Abstract
Background
Women with a history of a hypertensive disorder during pregnancy (HDP) have an increased risk of cardiovascular events. Guidelines recommend assessment of cardiovascular risk factors in these women later in life, but provide limited advice on how this follow-up should be organized.
Design
Systematic review and meta-regression analysis.
Methods
The aim of our study was to provide an overview of existing knowledge on the changes over time in three major modifiable components of cardiovascular risk assessment after HDP: blood pressure, glucose homeostasis and lipid levels. Data from 44 studies and up to 6904 women with a history of a HDP were compared with risk factor levels reported for women of corresponding age in the National Health And Nutrition Examination Survey, Estudio Epidemiólogico de la Insuficiencia Renal en España and Hong Kong cohorts (N = 27,803).
Results
Compared with the reference cohort, women with a HDP presented with higher mean blood pressure. Hypertension was present in a higher rate among women with a previous HDP from 15 years postpartum onwards. At 15 years postpartum (±age 45), one in five women with a history of a HDP suffer from hypertension. No differences in glucose homeostasis parameters or lipid levels were observed.
Conclusions
Based on our analysis, it is not possible to point out a time point to commence screening for cardiovascular risk factors in women after a HDP. We recommend redirection of future research towards the development of a stepwise approach identifying the women with the highest cardiovascular risk.
Keywords: Pregnancy, pre-eclampsia, hypertension, pregnancy-induced, hypertension, cardiovascular diseases, preventive medicine
Introduction
Large cohort studies have consistently demonstrated an increased risk – up to seven-fold – of cardiovascular disease (CVD) later in life in women with a history of hypertensive disorders of pregnancy (HDPs) compared with women with uncomplicated pregnancies.1,2 Manifestation of CVD occurs earlier in HDPs: approximately 6–8 years before controls.1,2 Consensus on which mechanisms contribute to this increased risk has not been established. Current opinion is that HDP and CVD share common risk factors, including sympathetic driven hypertension, insulin resistance, inflammation and obesity.1,3 Also pregnancy itself has been proven to enlarge future CVD risk: parity was independently associated in a J-shaped fashion, with two births representing the lowest risk.4,5
Pregnancy induces an extensive adaptation of the circulatory system, including major cardiac output and renal glomerular changes. Pregnancy may unmask limited cardiovascular reserves resulting in HDP and thus pregnancy complicated by HDP can be considered as a failed cardiovascular stress test.6 Therefore, positive history of HDP may allow for early identification of women at risk of CVD and provide opportunities for prevention and intervention.7
The last three decades, there is an increasing imperative for primary CVD prevention in women.8 Unfortunately, guidelines still fail to provide a uniform recommendation on risk stratification based on sex specific risk factors, such as HDP and subtypes.4 The American Colleges of Obstetrics and Gynaecologists’ Task Force on Hypertension in Pregnancy emphasizes the opportunity that early identification of a group of young women at risk offers for prevention, but appoints solely women with preterm pre-eclampsia or recurrent pre-eclampsia to be eligible for screening, whereas other guidelines do not discriminate between HDP phenotypes.9–12 All guidelines suggest evaluation of blood pressure after HDP, although their recommendation regarding the commencement and the time interval of screening differs.9–13 The recently published Dutch multidisciplinary guideline suggests optimization of modifiable cardiovascular risk factors to reduce risk of future CVD. The recommendation to screen at age 50 was obtained by consensus between the different participating disciplines rather than based on evidence.
Identification of deviating risk factor patterns is required to find a window of opportunity for screening and possibly allows for the creation of preventative programmes to reverse the increased risk of CVD. Established risk factors for CVD as adopted by the American Heart Association and the European Society of Cardiology risk assessment tools are age, sex, blood pressure, diabetes, lipid level, smoking, family history, impaired renal function, physical inactivity and body weight.11,14 Existing evidence on the development of these risk factors in HDPs lacks longer follow-up. Only few studies reported on the fourth and fifth decade of life, the time frame in which the first clinical signs of cardiovascular disease is expected.7
We aim to provide an insight into the risk profile development of women who experienced HDP by performing a systematic review and meta-regression analysis on the course of blood pressure, fasting glucose and homeostasis model assessment of insulin resistance (HOMA-IR) as parameters for glucose metabolism and high-density lipoprotein cholesterol (HDL-C) and low-density lipoprotein cholesterol (LDL-C) in HDPs compared with a reference cohort.
Methods
Data sources and searches
A systematic literature search in PubMed, Embase and The Cochrane Library was conducted. No filters were used, nor restrictions on publication year. Relevant synonyms for [history of hypertensive disorders of pregnancy] and [risk factor#] (#blood pressure OR glucose OR insulin OR HOMA-IR OR HDL-C OR LDL-C) were combined. After title and abstract screening by TKJG, remaining articles were screened on full text. Screening was performed based on the article’s content meeting our inclusion criteria. The reference lists of selected articles, related reviews and meta-analyses were manually searched for additional eligible articles. Detailed search strategies are described in supplement 1 in the Supplementary Material online.
Eligibility criteria
We included prospective and retrospective cohort studies assessing women of any parity or age or with a history of a HDP. A cohort study was defined as a study that identified HDP as determinant and reported on selected risk factors as outcome. The relevance and validity of the studies was assessed using the Newcastle–Ottawa Scale15 with some additions according to the evidence-based medicine guidelines as stated by Scholten et al.16 (Supplementary Material supplement 2 – critical appraisal table and legend). Relevance of the study findings for applicability involved evaluation of the study population and reported outcomes (the prementioned risk factors). Studies in women with pre-existing cardiovascular co-morbidities – such as pre-existent hypertension, renal failure and cardiac disease – prior to the index pregnancy were excluded from the analysis. Also studies conducted in women with previous early pre-eclampsia were excluded, for the pathophysiological mechanisms including vascular remodelling and risk factors associated with this type of HDP are thought to be different and thus the course to later CVD might be too.17,18 Classification of the validity of the studies consisted of the evaluation of: selective inclusion, standardization of HDP and outcome and completeness of data, respectively loss to follow-up and missing data. Minimally, HDP status assessment was conducted through clinical and laboratory diagnostics. Assessment of HDP status based on recall through a questionnaire was considered of insufficient reliability. We also excluded articles that reported on only prevalence of the risk factor (e.g. hypertension, diabetes) rather that mean values for the derivative measurement (blood pressure, fasting glucose). Due to differences in the definition of the risk factors, these were considered too heterogenic to incorporate into a reliable meta-analysis. Each relevance and validity criterion was classified with a two or three point scoring system. Only articles of sufficient methodological quality were included in the meta-analysis.
