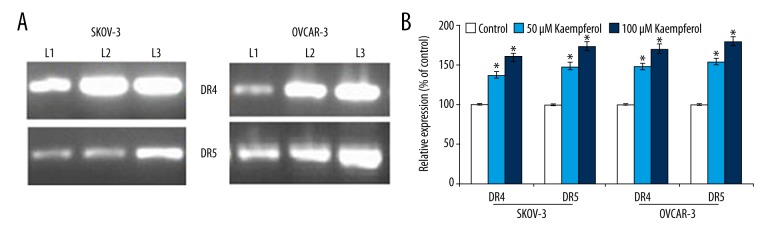
TRAIL is known to induce apoptosis through its interaction with death receptors – DR4 and DR5 which eventually interact with FADD leading to the sequential activation of initiator caspase-8 and caspase-3 [29]. We were particularly interested to know if kaempferol-induced apoptosis is associated with up-regulation of the cell surface expression of DR4 and DR5 in the ovarian cancer cells. Exposure to kaempferol resulted in a significant (p<0.05) increase in the expression of DR4 and DR5 in a dose-dependent manner. However, kaempferol at both the doses were more effective on the expression of DR5 than DR4. Further, SKOV-3 cells presented striking expression of DR5 than the OVCAR-3 cells following exposure to 50 and 100 μM of kaempferol (Figure 1).
Figure 1.
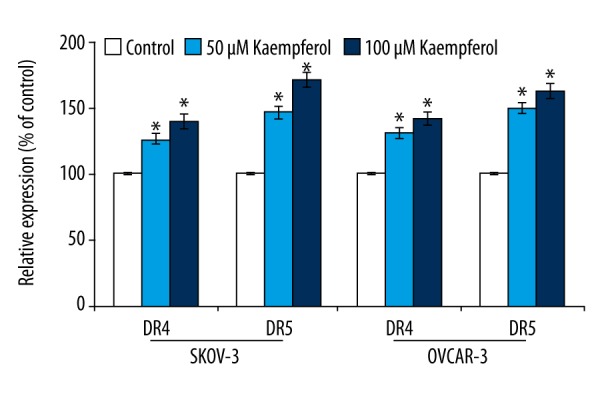
Influence of kaempferol on cell surface expression of DR4 and DR5. Kaempferol caused markedly up-regulated the expressions of death receptors – DR4 and DR5 on the cell surface of the ovarian cancer cells. Values are represented as mean ±SD, n=6. * Denotes statistical significance at p<0.05 compared against control as determined by one-way ANOVA
Furthermore, to examine the influence of kaempferol on the expression of DR4 at the mRNA level, RT-PCR was performed. Kaempferol potentially induced the expression of DR5 and DR4 in dose-dependent way (Figure 2A, 2B). Kaempferol induced significant effects on the mRNA expression levels of DR4 and DR5 and the same was observed to be in line with the protein level of the death receptors as determined by western blot analysis (Figure 3). However, the up-regulation of DR5 was more pronounced than DR4.
Figure 2.
(A) Kaempferol enhanced the expression of DR4 and DR5. RT-PCR analysis revealed that kaempferol exposure significantly enhanced the expression of DR4 and DR5 at mRNA levels in ovarian cancer cells SKOV-3 and OVCAR-3. (L1 – Control; L2 – 50 μM Kaempferol; L3 – 100 μM Kaempferol). (B) Relative expression of kaempferol on DR4 and DR5. Values are represented as mean ± SD, n=6. * Denotes statistical significance at p<0.05 compared against control as determined by one-way ANOVA.
Figure 3.
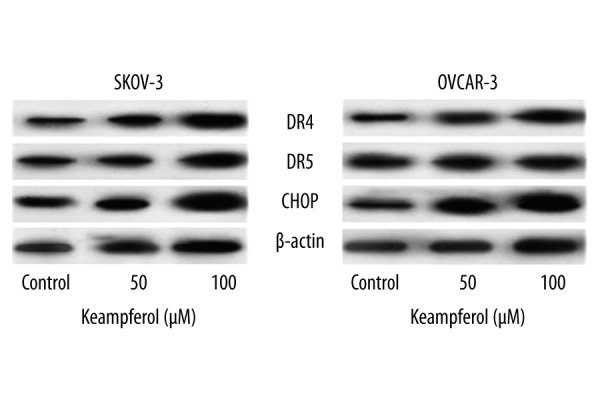
Influence of kaempferol on the expression of death receptors and CHOP. Immunoblotting studies indicated that kaempferol dose-dependently elevated the expressions of DR4, DR5 and CHOP in SKOV-3 and OVCAR-3 cells.
