Abstract
Introduction
CKD273 is a urinary biomarker, which in advanced chronic kidney disease predicts further deterioration. We investigated whether CKD273 can also predict a decline of estimated glomerular filtration rate (eGFR) to <60 ml/min per 1.73 m2.
Methods
In analyses of 2087 individuals from 6 cohorts (46.4% women; 73.5% with diabetes; mean age, 46.1 years; eGFR ≥ 60 ml/min per 1.73 m2, 100%; urinary albumin excretion rate [UAE] ≥20 μg/min, 6.2%), we accounted for cohort, sex, age, mean arterial pressure, diabetes, and eGFR at baseline and expressed associations per 1-SD increment in urinary biomarkers.
Results
Over 5 (median) follow-up visits, eGFR decreased more with higher baseline CKD273 than UAE (1.64 vs. 0.82 ml/min per 1.73 m2; P < 0.0001). Over 4.6 years (median), 390 participants experienced a first renal endpoint (eGFR decrease by ≥10 to <60 ml/min per 1.73 m2), and 172 experienced an endpoint sustained over follow-up. The risk of a first and sustained renal endpoint increased with UAE (hazard ratio ≥ 1.23; P ≤ 0.043) and CKD273 (≥ 1.20; P ≤ 0.031). UAE (≥20 μg/min) and CKD273 (≥0.154) thresholds yielded sensitivities of 30% and 33% and specificities of 82% and 83% (P ≤ 0.0001 for difference between UAE and CKD273 in proportion of correctly classified individuals). As continuous markers, CKD273 (P = 0.039), but not UAE (P = 0.065), increased the integrated discrimination improvement, while both UAE and CKD273 improved the net reclassification index (P ≤ 0.0003), except for UAE per threshold (P = 0.086).
Discussion
In conclusion, while accounting for baseline eGFR, albuminuria, and covariables, CKD273 adds to the prediction of stage 3 chronic kidney disease, at which point intervention remains an achievable therapeutic target.
Keywords: biomarker, chronic kidney disease, clinical science, glomerular filtration rate, peptidomics, proteomics
Chronic kidney disease (CKD), defined as abnormalities of kidney structure or function lasting for more than 3 months,1 is a major health problem affecting 10% of the general population and thereby decreases the quality of life of millions of people.2, 3, 4 Compared with other chronic age-related illnesses, relatively few clinical trials have addressed the prevention of progression in patients with CKD.5 One of the underlying reasons is the long follow-up required to reach severe endpoints, such as doubling of serum creatinine6 or a 50% decrease in the estimated glomerular filtration rate (eGFR),7 the need for renal replacement therapy,6, 7 or death.6 In 2016, the European Medicines Agency (EMA) proposed that primary efficacy endpoints in trials with renal outcomes can be the prevention or delay of renal function decline defined as time to or the incidence rate of stage 3 CKD with or without albuminuria or proteinuria.8 A recent meta-analysis of 1.7 million subjects demonstrated that a 30% reduction in eGFR over 2 years predicted end-stage renal disease and death9 and thereby supported the concept of using shorter-term endpoints as proposed by the EMA.
Capillary electrophoresis coupled with high-resolution mass spectrometry (CE-MS) enables detection of more than 5000 peptide fragments in urine samples.10, 11, 12 CKD273 is a multidimensional urinary biomarker consisting of 273 peptide fragments,10, 13 which in patients with advanced CKD predicts further deterioration of renal function.14, 15 The Food and Drug Administration recently encouraged further studies of CKD273 as a tool for the diagnosis and risk prediction in CKD.13 In keeping with the EMA recommendation8 and the Food and Drug Administration statement,13 the objective of the present study was to investigate whether CKD273 also predicts the incidence of eGFR to less than 60 ml/min per 1.73 m2, at which point intervention16 is still an option before structural alterations associated with later stages of CKD render stopping or reversing the disease processes difficult, if not impossible.
Methods
Participants
The Human Urine Proteome Database available at Mosaiques Diagnostics (Hannover, Germany)17 includes anonymized clinical information on participants enrolled in several studies, as well as urinary proteomic signatures, including CKD273. These studies comply with the Declaration of Helsinki18 and received ethical approval from the pertinent institutional review boards. All participants gave informed written consent. The Ethics Committee of the University of Glasgow authorized the current analysis (approval number 3115-2016). To be eligible for analysis, the following criteria had to be fulfilled: (i) eGFR at baseline of 60 ml/min per 1.73 m2 or higher; (ii) repeat assessment of eGFR during a follow-up of at least 2 years; and (iii) information available on clinically relevant covariables, including sex, age, systolic and diastolic blood pressure, serum creatinine, and albuminuria.
The number of participants in the Human Urine Proteome Database complying with the aforementioned eligibility criteria totaled 2087. Participants were: (i) patients enrolled in the Diabetes Retinopathy Candesartan Trials with type 1 (DIRECT1; n = 740)19 and type 2 (DIRECT2; n = 618)20 diabetes; (ii) patients with type 2 diabetes recruited into a Dutch study (PREvention of DIabetic ComplicaTIONS [PREDICTIONS])21 aimed at identifying disease pathway–specific biomarkers (n = 96), (iii) and patients with diabetes recruited from clinics in Australia (n = 47)22 and Hannover, Germany (n = 22).16 The remaining 564 analyzed individuals were enrolled in the Flemish Study on Environment, Genes and Health Outcomes (FLEMENGHO).23
Assessment of Renal Function
Estimated GFR was calculated from serum creatinine by the Chronic Kidney Disease Epidemiology Collaboration equation.24 The primary renal endpoint was a decrease in eGFR from baseline to follow-up by at least 10 ml/min per 1.73 m2 to less than 60 ml/min per 1.73 m2. A sustained renal endpoint required that the impairment of glomerular function be maintained for at least 3 months with no increase above 60 ml/min per 1.73 m2 at any time during further follow-up. Estimated GFR categories, defined according to the National Kidney Foundation Kidney Disease Outcomes Quality Initiative guidelines,25 were eGFR ≥90, 89–60, 59–45, 44–30, 29–15, and <15 ml/min per 1.73 m2 for stages 1, 2, 3A, 3B, 4, and 5, respectively. In keeping with the suggestion to use declines in eGFR smaller than those associated with a doubling of serum creatinine,9 in a sensitivity analysis, we redefined the renal endpoint as a decrease in eGFR by 30% or more over 2 years.