Statistical analysis
Studies were divided into groups based on years of follow-up from index pregnancy. Per category weighted mean values (χw = ) and standard deviations (variance σ2 ; standard deviation σ = ) were calculated. Given weighted means and standard deviations, number of participants with values above treatment thresholds could be calculated using Excel normal distribution formulas.19
Per risk factor, weighted mean values of women with a history of HDP were compared with reference cohorts. Blood pressure data were compared with the data of the Third National Health And Nutrition Examination Survey (NHANES III; 1988–1994), which consisted of 15.326 female US natives of 18–74 years old.20 Officially, the definition of hypertension contains both a systolic (140 mmHg) and diastolic (90 mmHg) threshold. However, since we rely on reported cohort means, we were unable to extract individual data to see if patients would meet either of these criteria. As cardiovascular risk scores such as SCORE and SMART have their risk estimation based on systolic pressure we decided to define hypertension based on the systolic blood pressure only.11,21
The weighted mean fasting glucose values of all cases calculated as described above are compared with 11,148 women, citizens of Hong Kong.22 Age category specific weighted mean fasting insulin and glucose were used to calculate homeostasis model assessment of insulin resistance (HOMA-IR), if not already reported by the selected articles. The ATP III (Third Adult Treatment Panel) age specific HOMA-IR values for non-diabetic women were used as reference, as these were reported most sensitive and specific in the EPIRCE study (N = 1329).23 The data from the 1994–2002 NHANES cohort were used as control cohort for HDL-C and LDL-C levels data based on 4549 female adults of 20 years and older.24
Results
Study selection
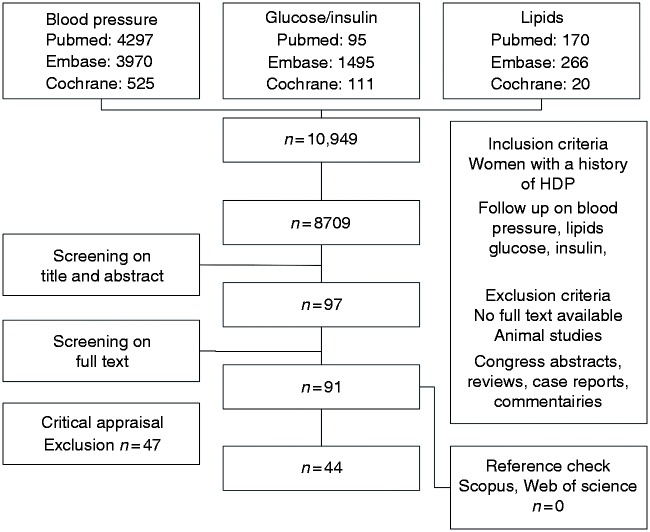
The systematic search (supplement 1, Supplementary Material) yielded 8709 unique studies. Figure 1 shows the articles retrieved from the searches, reviewed and included in the analysis. After selection based on title and abstract and subsequent full-text screening, 91 articles were considered potentially eligible for answering our question. Thirteen articles were excluded based on determinant discrepancies25–37 (supplement 2 – critical appraisal Table 1). Four studies were at risk for selection bias.38–41 The eligible outcomes were missing or reported with incompatible stratification in seven studies.42–49 Three studies used a questionnaire to establish previous HDP, which was thought to be of insufficient reliability.37,50,51 Fifteen articles were excluded from analysis for they reported only prevalences of hypertension, diabetes or dyslipidaemia and not mean values.52–67 Last, two studies reported too large proportion of loss to follow-up.68,69 When two manuscripts were published on the same cohort, the oldest was excluded.70–72
Figure 1.
Flowchart (last search 20 October 2015).
HDP: hypertensive disorder during pregnancy
Study characteristics
Table 2a in supplement 3 lists the characteristics of the 36 studies reporting on blood pressure. The majority of the studies report on women with a history of pre-eclampsia.35,72–96 Sample sizes differ from 2679 to 322588 participants. In total the analysis was performed with 36 studies, consisting of 6904 HDPs.35,65,72–95,97–107 Only two studies reported on women over the age of 55 years.80,96 All studies report on non-recurrent complications of pregnancy. If reported, phenotype of HDP is specified: pre-eclampsia, pregnancy induced hypertension (PIH) or both (HDP).
Baseline characteristics of the studies on fasting glucose, fasting insulin, HOMA-IR, HDL-C and LDL-C are displayed in Tables 2b and 2c (supplement 3).
Blood pressure
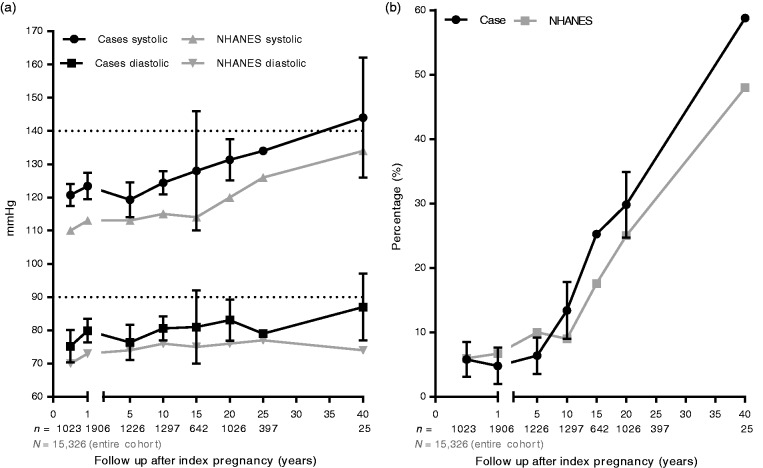
HDPs had an overall higher mean blood pressure (Figure 2(a)) compared with controls.35,65,72–95,97–107 The large standard deviation in follow-up categories 15–20 years and 40–45 years post-partum can be explained by the small sample sizes, for both categories consist of only two studies.65
Figure 2.
(a) Weighted mean blood pressure. (b) Prevalence of hypertension. Sample sizes are shown below the x-axis. Ranges or standard deviations are missing in the plots, since these are not reported in the NHANES manuscript.