DR5 expression is reported to be regulated at the transcriptional level through CHOP upon interacting at CHOP-binding site in the DR5 [30]. Previous studies have shown that the induction of death receptor by chemotherapeutic agents is mediated through the activation of CHOP [31]. Kaempferol at 50 and 100 μM caused marked (p<0.05) increase in the expression of CHOP. Enhanced expression of CHOP as observed was in line with expression level of DR5, suggesting that elevated CHOP also might had probably induced the expression of DR5 (Figure 3). The results suggest that kaempferol-enhanced TRAIL-induced apoptosis was possibly due its effect on up-regulation of the death receptor expressions.
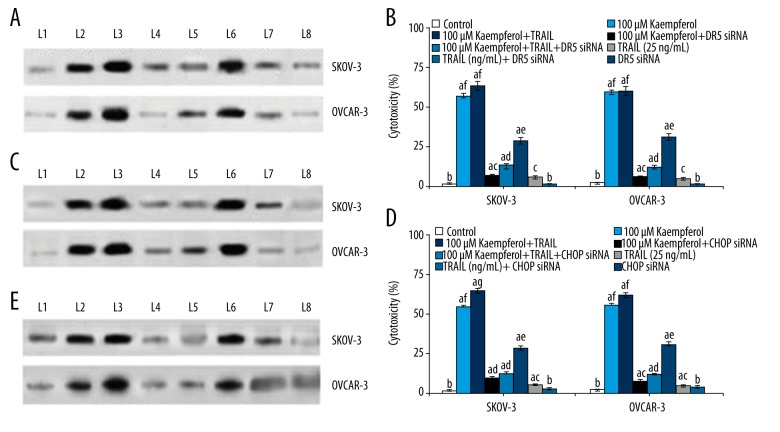
CHOP mediates kaempferol-induced up-regulation of DR5 is required for TRAIL-induced apoptosis
To further assess the involvement of CHOP and DR5 in kaempferol-induced increase in TRAIL-induced apoptosis, siRNAs specific to DR5 and CHOP were employed. Transfection of the cancer cells with siRNA for DR5 caused reduction in DR5 expression (Figure 4A–4E). However, TRAIL and 100 μM kaempferol caused slight up-regulation of DR5. In the cells that were not exposed to DR5 siRNA, elevated DR5 expression was observed. In cells transfected with siRNA for CHOP, decrease in DR5 expression level was found indicating the involvement of CHOP in DR5 expression. While kaempferol caused elevated DR5 levels, the cells not transfected with CHOP siRNA exhibited pronounced expression than cells exposed, resulting in apoptosis. This was further supported by the increase in the apoptosis counts of the cancer cells following cell viability assay. In cells transfected with siRNA, the viability percentage of the cancer cells was high. The results suggest that CHOP and DR5 are needed for TRAL-induced apoptosis of the cancer cells.
Figure 4.