To harmonize baseline albuminuria across cohorts, we expressed urinary albumin excretion rate (UAE) in micrograms per minute. This involved a conversion from milligrams per 24 hours in the Flemish Study on Environment, Genes and Health Outcomes (FLEMENGHO)23 and a conversion from milligrams per liter in the PREvention of DIabetic ComplicaTIONS (PREDICTIONS) (n = 96) and in the Australian22 (n = 47) and German16 (n = 22) patients, assuming a diuresis of 1500 ml/day. The cutoff threshold for UAE was 20 μg/min, approximately corresponding to a 24-hour albuminuria of 30 mg.26
Measurement of CKD273
Detailed information about urine sample preparation, proteome analysis by capillary-electrophoresis online coupled to electrospray micro time-of-flight mass spectrometry, data processing, and sequencing is available in previous publications.27, 28 CKD273, measured at baseline, is a multidimensional classifier based on 273 urinary peptides derived by support vector machine modeling.10, 13 For normalization of analytical and urine dilution variances, the mass spectrometric signals were normalized by using 29 internal standard peptides present in at least 90% of all urine samples with small SDs.29 In previous studies,11, 30 investigators proposed cutoff thresholds for CKD273 of 0.154 and 0.343 to predict early and advanced CKD.
Statistical Analyses
For database management and statistical analysis, we used the SAS system, version 9.4 (SAS Institute Inc., Cary, NC). Means were compared by using the large-sample z-test, and proportions, by the Fisher exact test. We rank normalized the distributions of albuminuria by sorting measurements from the smallest to the highest and then applying the inverse cumulative normal function.31
Changes in eGFR during follow-up, as predicted by baseline UAE and CKD273, were assessed from mixed models adjusted for sex, age, mean arterial pressure (i.e., diastolic blood pressure plus one-third of the difference between systolic and diastolic blood pressure), and history of diabetes mellitus (categorical variable coded 0 or 1). These mixed models accounted for the varying numbers of follow-up eGFR measurements and clustering of the observations within individuals and study centers. In exploratory analyses, we assessed the cumulative incidence of the primary (first) renal endpoint by quartiles of the distributions of UAE and CKD273, while stratifying for center and adjusting for baseline eGFR, sex, age, mean arterial pressure, and history of diabetes mellitus. Next, we used similarly adjusted proportional hazard regression to model time to the first and sustained renal endpoints. For the calculation of relative risk in the sensitivity analysis, we applied logistic regression.
We assessed the added value of UAE and CKD273 to predict study endpoints from nested Cox models and the log likelihood ratio test and from the integrated discrimination improvement (IDI) and the net reclassification improvement (NRI).32 IDI is the difference between the discrimination slopes of the basic model and the basic model extended with the biomarker. The discrimination slope is the difference in predicted probabilities (%) between subjects with and without endpoint. To calculate NRI, we predicted in each participant the 5-year risk of a renal event from a Cox model with and without the biomarker.32 If P(up/event) is the percentage of subjects with events whose predicted probability is increased by adding the biomarker to the model and if P(up/nonevent) is the percentage of subjects without events whose predicted probability is increased, then NRI = 2 × (P[up/event] – P[up/nonevent]).
Finally, we assessed the performance of published thresholds of UAE26 and CKD27311, 30 from 2-×-2 tables and applied the McNemar test for the pairwise comparison of proportions. Running a published SAS macro33 over our current data, we determined thresholds for UAE and CKD273, yielding a specificity of 90%, and we compared the sensitivity of the so derived UAE and CKD273 thresholds.
Results
Baseline Characteristics of Participants
The entire study group (n = 2087) included 968 women (46.4%), 1533 (73.5%) patients with diabetes mellitus, and 388 (18.6%) patients with hypertension. At baseline, 1959 participants (93.9%) and 1824 (87.4%) did not exceed the thresholds for albuminuria (UAE ≥20 μg/min) or a CKD273 signal indicative of CKD (≥0.154), respectively. Mean values in the entire study population were 46.1 ± 15.2 years for age; 125.9 ± 14.7 mm Hg and 76.9 ± 8.2 mm Hg for systolic and diastolic blood pressure, respectively; 87.5 ± 12.4 μmol/l for serum creatinine; and 81.0 ± 13.0 ml/min per 1.73 m2 for eGFR. Progressors experienced a decrease in eGFR during follow-up of at least 10 ml/min per 1.73 m2 to less than 60 ml/min per 1.73 m2. Progressors compared with nonprogressors (Table 1) were older (P < 0.0001), had higher systolic and mean arterial blood pressure (P < 0.0001), higher UAE (P < 0.0001), and stronger CKD273 signal intensity (P < 0.0001) but had lower eGFR (P < 0.0001).
Table 1.
Baseline characteristics of participants by renal function
| Characteristic | Progressors | Nonprogressors |
|---|---|---|
| Number of participants (%) | ||
| All participants in category | 390 | 1697 |
| Women | 236 (60.5)a | 732 (43.1) |
| Diabetes mellitus | 358 (91.8)a | 1175 (69.2) |
| Hypertension | 110 (28.2)a | 278 (16.4) |
| UAE ≥20 μg/min | 40 (10.3)b | 89 (5.2) |
| CDK273 signal ≥0.154 and <0.343 | 34 (8.7)c | 99 (5.8) |
| CKD273 signal ≥0.343 | 54 (13.8)a | 76 (4.5) |
| Mean of characteristic | ||
| Age (yr) | 55.2 ± 11.7a | 44.0 ± 15.0 |
| Systolic pressure (mm Hg) | 131.4 ± 14.8a | 124.7 ± 14.4 |
| Diastolic pressure (mm Hg) | 77.3 ± 7.5 | 76.8 ± 8.3 |
| Mean arterial pressure (mm Hg) | 95.4 ± 8.8a | 92.7 ± 9.2 |
| Serum creatinine (μmol/L) | 87.5 ± 12.4 | 88.4 ± 12.4 |
| eGFR (ml/min per 1.73 m2) | 73.0 ± 9.93a | 82.8 ± 12.9 |
| UAE (μg/min)c | 6.76 (3.50 to 9.51)a | 5.24 (3.12 to 7.08) |
| CKD273 | –0.17 ± 0.43a | –0.40 ± 0.41 |
| Follow-up (yr) | 4.73 (4.22–4.94)a | 4.50 (4.16–5.11) |
| eGFR data points | 6 (5–6)a | 5 (2–6) |
Averages are arithmetic means ± SD or geometric means (interquartile range). Estimated glomerular filtration rate was derived from serum creatinine by the Chronic Kidney Disease Epidemiology Collaboration formula.
CKD273, urinary proteomic biomarker; eGFR, estimated glomerular filtration rate; UAE, urinary albumin excretion rate.