NHANES: National Health And Nutrition Examination Survey
From 15 years after the index pregnancy onwards, HDPs show hypertension at a higher rate compared with reference populations. At 15 year follow-up, which is approximately at age 45, one in five (+/− 20%) of HDPs suffer from hypertension versus 17.6% in the NHANES cohort (p < 0.0001; estimated with the standard deviation of our analysis applied to NHANES for NHANES did not report on standard deviations). This is illustrated in Figure 2(b), which displays the calculated percentages of hypertension, derived from the raw data on mean values and deviations per follow-up category. The NHANES III cohort is used as reference, displaying values from the age corresponding to the mean age per follow-up category.20
Glucose metabolism and lipids
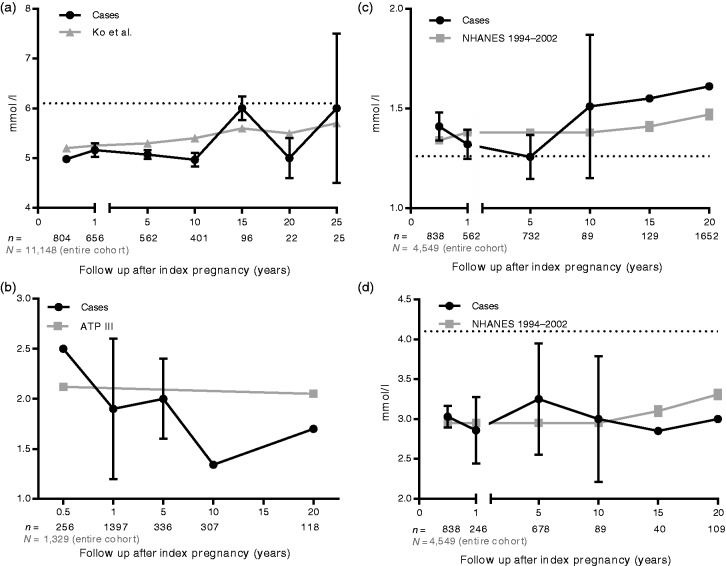
A total of 22 studies in 3032 post-HDP women reporting on glucose homeostasis metabolites were included in the analysis. Sixteen studies reported on post-pre-eclampsia women, four on multiple phenotypes of HDP and two combined all phenotypes into the category HDP. Sample sizes varied from 13108 to 698109 women with previous HDP. Only Haukkamaa et al.80 and Collén et al.96 reported on fasting glucose and insulin after the age of 50.
Figure 3(a) and (b) displays mean fasting glucose and HOMA-IR over follow-up time after index pregnancy.35,73,80,81,83,84,89,94–96,98,99,102,103,107–115 In the analysis of the course of fasting glucose development over time, no difference between HDPs and controls can be detected. The control cohort for the fasting glucose is formed by 11,148 Chinese women from the Hong Kong cohort of Ko et al.22 The age specific thresholds according to ATP III as calculated in the EPIRCE trial were used to compare with HOMA-IR data in the HDPs.116
Figure 3.
(a) Weighted mean fasting glucose. (b) Homeostasis model assessment of insulin resistance. (c) Weighted mean high-density lipoprotein cholesterol. (d) Weighted mean low-density lipoprotein cholesterol. Sample sizes are shown below the x-axis.
NHANES: National Health And Nutrition Examination Survey; ATP III: Third Adult Treatment Panel
In all 24 studies combined, 5304 former HDP women contributed to the analysis on lipid spectrum. Weighted mean values for HDL-C and LDL-C are displayed in Figure 3(c) and (d).35,71,72,74,80,81,84,89,91,95,99–103,106–111,113,114,117–119 The large standard deviation for mean HDL-C in the 10-year post-partum group is because this category yields only one study.89
Discussion
This meta-regression analysis shows that HDPs have an overall higher blood pressure during post-partum follow-up. Furthermore, HDPs develop hypertension at a faster pace compared with the reference cohort, reporting on 20% hypertension in HDPs at 15 years post-partum (approximately age 45).20 In current practice, women with a history of HDP are discharged from the obstetrics department without a strategy for cardiovascular follow-up although the increased risk of future CVD is evident.120 Current guidelines are not uniform in their advice on screening and treatment in women with a history of HDP.9-13 These data do not show a specific time-frame for screening. A stepwise approach in screening starting at a young age seems to be the most appropriate strategy. Possibly also other risk factors then the classical cardiovascular factors could be of value in this high risk female population.
First we show an overall higher blood pressure without increase in difference over time after HDP. Second, our analysis on blood pressure and subsequently calculated hypertension rates shows hypertension prevalence from 5% up to 59% at 40 year follow-up after index pregnancy. Interestingly, when looking at hypertension rates until 10 years post-partum the NHANES cohort shows higher rates.20 This could be due to a variable number of factors: medication use, pooling of data, non-pregnant subjects (including infertile women). Growing evidence suggests that female infertility is associated with increased cardiovascular risk.121
As explained, we excluded studies that reported on hypertension only, because of differences in definition. These excluded studies report hypertension rates of 10–65%.35,65,72,73,77–81,84,88,89,93,96,98,99,112,114,122,123 The difference between reported prevalence of hypertension is striking; it could imply that our result underestimates the number of women with hypertension after a HDP. Also, prevalence in the excluded studies might have been subject to publication bias. The results presented on glucose homeostasis parameters did not show any difference in course of development when comparing post-HDP women with the general population. Feig et al. did find a two-fold increased risk of developing diabetes when followed up to 16.5 years after pregnancy complicated by HDP.56 Our analysis is possibly restricted by sample size in higher age categories, reporting on measurements in only 22–26 participants. Furthermore, the discrepancy might be explained by confounding variables, in particular, obesity, which in itself is associated with insulin resistance, and is a well-known risk factor of HDP and hypertension.124 HOMA-IR development shows a decline in the first year post-partum, possibly indicating a remission period after physiological insulin resistance during pregnancy.125 The World Health Organization global report on diabetes shows prevalence of diabetes in Asia to be 8.7% versus 7.3% in Europe.126 Our meta-regression analysis involves 26 studies from Europe, two from Asia, 10 from the Americas and three from the Western Pacific. The risk of glucose homeostasis disturbances in the pooled population of our analysis possibly is more towards the European average. Comparing this with the Hong Kong cohort might thus have underestimated the difference between HDPs and the ‘general’ population.
Regarding lipid levels, small studies conducted pre-pregnancy and shortly post-partum reported unfavourable alterations in lipid spectrum in HDPs compared with controls.31,81,84,127 However, in larger cohort studies with longer follow-up, this difference resolves.64 Finally, in our analysis, with a mean follow-up of eight years (range 0.1–23 years) the course of HDL-C and LDL-C over time did not deviate in HDPs compared with reference cohorts. Dyslipidaemia rates among all female residents are 52% in Europe and 48% in the Americas, resulting in an overestimation of our analysis. But of the 23 studies on lipids in our analysis, eight were of Dutch origin, with an average prevalence 22.4% of dyslipidaemia in the female population.128
The interpretation of our findings is hindered by a few limitations. The studies included in our analysis mostly report on pre-eclampsia. Hypertension in pregnancy is present in approximately 10% of pregnancy; pre-eclampsia in 2–8% of pregnancy.129 It is thought that the severity of the event resembles the amount of cardiovascular distress and consecutive risk of CVD later in life.130 By pooling all studies, containing different phenotypes of HDP assessed at different moments in time, we might have overestimated the real effect of HDP. Separate analysis of the HDP phenotypes (early onset pre-eclampsia, late onset pre-eclampsia, pregnancy induced hypertension) resulted in insufficient sample size for meta-analysis. Therefore, we chose to merge the results into one group of post-HDP women. Meta-regression does not allow correction for confounding (including difference in body mass index, age, race, smoking, family history). Methodological limitations, embedded in the nature of a meta-regression analysis, including publication bias, have to be taken into consideration.