(A) Cytotoxicity exhibited after transfection with siRNA specific for DR5 under the influence of kaempferol. Transfection with siRNA specific for DR5 resulted in a significant decrease in the expression of DR5. (L1 – Control; L2 – 100 μM Kaempferol; L3 – 100 μM Kaempferol+TRAIL; L4 – 100 μM Kaempferol+D5 siRNA; L5 – 100 μM Kaempferol+TRAIL+D5 siRNA; L6 – TRAIL (25 ng/mL); L7 – TRAIL (25 ng/mL)+D5 siRNA; L8 – D5 siRNA). (B) Cytotoxicity exhibited after transfection with siRNA under the influence of kaempferol. Transfection with siRNA specific for DR5 resulted in a significant decrease in the expression of DR5 that consequently resulted in a marked decline in cytotoxicity percentage. Values are represented as mean ±SD, n=6; a denotes statistical significance at p<0.05 compared against control as determined and b–f denotes values within the same group that differ from each other at p<0.05 as determined by one-way ANOVA followed by DMRT analysis. (C) Cytotoxicity exhibited after transfection with siRNA under the influence of kaempferol. Transfection with siRNA specific for CHOP resulted in a significant decrease in the expression of CHOP. (L1 – Control; L2 – 100 μM Kaempferol; L3 – 100 μM Kaempferol+TRAIL; L4 – 100 μM Kaempferol+D5 siRNA; L5 – 100 μM Kaempferol+TRAIL+D5 siRNA; L6 – TRAIL (25 ng/mL); L7 – TRAIL (25 ng/mL)+D5 siRNA; L8 – D5 siRNA). (D) Cytotoxicity exhibited after transfection with siRNA under the influence of kaempferol. Transfection with siRNA specific for CHOP resulted in a significant decrease in the expression of CHOP that consequently resulted in a marked decline in cytotoxicity percentage. Values are represented as mean ±SD, n=6; a denotes statistical significance at p<0.05 compared against control as determined and b–g denotes values within the same group that differ from each other at p<0.05 as determined by one-way ANOVA followed by DMRT analysis. (E) Cytotoxicity exhibited after transfection with siRNA under the influence of kaempferol. Transfection with CHOP siRNA strikingly reduced the DR5 expression levels that was in line with cytotoxicity levels observed with CHOP siRNA, suggesting the involvement of CHOP in DR5 up-regulation and expression. (L1 – Control; L2 – 100 μM Kaempferol; L3 – 100 μM Kaempferol+TRAIL; L4 – 100 μM Kaempferol+D5 siRNA; L5 – 100 μM Kaempferol+TRAIL+D5 siRNA; L6 – TRAIL (25 ng/mL); L7 – TRAIL (25 ng/mL)+D5 siRNA; L8 – D5 siRNA).
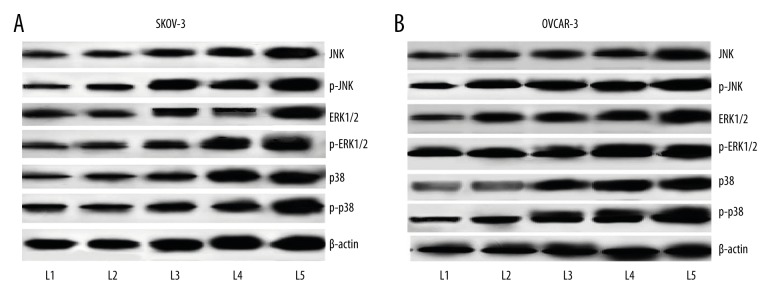
Kaempferol-induced death receptor up-regulation requires MAP kinases
We studied whether kaempferol influenced the activation of MAP kinases – ERK1/2, p38 MAPK, and JNK. Incubation with kaempferol caused significant (p<0.05) up-regulation in the expression of ERK1/2, JNK and p38. Kaempferol caused multi-fold increase in the level of phosphorylated ERK1/2 and JNK. Though we observed a considerable increase in p-p38 levels, it was not significant. Kaempferol induced enhancement in the phosphorylation level was more following exposure to 100 μM, than other lower doses used (Figure 5A, 5B).
Figure 5.
(A) Influence of kaempferol on MAPkinase expression on SKOV-3 human ovarian cancer cells. Kaempferol exposure caused significant increase in the expression levels of MAP kinases – JNK, ERK1/2 and p-38. Striking elevations in the phosphorylated forms of JNK, ERK1/2 and p-38 were observed in SKOV-3 cells. (L1 – Control; L2 – 50 μM Kaempferol; L3 – 100 μM Kaempferol; L4 – 50 μM Kaempferol+TRAIL; L5 – 100 μM Kaempferol+TRAIL). (B) Influence of kaempferol on MAPkinase expression on OVCAR-3 human ovarian cancer cells. Kaempferol exposure caused significant increase in the expression levels of MAP kinases – JNK, ERK1/2 and p-38. Striking elevations in the phosphorylated forms of JNK, ERK1/2 and p-38 were observed in OVCAR-3cells. (L1 – Control; L2 – 50 μM Kaempferol; L3 – 100 μM Kaempferol; L4 – 50 μM Kaempferol+TRAIL; L5 – 100 μM Kaempferol+TRAIL)