Progressors had an eGFR decrease by at least 10 ml/min per 1.73 m2 to less than 60 ml/min per 1.73 m2. P values indicate significance of the difference between progressors and nonprogressors.
P ≤ 0.0001.
P ≤ 0.001.
P ≤ 0.05.
Change in eGFR
The median number of eGFR data points was 6 (interquartile range, 5–6) in progressors and 5 (interquartile range, 2–6) in nonprogressors. From baseline to last follow-up, the annual decrease in eGFR averaged 3.97 ml/min per 1.73 m2 (95% confidence interval [CI], 3.62–4.29) in progressors and 1.27 ml/min per 1.73 m2 (95% CI, 1.07–1.49) in nonprogressors. In repeated-measures analyses of all participants covering the entire follow-up, we stratified for center and adjusted estimates for baseline eGFR, sex, age, mean arterial pressure, history of diabetes mellitus, and follow-up duration; and we expressed effect sizes per 1-SD increments in the urinary biomarkers. In models including a single marker, eGFR decreased by 1.20 ml/min per 1.73 m2 (95% CI, 1.00–1.40; P < 0.0001) in relation to UAE and by 1.84 ml/min per 1.73 m2 (CI, 1.64–2.04; P < 0.0001) in relation to CKD273. In models including both biomarkers, the decline in eGFR averaged 1.64 ml/min per 1.73 m2 (95% CI, 1.43–1.84; P < 0.0001) for CKD273 and 0.82 ml/min per 1.73 m2 (95% CI, 0.62–1.02; P < 0.0001) for UAE (P for difference between the 2 markers <0.0001).
Baseline UAE and CKD273 as Continuous Predictors
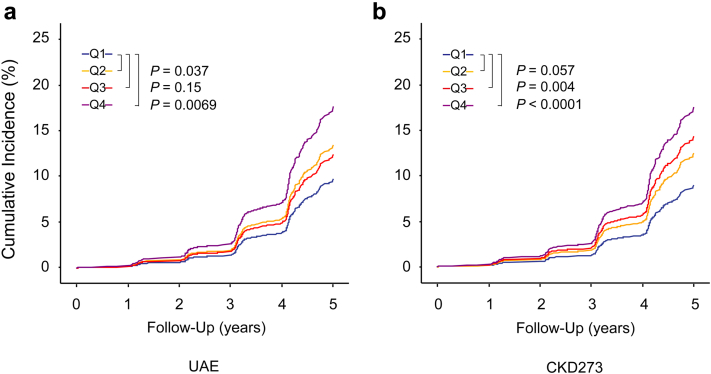
Over a median follow-up of 4.6 years (interquartile range, 4.1–5.1; 5th to 95th percentile interval, 2.1 to 5.7), the 2087 participants experienced 390 primary renal endpoints at a rate of 43.2 per 1000 person-years (95% CI, 41.1–43.3). After the 564 participants with only a single follow-up eGFR assessment were excluded, 172 sustained renal endpoints occurred at a rate of 26.5 (95% CI, 26.4–26.6) per 1000 person-years. Among 390 patients with a primary renal endpoint, at the end of follow-up, 340 had progressed to stage 3A, 44 to stage 3B, 4 to stage 4, and 2 to stage 5. When we had accounted for center, sex, age, mean arterial pressure, the presence of diabetes mellitus, and baseline eGFR, the cumulative incidence of the first (primary) endpoint did not show a coherent gradient across quartiles of the UAE distribution, with the incidence for the low-medium quartile running above that of the medium-high group. In contrast, the cumulative incidence of a first renal endpoint consistently increased from the lowest to highest quartile of CKD273 (Figure 1).
Figure 1.
Cumulative incidence of a first renal endpoint by quartiles of the distributions of urinary albumin excretion rate (UAE) (a) and CKD273 (b). Midpoints of the quartiles (Q1, Q2, Q3, and Q4) were 2.0, 3.5, 6.0, and 13.0 μg/min for UAE and -0.81, -0.52, -0.25, and 0.16 for CKD273. Incidence rates were adjusted for center, sex, age, mean arterial pressure, presence of diabetes mellitus, and baseline estimated glomerular filtration rate. The cumulative incidence of the first (primary) endpoint did not show a consistent gradient across quartiles of the UAE distribution, whereas the cumulative incidence of a first renal endpoint consistently increased from the lowest to highest quartile of CKD273. For UAE, the Q2 line runs above the Q3 line, whereas for CKD273, the 4 lines run according to the order of the quartiles.
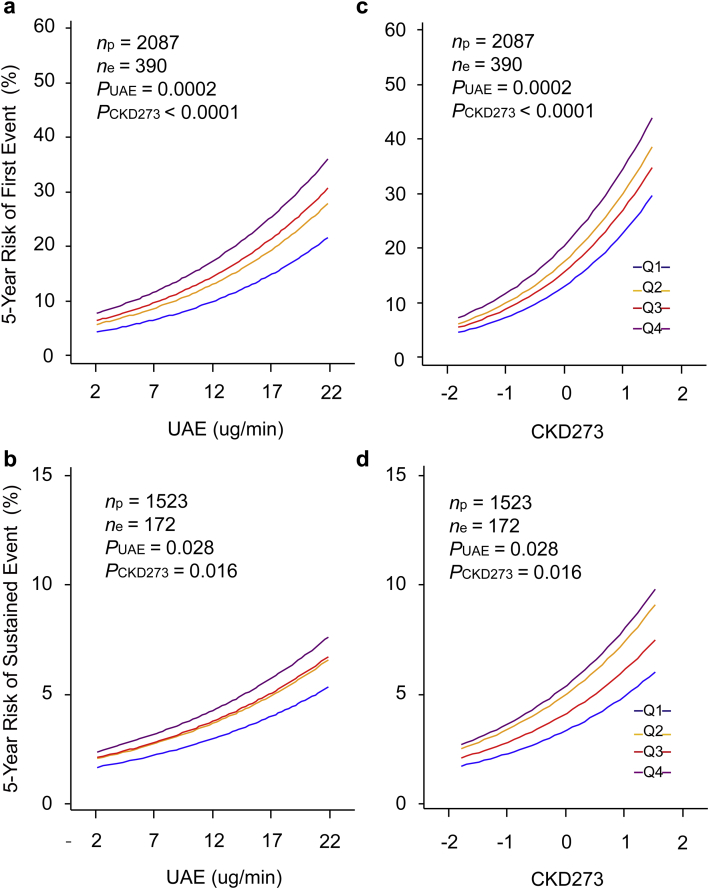
Next, we modeled the risk associated with eGFR, UAE, and CKD273 analyzed as continuous variables. We expressed the risk per 1-SD increments in the biomarkers (Table 2). All models accounted for study center, sex, age, mean arterial pressure, and the presence of diabetes mellitus. If we introduced eGFR, UAE, and CKD273 as single predictors in the multivariable-adjusted models, the hazard ratios for the first renal endpoint were 0.71 (P < 0.0001) for eGFR, 1.29 (P = 0.0002) for UAE, and 1.29 (P < 0.0001) for CKD273; the corresponding point estimates for a sustained renal endpoint were 0.38 (P < 0.0001), 1.19 (P = 0.096), and 1.18 (P = 0.050), respectively. In models additionally accounting for baseline eGFR, the hazard ratios for a first renal endpoint associated with UAE and CKD273 were 1.31 (P < 0.0001) and 1.30 (P < 0.0001), and for a sustained renal endpoint, 1.25 (P = 0.027) and 1.21 (P = 0.020), respectively. The χ2 statistics of the log likelihood ratios (Table 3) confirmed that CKD273 improved the model fit over and beyond baseline eGFR for the first (P < 0.0001) and sustained (P ≤ 0.049) renal endpoints, whereas UAE did so for the first renal endpoint (P ≤ 0.0002) but required the presence in the model of