The consensus for screening in the HDP population is inconsistent. The evidence presented in this paper and other meta-analyses is consistent in terms of blood pressure, showing increased hypertension risk from the age of 45 years onwards. The absolute risk for a cardiovascular event remains low because these are age driven and our population of interest is young. It is understandable that screening strategies are so diverse since this absolute risk is leading in screening implementation.131 Second, treatment strategy is not well investigated. Furthermore, it is unclear who is the highest risk (PIH versus pre-eclampsia, early versus late onset, with and without premature birth and foetal growth restriction). We question whether future research should not be redirected towards the development of a stepwise screening programme taking all different mechanisms (classical risk factors, HDP phenotype, genetics, etc.) into account. Combined with lifestyle behaviour and family history of CVD, risk stratification and consecutive treatment (non-pharmacological and pharmacological) targets and follow-up can be established. Since the absolute risk is so low, the first appropriate step would be lifestyle intervention.132–134
Perspectives
In conclusion, this systematic review and meta-regression analysis confirmed an altered onset and development of hypertension in women with a history of HDP as compared with women without these pregnancy complications. We could not identify an optimal time frame for screening. Interestingly, guidelines published in the last six years have made different recommendations. We could not perform sub analysis on HDP phenotype. Phenotyping and other (non-)classical and/or stepwise CVD risk factor screening and, possibly, incorporating more mechanistic research could be the future direction to improve CVD health in this high risk female population.
Supplementary Material
Author contribution
TKJG, BBR, JERL, MLB and ATL contributed to the conception or design of the work. TKJG performed searches and article selections. TKJG, BBR, AF, JERL, MLB and ATL contributed to the analysis and/or interpretation of data for the work. TKJG drafted the manuscript. BBR, AF, JERL, MLB and ATL critically revised the manuscript. All gave final approval and agree to be accountable for all aspects of work ensuring integrity and accuracy.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: AT Lely is supported by the ZonMW Clinical Fellowship grant.
References
- 1.Brown MC, Best KE, Pearce MS, et al. Cardiovascular disease risk in women with pre-eclampsia: Systematic review and meta-analysis. Eur J Epidemiol 2013; 28: 1–19. [DOI] [PubMed] [Google Scholar]
- 2.Bellamy L, Casas JP, Hingorani AD, et al. Pre-eclampsia and risk of cardiovascular disease and cancer in later life: Systematic review and meta-analysis. BMJ 2007; 335: 974–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hermes W, van Kesteren F, de Groot CJ. Preeclampsia and cardiovascular risk. Minerva Ginecol 2012; 64: 281–292. [PubMed] [Google Scholar]
- 4.Staff AC, Redman CW, Williams D, et al. Pregnancy and long-term maternal cardiovascular health: Progress through harmonization of research cohorts and biobanks. Hypertension 2016; 67: 251–260. [DOI] [PubMed] [Google Scholar]
- 5.Parikh NI, Cnattingius S, Dickman PW, et al. Parity and risk of later-life maternal cardiovascular disease. Am Heart J 2010; 159: 215–221.e6. [DOI] [PubMed] [Google Scholar]
- 6.Sattar N, Greer I. Pregnancy complications and maternal cardiovascular risk: Opportunities for intervention and screening? BMJ 2002; 323: 157–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zoet GA, Koster MP, Velthuis BK, et al. Determinants of future cardiovascular health in women with a history of preeclampsia. Maturitas 2015; 82: 153–161. [DOI] [PubMed] [Google Scholar]
- 8.Mosca F, Benjamin EJ, Berra K. Guide to Preventive cardiology for women. Circulation 1999; 99: 2480–2484. [DOI] [PubMed] [Google Scholar]
- 9.Mosca L, Benjamin EJ, Berra K, et al. Effectiveness-based guidelines for the prevention of cardiovascular disease in women – 2011 update: A guideline from the American Heart Association. Circulation 2011; 123: 1243–1262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.National Institute for Health and Clinical Excellence. Hypertension in pregnancy: The management of hypertensive disorders during pregnancy, London: RCOG Press, 2010. [PubMed] [Google Scholar]
- 11.Piepoli MF, Hoes AW, Agewall S, et al. 2016 European Guidelines on cardiovascular disease prevention in clinical practice: The Sixth Joint Task Force of the European Society of Cardiology and Other Societies on Cardiovascular Disease Prevention in Clinical Practice (constituted by representatives of 10 societies and by invited experts): Developed with the special contribution of the European Association for Cardiovascular Prevention & Rehabilitation (EACPR). Eur J Prev Cardiol 2016; 23: NP1–NP96. [DOI] [PubMed] [Google Scholar]
- 12.Heida KY, Bots ML, De Groot CJ, et al. Cardiovascular risk management after reproductive and pregnancy-related disorders: A Dutch multidisciplinary evidence-based guideline. Eur J Prev Cardiol 2016; 23: 1863–1879. [DOI] [PubMed] [Google Scholar]
- 13.ACOG. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol 2013; 122: 1122–1131. [DOI] [PubMed] [Google Scholar]
- 14.Goff DC, Lloyd-Jones DM, Bennett G, et al. 2013 ACC/AHA Guideline on the assessment of cardiovascular risk. J Am Coll Cardiol 2014; 63: 2935–2959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wells GA, Shea B, O’Connell D, et al. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomized studies in meta-analyses, http://www.ohri.ca/programs/clinical_epidemiology/oxford.htm (2009, accessed 20 October 2015).
- 16.Scholten RJPM, Offringa M, Assendelft WJJ. Inleiding in evidence-based medicine. Klinisch handelen gebaseerd op bewijsmateriaal, Houten: Bohn Stafleu van Loghum, 2013. [Google Scholar]
- 17.Lisonkova S, Joseph KS. Incidence of preeclampsia: Risk factors and outcomes associated with early- versus late-onset disease. Am J Obstet Gynecol 2013; 209: 544.e1–e12. [DOI] [PubMed] [Google Scholar]
- 18.Stergiotou I, Crispi F, Valenzuela-Alcaraz B, et al. Patterns of maternal vascular remodeling and responsiveness in early- versus late-onset preeclampsia. Am J Obstet Gynecol 2013; 209: 558.e1–e14. [DOI] [PubMed] [Google Scholar]
- 19.Carlberg C. Statistical analysis: Microsoft Excel 2010. Indianapolis: QUE, 2011.