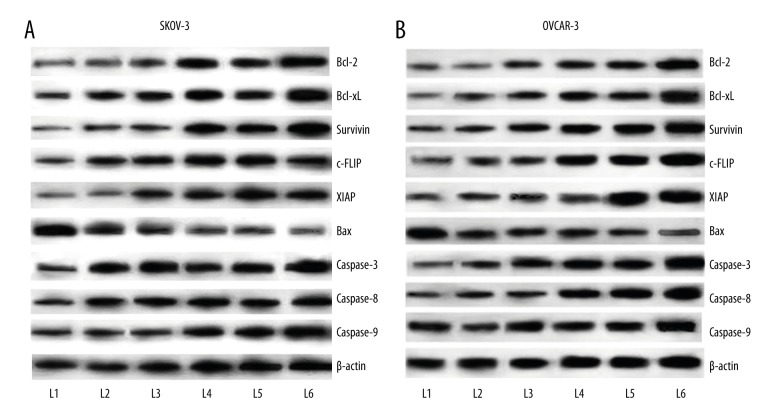
Kaempferol modulates the expression of apoptotic pathway proteins
Bonding of TRAIL to the death receptors (DR4 and DR5) is pivotal for initiation of apoptosis. This interaction results in the initiation of the caspase cascade. The effects of kaempferol on TRAIL-induced activation of caspases was assessed where kaempferol at 50 and 100 μM strikingly increased (p<0.05) the expression of caspase-3,-8 and -9 in a dose-dependent manner in both OVCAR-3 and SKOV-3 cell lines. However, the combined exposure of TRAIL and kaempferol had more impact in the caspase expression with 100 μM presenting significant effect than the lower doses (Figure 6A, 6B).
Figure 6.
(A) Influence of kaempferol on expression of apoptotic pathway proteins in SKOV-3 human ovarian cancer cells. Kaempferol exposure caused significant increase in the expression levels of apoptotic proteins Bax and caspases, and strikingly down-regulated the expressions of cell survival proteins (Bcl-xL, Bcl-2, survivin, c-FLIP and XIAP) in SKOV-3 cells. Combined exposure to TRAIL and kaempferol resulted in more pronounced modulations in the expressions of the proteins. (L1 – Control; L2 – TRAIL; L3 – 50 μM Kaempferol; L4 – 100 μM Kaempferol; L5 – 50 μM Kaempferol+TRAIL; L6 – 100 μM Kaempferol+TRAIL). (B) Influence of kaempferol on expression of apoptotic pathway proteins in OVCAR-3 human ovarian cancer cells. Kaempferol exposure caused significant increase in the expression levels of apoptotic proteins Bax and caspases, and strikingly down-regulated the expressions of cell survival proteins (Bcl-xL, Bcl-2, survivin, c-FLIP and XIAP) in OVCAR-3cells. Combined exposure to TRAIL and kaempferol resulted in more pronounced modulations in the expressions of the proteins. (L1 – Control; L2 – TRAIL; L3 – 50 μM Kaempferol; L4 – 100 μM Kaempferol; L5 – 50 μM Kaempferol+TRAIL; L6 – 100 μM Kaempferol+TRAIL).
OVCAR-3 and SKOV-3 cells treated with different concentrations of kaempferol and/or TRAIL were evaluated for expression of cell survival proteins. Kaempferol caused significant (p<0.05) inhibition on the expressions of Bcl-xL, Bcl-2, survivin, c-FLIP and XIAP, while up-regulating the pro-apoptotic protein, Bax (Figure 6A, 6B). While 100 μM brought out a decrease in the expression of these anti-apoptotic proteins, the combined effects of TRAIL and kaempferol was more significant than kaempferol or TRAIL when used alone. Collectively, the results suggest that kaempferol potentiated the down-regulation of cell survival proteins probably by similar mechanisms as TRAIL, suggesting that kaempferol potentiated TRAIL-induced apoptosis.
Discussion
Despite promising effects of TRAIL on cancer cells, accumulating reports have made it evident that human tumors are gaining resistant to TRAIL and occurrences of acquiring resistance to TRAIL-mediated cell death [32]. It has been postulated that in tumors resistant to TRAIL, the cell survival signals normally become prominent due to deficiencies in the apoptotic pathways. Signaling pathway dysfunctions including the altered expression of the DRs, the Fas-associated death domain, or caspase-8 [33], overexpression of anti-apoptotic Bcl-2 family proteins, Mcl-1, survivin and cFLIP, a caspase-8 inhibitor [34], and activation of NF-κB [35], enhanced expressions of XIAP have been observed in many tumor cell lines that may lead to TRAIL resistance by inhibiting caspases [36].