baseline eGFR to reach significance for the sustained renal endpoint (P = 0.027). Similarly, in models that already included baseline eGFR and UAE (Tables 2 and 3), introduction of CKD273 improved the model fit (P ≤ 0.030), yielding hazard ratios of 1.28 (P < 0.0001) and 1.20 (P = 0.031) for the first and sustained renal outcomes, respectively. Figure 2 shows the 5-year absolute risk of the renal endpoints in relation to UAE (Figure 2a and 2c) at different levels of CKD273 and vice versa. It illustrates that in fully adjusted models, both UAE and CKD273 predicted first and sustained renal outcomes (P ≤ 0.028). As continuous variables (Table 4), CKD273 (P = 0.039), but not UAE (P = 0.065), improved the IDI, while both markers increased the NRI (P ≤ 0.0003).
Table 2.
Multivariable-adjusted hazard ratios predicting progression of chronic kidney disease
| Models | First renal endpoint (390 vs. 1697) |
Sustained renal endpoint (172 vs. 1351) |
||
|---|---|---|---|---|
| Hazard ratio | P value | Hazard ratio | P value | |
| Single-biomarker models | ||||
| Baseline eGFR | 0.71 (0.61 to 0.84) | <0.0001 | 0.38 (0.29 to 0.52) | <0.0001 |
| UAE | 1.29 (1.13 to 1.47) | 0.0002 | 1.19 (0.97 to 1.45) | 0.096 |
| CKD273 | 1.29 (1.15 to 1.44) | <0.0001 | 1.18 (1.00 to 1.40) | 0.050 |
| Two-biomarker models | ||||
| Baseline eGFR | 0.70 (0.60 to 0.83) | <0.0001 | 0.37 (0.27 to 0.50) | <0.0001 |
| UAE | 1.31 (1.14 to 1.50) | <0.0001 | 1.25 (1.03 to 1.53) | 0.027 |
| Baseline eGFR | 0.71 (0.61 to 0.83) | <0.0001 | 0.38 (0.28 to 0.51) | <0.0001 |
| CKD273 | 1.30 (1.16 to 1.45) | <0.0001 | 1.21 (1.03 to 1.43) | 0.020 |
| Three-biomarker model | ||||
| Baseline eGFR | 0.50 (0.60 to 0.82) | <0.0001 | 0.37 (0.27 to 0.50) | <0.0001 |
| UAE | 1.27 (1.11 to 1.46) | 0.0004 | 1.23 (1.01 to 1.51) | 0.043 |
| CKD273 | 1.28 (1.14 to 1.43) | <0.0001 | 1.20 (1.02 to 1.42) | 0.031 |
CKD273, urinary proteomic biomarker; eGFR, estimated glomerular filtration rate derived from serum creatinine by the Chronic Kidney Disease Epidemiology Collaboration formula; UAE, urinary albumin excretion rate. Hazard ratios express the increase in risk associated with a 1-SD increase in the baseline biomarkers: 13.1 ml/min per 1.73 m2 for estimated glomerular filtration rate, 1.00 for urinary albumin excretion rate, and 0.41 for CKD273. Associations were stratified by center and accounted for sex, age, mean arterial pressure, and prevalence of diabetes at baseline. Five hundred sixty-four participants had only 1 eGFR follow-up measurement and were not included in the analysis of sustained incidence.
Table 3.
Predictive value of nested Cox regression models
| Model (variables included) | First renal endpoint |
Sustained renal endpoint |
||
|---|---|---|---|---|
| χ2 | P value | χ2 | P value | |
| Basic model (sex, age, MAP, DM) | ||||
| + Baseline eGFR | 17.8 | <0.0001 | 46.6 | <0.0001 |
| + UAE | 13.6 | 0.0002 | 2.79 | 0.094 |
| + CKD273 | 19.4 | <0.0001 | 3.88 | 0.049 |
| Basic model (sex, age, MAP, DM, eGFR) | ||||
| + UAE | 15.4 | <0.0001 | 4.90 | 0.027 |
| + CKD273 | 20.7 | <0.0001 | 5.48 | 0.019 |
| Basic model (sex, age, MAP, DM, eGFR, UAE) | ||||
| + CKD273 | 17.8 | <0.0001 | 4.70 | 0.030 |
CKD273, urinary proteomic biomarker; DM, diabetes mellitus; eGFR, estimated glomerular filtration rate derived from serum creatinine by the Chronic Kidney Disease Epidemiology Collaboration formula; MAP, mean arterial pressure; UAE, urinary albumin excretion rate.
χ2 is the test statistic for the log likelihood ratio with 1 degree of freedom. All basic Cox models were stratified by study center. P values are for the improvement of the fit across nested models.
Figure 2.
Five-year absolute risk of a first (a,c) or sustained (b,d) renal endpoint in relation to urinary albumin excretion rate (UAE) at different levels of CKD273 (a,b) and in relation to CKD273 at different levels of UAE (c,d). The analyses accounted for center, sex, age, mean arterial pressure, presence of diabetes, and estimated glomerular filtration rate at baseline. In (a) and (b), risk functions span the 5th to 95th percentile interval of UAE, and lines represent quartiles of the CKD273 distribution. In (c) and (d), risk functions span the 5th to 95th percentile interval of CKD273 and lines represent quartiles of the UAE distribution. Midpoints of the quartiles (Q1, Q2, Q3, and, Q4) were 2.0, 3.5, 6.0, and 13.0 μg/min for UAE and -0.81, -0.52, -0.25, and 0.16 for CKD273. P values are for the independent effect of UAE (PUAE) and CDK273 (PCKD). np and ne indicate the number of participants at risk and the number of renal endpoints, respectively.