- 20.Joffres MR, Hamet P, MacLean DR, et al. Distribution of blood pressure and hypertension in Canada and the United States. Am J Hypertens 2001; 14: 1099–1105. [DOI] [PubMed] [Google Scholar]
- 21.Dorresteijn JA, Visseren FL, Wassink AM, et al. Development and validation of a prediction rule for recurrent vascular events based on a cohort study of patients with arterial disease: The SMART risk score. Heart 2013; 99: 866–872. [DOI] [PubMed] [Google Scholar]
- 22.Ko GTC, Wai HPS, Tang JSF. Effects of age on plasma glucose levels in non-diabetic Hong Kong Chinese. Croat Med J 2006; 47: 709–713. [PMC free article] [PubMed] [Google Scholar]
- 23.Gayoso-Diz P, Otero-Gonzalez A, Rodriguez-Alvares MX, et al. Insulin resistance (HOMA-IR) cut-off values and the metabolic syndrome in a general adult population: Effect of gender and age: EPIRCE cross-sectional study. BMC Endocr Disord 2013; 13. [DOI] [PMC free article] [PubMed]
- 24.Carroll MD, Lacher DA, Sorlie P, et al. Trends in serum lipids and lipoproteins of adults, 1960–2002. JAMA 2005; 294: 1773–1781. [DOI] [PubMed] [Google Scholar]
- 25.Gaugler-Senden IP, Berends AL, de Groot CJ, et al. Severe, very early onset preeclampsia: Subsequent pregnancies and future parental cardiovascular health. Eur J Obstet Gynecol Reprod Biol 2008; 140: 171–177. [DOI] [PubMed] [Google Scholar]
- 26.Goynumer G, Yucel N, Adali E, et al. Vascular risk in women with a history of severe preeclampsia. J Clin Ultrasound 2013; 41: 145–150. [DOI] [PubMed] [Google Scholar]
- 27.Gratacos E, Casals E, Gomez O, et al. Increased susceptibility to low density lipoprotein oxidation in women with a history of pre-eclampsia. Br J Obstet Gynaecol 2003; 110: 400–404. [PubMed] [Google Scholar]
- 28.Hubel CA, Powers RW, Snaedal S, et al. C-reactive protein is elevated 30 years after eclamptic pregnancy. Hypertension 2008; 51: 1499–1505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hubel CA, Snaedal S, Ness RB, et al. Dyslipoproteinaemia in postmenopausal women with a history of eclampsia. Br J Obstet Gynaecol 2000; 107: 776–784. [DOI] [PubMed] [Google Scholar]
- 30.Kaze FF, Njukeng FA, Kengne AP, et al. Post-partum trend in blood pressure levels, renal function and proteinuria in women with severe preeclampsia and eclampsia in Sub-Saharan Africa: A 6-months cohort study. BMC Pregnancy Childbirth 2014; 14: 134–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Germain AM, Romanik MC, Guerra I, et al. Endothelial dysfunction: A link among preeclampsia, recurrent pregnancy loss, and future cardiovascular events? Hypertension 2007; 49: 90–95. [DOI] [PubMed] [Google Scholar]
- 32.Scholten RR, Hopman MT, Sweep FC, et al. Co-occurrence of cardiovascular and prothrombotic risk factors in women with a history of preeclampsia. Obstet Gynecol 2013; 121: 97–105. [DOI] [PubMed] [Google Scholar]
- 33.Habli M, Eftekhari N, Wiebracht E, et al. Long-term maternal and subsequent pregnancy outcomes 5 years after hemolysis, elevated liver enzymes, and low platelets (HELLP) syndrome. Am J Obstet Gynecol 2009; 201: 385.e1–e5. [DOI] [PubMed] [Google Scholar]
- 34.Van Rijn BB, Heida KY, Veerbeek JH, et al. Pregnancy as a cardiovascular challenge test: Evaluation of hypertension and diabetes in pregnancy as predictors for cardiovascular disease among 15,175 women aged 49–70 years. Reprod Sci 2013; 20: 105A–105A. [Google Scholar]
- 35.Van Rijn BB, Nijdam ME, Bruinse HW, et al. Cardiovascular disease risk factors in women with a history of early-onset preeclampsia. Obstet Gynecol 2013; 121: 1040–1048. [DOI] [PubMed] [Google Scholar]
- 36.Van Rijn BB, Veerbeek JH, Scholtens LC, et al. C-reactive protein and fibrinogen levels as determinants of recurrent preeclampsia: A prospective cohort study. J Hypertens 2014; 32: 408–414. [DOI] [PubMed] [Google Scholar]
- 37.Catov JM, Newman AB, Sutton-Tyrrell K, et al. Parity and cardiovascular disease risk among older women: How do pregnancy complications mediate the association? Ann Epidemiol 2008; 18: 873–879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chambers JC, Fusi L, Malik IS, et al. Association of maternal endothelial dysfunction with preeclampsia. JAMA 2011; 285: 1607–1612. [DOI] [PubMed] [Google Scholar]
- 39.He S, Silveira A, Hamsten A, et al. Haemostatic, endothelial and lipoprotein parameters and blood pressure levels in women with a history of preeclampsia. Thromb Haemost 1999; 81: 538–542. [PubMed] [Google Scholar]
- 40.Henriques AC, Carvalho FH, Feitosa HN, et al. Endothelial dysfunction after pregnancy-induced hypertension. Int J Gynaecol Obstet 2014; 124: 230–234. [DOI] [PubMed] [Google Scholar]
- 41.Paradisi G, Biaggi A, Savone R, et al. Cardiovascular risk factors in healthy women with previous gestational hypertension. J Clin Endocrinol Metab 2006; 91: 1233–1238. [DOI] [PubMed] [Google Scholar]
- 42.Agatisa PK, Ness RB, Roberts JM, et al. Impairment of endothelial function in women with a history of preeclampsia: An indicator of cardiovascular risk. Am J Physiol Heart Circ Physiol 2004; 286: H1389–H1393. [DOI] [PubMed] [Google Scholar]
- 43.Ghossein-Doha C, Spaanderman M, van Kuijk SMJ, et al. Long-term risk to develop hypertension in women with former preeclampsia: A longitudinal pilot study. Reprod Sci 2014; 21: 846–853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Magnussen EB, Vatten LJ, Smith GD, et al. Hypertensive disorders in pregnancy and subsequently measured cardiovascular risk factors. Obstet Gynecol 2009; 114: 961–970. [DOI] [PubMed] [Google Scholar]
- 45.Melchiorre K, Sutherland G, Baltabaeva A, et al. Preeclampsia is associated to persistent long-term postpartum left ventricular myocardial injury. Pregnancy Hypertens 2011; 1: 270–270. [DOI] [PubMed] [Google Scholar]
- 46.Stekkinger E, Zandstra M, Peeters LL, et al. Early-onset preeclampsia and the prevalence of postpartum metabolic syndrome. Obstet Gynecol 2009; 114: 1076–1084. [DOI] [PubMed] [Google Scholar]
- 47.Srinivas SK, Sammel MD, Bastek J, et al. Evaluating the association between all components of the metabolic syndrome and pre-eclampsia. J Matern Fetal Neonat Med 2009; 22: 501–509. [DOI] [PubMed] [Google Scholar]