Depending on the survival pathway elicited and/or the factors contributing to resistance, TRAIL-resistant cancers possibly lead to an improved survival and even tumor cells migration despite treatment with TRAIL [37]. Thus, it becomes pivotal to identify novel compounds that could effectively sensitize tumor cells to TRAIL-induced apoptosis and can be possibly combined with TRAIL to amplify its apoptotic effects [34].
Studies indicate that ovarian cancers also exhibit resistance to TRAIL through various molecular events [38]. Recent studies have demonstrated that exposure to gingerol and quercetin could sensitize cancer cells [39].
We investigated whether kaempferol was able to sensitize SKOV-3 and OVCAR-3 human ovarian cancer cells to TRAIL.
Kaempferol at 20–100 μM decreased SKOV-3 and OVCAR-3 cells viability, suggesting its anti-proliferative efficacy. Furthermore, the combined exposure to kaempferol and TRAIL dramatically increased the apoptotic cell counts as compared to cells treated with either kaempferol or TRAIL, suggesting that kaempferol effectively potentiated the effects of TRAIL. In addition, down-regulated expression of anti-apoptotic proteins, Bcl-2, Bcl-xL and survivin were observed following exposure to kaempferol. The ratio between pro-apoptotic and anti-apoptotic members of the Bcl-2 family determines the transduction of death signal. Down-regulation of Bcl-xL, Bcl-2 and survivin expression is known to promote sensitivity to TRAIL-mediated cell death [40]. Further, resistance to TRAIL has also been correlated with over-expressions of c-FLIP and XIAP [34,40]. It has been shown that the phytochemical drugs, casticin and cardamonin inhibit XIAP and enhanced TRAIL-mediated apoptosis [41]. Thus, kaempferol-induced down-regulation of XIAP and c-FLIP could also have contributed to the caspase cascade activation, that is evident by the significant up-regulation of caspase-3, caspase-8, caspase-9 and pro-apoptotic protein (Bax) as well. The differences observed in the expression patterns were dose-dependent, where 100 μM kaempferol combined with TRAIL had greater influence.
Interaction with DR4 and DR5 receptors triggers the transduction of the apoptotic pathway. The higher expression of these receptors on tumor cells contributes to TRAIL-induced cell death [42]. Thus, for any changes either at the protein level and/or cell surface level, TRAIL receptors have profound effects on TRAIL, as a death-inducing ligand. Dysregulated expression levels have been reported in several TRAIL-resistant cancers [43] subsequent up-regulation of the receptors contributes to promote apoptosis. Kaempferol caused significant up-regulation in the expression of DR4 and DR5 on the surface of SKOV-3 and OVCAR-3 cells and also enhanced expression at the gene and protein level. Silencing of DR5 gene was executed to understand the contribution of DR5 up-regulation in kaempferol induced TRAIL-mediated apoptosis. Transfection of the cells with DR5 siRNA affected DR5 expression and apoptotic cell counts. While combined exposure to kaempferol and TRAIL presented a slight increase in apoptotic cells, increase in apoptosis in cancer cells that were not transfected with siRNA was more robust, indicating the contribution of DR5 in TRAIL-mediated cell death.
CHOP, a chaperone in the ER, is a key regulator of DR5 [30]. It binds to DR5 promoter region and regulates the expression of DR5 [30]. Our observations revealed that induction of DR5 expression by kaempferol is mediated through CHOP up-regulation. While kaempferol caused striking increase in the expression level of CHOP, transfection with siRNA specific to CHOP reduced the expression of CHOP and DR5 even on exposure to kaempferol that well correlated with the apoptotic cell counts observed [44,45].
MAP kinases transduce various extracellular stimuli and are vital for the sustainment of various intracellular processes like that of cell development and growth, apoptosis and reactions to various external stresses [14]. The MAP kinases – JNK, ERK and p38 kinase have been found to be activated in response to TRAIL stimulation [14] and also regulate the expression of DR4 and DR5 [28]. Kaempferol caused up-regulation of the phosphorylated forms indicating activation of JNK, p-38 and ERK1/2 in a dose-dependent way. Gingerol, gossypol and quercetin enhanced TRAIL-induced apoptosis through activation of MAP kinases [28, 39].
Conclusions
Observations of our study suggest that kaempferol effectively sensitized the ovarian cancer cells and enhanced TRAIL-induced apoptosis by significantly upregulating the death receptors and through the activation of CHOP and MAP kinases. Thus, kaempferol could possibly be employed in combined therapy with TRAIL in the treatment of TRAIL-resistant tumors.