Table 4.
Integrated discrimination improvement and net reclassification improvement by adding CKD273 to the basic model including covariables
| Biomarkers (threshold) | Integrated discrimination improvement |
Net reclassification Improvement |
||||
|---|---|---|---|---|---|---|
| IDI (%) | CI (%) | P value | NRI (%) | CI (%) | P value | |
| UAE continuous | 0.69 | –0.04 to 1.41 | 0.065 | 20.1 | 9.14 to 31.1 | 0.0003 |
| UAE (20 μg/min) | 0.50 | –0.17 to 1.18 | 0.14 | 8.22 | –1.16 to 17.6 | 0.086 |
| CKD273 continuous | 0.86 | 0.04 to 1.68 | 0.039 | 25.8 | 14.9 to 36.8 | <0.0001 |
| CKD273 (0.154) | 0.34 | –0.21 to 0.88 | 0.23 | 23.3 | 14.5 to 32.1 | <0.0001 |
| CKD273 (0.343) | 0.57 | –0.08 to 1.23 | 0.085 | 17.9 | 10.8 to 25.1 | <0.0001 |
CI, confidence interval; CKD273, urinary proteomic biomarker; IDI, integrated discrimination improvement; NRI, net reclassification improvement; UAE, urinary albumin excretion rate.
The basic reference models were stratified by study center and included sex, age, mean arterial pressure, estimated glomerular filtration rate, and prevalence of diabetes at baseline as covariables. The integrated discrimination improvement is the difference between the discrimination slopes of basic models and basic models extended with CKD273. The discrimination slope is the difference in predicted probabilities (%) between patients and control subjects. Control subjects are participants without incident chronic kidney disease. The net reclassification improvement is the sum of the percentages of subjects reclassified correctly in the groups of cases and control subjects. All estimates are provided with 95% confidence intervals.
Baseline UAE and CKD273 as Categorical Predictors
In the next step of our analysis, we evaluated established thresholds of UAE26 and CKD27311, 30 as predictors of CKD progression. In all participants (Table 5), the UAE cutoff value of 20 μg/min and the CKD273 thresholds of 0.154 and 0.343 yielded sensitivities of 30%, 33%, and 42% and specificities of 82%, 83%, and 83%, respectively. The corresponding proportions in patients with diabetes were 8%, 23%, and 13% for sensitivity and 93%, 86%, and 94% for specificity (Table 5). In all participants and in patients with diabetes, differences between the UAE threshold and both CKD273 thresholds in the proportion of correctly versus incorrectly classified individuals were significant (P < 0.0001). With specificity set at 90%, thresholds derived from the current dataset were 14 μg/min for UAE and 0.163 for CKD273. The sensitivity of these thresholds was lower for UAE than for CKD273 (15.6% vs. 20.8%; P = 0.004). Assessed per threshold, no marker improved IDI, but CKD273 (P < 0.0001), not UAE (P = 0.086), enhanced NRI.
Table 5.
Classification parameters by categories of urinary albumin excretion rate and CKD273 at baseline
| Biomarkers (threshold) | Correctly classified |
Incorrectly classified |
Classification parameters |
|||||
|---|---|---|---|---|---|---|---|---|
| Progressor | Nonprogressor | Progressor | Nonprogressor | Sensitivity (%) | Specificity (%) | Positive predictive value (%) | Negative predictive value (%) | |
| All participants | ||||||||
| UAE (20 μg/min) | 38 | 1607 | 352 | 90 | 30 | 82 | 10 | 95 |
| CKD273 (0.154) | 88 | 1522 | 302 | 175 | 33 | 83 | 23 | 90 |
| CKD273 (0.343) | 54 | 1621 | 336 | 76 | 42 | 83 | 14 | 96 |
| Patients with diabetes | ||||||||
| UAE (20 μg/min) | 30 | 1097 | 328 | 78 | 28 | 77 | 8 | 93 |
| CKD273 (0.154) | 82 | 1014 | 276 | 161 | 34 | 79 | 23 | 86 |
| CKD (0.343) | 48 | 1103 | 310 | 72 | 40 | 78 | 13 | 94 |
CKD273, urinary proteomic biomarker; UAE, urinary albumin excretion rate.
Sensitivity Analysis
In a sensitivity analysis, we applied a decline in eGFR by 30% or more over 2 years as the renal endpoint.9 There were 33 cases among 2087 participants at risk. After stratification by center and while accounting for sex, age, mean arterial pressure, and prevalence of diabetes, we constructed the 3-biomarker model as in Table 2. In this model, the odds ratios were 2.03 (95% CI, 1.36–3.03; P = 0.0005) for UAE and 1.83 (95% CI, 1.22–2.74; P = 0.0033) for CKD273. In all participants, the UAE cutoff value of 20 μg/min and the CKD273 thresholds of 0.154 and 0.343 yielded sensitivities of 45%, 46%, and 42% and specificities of 95%, 88%, and 94%, respectively.
Discussion
In keeping with research priorities endorsed by the EMA8 and the Food and Drug Administration,13 we investigated whether CKD273 can predict decline of eGFR below 60 ml/min per 1.73 m2. The key findings of the present study can be summarized as follows: (i) per 1-SD increment in the urinary markers, eGFR decreased 0.82 ml/min per 1.73 m2 more with baseline CKD273 than with baseline UAE; (ii) the risk of a first and a sustained renal endpoint increased with UAE and CKD273; (iii) when published thresholds were used,11, 26, 30 the sensitivity of both markers was low with a specificity of approximately 80%, but the proportion of correctly classified individuals was higher for CKD273 than UAE; (iv) there was agreement between the CKD273 thresholds for early CKD published before in patients with diabetes (0.154)11, 30 and derived from the current data (0.163); and (v) CKD273 continuous, but not UAE continuous, increased IDI, while both markers continuous and CKD273 also per threshold improved NRI. The IDI and NRI indices provide complementary information. Indeed, if addition of a biomarker to a model increases the predicted probability in cases, this is reflected by a significant increase in IDI, as was the case for CKD273 continuous (Table 4). NRI indicates the extent to which a biomarker improves diagnostic accuracy, which in the current analyses amounted to 20.1% and 8.22% for UAE continuous and per threshold and to 25.8%, 23.3%, and 17.9% for CKD273 continuous and per 0.154 and 0.343 threshold. Expert statisticians have suggested that IDI and NRI have limitations.34 They recommend retaining existing descriptive terms, such as the true-positive and false-positive classification rates (Table 5), and testing the null hypothesis of no prediction increment from modeled regression coefficients (Table 2 and Figure 2). In our current analyses, we implemented these different approaches.34 Whatever method was used, CKD273 provided a meaningful improvement in risk stratification.