- 48.Tooher J, Chiu CL, Yeung K, et al. High blood pressure during pregnancy is associated with future cardiovascular disease: An observational cohort study. BMJ Open 2013; 3. [DOI] [PMC free article] [PubMed]
- 49.Visser VS, Hermes W, Franx A, et al. High blood pressure six weeks postpartum after hypertensive pregnancy disorders at term is associated with chronic hypertension. Pregnancy Hypertens 2013; 3: 242–247. [DOI] [PubMed] [Google Scholar]
- 50.Kharazmi E, Kaaja R, Fallah M, et al. Pregnancy-related factors and the risk of isolated systolic hypertension. Blood Press 2007; 16: 50–55. [DOI] [PubMed] [Google Scholar]
- 51.Brown CM, Turner ST, Bailey KR, et al. Hypertension in pregnancy is associated with elevated C-reactive protein levels later in life. J Hypertens 2013; 31: 2213–2219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Carr DB, Newton KM, Utzschneider KM, et al. Preeclampsia and risk of developing subsequent diabetes. Hypertens Pregnancy 2009; 28: 435–447. [DOI] [PubMed] [Google Scholar]
- 53.Drost JT, Arpaci G, Ottervanger JP, et al. Cardiovascular risk factors in women 10 years post early preeclampsia: Baseline data from the preeclampsia risk evaluation in females study (prevfem). J Am Coll Cardiol 2011; 57: E487–E487. [DOI] [PubMed] [Google Scholar]
- 54.Edlow AG, Srinivas SK, Elovitz MA. Investigating the risk of hypertension shortly after pregnancies complicated by preeclampsia. Am J Obstet Gynecol 2009; 200: e60–e62. [DOI] [PubMed] [Google Scholar]
- 55.Engeland A, Bjorge T, Daltveit AK, et al. Risk of diabetes after gestational diabetes and preeclampsia. A registry-based study of 230,000 women in Norway. Eur J Epidemiol 2011; 26: 157–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Feig DS, Shah BR, Lipscombe LL, et al. Preeclampsia as a risk factor for diabetes: A population-based cohort study. PLoS Med 2013; 10: e1001425–e1001425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hannaford P, Ferry S, Hirsch S. Cardiovascular sequelae of toxaemia of pregnancy. Heart 1997; 77: 154–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Koual M, Abbou H, Carbonnel M, et al. Short-term outcome of patients with preeclampsia. Vasc Health Risk Manag 2013; 9: 143–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Libby G, Murphy DJ, McEwan NF, et al. Pre-eclampsia and the later development of type 2 diabetes in mothers and their children: An intergenerational study from the Walker cohort. Diabetologia 2007; 50: 523–530. [DOI] [PubMed] [Google Scholar]
- 60.Lykke JA, Langhoff-Roos J, Sibai BM, et al. Hypertensive pregnancy disorders and subsequent cardiovascular morbidity and type 2 diabetes mellitus in the mother. Hypertension 2009; 53: 944–951. [DOI] [PubMed] [Google Scholar]
- 61.Mannisto T, Karumanchi SA, Pouta A, et al. Preeclampsia, gestational hypertension and subsequent hypothyroidism. Pregnancy Hypertens 2013; 3: 21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Wilson BJ, Watson MS, Prescott GJ, et al. Hypertensive diseases of pregnancy and risk of hypertension and stroke in later life: Results from cohort study. BMJ 2003; 326: 845–845. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Callaway LK, Mamun A, McIntyre HD, et al. Does a history of hypertensive disorders of pregnancy help predict future essential hypertension? Findings from a prospective pregnancy cohort study. J Hum Hypertens 2013; 27: 309–314. [DOI] [PubMed] [Google Scholar]
- 64.Marin R, Gorostidi M, Portal CG, et al. Long-term prognosis of hypertension in pregnancy. Hypertens Pregnancy 2000; 19: 199–209. [DOI] [PubMed] [Google Scholar]
- 65.Drost JT, van der Schouw YT, Maas AH, et al. Longitudinal analysis of cardiovascular risk parameters in women with a history of hypertensive pregnancy disorders: The Doetinchem Cohort Study. Br J Obstet Gynaecol 2013; 120: 1333–1339. [DOI] [PubMed] [Google Scholar]
- 66.Kesteren FV, Visser VS, Hermes W, et al. Secondary preventive interventions of cardiovascular risk in women who had hypertension during pregnancy after 36 weeks gestation. Pregnancy Hypertens 2011; 1: 267–268. [DOI] [PubMed] [Google Scholar]
- 67.Wang IK, Tsai IJ, Chen PC, et al. Hypertensive disorders in pregnancy and subsequent diabetes mellitus: A retrospective cohort study. Am J Med 2012; 125: 251–257. [DOI] [PubMed] [Google Scholar]
- 68.Aykas F, Solak Y, Erden A, et al. Persistence of cardiovascular risk factors in women with previous preeclampsia: A long-term follow-up study. J Investig Med 2015; 63: 641–645. [DOI] [PubMed] [Google Scholar]
- 69.Kurabayashi T, Mizunuma H, Kubota T, et al. Pregnancy-induced hypertension is associated with maternal history and a risk of cardiovascular disease in later life: Japanese cross-sectional study. Maturitas 2013; 75: 227–231. [DOI] [PubMed] [Google Scholar]
- 70.Lampinen KH, Rönnback M, Kaaja RJ, et al. Impaired vascular dilatation in women with a history of pre-eclampsia. J Hypertens 2006; 24: 751–756. [DOI] [PubMed] [Google Scholar]
- 71.Portelinha A, Belo L, Cerdeira AS, et al. Lipid levels including oxidized LDL in women with history of preeclampsia. Hypertens Pregnancy 2010; 29: 93–100. [DOI] [PubMed] [Google Scholar]
- 72.Forest JC, Girouard J, Masse J, et al. Early occurrence of metabolic syndrome after hypertension in pregnancy. Obstet Gynecol 2005; 105: 1373–1380. [DOI] [PubMed] [Google Scholar]
- 73.Alsnes IV, Janszky I, Forman MR, et al. A population-based study of associations between preeclampsia and later cardiovascular risk factors. Am J Obstet Gynecol 2014; 211: 657.e1–7. [DOI] [PubMed] [Google Scholar]
- 74.Barry DR, Utzschneider KM, Tong J, et al. Intraabdominal fat, insulin sensitivity, and cardiovascular risk factors in postpartum women with a history of preeclampsia. Am J Obstet Gynecol 2015; 213: 104.e1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Blaauw J, Souwer ETD, Coffeng S, et al. Longitudinal study on cardiovascular risk markers in women with a history of severe early-onset preeclampsia. Pregnancy Hypertens 2010; 1: S58–S59. [Google Scholar]
- 76.Breetveld NM, Ghossein-Doha C, van Kuijk SMJ, et al. Cardiovascular disease risk is only elevated in hypertensive, formerly preeclamptic women. Br J Obstet Gynaecol 2015; 122: 1092–1100. [DOI] [PubMed]