Footnotes
Conflicts of interest
None.
Source of support: Departmental sources
References
- 1.Ferlay J, Shin H, Bray F, et al. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Buys SS, Partridge E, Black A, et al. Effect of screening on ovarian cancer mortality: The Prostate, Lung Colorectal and Ovarian (PLCO) cancer screening randomized controlled trial. JAMA. 2011;305:2295–303. doi: 10.1001/jama.2011.766. [DOI] [PubMed] [Google Scholar]
- 3.Deraco M, Rossi CR, Pennacchioli E, et al. Cytoreductive surgery followed by intraperitoneal hyperthermic perfusion in the treatment of recurrent epithelial ovarian cancer: A phase II clinical study. Tumori. 2001;87:120–26. doi: 10.1177/030089160108700302. [DOI] [PubMed] [Google Scholar]
- 4.Agarwal R, Kaye SB. Ovarian cancer: Strategies for overcoming resistance to chemotherapy. Nat Rev Cancer. 2003;3:502–16. doi: 10.1038/nrc1123. [DOI] [PubMed] [Google Scholar]
- 5.Zhang C, Zhang Z, Zhang S, et al. Targeting of Wnt/β-catenin by anthelmintic drug pyrvinium enhances sensitivity of ovarian cancer cells to chemotherapy. Med Sci Monit. 2017;23:266–75. doi: 10.12659/MSM.901667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ashkenazi A, Pai RC, Fong S, et al. Safety and antitumor activity of recombinant soluble Apo2 ligand. J Clin Investigation. 1999;104:155–62. doi: 10.1172/JCI6926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pan G, O’Rourke K, Chinnaiyan AM, et al. The receptor for the cytotoxic ligand TRAIL. Science. 1997;276:111–13. doi: 10.1126/science.276.5309.111. [DOI] [PubMed] [Google Scholar]
- 8.Pan G, Ni J, Wei YF, et al. An antagonist decoy receptor and a death domain-containing receptor for TRAIL. Science. 1997;277:815–18. doi: 10.1126/science.277.5327.815. [DOI] [PubMed] [Google Scholar]
- 9.Marsters SA, Sheridan JP, Pitti RM, et al. A novel receptor for Apo2L/TRAIL contains a truncated death domain. Curr Biol. 1997;7:1003–6. doi: 10.1016/s0960-9822(06)00422-2. [DOI] [PubMed] [Google Scholar]
- 10.Emery JG, McDonnell P, Burke MB, et al. Osteoprotegerin is a receptor for the cytotoxic ligand TRAIL. J Biol Chem. 1998;273:14363–67. doi: 10.1074/jbc.273.23.14363. [DOI] [PubMed] [Google Scholar]
- 11.Medema JP, Scaffidi C, Kischkel FC, et al. FLICE is activated by association with the CD95 death inducing signaling complex (DISC) EMBO J. 1997;16:2794–804. doi: 10.1093/emboj/16.10.2794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kischkel FC, Hellbardt S, Behrmann I, et al. Cytotoxicity-dependent APO-1 (Fas/CD95)-associated proteins form a death-inducing signaling complex (DISC) with the receptor. EMBO J. 1995;14:5579–88. doi: 10.1002/j.1460-2075.1995.tb00245.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kischkel FC, Lawrence DA, Chuntharapai A, et al. Apo2L/TRAIL-dependent recruitment of endogenous FADD and caspase-8 to death receptors 4 and 5. Immunity. 2000;12:611–20. doi: 10.1016/s1074-7613(00)80212-5. [DOI] [PubMed] [Google Scholar]
- 14.Mellier G, Huang S, Shenoy K, Pervaiz S. TRAILing death in cancer. Mol Aspects Med. 2010;31:93–112. doi: 10.1016/j.mam.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 15.Inoue T, Shiraki K, Fuke H, et al. Proteasome inhibition sensitizes hepatocellular carcinoma cells to TRAIL by suppressing caspase inhibitors and AKT pathway. Anticancer Drugs. 2006;17:261–68. doi: 10.1097/00001813-200603000-00004. [DOI] [PubMed] [Google Scholar]