While adjusting for covariables and baseline eGFR, our study suggests that CKD273, independent from UAE, predicts decline in glomerular filtration and progression to stage 3 CKD. The clinical relevance of our observations pertains to the clinical management of patients and the design of intervention trials for patients at risk for CKD. In the present study, we intentionally recruited participants with an eGFR of 60 ml/min per 1.73 m2 or higher and implemented eGFR <60 ml/min per 1.73 m2 as the primary endpoint. At this relatively early phase, interventions aimed at reversing or stopping the disease process before irreversible renal anatomic disruption occurs remain a potentially achievable therapeutic target.35 Timely intervention would substantially increase the quality of life of patients while reducing health care costs. For instance, type 2 diabetes currently accounts for 15% of the health care budget in Europe.36 Preventing or delaying the complications of diabetic nephropathy might reduce health care costs 6 fold.36 From the viewpoint of clinical trial design, measuring CKD273 will help in selecting patients at risk for progressive CKD for enrollment in intervention studies, thereby reducing the number of patients to be randomized and curtailing the follow-up duration required to achieve an intermediate endpoint (e.g., CKD stage 3 as recommended by the EMA).8 This approach is presently being implemented in the multicenter, double-blind, placebo-controlled PRIORITY trial (proteomic prediction and renin angiotensin aldosterone system inhibition prevention of early diabetic nephropathy in type 2 diabetic patients with normal albumin excretion).30
Renal interstitial fibrosis is a universal predictor of the decline in renal function37 and is characterized by exaggerated deposition of extracellular matrix by an expanding population of fibroblasts and myofibroblasts.38 Consistent with the pathophysiology of CKD,37, 38 the majority of peptide fragments included in CKD273 are dysregulated collagen fragments. Examination of the top 20 sequenced peptide fragments with a differential signal amplitude between progressors and nonprogressors revealed that 15 were fragments of collagen I or III, which were all downregulated in cases (Table S1). Higher levels of tissue inhibitor of matrix metalloproteinase type 1, as observed in patients with renal dysfunction,39 might inhibit the breakdown of collagen. This process might be further enhanced by increased crosslinking of collagen, inhibiting physiologic degradation of collagen by matrix metalloproteases.40 In addition, 4 fibrinogen fragments were upregulated. Fibrinogen plays a role in the pathogenesis of fibrotic disorders by acting as a profibrotic ligand for a variety of cellular surface receptors. In a mouse model of renal interstitial fibrosis induced by obstruction of the ureter,41, 42 pharmacologic or genetic depletion of fibrinogen protected the kidneys from fibrosis. In selected patients with hypertensive nephropathy, the urinary excretion of the fibrinogen α chain was 15-fold higher compared with that of healthy control subjects and was associated with a rapid decline in renal function (6.7 ml/min per 1.73 m2 per year).43 Of particular interest among the sequenced peptides (Table S1) is a fragment of the mucin-1 subunit α, an extracellular protein that is shed from renal tubular epithelium and, which as previously reported in this journal,44 is a strong predictor of renal dysfunction.
The present study must also be interpreted within the context of its limitations. First, in line with the EMA's recommendations,8 we chose as primary renal endpoint a decrease in eGFR from baseline to follow-up by at least 10 ml/min per 1.73 m2 to less than 60 ml/min per 1.73 m2. This endpoint has not yet been firmly validated in terms of adverse health outcomes. However, a sensitivity analysis based on a decrease in eGFR by 30% or more over 2 years9 confirmed the predictive value of CKD273. This relatively small decline in eGFR is a strong predictor of progression to end-stage renal disease and death.9 Second, selection of individuals with an eGFR of 60 ml/min per 1.73 m2 or higher resulted in a prevalence of albuminuria (≥20 μg/min)26 of only 6.2%. Therefore our current results cannot be extrapolated to patients with albuminuria or proteinuria. Third, the participants available for analysis from the Human Urine Proteome Database17 were enrolled in studies with different designs, including 2 randomized clinical trials,19, 20 1 population study,23 and 3 smaller subgroups of patients with diabetes recruited in Western Europe16, 21 and Australia.22 Although heterogeneity might be considered a limitation, it might also facilitate generalizability. Fourth, for 564 participants, only a single follow-up measurement of eGFR was available. However, results involving first and sustained renal endpoints were consistent (Tables 2 and 3). Finally, to compute albuminuria, we had to assume a urinary volume of 1500 ml/day in 165 participants (7.9%). However, excluding these participants did not materially alter our results.
In conclusion, CKD273 adds to the prediction of stage 3 CKD, at which intervention remains an achievable therapeutic target. Our study confirms a CKD273 threshold of 0.154 for clinical application. Nevertheless, future translation of our current results into widespread day-to-day practice will be challenging.13, 45 Urinary proteomic analysis is complex, requires expertise only available in few centers,46, 47 and for now remains costly compared with other diagnostic tests employed in the management of patients who have or are at risk for having CKD. However, a recent health-economic assessment48 indicated that the application of CKD273 in type 2 diabetic patients is cost effective. Future trials involving CKD273 should demonstrate the exact magnitude of the cost–benefit ratio supporting implementation of CKD273 in clinical practice. In addition, CKD273 is a biomarker reflecting the pathophysiology of CKD and is thereby generating insights into potentially effective pharmacologic approaches to manage this high-risk condition affecting millions of people worldwide.2, 3, 4
Disclosure
HM is cofounder and a shareholder of Mosaiques Diagnostics GmbH. CP and PZ are employees of Mosaiques Diagnostics GmbH. All the other authors declared no competing interests.
Acknowledgments
JPS, GG, RV, HM, and JJ are members of the European Uraemic Toxin Working Group. The European Union Seventh Framework Programme (FP7/2007-2013) under grant agreements 279277 (PRIORITY), 278249 (EU-MASCARA), 305507 (HOMAGE) and 608332 (iMODE-CKD) supported research on which the current results are based. The European Union (HEALTH-FP7-278249-EUMASCARA, HEALTH-F7-305507 HOMAGE and the European Research Council (Advanced Researcher Grant 2011-294713-EPLORE and Proof-of-Concept Grant 713601-uPROPHET) and the Fonds voor Wetenschappelijk Onderzoek Vlaanderen, Ministry of the Flemish Community, Brussels, Belgium (G.0881.13, G.088013, and 11Z0916N) currently support the Studies Coordinating Centre in Leuven.