- 77.Canti IC, Komlos M, Martins-Costa SH, et al. Risk factors for cardiovascular disease ten years after preeclampsia. Sao Paulo Med J 2010; 128: 10–13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Evans CS, Gooch L, Flotta D, et al. Cardiovascular system during the postpartum state in women with a history of preeclampsia. Hypertension 2011; 58: 57–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fuh MM, Yin CS, Pei D, et al. Resistance to insulin-mediated glucose uptake and hyperinsulinemia in women who had preeclampsia during pregnancy. Am J Hypertens 1995; 8: 768–771. [DOI] [PubMed] [Google Scholar]
- 80.Haukkamaa L, Moilanen L, Kattainen A, et al. Pre-eclampsia is a risk factor of carotid artery atherosclerosis. Cerebrovasc Dis 2009; 27: 599–607. [DOI] [PubMed] [Google Scholar]
- 81.Heidema WM, Scholten RR, Lotgering FK, et al. History of preeclampsia is more predictive of cardiometabolic and cardiovascular risk factors than obesity. Eur J Obstet Gynecol Reprod Biol 2015; 194: 189–193. [DOI] [PubMed] [Google Scholar]
- 82.Kaaja R, Laivuori H, Laakso M, et al. Evidence of a state of increased insulin resistance in preeclampsia. Metabolism 1999; 48: 892–896. [DOI] [PubMed] [Google Scholar]
- 83.Lampinen KH, Ronnback M, Groop PH, et al. A relationship between insulin sensitivity and vasodilation in women with a history of preeclamptic pregnancy. Hypertension 2008; 52: 394–401. [DOI] [PubMed] [Google Scholar]
- 84.Manten GT, Sikkema MJ, Voorbij HA, et al. Risk factors for cardiovascular disease in women with a history of pregnancy complicated by preeclampsia or intrauterine growth restriction. Hypertens Pregnancy 2007; 26: 39–50. [DOI] [PubMed] [Google Scholar]
- 85.McDonald SD, Yusuf S, Walsh MW, et al. Increased cardiovascular risk after pre-eclampsia in women with dysglycaemia. Diabet Med 2013; 30: e1–7. [DOI] [PubMed] [Google Scholar]
- 86.North RA, Simmons D, Barnfather D, et al. What happens to women with preeclampsia? Microalbuminuria and hypertension following preeclampsia. Aust N Z J Obstet Gynaecol 1996; 36: 233–238. [DOI] [PubMed] [Google Scholar]
- 87.Pouta A, Hartikainen AL, Sovio U, et al. Manifestations of metabolic syndrome after hypertensive pregnancy. Hypertension 2004; 43: 825–831. [DOI] [PubMed] [Google Scholar]
- 88.Romundstad PR, Magnussen EB, Smith GD, et al. Hypertension in pregnancy and later cardiovascular risk: common antecedents? Circulation 2010; 122: 579–584. [DOI] [PubMed] [Google Scholar]
- 89.Sandvik MK, Leirgul E, Nygard O, et al. Preeclampsia in healthy women and endothelial dysfunction 10 years later. Am J Obstet Gynecol 2013; 209: 569.e1–e10. [DOI] [PubMed] [Google Scholar]
- 90.Schreurs MP, Cipolla MJ, Al-Nasiry S, et al. Formerly eclamptic women have lower nonpregnant blood pressure compared with formerly pre-eclamptic women: A retrospective cohort study. Br J Obstet Gynaecol 2015; 122: 1403–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Soonthornpun K, Soonthornpun S, Wannaro P, et al. Insulin resistance in women with a history of severe pre-eclampsia. J Obstet Gynaecol Res 2009; 35: 55–59. [DOI] [PubMed] [Google Scholar]
- 92.Smith GN, Walker MC, Liu A, et al. A history of preeclampsia identifies women who have underlying cardiovascular risk factors. Am J Obstet Gynecol 2009; 200: 58.e1–8. [DOI] [PubMed] [Google Scholar]
- 93.Spaan JJ, Ekhart T, Spaanderman ME, et al. Remote hemodynamics and renal function in formerly preeclamptic women. Obstet Gynecol 2009; 113: 853–859. [DOI] [PubMed] [Google Scholar]
- 94.Spaan JJ, Houben AJ, Musella A, et al. Insulin resistance relates to microvascular reactivity 23 years after preeclampsia. Microvasc Res 2010; 80: 417–421. [DOI] [PubMed] [Google Scholar]
- 95.Veerbeek JH, Hermes W, Breimer AY, et al. Cardiovascular disease risk factors after early-onset preeclampsia, late-onset preeclampsia, and pregnancy-induced hypertension. Hypertension 2015; 65: 600–606. [DOI] [PubMed] [Google Scholar]
- 96.Collén AC, Hellgren M, Gustafsson H, et al. Cardiovascular and metabolic characteristics 40 years after hypertensive pregnancies: A long-term follow-up study of mothers. J Hypertens 2013; 31: 758–765. [DOI] [PubMed] [Google Scholar]
- 97.Collén AC, Johansson MC, Guron CW, et al. Cardiac structure and function is related to current blood pressure rather than to previous hypertensive pregnancy. J Hum Hypertens 2015; 29: 702–704. [DOI] [PubMed] [Google Scholar]
- 98.Ehrenthal DB, Rogers S, Goldstein ND, et al. Cardiovascular risk factors one year after a hypertensive disorder of pregnancy. J Womens Health 2015; 24: 23–29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Hashemi S, Ramezani Tehrani F, Mehrabi Y, et al. Hypertensive pregnancy disorders as a risk factor for future cardiovascular and metabolic disorders (Tehran Lipid and Glucose Study). J Obstet Gynaecol Res 2013; 39: 891–897. [DOI] [PubMed] [Google Scholar]
- 100.Hermes W, Franx A, Pampus MG, et al. Cardiovascular risk factors in women who had hypertensive disorders late in pregnancy: A cohort study. Am J Obstet Gynecol 2013; 208: 474.e1–8. [DOI] [PubMed] [Google Scholar]
- 101.Mahmud A, Jatoi M, Chee YR, et al. History of gestational hypertension is associated with the metabolic syndrome and masked hypertension but not arterial stiffness in women with essential hypertension. J Clin Hypertens 2008; 10: 21–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Watanabe K, Kimura C, Iwasaki A, et al. Pregnancy-induced hypertension is associated with an increase in the prevalence of cardiovascular disease risk factors in Japanese women. Menopause 2015; 22: 656–659. [DOI] [PubMed] [Google Scholar]
- 103.Girouard J, Giguere Y, Moutquin JM, et al. Previous hypertensive disease of pregnancy is associated with alterations of markers of insulin resistance. Hypertension 2007; 49: 1056–1062. [DOI] [PubMed] [Google Scholar]
- 104.Freeman DJ, McManus F, Brown EA, et al. Short- and long-term changes in plasma inflammatory markers associated with preeclampsia. Hypertension 2004; 44: 708–714. [DOI] [PubMed] [Google Scholar]
- 105.Hamad RR, Eriksson MJ, Silveira A, et al. Decreased flow-mediated dilation is present 1 year after a pre-eclamptic pregnancy. J Hypertens 2007; 25: 2301–2307. [DOI] [PubMed] [Google Scholar]