- 16.Zhang L, Zhu H, Teraishi F, et al. Accelerated degradation of caspase-8 protein correlates with TRAIL resistance in a DLD1 human colon cancer cell line. Neoplasia. 2005;7:594–602. doi: 10.1593/neo.04688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jung YH, Heo J, Lee YJ, et al. Quercetin enhances TRAIL-induced apoptosis in prostate cancer cells via increased protein stability of death receptor 5. Life Sci. 2010;86:351–57. doi: 10.1016/j.lfs.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Han MA, Woo SM, Min KJ, et al. 6-Shogaol enhances renal carcinoma Caki cells to TRAIL-induced apoptosis through reactive oxygen species-mediated cytochrome c release and down-regulation of c-FLIP(L) expression. Chem Biol Interact. 2015;228:69–78. doi: 10.1016/j.cbi.2015.01.020. [DOI] [PubMed] [Google Scholar]
- 19.Henrich CJ, Brooks AD, Erickson KL, et al. Withanolide E sensitizes renal carcinoma cells to TRAIL-induced apoptosis by increasing cFLIP degradation. Cell Death Dis. 2015;6:e1666. doi: 10.1038/cddis.2015.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harborne JB, Williams CA. Advances in flavonoid research since 1992. Phytochemistry. 2000;55:481–504. doi: 10.1016/s0031-9422(00)00235-1. [DOI] [PubMed] [Google Scholar]
- 21.Ross JA, Kasum CM. Dietary flavonoids: Bioavailability, metabolic effects, and safety. Annu Rev Nutr. 2002;22:e19–e34. doi: 10.1146/annurev.nutr.22.111401.144957. [DOI] [PubMed] [Google Scholar]
- 22.Casagrande F, Darbon JM. Effects of structurally related flavonoids on cell cycle progression of human melanoma cells: Regulation of cyclin-dependent kinases CDK2 and CDK1. Biochem Pharmacol. 2001;61:1205–15. doi: 10.1016/s0006-2952(01)00583-4. [DOI] [PubMed] [Google Scholar]
- 23.Prasad S, Ravindran J, Sung B, et al. Garcinol potentiates TRAIL-induced apoptosis through modulation of death receptors and antiapoptotic proteins. Mol Cancer Ther. 2010;9:856–68. doi: 10.1158/1535-7163.MCT-09-1113. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Wei G, Zhang GM, Liu Y, et al. IFN-γ withdrawal after immunotherapy potentiates B16 melanoma invasion and metastasis by intensifying tumor integrin αvβ3 signaling. Int J Cancer. 2008;123:702–8. doi: 10.1002/ijc.23553. [DOI] [PubMed] [Google Scholar]
- 25.Frese S, Frese-Schaper M, Andres AC, et al. Cardiac glycosides initiate Apo2L/TRAIL induced apoptosis in non-small cell lung cancer cells by upregulation of death receptors 4 and 5. Cancer Res. 2006;66:5867–74. doi: 10.1158/0008-5472.CAN-05-3544. [DOI] [PubMed] [Google Scholar]
- 26.Yang XC, Tu CX, Luo PH, et al. Antimetastatic activity of MONCPT in preclinical melanoma mice model. Invest New Drugs. 2010;28:800–11. doi: 10.1007/s10637-009-9323-8. [DOI] [PubMed] [Google Scholar]
- 27.Yadav VR, Sung B, Prasad S, et al. Celastrol suppresses invasion of colon and pancreatic cancer cells through the down-regulation of expression of CXCR4 chemokine receptor. J Mol Med. 2010;88:1243–53. doi: 10.1007/s00109-010-0669-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sung B, Ravindran J, Prasad S, et al. Gossypol induces death receptor-5 through activation of the ROS-ERK-CHOP pathway and sensitizes colon cancer cells to TRAIL. J Biol Chem. 2010;285:35418–27. doi: 10.1074/jbc.M110.172767. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 29.Aggarwal BB. Signalling pathways of the TNF superfamily: A double-edged sword. Nat Rev Immunol. 2003;3:745–56. doi: 10.1038/nri1184. [DOI] [PubMed] [Google Scholar]
- 30.Yamaguchi H, Wang HG. CHOP is involved in endoplasmic reticulum stress-induced apoptosis by enhancing DR5 expression in human carcinoma cells. J Biol Chem. 2004;279:45495–502. doi: 10.1074/jbc.M406933200. [DOI] [PubMed] [Google Scholar]