The authors gratefully acknowledge the clerical staff at the Studies Coordinating Centre (Vera De Leebeeck and Renilde Wolfs) and Dr. George Jerums, University of Melbourne, Australia, for providing the data.
Footnotes
Table S1. Top 20 peptide fragments predictive of stage 3 chronic kidney disease.
Table S2. Baseline characteristics of participants enrolled in 6 studies.
Supplementary material is linked to the online version of the paper at www.kireports.org.
Supplementary Material
Top 20 peptide fragments predictive of stage 3 chronic kidney disease.
Baseline characteristics of participants enrolled in 6 studies.
References
- 1.Chapter 1: Definition and classification of CKD. Kidney Int Suppl. 2013;3:19–62. doi: 10.1038/kisup.2012.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Eckardt K.U., Coresh J., Devuyst O. Evolving importance of kidney disease: from subspecialty to global health burden. Lancet. 2013;382:158–169. doi: 10.1016/S0140-6736(13)60439-0. [DOI] [PubMed] [Google Scholar]
- 3.Wang H., Dwyer-Lindgren L., Lofgren K.T. Age-specific and sex-specific mortality in 187 countries, 1970-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2071–2094. doi: 10.1016/S0140-6736(12)61719-X. [DOI] [PubMed] [Google Scholar]
- 4.Coresh J., Selvin E., Stevens L.A. Prevalence of chronic kidney disease in the United States. JAMA. 2007;298:2038–2047. doi: 10.1001/jama.298.17.2038. [DOI] [PubMed] [Google Scholar]
- 5.Palmer S.C., Sciancalepore M., Strippoli G.F. Trial quality in nephrology: how are we measuring up? Am J Kidney Dis. 2011;58:335–337. doi: 10.1053/j.ajkd.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 6.Lewis E.J., Hunsicker L.G., Clarke W.R. Renoprotective effect of the angiotensin-receptor antagonist irbesartan in patients with nephropathy due to type 2 diabetes. N Engl J Med. 2001;345:851–860. doi: 10.1056/NEJMoa011303. [DOI] [PubMed] [Google Scholar]
- 7.SPRINT Research Group. Wright J.T., Jr., Williamson J.D. A randomized trial of intensive versus standard blood pressure control. N Engl J Med. 2015;373:2103–2116. doi: 10.1056/NEJMoa1511939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.European Medicines Agency . September 15, 2016. Guideline on the clinical investigation of medicinal products to prevent development/slow progression of chronic renal insufficiency.http://www.ema.europa.eu/docs/en_GB/document_library/Scientific_guideline/2016/10/WC500214980.pdf Available at: [Google Scholar]
- 9.Coresh J., Turin T.C., Matsushita K. Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA. 2014;311:2518–2531. doi: 10.1001/jama.2014.6634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mischak H., Delles C., Vlahou A. Proteomic biomarkers in kidney disease: issues in development and implementation. Nat Rev Nephrol. 2015;11:221–232. doi: 10.1038/nrneph.2014.247. [DOI] [PubMed] [Google Scholar]
- 11.Good D.M., Zürbig P., Argilés A. Naturally occurring human urinary peptides for use in diagnosis of chronic kidney disease. Mol Cell Proteomics. 2010;9:2424–2437. doi: 10.1074/mcp.M110.001917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klein J., Bascands J.L., Mischak H. The role of urinary peptidomics in kidney disease research. Kidney Int. 2016;89:539–545. doi: 10.1016/j.kint.2015.10.010. [DOI] [PubMed] [Google Scholar]
- 13.Nkuipou-Kenfack E., Zürbig P., Mischak H. The long path towards implementation of clinical proteomics: exemplified based on CKD273. Proteomics Clin Appl. 2017;11:5–6. doi: 10.1002/prca.201600104. [DOI] [PubMed] [Google Scholar]
- 14.Argilés A., Siwy J., Duranton F. CKD273, a new proteomics classifier assessing CKD and its prognosis. PLoS One. 2013;8:e62837. doi: 10.1371/journal.pone.0062837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schanstra J.P., Zürbig P., Alkhalaf A. Diagnosis and prediction of CKD progression by assessment of urinary peptides. J Am Soc Nephrol. 2015;26:1999–2010. doi: 10.1681/ASN.2014050423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Haller H., Ito S., Izzo J.L., Jr. Olmesartan for the delay or prevention of microalbuminuria in type 2 diabetes. N Engl J Med. 2011;364:907–917. doi: 10.1056/NEJMoa1007994. [DOI] [PubMed] [Google Scholar]
- 17.Stalmach A., Albalat A., Mullen W. Recent advances in capillary electrophoresis coupled to mass spectrometry for clinical proteomic applications. Electrophoresis. 2013;34:1452–1464. doi: 10.1002/elps.201200708. [DOI] [PubMed] [Google Scholar]
- 18.World Medical Association Declaration of Helsinki. JAMA. 2013;227:184–189. [Google Scholar]
- 19.Chaturvedi N., Porta M., Klein R. Effect of candesartan on prevention (DIRECT-Prevent 1) and progression (DIRECT-Protect 1) of retinopathy in type 1 diabetes: randomised placebo-controlled trials. Lancet. 2008;372:1394–1402. doi: 10.1016/S0140-6736(08)61412-9. [DOI] [PubMed] [Google Scholar]
- 20.Sjølie A.K., Klein R., Porta M. Effect of candesartan on progression and regression of retinopathy in type 2 diabetes (DIRECT-Protect 2): a randomised placebo-controlled trial. Lancet. 2008;372:1385–1393. doi: 10.1016/S0140-6736(08)61411-7. [DOI] [PubMed] [Google Scholar]
- 21.Pena M.J., Heinzel A., Heinze G. A panel of novel biomarkers representing different disease pathways improves prediction of renal function decline in type 2 diabetes. PLoS One. 2015;10:e0120995. doi: 10.1371/journal.pone.0120995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wood A.J., Churilov L., Perera N. Estimating glomerular filtration rate: performance of the CKD-EPI equation over time in patients with type 2 diabetes. J Diabetes Complications. 2016;30:49–54. doi: 10.1016/j.jdiacomp.2015.08.025. [DOI] [PubMed] [Google Scholar]
- 23.Gu Y.M., Thijs L., Liu Y.P. The urinary proteome as correlate and predictor of renal function in a population study. Nephrol Dial Transplant. 2014;29:2260–2268. doi: 10.1093/ndt/gfu234. [DOI] [PubMed] [Google Scholar]