- 106.Kvehaugen AS, Dechend R, Ramstad HB, et al. Endothelial function and circulating biomarkers are disturbed in women and children after preeclampsia. Hypertension 2011; 58: 63–69. [DOI] [PubMed] [Google Scholar]
- 107.Vallejo Vaz AJ, Guisado ML, Garcia-Junco PS, et al. Differences in the prevalence of metabolic syndrome and levels of C-reactive protein after puerperium in women with hypertensive disorders during pregnancy. Hypertens Res 2010; 33: 1012–1017. [DOI] [PubMed] [Google Scholar]
- 108.Innes KE, Weitzel L, Laudenslager M. Altered metabolic profiles among older mothers with a history of preeclampsia. Gynecol Obstet Invest 2005; 59: 192–201. [DOI] [PubMed] [Google Scholar]
- 109.Schreurs MP, Cipolla MJ, Al-Nasiry S, et al. Formerly eclamptic women have lower nonpregnant blood pressure compared with formerly pre-eclamptic women: A retrospective cohort study. Br J Obstet Gynaecol 2015; 122: 1403–1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Al-Nasiry S, Ghossein-Doha C, Polman S, et al. Metabolic syndrome after pregnancies complicated by pre-eclampsia or small for gestational age: A retrospective cohort. Br J Obstet Gynaecol 2015; 122: 1818–1823. [DOI] [PubMed]
- 111.Breetveld NM, Ghossein-Doha C, van Kuijk S, et al. Cardiovascular disease risk is only elevated in hypertensive, formerly preeclamptic women. Br J Obstet Gynaecol 2015; 122: 1092–1100. [DOI] [PubMed] [Google Scholar]
- 112.Hermes W, Tamsma JT, Grootendorst DC, et al. Cardiovascular risk estimation in women with a history of hypertensive pregnancy disorders at term: A longitudinal follow-up study. BMC Pregnancy Childbirth 2013; 13: 126–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Mangos GJ, Spaan JJ, Pirabhahar S, et al. Markers of cardiovascular disease risk after hypertension in pregnancy. J Hypertens 2012; 30: 351–358. [DOI] [PubMed] [Google Scholar]
- 114.Sattar N, Ramsay J, Crawford L, et al. Classic and novel risk factor parameters in women with a history of preeclampsia. Hypertension 2003; 42: 39–42. [DOI] [PubMed] [Google Scholar]
- 115.Wolf M, Hubel CA, Lam C, et al. Preeclampsia and future cardiovascular disease: Potential role of altered angiogenesis and insulin resistance. J Clin Endocrinol Metab 2004; 89: 6239–6243. [DOI] [PubMed] [Google Scholar]
- 116.Gayoso-Diz P, Otero-González A, Rodriguez-Alvarez MX, et al. Insulin resistance (HOMA-IR) cut-off values and the metabolic syndrome in a general adult population: Effect of gender and age: EPIRCE cross-sectional study. BMC Endocr Disord 2013; 13: 47–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Andersgaard AB, Acharya G, Mathiesen EB, et al. Recurrence and long-term maternal health risks of hypertensive disorders of pregnancy: A population-based study. Am J Obstet Gynecol 2012; 206: 143.e1–8. [DOI] [PubMed] [Google Scholar]
- 118.Barden AE, Beilin LJ, Ritchie J, et al. Does a predisposition to the metabolic syndrome sensitize women to develop pre-eclampsia? J Hypertens 1999; 17: 1307–1315. [DOI] [PubMed] [Google Scholar]
- 119.McDonald SD, Ray J, Teo K, et al. Measures of cardiovascular risk and subclinical atherosclerosis in a cohort of women with a remote history of preeclampsia. Atherosclerosis 2013; 229: 234–239. [DOI] [PubMed] [Google Scholar]
- 120.Nijdam ME, Timmerman MR, Franx A, et al. Cardiovascular risk factor assessment after pre-eclampsia in primary care. BMC Fam Pract 2009; 10: 77–77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Verit FF, Yildiz Zeyrek F, Zebitay AG, et al. Cardiovascular risk may be increased in women with unexplained infertility. Clin Exp Reprod Med 2017; 44: 28–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Drost JT, Arpaci G, Ottervanger JP, et al. Cardiovascular risk factors in women 10 years post early preeclampsia: The Preeclampsia Risk EValuation in FEMales study (PREVFEM). Eur J Prev Cardiol 2012; 19: 1138–1144. [DOI] [PubMed] [Google Scholar]
- 123.Kaaja R, Kinnunen T, Luoto R. Regional differences in the prevalence of pre-eclampsia in relation to the risk factors for coronary artery disease in women in Finland. Eur Heart J 2005; 26: 44–50. [DOI] [PubMed] [Google Scholar]
- 124.O’Brien TE, Ray JG, Chan WS. Maternal body mass index and the risk of preeclampsia: A systematic overview. Epidemiology 2003; 14: 368–374. [DOI] [PubMed] [Google Scholar]
- 125.Catalano PM, Tyzbir MS, Roman N, et al. Longitudinal changes in insulin release and insulin resistance in nonobese pregnant women. Am J Obstet Gynecol 1991; 165: 1667–1672. [DOI] [PubMed] [Google Scholar]
- 126.World Health Organization. Global report on diabetes, http://apps.who.int/iris/bitstream/10665/204871/1/9789241565257_eng.pdf (2016, accessed 12 May 2017).
- 127.Innes KE, Weitzel L, Laudenslager M. Altered metabolic profiles among older mothers with a history of preeclampsia. Obstet Gynecol Surv 2005; 60: 778–779. [DOI] [PubMed] [Google Scholar]
- 128.World Health Organization. Global health observatory data – raised cholesterol, http://www.who.int/gho/ncd/risk_factors/cholesterol_text/en/ (2008, accessed 12 May 2017).
- 129.Duley L. The global impact of pre-eclampsia and eclampsia. Semin Perinatol 2009; 33: 130–137. [DOI] [PubMed] [Google Scholar]
- 130.Newstead J, von Dadelszen P, Magee LA. Preeclampsia and future cardiovascular risk. Expert Rev Cardiovasc Ther 2007; 5: 283–294. [DOI] [PubMed] [Google Scholar]
- 131.Andermann A, Blancquaert I, Beauchamp S, et al. Revisiting Wilson and Jungner in the genomic age: A review of screening criteria over the past 40 years. Bull World Health Organ 2008; 86: 317–319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Safdar A, Saleem A, Tarnopolsky MA. The potential of endurance exercise-derived exosomes to treat metabolic diseases. Nat Rev Endocrinol 2016; 12: 504–517. [DOI] [PubMed] [Google Scholar]
- 133.Moran AE, Odden MC, Thanataveerat A, et al. Cost-effectiveness of hypertension therapy according to 2014 guidelines. N Engl J Med 2015; 372: 447–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Berks D, Hoedjes M, Raat H, et al. Risk of cardiovascular disease after pre-eclampsia and the effect of lifestyle interventions: A literature-based study. Br J Obstet Gynaecol 2013; 120: 924–931. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.