- 31.Lee TJ, Um HJ, Mindo S, et al. Withaferin A sensitizes TRAIL-induced apoptosis through reactive oxygen species-mediated up-regulation of death receptor 5 and down-regulation of c-FLIP. Free Radic Biol Med. 2009;46:1639–49. doi: 10.1016/j.freeradbiomed.2009.03.022. [DOI] [PubMed] [Google Scholar]
- 32.LeBlanc HN, Ashkenazi A. Apo2L/TRAIL and its death and decoy receptors. Cell Death Differ. 2003;10:66–75. doi: 10.1038/sj.cdd.4401187. [DOI] [PubMed] [Google Scholar]
- 33.Wagner KW, Punnoose EA, Januario T, et al. Death-receptor O-glycosylation controls tumor-cell sensitivity to the proapoptotic ligand Apo2L/TRAIL. Nat Med. 2007;13:1070–77. doi: 10.1038/nm1627. [DOI] [PubMed] [Google Scholar]
- 34.Zhang L, Fang B. Mechanisms of resistance to TRAIL-induced apoptosis in cancer. Cancer Gene Ther. 2005;12:228–37. doi: 10.1038/sj.cgt.7700792. [DOI] [PubMed] [Google Scholar]
- 35.Ravi R, Bedi GC, Engstrom LW, et al. Regulation of death receptor expression and TRAIL/Apo2L-induced apoptosis by NF-kappa-B. Nat Cell Biol. 2001;3:409–16. doi: 10.1038/35070096. [DOI] [PubMed] [Google Scholar]
- 36.Deveraux QL, Takahashi R, Salvesen GS, Reed JC. X-linked IAP is a direct inhibitor of cell-death proteases. Nature. 1997;388:300–4. doi: 10.1038/40901. [DOI] [PubMed] [Google Scholar]
- 37.Secchiero P, Gonelli A, Carnevale E, et al. TRAIL promotes the survival and proliferation of primary human vascular endothelial cells by activating the Akt and ERK pathways. Circulation. 2003;107:2250–56. doi: 10.1161/01.CIR.0000062702.60708.C4. [DOI] [PubMed] [Google Scholar]
- 38.Horak P, Pils D, Kaider A, et al. Perturbation of the tumor necrosis factor-related apoptosis-inducing ligand cascade in ovarian cancer: Overexpression of FLIPL and deregulation of the functional receptors DR4 and DR5. Clin Cancer Res. 2005;11:8585–91. doi: 10.1158/1078-0432.CCR-05-1276. [DOI] [PubMed] [Google Scholar]
- 39.Lee DH, Kim DW, Jung CH, et al. Gingerol sensitizes TRAIL-induced apoptotic cell death of glioblastoma cells. Toxicol Appl Pharmacol. 2014;279:253–65. doi: 10.1016/j.taap.2014.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Fulda S, Meyer E, Debatin KM. Inhibition of TRAIL-induced apoptosis by Bcl-2 overexpression. Oncogene. 2002;21:2283–94. doi: 10.1038/sj.onc.1205258. [DOI] [PubMed] [Google Scholar]
- 41.Tang SY, Zhong MZ, Yuan GJ, et al. Casticin, a flavonoid, potentiates TRAIL-induced apoptosis through modulation of anti-apoptotic proteins and death receptor 5 in colon cancer cells. Oncol Rep. 2013;29:474–80. doi: 10.3892/or.2012.2127. [DOI] [PubMed] [Google Scholar]
- 42.Kruyt FA. TRAIL and cancer therapy. Cancer Lett. 2008;263:14–25. doi: 10.1016/j.canlet.2008.02.003. [DOI] [PubMed] [Google Scholar]
- 43.Fisher MJ, Virmani AK, Wu L, et al. Nucleotide substitution in the ectodomain of trail receptor DR4 is associated with lung cancer and head and neck cancer. Clin Cancer Res. 2001;7:1688–97. [PubMed] [Google Scholar]
- 44.Jiang M, Lin X, He R, et al. Decoy Receptor 3 (DcR3) as a biomarker of tumor deterioration in female reproductive cancers: A Meta-Analysis. Med Sci Monit. 2016;22:1850–57. doi: 10.12659/MSM.896226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang H, Gao W. DJ-1 Expression in cervical carcinoma and its effects on cell viability and apoptosis. Med Sci Monit. 2016;22:2943–49. doi: 10.12659/MSM.896861. [DOI] [PMC free article] [PubMed] [Google Scholar]