- 24.Levey A.S., Stevens L.A., Schmid C.H. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Kidney Foundation KDOQI clinical practice guidelines for diabetes and CKD: 2012 update. Am J Kidney Dis. 2012;60:850–886. doi: 10.1053/j.ajkd.2012.07.005. [DOI] [PubMed] [Google Scholar]
- 26.Basi S., Mimran A., Fesler P. Microalbuminuria in type 2 diabetes and hypertension: a marker, treatment target, or innocent bystander? Diabet Care. 2008;31:S194–S201. doi: 10.2337/dc08-s249. [DOI] [PubMed] [Google Scholar]
- 27.Jantos-Siwy J., Schiffer E., Brand K. Quantitative urinary proteome analysis for biomarker evaluation in chronic kidney disease. J Proteome Res. 2009;8:268–281. doi: 10.1021/pr800401m. [DOI] [PubMed] [Google Scholar]
- 28.Mischak H., Kolch W., Aivalotis M. Comprehensive human urine standards for comparability and standardization in clinical proteome analysis. Proteomics Clin Appl. 2010;4:464–478. doi: 10.1002/prca.200900189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Theodorescu D., Wittke S., Ross M.M. Discovery and validation of new protein biomarkers for urothelial cancer: a prospective analysis. Lancet Oncol. 2006;7:230–240. doi: 10.1016/S1470-2045(06)70584-8. [DOI] [PubMed] [Google Scholar]
- 30.Lindhardt M., Persson F., Currie G. Proteomic prediction and Renin angiotensin aldosterone system Inhibition prevention Of early diabetic nephRopathy in TYpe 2 diabetic patients with normoalbuminuria (PRIORITY): essential study design and rationale of a randomised clinical multicentre trial. BN+MJ Open. 2016;2016:e010310. doi: 10.1136/bmjopen-2015-010310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Blom G. Statistical estimates and transformed beta-variables. Biom J. 1961;3:285. [Google Scholar]
- 32.Pencina M.J., D'Agostino R.B., Sr, D'Agostino R.B., Jr. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–172. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 33.Shin S. ROC analysis for the evaluation of continuous biomarkers: existing tools and new features in SAS® 9.2 (paper 210-31). 2009;1-15. Available at: http://www.lexjansen.com/pharmasug/2009/sp/SP09.pdf.
- 34.Kerr K.F., Wang Z., Janes H. Net reclassification indices for evaluating risk-prediction instruments: a critical review. Epidemiology. 2014;25:114–121. doi: 10.1097/EDE.0000000000000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Schievink B., Kröpelin T., Mulder S. Early renin-angiotensin system intervention is more beneficial than late intervention in delaying end-stage renal disease in patients with type 2 diabetes. Diabetes Obes Metab. 2016;18:64–71. doi: 10.1111/dom.12583. [DOI] [PubMed] [Google Scholar]
- 36.Li R., Bilik D., Brown M.B. Medical costs associated with type 2 diabetes complications and comorbidities. Am J Manag Care. 2013;19:421–430. [PMC free article] [PubMed] [Google Scholar]
- 37.Barnes J.L., Glass W.F., 2nd Renal interstitial fibrosis: a critical evaluation of the origin of myofibroblasts. Contrib Nephrol. 2011;169:73–93. doi: 10.1159/000313946. [DOI] [PubMed] [Google Scholar]
- 38.Meran S., Steadman R. Fibroblasts and myofibroblasts in renal fibrosis. Int J Exp Pathol. 2011;92:158–167. doi: 10.1111/j.1365-2613.2011.00764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Hörstrup J.H., Gehrmann M., Schneider B. Elevation of serum and urine levels of TIMP-1 and tenascin in patients with renal disease. Nephrol Dial Transplant. 2002;17:1005–1013. doi: 10.1093/ndt/17.6.1005. [DOI] [PubMed] [Google Scholar]
- 40.Fisher M., Jones R.A., Huang L. Modulation of tissue transglutaminase in tubular epithelial cells alters extracellular matrix levels: a potential mechanism of tissue scarring. Matrix Biol. 2009;28:20–31. doi: 10.1016/j.matbio.2008.10.003. [DOI] [PubMed] [Google Scholar]
- 41.Sörensen I., Susnik N., Inhester T. Fibrinogen, acting as a mitogen for tubulointerstitial fibroblasts, promotes renal fibrosis. Kidney Int. 2011;80:1035–1044. doi: 10.1038/ki.2011.214. [DOI] [PubMed] [Google Scholar]
- 42.Craciun F.L., Ajay A.K., Hoffmann D. Pharmacological and genetic depletion of fibrinogen protects from kidney fibrosis. Am J Physiol Renal Physiol. 2014;307:F471–F484. doi: 10.1152/ajprenal.00189.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Øvrehus M.A., Zürbig P., Vikse B.E. Urinary proteomics in chronic kidney disease: diagnosis and risk of progression beyond albuminuria. Clin Proteomics. 2015;12:21. doi: 10.1186/s12014-015-9092-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zhang ZY, Ravassa S, Pejchinovski M, et al. A urinary fragment of mucin-1 subunit a is a novel biomarker associated with renal dysfunction in the general population [e-pub ahead of print]. Kidney Int Rep. http://dx.doi.org/10.1016/j.ekir.2017.03.012. Accessed July 7, 2017. [DOI] [PMC free article] [PubMed]
- 45.Mischak H., Ioannidis J.P., Argiles A. Implementation of proteomic biomarkers: making it work. Eur J Clin Invest. 2012;42:1027–1036. doi: 10.1111/j.1365-2362.2012.02674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mischak H., Vlahou A., Ioannidis J.P. Technical aspects and inter-laboratory variability in native peptide profiling: the CE-MS experience. Clin Biochem. 2013;46:432–443. doi: 10.1016/j.clinbiochem.2012.09.025. [DOI] [PubMed] [Google Scholar]
- 47.Klein J., Lacroix C., Caubet C. Fetal urinary peptides to predict postnatal outcome of renal disease in fetuses with posterior urethral valves (PUV) Sci Transl Med. 2013;5:198ra106. doi: 10.1126/scitranslmed.3005807. [DOI] [PubMed] [Google Scholar]
- 48.Critselis E., Vlahou A., Stel V.S. Cost-effectiveness of screening type 2 diabetes patients for chronic kidney disease progression with the CKD273 urinary peptide classifier as compared to urinary albumin excretion. Nephrol Dial Transplant. 2017:1–9. doi: 10.1093/ndt/gfx068. Accessed July 7, 2017. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Top 20 peptide fragments predictive of stage 3 chronic kidney disease.
Baseline characteristics of participants enrolled in 6 studies.